Abstract
An aqueous solution of Pectinex (containing cellulase, hemicellulase, and pectinase) at 1%, 2.5%, 5%, 7%, and 10% concentrations and 40°C was used to extract anthocyanins (Acys) of saffron tepals at 20, 40, 60, 120 and 180 min reaction times and compared with ethanol solvent under similar conditions. The Acys of the Pectinex solution reached 6.7 mg/g of tepal powder (~40% more than the ethanol method) when the enzyme concentrations and extraction times were, respectively, 5% and 60 min. The Acys of aqueous enzymes had three times slower degradation rates and 50% more attractive chroma color than the ones recovered by ethanol solution after 3 h of extraction time. Additionally, the Acys of the ethanol solution lost its content sharply (>45%) and its chroma changed quickly (due to the browning and polymerization). High performance liquid chromatography (HPLC) analysis showed that Acys extracted with mixed enzymes had about 80% more cyanidin 3-glucosides and 20% less pelargonidin 3,5-glucosides than with the ethanol method. Most probably, the high content of cyanidin 3-glycosides in enzyme-extracted Acys of saffron tepals was the key factor for its high stability.
Keywords: anthocyanins, color properties, degradation index, Pectinex enzymes, saffron tepals
1. Introduction
The flowers of saffron (Crocus sativus L.) contains anthocyanins (Acys); it has a mixture of natural colorants and is used as a food additive. The delicate combination of color, flavor, and aroma in this spice is due to the existence of crocin, picrocrocin, and safranal compounds, respectively [1]. Spectrophotometric and high performance liquid chromatography (HPLC) methods were used to identify three flavonol aglycones including myricetin, quercetin, and kaempferol in hydrolyzed tepals of Saffron [2]. The color properties and stability of these pigments depends on several factors, including their structure, concentration, pH, temperature, light intensity, attached copigments, metallic ions, enzymes, oxygen, ascorbic acid, sugars, degradation products, and sulfur dioxide in the solution [3,4]. Saffron is cultivated for its red stigmatic lobes and blooms only once/y. After harvesting flowers, usually the stigmas are removed and their tepals discarded or used as feed or for composting. The tepals of saffron are defined as the free segment of saffron flower (perianth) that is not clearly differentiated into calyx (sepals) and corolla (petals). While the delicate colors (mainly gold, red, and orange), flavor, and aroma of saffron stigma are due to, respectively, crocin, picrocrocin, and safranal compounds [1,5], the tepals of saffron have an attractive color and are the source of Acys and flavanols [6–8]. Various phytochemical studies confirmed that the rich colors and flavonoids of saffron tepals have protective carotenoid activity against cancer [9–12]. Additionally, they have antidepressant effects [13], and an extract mixed with stigmas is effective in the cure of mild to moderate depression [5,14].
Different enzymes (alone or in combination) have been employed to enhance and accelerate pigment extraction of various plant materials. While pectin enzymes have been used to cut down maceration, settling, and filtration time of red grape tissue, they were able to extract and release its red pigments quicker and with higher efficiency than conventional ethanol extraction [15]. Some researchers could separate the polyphenols from the needles of two plant pigments (Pinus taiwanensis and Pinus morrisonicola) successfully when they used mixed enzymes of cellulase, hemicellulase, pectinase, and protease, and also hot pressurized water for extraction [16].
The main goal of this project was to select the suitable food-grade enzyme between Pectinex Ultra SP-L and Cellubrix for aqueous recovery of saffron tepals Acys, study its quantity (yield) and quality criteria (mainly color properties and stability), and compare its properties with the ones extracted by the conventional ethanol method.
2. Materials and methods
2.1. Samples preparation
After detaching stigmas (real saffron) from saffron flowers harvested from a farm located in the Northeast of Iran, the remaining parts (mainly tepals) were carefully sorted, collected, and transferred quickly to a cold storage at <5°C and frozen in a kitchen freezer at −20°C. Later, the frozen tepals were defrosted at room temperature and dried in a thermostatic vacuum oven (Townson & Mercer, Manchester, England) at 40°C for 36 hours to <3% moisture. Dried tepals were crushed in a coffee grinder (Moulinex International Corp., A member of SEB Groupe, Ecully Cedex, France) and passed through a 40 mesh sieve. The resulting fine powder was placed in a brown vial and stored at −20°C. The Pectinex Ultra SP-L was produced from the strain of Aspergillus aculeatus and had several enzymes including pectinase, cellulase, hemicellulase, pectinesterase, protease, fructosyltransferase, and β-galactosidase [17,18]. Cellubrix was a liquid form of two mixed enzymes of cellulase and cellobiase produced by separate fermentation of a Trichoderma and Aspergillus strains [19]. Both Pectinex Ultra SP-L and Cellubrix were supplied by Novozymes (Bagsverd, Denmark). All chemicals and reagents used in this study were analytical grade and obtained from Merck KGaA (a pharmaceutical and chemical company, Darmstadt, Germany).
