Abstract
Terminalia catappa Linn (TC) is an ornamental tree planted extensively in many countries. It has been known for a long time that the seeds are edible but no research has focused on the realm of its use as food. Our previous data showed that the seed contains high levels of oil content (600 g/kg) and possesses the optimum fatty acid balance indicated in fat dietary guidelines. This study aims to investigate the physical and chemical properties and the possibility of using TC seed oil as a new dietary lipid. The effects of extraction conditions, partial refining process, and storage stability on TC oil properties were conducted compared with soybean oil. The results showed that physicochemical properties including the density, refractive index, melting point, acidity, free fatty acid, saponification value, unsaponifiable, peroxide, and fatty acid composition of the extracted oil were comparable with soybean oil and their values followed the dietary standard of edible oil.
Keywords: edible oil, oil refining, storage stability
1. Introduction
Belonging to the family Combretaceae, Terminalia catappa Linn (TC) is naturally occurring and widespread in the subtropical and tropical zones of the Indian and Pacific Oceans, and is planted extensively in many countries as an ornamental tree. Most of the research on TC has mainly focused on biological and phytochemical studies of leaves, bark, and whole fruit extracts as a database for medicinal benefits, not in the realm of food application [1–5]. TC fruit extracted by petroleum ether, methanol, and aqueous solution possess antidiabetic activity [6], and the kernel of TC also has aphrodisiac properties [7]. Ajayi et al [8] evaluated the short-term toxicology of three kinds of oilseeds in rats, and found that oil obtained from TC seeds had the least deleterious effect, and was more suitable for consumption. In addition, Oliveria [9] indicated that the oil content of T. catappa (583.0 g/kg dry matter) is comparable to that of other oilseeds such as peanut, rapeseed, and sunflower.
Our previous report [10] also showed that the seed kernels of this plant contain high amounts of oil—about 60%. The main fatty acid contents are the monounsaturated fatty acid (MUFA), oleic acid (C18:1), and the polyunsaturated fatty acid (PUFA0, linoleic acid (C18:2), which were determined at 32.4% and 30.3%, respectively. In addition, the fatty acid balance of TC seeds—1.2:1.1:1 for saturated fatty acid (SFA):MUFA:PUFA—is close to the fat dietary guideline of the current National Cholesterol Education Program (NCEP) and American Heart Association (AHA) compared with other dietary vegetable oils such as palm, soybean oil, sesame, olive, and coconut oils [11]. The oil is a pale yellow liquid, and is also similar to almond oil in flavor and odor. To date, there are no dietary oils containing the fatty acid balance following the fat dietary guidelines of the NCEP and AHA. These finding suggest a promising potential capacity to develop TC oil as a healthy dietary oil.
In oil manufacturing, the solvent extraction process is typically done using hexane due to its extraction power, and low boiling point and cost. After removing hexane, the crude oil goes through the refining process to remove undesirable components with minimal damage to desirable components. The common oil refining process includes degumming, neutralization, bleaching, dewaxing, and deodorization [12]. Degumming removes phospholipids and lipoproteins, through hydration, using water and either citric or phosphoric acid, followed by centrifugation [13]. For neutralization, free fatty acids are removed by a sodium hydroxide solution and the sodium salts of the free fatty acids (soaps) are separated by centrifugation [14]. The pigments naturally present in the crude oil (including chlorophylls and carotenoids) are removed by adsorption on bleaching earth [14]. In this study, we focused on partial refining processes including degumming, neutralization, and bleaching.
To evaluate the possible use as dietary oil, the physicochemical characteristics of the oil, effects of extraction and refining on oil quality, and the shelf-life storage test are very important factors. No compositional data of TC oil in terms of being dietary oil have been reported, and no studies have been conducted on its potential as a new oilseed. Therefore, the present study analyzed the physicochemical characteristics of TC oil extracted under different conditions and to determine the effect of partially refining including degumming and neutralization on chemical properties of TC oil. The shelf-life storage study of partially refined TC oil was also evaluated compared with soybean oil.
2. Methods
2.1. Seed materials and chemical materials
Natural samples of TC with partially dried fruits (with gray-colored pericarp) were collected from the campus of Naresuan University, Phitsanulok, Thailand during July–August and October–November. The fruit was cleaned and oven-dried at 60°C for 12 hours. The shell was cracked to remove the single seed (kernel) and dried at 60°C for 2 hours. The seeds were then ground in a coffee grinder (CBG5 series, Black and Decker Canada, Brockville, Ontario, Canada) for 10 seconds to a fine powder followed by lipid extraction. Soybeans (Raitip; Thai Cereal World, Nonthaburi, Thailand) were bought from a Makro supermarket (Siam Makro Public Company, Phitsanulok, Thailand) and they were directly ground and kept under the same conditions as TC fine powder.
2.2. Lipid extraction
Lipid extraction was carried out with n-hexane (250 mL) and 50 g samples (ground dried seeds). Soxhlet extraction was done using the Soxhlet apparatus while maceration extraction conditions were conducted with and without Grant OLS 200 orbital shaker (175 rpm) and temperature at 55°C as adapted from Yong and Salimon’s study [15]. The oil extraction by maceration was carried out for 4 hours. The extracted lipids were obtained by filtering the solvent to remove the solid before the hexane was removed using rotary evaporator apparatus at 40°C. The extracted seed oil was subsequently analyzed physicochemically.
2.3. Analytical methods
All analytical determinations were achieved in triplicate. Values of each parameter are expressed as mean ± standard deviation. The samples of the lipid were analyzed for their quality characteristics using the following standard procedures.
2.4. Physical analysis of seed oil
The viscosity of seed oil was determined using Brookfield DV-I with a spindle of S00 at 100 rpm at room temperature. To determine its color, the CIELab coordinates (L*, a*, and b*) were directly read using Hunter Lab DP9000 S/N 90905.
Specific gravity was determined according to the Association of Official Analytical Chemists (AOAC) method No. 40.1.08 (1990) [16]. A 25 mL specific gravity bottle was used. The bottle was weighed (W0) and then filled with oil, a stopper inserted and then reweighed to give W1. The oil was substituted with water after washing and drying the bottle and weighed to give W2. The specific gravity was calculated from the following equation:
| (1) |
A differential scanning calorimeter (DSC; Diamond; PerkinElmer, Waltham, MA, USA) was used to determine the melting point of the sample. The