2.2. Mixing ratios of dried saffron tepals with water
To determine the best ratios of mixing tepals with water, 1 g of tepal powder was homogenized with 10, 15, and 25 mL water to make tepal solutions with different soluble and insoluble concentrations of 10, 6.7 and 4%, respectively. The highest concentration was kept at 10% to obtain absorbance units (measured in 10 mL of cuvette with 1 cm depth) in a range of 0.1–2.0 and the absorbance versus soluble concentration of tepals does not deviate from the linear trend (Lambert-Beers’ Law). All the water and ethanol extracts were stirred in a dark room around 30 g force for 1 h and a Universal 320 bench-top centrifuge (A. Daigger & Company, Vernon Hills, IL, USA) was used at around 3000 × g force for 15 min to separate the total insoluble solids. Then pure water was used to adjust the brix of each clear sample at 1, 2, 3, 4 and 6% and their corresponding optical densities were read after 15 min resting time.
2.3. Acys extraction and measurement
Extraction of Acys available in saffron tepals was done with separate acidified ethanol (AE) and enzymatic water (EW). In the AE method 1 g of the sample was mixed with 10 mL solution of 1.5N hydrochloric acid in ethanol (15:85 v/v) and held in a thermostatic chamber at 40°C for various duration times of 5, 10, 20, 60, 120 and 180 min. After ethanol washing of solid sediment, the resulting solution of each sample was centrifuged and then its ethanol portion (containing Acys) was evaporated at negative pressure of 0.1 MPa at 40°C. The stock of alcohol-free thick solution was kept in a refrigerator at 0–5°C for the Acys determination. In the EW method, again 1g powder of saffron tepals was mixed with 10 mL of prepared buffer solution. The buffer solution was prepared by adding 21.01 g of citric acid to 200 mL NaOH (1N). Then 20 mL of resulting solution was mixed with 80 mL of HCl (0.1N) to make an aqueous solution with pH of 3.5. Then Cellubrix and Pectinex enzymes were added separately to the buffer solution at different concentrations of 1, 2.5, 5, 7, 10 and 12.5% and held for 2 h reaction time at an ambient temperature of 40°C. Although the optimum temperature for extraction of phenolic compounds from plant materials is around 50°C [20], the temperature was adjusted at 40°C in our experiment to prevent its degradation during the recovery process. The Acys yields extracted by Pectinex Ultra SP-L and Cellubrix samples were measured at different enzyme concentrations. Then, the highest content of Acys yield between two groups of enzymes and among different concentrations was chosen to find out the most suitable extraction time. In this stage, the Acys experiment was repeated with different extraction times of 20, 40, 60, 120 and 180 min. After centrifugation of each sample, its supernatant was collected, and after washing the resulting sediment with enough AE, its Acys content was determined in three replicates and quantified in cyanidin 3-glucoside by using the following formulae and its updated version for Acys measurement [21,22].
where Aλmax is the maximum absorbance at wavelength of 520, A700 is the absorbance of at wave length of 700 to eliminate the turbidity effects of foreign materials, A is the optical density of Acys, DF is dilution factor and L is the cuvette thickness (1 cm) of each sample. MV and ɛ are, respectively, the molecular weight (449.2) and molar absorbance (26,900) of cyanidin 3-glycoside.
2.4. Monomeric, polymeric, and degradation index of Acys
A bisulfate bleaching method [23] used to determine polymeric and monomeric colors of extracted Acys. In this method, 0.2 mL of 20% K2O5S2 and 0.2 mL of distilled water were added to two different 2.8 mL samples of extracted Acys separately to make bleached and unbleached samples. Then, a DR/4000 U Spectrophotometer (Hach Company, Loveland, CO, USA) was used to read and record the absorbance of bleached and unbleached samples of Acys at the λmax, 420 nm and at 700 nm (for correction of turbidity). Polymeric and monomeric Acys of saffron tepals were figured out by using following equations [24]:
Usually, the ratio of absorbance readings of Acys at 420 nm and 520 nm (A420nm/A520nm) is used to evaluate its degradation index (DI) or stability [25]. However, this formula is very similar with the following equation of DI or the ratio of total optical density (T.O.D.) of each Acys sample at pH of 1 to the absorbency difference (ΔD.O) between pH 4.5 and pH 1 [26].
The spectrophotometer was also used to measure color characteristics of different extracted Acys solutions. Each sample was placed in a 1 cm path length of optical glass cell and in a total transmission mode (using illuminant C and 2° observer angle) and its L, a, and b values were read and noted in duplicates. The formula of C = (a + b)1/2 was applied to determine the related chromas.
2.5. Quantification of different anthocyanidins in Saffron’s tepals
A Knauer HPLC system (Berlin, Germany) equipped with a Triathlon autosampler, a K-1001 pump, and a UV–visible detector (K-2600) was used to recognize and measure different anthocyanidins in saffron tepals by chromatographic analysis [27]. Each Acys sample (extracted by either enzymes or ethanol) centrifuged in an Eppendorf tube (4 minutes ~2800 g force) and its supernatant was passed through a 0.45μM pol-ytetrafluoroethylene (PTFE) filter (prepared from Chromafil CA-45/25 S, Duren, Germany) before 50 μL injection. To separate the components of each sample, an RP C18 Nucleosil 100 (Machery-Nagel GmbH & Co. KG, Düren, Germany) (125 and 5.0 mm, respectively internal diameter and length of column containing 5.0 μm particles) column was used. The mobile phase of the HPLC system had two solvents of A (2.5% v/v, solution of acetic acid in water) and B (2.5% v/v, solution of acetic acid in methanol) at different ratios. The gradient profiles of solvent A were 100, 90, 50 and 100% at 5, 15, 45 and 55 min, respectively. Furthermore, the flow rate was 1.0 mL/min, and chromatograms recorded at 510nM. In order to record and process the resulting data, software of Chrome Gate (version 3.1, build 3.1.0.2384; Knauer, Berlin, Germany) was used. Five different standard solutions of anthocyanidins including cyanidin 3,5-diglucoside (Cy 3,5), pelargonidin 3,5-diglucoside (Pg 3,5), delphinidin 3-glucoside (Dp3), pelargonidin 3-glucoside (Pg 3), and petunidin with different concentrations (0.01–0.04 mg/100g) were injected and their related curves obtained. The peak area of each anthocyanidin was compared with its standard curve, and the concentration in the sample was quantified.
2.6. Statistical analysis
Minitab Statistical Software (Minitab Inc., State College, PA, USA) [furnished with one-way analysis of variance (ANOVA) or ANOVA] was used to evaluate the effects of enzyme concentration and extraction time on total Acys of saffron tepals extracted by aqueous solution of Pectinex (mixed) enzymes and AE. The means of three replicates (each had 2 measurements of the same solution) were reported in this study. A statistical package of Student test and ANOVA program available in Microsoft Office Software (Microsoft Building, Redmond, WA, USA) was used to assess significance levels at probability of 0.05.
3. Results and discussion
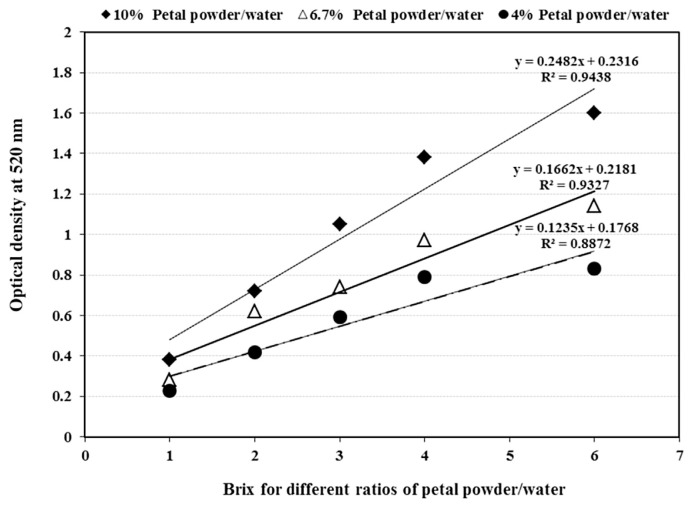
When the concentration (soluble and insoluble) of saffron tepals changed from 4% to 10% (at pH = 1), the slope of linear relationship between pigment concentration and its optical density increased considerably and reached its highest amount (Fig. 1). The absorbency of saffron tepal solution did not increase with the same trend linearly once the tepals to water ratio (w/v) was exceeded from the 10%, and therefore this concentration was used for measuring the Acys yield and its qualitative criteria. Giusti and Wrolstad [23] found a deviation from linearity when they used higher aqueous concentrations of other plant materials for Acys extraction.
Fig. 1.
The effects of soluble pigments concentration on optical density of aqueous solution extracted from saffron tepals at different original ratios of powder/water (w/w).
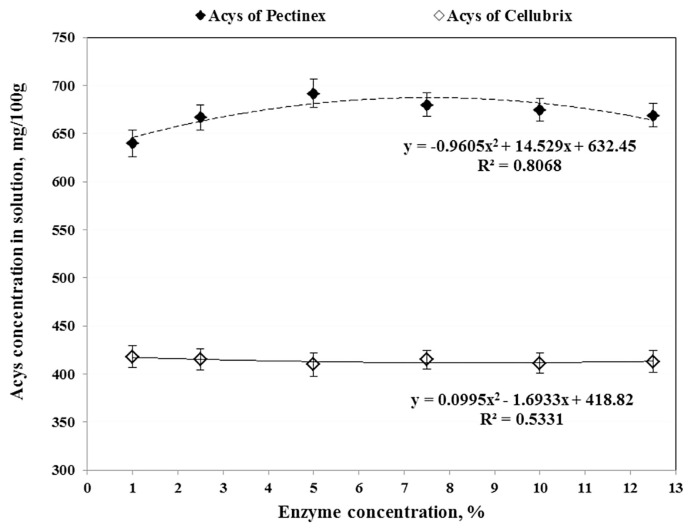
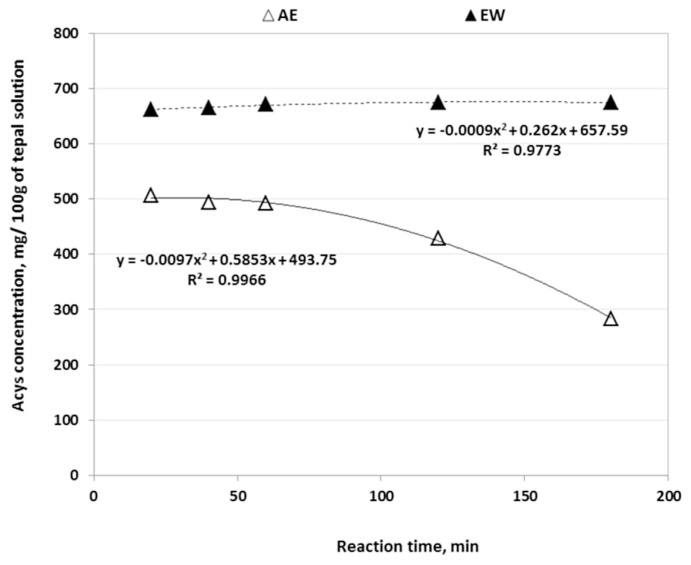
Due to the fact that the Pectinex had a combination of different enzymes, it was able to extract Acys of saffron tepals with about 50% more recovery than the Cellubrix after 2 hours at 40°C (Fig. 2). Because the increase of Pectinex Ultra SP-L in water did not improve Acys recovery, the concentration of EW solution was adjusted at 5% (v/v). The higher enzyme concentration could destruct the pigment cells due to the release of proteins from plant materials [20,28,29]. There was a considerable and significant difference in trends of Acys content of tepals extracted by Pectinex enzymes at 5% concentration and the AE solution (Fig. 3, Tables 1 and 2). While the Acys content of EW solution increased very quickly after 20 min and reached 675mg/100 g of solution after 3 h of extraction, the Acys extracted by AE solvent decreased sharply (>60%) at parallel conditions. Since fresh tepals had about 20% dried matter and the concentrations of tepal powder in both aqueous enzyme and ethanol solutions were 10%, the Acys contents in EW and AE methods after 180 min extraction were, respectively, about 13.3 mg/100 g and ~6 mg/100 g of fresh tepals. Munoz et al [15] obtained analogous results when they compared the Acys content of red grape skin extracted by aqueous solution of Pectinex and ethanol solvent. Landbo and Meyer [29] and Maier et al [28] reported that the combination of pectinase with other enzymes (similar to Pectinex) significantly enhanced the extraction and release of Acys and other phenolic compounds from black currant juice and grape pomace, respectively. They believe that the pectinase and cellulase have synergistic activities on cell wall degradation and they can render and break down the intracellular of plant materials and make the inside pigments more accessible for extraction.
Fig. 2.
The effects of enzyme concentration on the anthocyanins (Acys) content of saffron tepals extracted by aqueous solutions of Pectinex and Cellubrix at 40°C.
Fig. 3.
Effects of reaction time on anthocyanins (Acys) content of saffron tepals extracted by the acidified ethanol (AE) and enzymatic water (EW).
Table 1.
Effect of extraction time on instrumental color properties of saffron tepals extracted with acidified ethanol (AE) and enzymatic water (EW).a
| Time (min) | Total Acys (mg/100g) | Chroma = (a2+b2)0.5 | Lightness | Hue | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| AE | EW | AE | EW | AE | EW | AE | EW | |
| 20 | 507.5a | 662.8a | 39.2 ± 0.4a | 64.40 ± 0.5a | 12.8 ± 0.7a | 16.81 ± 0.6a | 38.4 ± 0.8a | 39.46 ± 0.7a |
| 40 | 494.3a | 665.4a | 39.8 ± 0.8b | 65.43 ± 0.4a | 12.7 ± 0.7a | 16.75 ± 0.5a | 37.4 ± 0.6a | 39.47 ± 0.6a |
| 60 | 492.2a | 671.1a | 40.4 ± 1.1c | 65.45 ± 0.2b | 16.2 ± 0.9b | 16.74 ± 0.1b | 40.3 ± 1.3b | 39.46 ± 0.8a |
| 120 | 429.1b | 675.4a | 41.3 ± 1.2c | 65.47 ± 0.2b | 19.6 ± 1.1c | 16.73 ± 0.2b | 44.5 ± 1.6c | 39.46 ± 0.9a |
| 180 | 283.1c | 674.9a | 41.1 ± 1.1c | 64.46 ± 0.1b | 22.6 ± 1.2c | 16.74 ± 0.2b | 48.2 ± 1.9d | 39.47 ± 0.7a |
The numbers in this table are the average of three replicates and values with different superscripts in each column are significantly (α = 0.05) different.
Acys = anthocyanins.
Table 2.
The effects of 3 hour extraction time on concentration and color properties of anthocyanins (Acys) extracted by separate acidified ethanol (AE) and enzymatic water (EW) using F test statistical analysis.
| Sources | DF dominator | F value | F from table | Probability |
|---|---|---|---|---|
| Acys mg/100g | 8 | 27.99a | 25.41 | p = 0.0009 < 0.001 |
| Degradation index | 8 | 24.94a | 14.96 | p = 0.003 < 0.005 |
| Chroma | 8 | 4065a | 25.41 | p = 6.2 × 10−6 < 0.001 |
The Acys of saffron tepals recovered by EW had significantly higher yield, chroma, and lower degradation than the similar Acys extracted by AE solution.
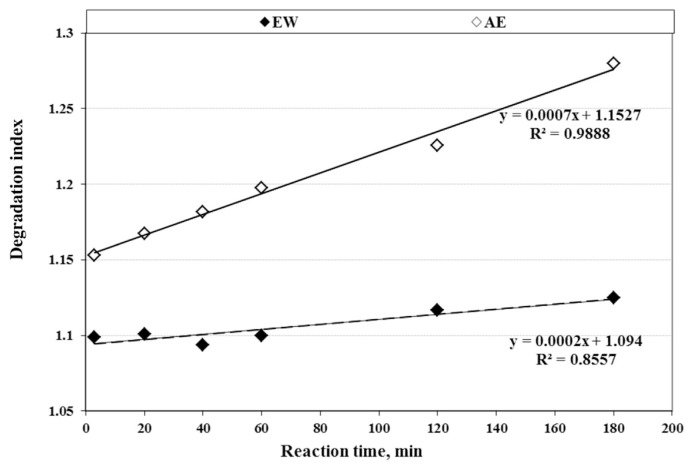
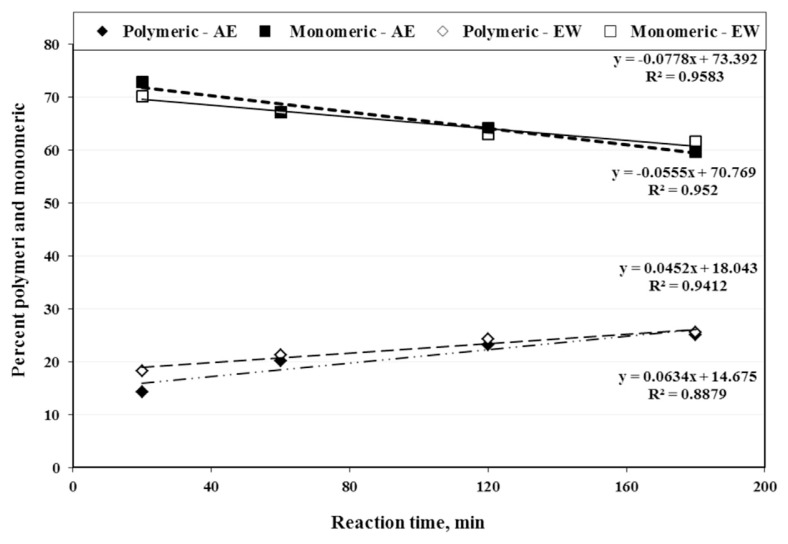
The DI of tepals Acys in EW had a slightly linear trend with <3% increase (with R2 = 0.86) after 3 h reaction time at 40°C (Fig. 4). However, the DI of Acys in AE solution increased significantly (Table 2) and went beyond 10% (with R2 = 0.99). Additionally, the Acys extracted by EW had three times slower degradation rate than the ones extracted by AE solution at comparable conditions. The changes of monomeric and polymeric Acys extracted by EW solution were similar (~7%) and were less than the ones recovered by AE solution (~12%) at a similar 3 h extraction time (Fig. 5).
Fig. 4.
Effects of reaction time on degradation index of anthocyanins (Acys) extracted by the acidified ethanol (AE) and enzymatic water (EW) solutions containing saffron tepals.
Fig. 5.
The effects of reaction time on monomeric and polymeric anthocyanins (Acys) extracted by the acidified ethanol (AE) and enzymatic water (EW) solutions containing saffron tepals.
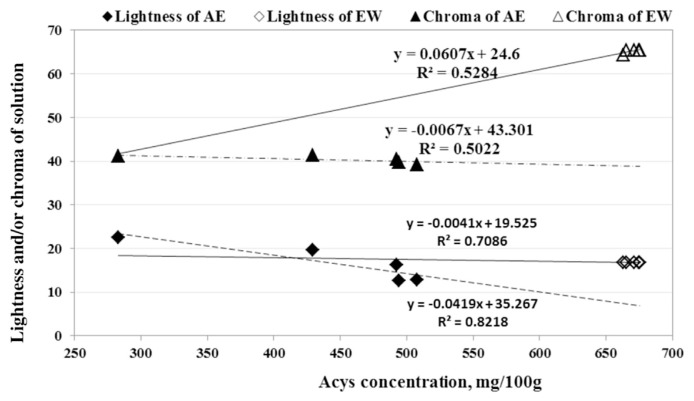
The color parameters of saffron tepal solutions [including lightness (L*), chroma (C*), and hue] were measured according to the method prescribed by [30,31]. When the Acys concentration of the sample solutions increased artificially from 300 to around 700 mg/100g solution, the chroma values of AE was remained constant (~40 unit), however the chroma of EW solution was significantly higher (Table 2) and reached >65 unit (Fig. 6). By contrast, the lightness of the EW solution was stable but the lightness of the AE solution decreased considerably in similar conditions. Additionally, the lightness reduction rate and chroma increasing rate of Acys recovered by EW solution were, respectively, 10 times slower and 10 times faster than AE solution at similar conditions (Fig. 6). Tsai and Huang [25] used the aqueous solution (without enzymes) to extract Acys from Roselle sepals (a kind of Hibiscus) and found that the degradation of Acys was increased along with a cumulative brown color (polymeric Acys), loss of attractive red color (monomeric Acys), and reduced antioxidant power. Byamukama et al. [32] also found a stronger browning reaction for Acys pigment of Hippeastrum when it was extracted by AE instead of EW solution.
Fig. 6.
The concentration effects of anthocyanins (Acys) on its lightness and chroma extracted by the acidified ethanol (AE) and enzymatic water (EW) solutions containing saffron tepals.
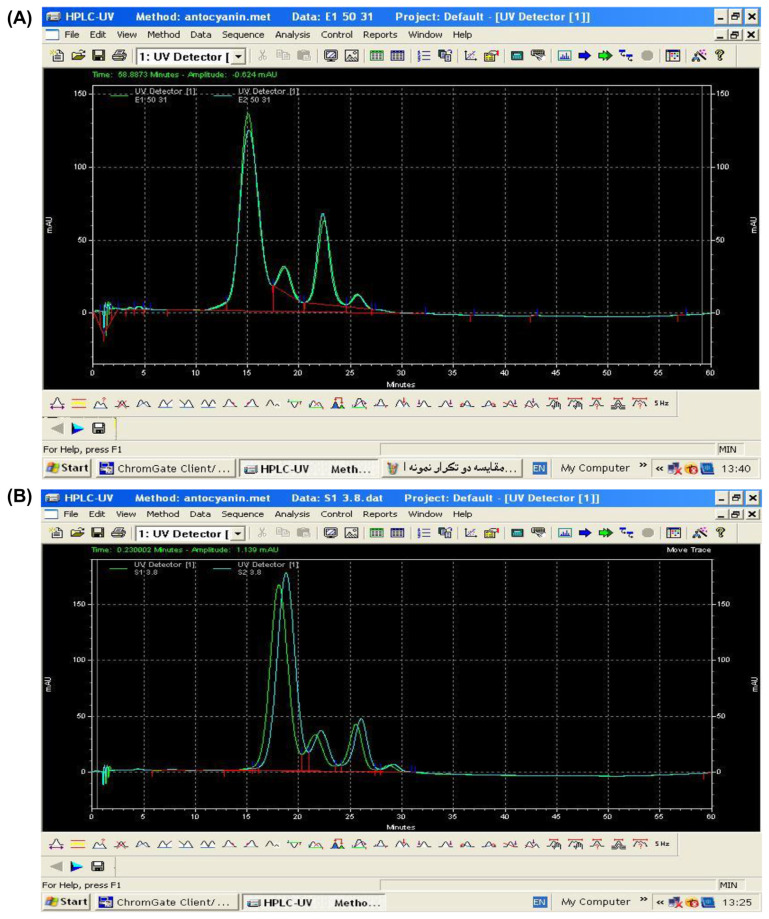
Byamukama et al [32] showed that the Acys quality extracted from flowers of Hippeastrum cultivars had a good correlation with its color values. Because a higher concentration of Acys is proportional to higher values of lightness and chroma, the Acys with high L* and C* values are more desirable. Table 3 and chromatograms of anthocyanidins (Fig. 7) show the Acys compositions of saffron tepals extracted with EW and AE solutions. Both solvents had Cyanidin (Cy) 3,5; Pelargonidin (Pg) 3,5; Delphinidin (Dp) 3; Pelargonidin (Pg) 3 and petunidin.
Table 3.
Anthocyanidins (%) in anthocyanins (Acys) of saffron tepals extracted by acidified ethanol (AE) and enzymatic water (EW) methodsa (n = 2).
| Extracting solvents | Pelargonidin 3-glycosides | Pelargonidin 3,5-glycosides | Petunidin (Pu) | 3,5 Cyanidin-diglycosides | Delphinidin (Del) 3-glycosides | Ratio of (Pu)/(Del) |
|---|---|---|---|---|---|---|
| AE | 3.4 ± 0.3a | 56.1 ± 2.9a | 15.5 ± 0.8a | 20.9 ± 1.1a | 4.1 ± 0.6a | 3.78 |
| EW | 3.6 ± 0.2a | 43.5 ± 1.6b | 14.4 ± 0.9a | 34.8 ± 2.2b | 3.7 ± 1.7b | 3.89 |
The numbers in this table are the average of two replicates and values with different superscripts in each column are significantly (α = 0.05) different.
Fig. 7.
Chromatograms of different anthocyanidins (from left to right pelargonidin 3-glycoside, pelargonidin 3,5-glycosides, petunidin, 3,5 cyanidin-diglycosides, and delphinidin 3-glycosides) in two solutions of anthocyanins (Acys) extracted by the (enzymatic water) EW (A) and (acidified ethanol) AE (B) containing saffron tepals.
Although few researchers [2,33] found only two anthocyanidins (delphinidin and petunidin) in the Acys of saffron tepals, our anthocyanidins results were comparable with them. Nørbæk et al [34] confirmed that petunidin and delphinidin are the two important anthocyanidins in saffron tepals which have considerable effects on pigment characteristics of perianth (the green outer plus fused petals) segment in Crocus sativus. While the Acys extracted by EW solution had about 80% more Cy 3,5 than the ones recovered by AE solution, it had about 20% less Pg 3,5 than AE solution. Most probably, this difference was due to a fact that EW solution had more stable Acys than AE solution. Since pelargonidin (one of the important anthocyanidins in saffron tepals) is converted to kaempferol through its hydrolysis or during pigment extraction [33], it can be concluded that most Pg 3,5 in EW solution was transformed to other compounds during aqueous enzyme Acys extraction. Since Cy 3,5 make color pigment more stable, the color strength of anthocyanin (at high acidic values) becomes higher, when cyanidin-3-O-glucoside is available in pigment of plant tissue [35]. Our results showed that the ratio of petunidin to delphinidin for Acys recovered from mixed colors (pink and violet) of saffron tepals and by AE and EW solutions were about 3.78:1 and 3.89:1, respectively. Early studies of saffron flower showed that the violet section of its tepals had a mixture of two Acys, including delphinidin diglucoside and petunidin glycoside, in a ratio of approximately 4:1 [36].
4. Conclusion
The mixed enzymes of Pectinex Ultra SP-L with 5% concentration in aqueous solution were able to extract Acys of saffron tepals with significantly higher efficiency than the ones extracted by ethanol solution. Furthermore, the enzyme-extracted Acys of saffron tepals was resistant against browning and decomposition processes and had much upper color values (more lightness and more chroma) and higher stability (less degradation and less polymerization) than the ones extracted with AE solution. Additionally, the enzyme-extracted Acys had a higher amount of Cy 3,5 (as a stable anthocyanidin) in comparison with Acys recovered by ethanol solution. Overall, the enzyme method utilizes saffron tepals (as a low-cost raw material) to produce a completely natural and healthy Acys with a valuable source of antioxidants and attractive natural color.
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1. Rios JL, Recio MC, Giner RM, et al. An update review of saffron and its active constituents. Phytother Res. 1996;10:189–93. [Google Scholar]
- 2. Garrido JL, Diez De Bethencourt C. Estudio des flavonoides contenidos en extractors hidrolizados de tepalos de Crocus Sativus L. Anal Bromatol. 1987;39:69–80. [in Spanish, English abstract] [Google Scholar]
- 3. Prior RL, Cao G. Antioxidant phytochemicals in fruits and vegetables: diet and health implications. HortScience. 2000;35:588–92. [Google Scholar]
- 4. Francis FJ. Food colorants: anthocyanins. Crit Rev Food Sci Nutr. 1989;28:273–314. doi: 10.1080/10408398909527503. [DOI] [PubMed] [Google Scholar]
- 5. Abdullaev FI. Cancer chemopreventive and tumoricidal properties of saffron (Crocus sativus L.) Exp Biol Med. 2000;227:20–5. doi: 10.1177/153537020222700104. [DOI] [PubMed] [Google Scholar]
- 6.Hosseini DK, Shariatmadar S. Iranian Institute of Science and Technology Reports. Khorasan Center. Tehran, Iran: Eslami Press; 1994. Identification of anthocyanins of Crocus sativus L. petals. [Google Scholar]
- 7. Tirillini B, Pagiotti R, Menghini L, et al. The volatile organic compounds from tepals and anthers of saffron flowers (Crocus sativus L.) J Essent Oil Res. 2006;18:298–300. [Google Scholar]
- 8. Hemati-Kakhki A. Stability of anthocyanin extracted from saffron (Crocus sativus L.) petals in a model beverage. Acta Hort. 2010;850:247–50. [Google Scholar]
- 9. Gainer JL, Walls DA, Jones JR. The effects of crocetin on skin papillomas and Rous sarcoma. Oncology. 1976;33:222–4. doi: 10.1159/000225150. [DOI] [PubMed] [Google Scholar]
- 10. Bendich A, Olson JA. Biological actions of carotenoids. FASEB J. 1989;3:1927–32. [PubMed] [Google Scholar]
- 11. Moon RC. Comparative aspects of carotenoids and retinoids as chemopreventive agents for cancer. J Nutr. 1989;119:127–34. doi: 10.1093/jn/119.1.127. [DOI] [PubMed] [Google Scholar]
- 12. Ziegler RG. A review of epidemiological evidence that carotenoids reduce the[13] risk of cancer. J Nutr. 1989;119:116–22. doi: 10.1093/jn/119.1.116. [DOI] [PubMed] [Google Scholar]
- 13. Karimi G, Hosseinzadeh H, Khaleghpanah P. Study of antidepressant effect of aqueous and ethanolic of Crocus sativus L. in mice. Iran J Basic Med Sci. 2001;4:11–5. [Google Scholar]
- 14. Noorbala AA, Akhondzadeh S, Tahmacebi-Pour N, et al. Hydro-alcoholic extract of Crocus sativus L. versus fluoxetine in the treatment of mild to moderate depression: a double-blind, randomized pilot trial. J Ethnopharm. 2005;97:281–4. doi: 10.1016/j.jep.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 15. Munoz O, Sepulveda M, Schwartz M. Effects of enzymatic treatment on anthocyanic pigments from grapes skin from Chilean wine. J Food Chem. 2004;87:487–90. [Google Scholar]
- 16. Lin S, Chang CJ, Deng T. Enzymatic hot pressurized fluids extraction of polyphenolics from Pinus taiwanensis and Pinus morrisonicola. J Taiw Ins Chem Eng. 2009;40:136–42. [Google Scholar]
- 17. Mutlu M, Sarioglu K, Demir N, et al. The use of commercial pectinase in fruit juice industry. Part I: viscosimetric determination of enzyme activity. J Food Eng. 1999;41:147–50. [Google Scholar]
- 18. Tanriseven A, Aslan Y. Immobilization of Pectinex Ultra SP-L to produce fructooligosaccharides. Enz Micro Tech. 2005;36:550–4. [Google Scholar]
- 19. Hanmoungjai P, Pyle DL, Niranjan K. Enzyme-assisted water-extraction of oil and protein from rice bran. J Chem Tech Biotech. 2002;77:771–6. [Google Scholar]
- 20. Li BB, Smith B, Hossain MdM. Extraction of phenolics from citrus peels: enzyme-assisted extraction method. Separ Purif Tech. 2006;48:189–96. [Google Scholar]
- 21. Fuleki T, Francis FJ. Quantitative methods for anthocyanins I. Extraction and determination of total anthocyanins in cranberry. J Food Sci. 1968;33:72–6. [Google Scholar]
- 22. Abdel-Aal ESM, Hucl P. A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chem. 1999;76:350–4. [Google Scholar]
- 23. Giusti MM, Wrolstad RE. Radish anthocyanin extract as a natural red colorant for maraschino cherries. J Food Sci. 1996;61:688–94. [Google Scholar]
- 24. Kalbasi A, Cisneros-Zevallos L. Fractionation of monomeric and polymeric anthocyanins from Concord grape (Vitis Labrusca L.) juice by membrane ultrafiltration. J Agric Food Chem. 2007;55:7036–42. doi: 10.1021/jf0706068. [DOI] [PubMed] [Google Scholar]
- 25. Tsai P, Huang H. Effect of polymerization on the antioxidant capacity of anthocyanins in Roselle. Food Res Intern. 2004;37:313–8. [Google Scholar]
- 26. Fuleki T, Francis FJ. Quantitative methods for anthocyanins II. Determination of total anthocyanins and degradation index for cranberry. J Food Sci. 1968;33:78–83. [Google Scholar]
- 27. Gil MI, Tomas-Barberan FA, Hess-Pierce B, et al. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J Agri Food Chem. 2000;48:4581–9. doi: 10.1021/jf000404a. [DOI] [PubMed] [Google Scholar]
- 28. Maier T, Goppert A, Kammerer R, et al. Optimization of a process for enzyme-assisted pigment extraction from grape (Vitis vinifera L.) pomace. Eur Food Res Tech. 2008;227:267–75. [Google Scholar]
- 29. Landbo K, Meyer SE. Enzyme assisted extraction of antioxidative phenols from black current juice press residue (Ribes nigrum) J Agric Food Chem. 2001;49:3169–77. doi: 10.1021/jf001443p. [DOI] [PubMed] [Google Scholar]
- 30. Bakker J, Bridle P, Bellworthy SJ, et al. Effect of sulphur dioxide and must extraction on colour, phenolic composition and sensory quality of red table wine. J Sci Food Agric. 1998;78:297–307. [Google Scholar]
- 31. Yang Z, Han Y, Gu Z, Fan G. Thermal degradation kinetics of aqueous anthocyanins and visual color of purple corn (Zea mays L.) cob. Innov Food Sci Emer Tech. 2008;9:341–7. [Google Scholar]
- 32. Byamukama R, Monica-Jordheim M, Kiremire B, et al. Anthocyanins from flowers of Hippeastrum cultivars. Sci Hort. 2006;109:262–6. [Google Scholar]
- 33. Hadizadeh F, Naaman Khalili N, Hosseinzadeh H, et al. Kaempferol from Saffron Petals. Iranian J Phar Res. 2003;2:251–2. [Google Scholar]
- 34. Nørbæk R, Brandt K, Nielsen JK, et al. Flower pigment composition of Crocus species and cultivars used for a chemotaxonomic investigation. Biochem Syst Eco. 2002;30:763–91. [Google Scholar]
- 35. Oliveira J, Fernandes V, Miranda C, et al. Color properties of four cyanidin-pyruvic acid adducts. J Agric Food Chem. 2006;54:6894–903. doi: 10.1021/jf061085b. [DOI] [PubMed] [Google Scholar]
- 36. Saito N, Mitsui S, Hayashi K. Delphinidin the anthocyanin of medicinal saffron and its identity with hyacin as showed by paper chromatography of partial hydrolysates. Studies on anthocyanins. Bot Mag Tokyo. 1960;36:340–5. [Google Scholar]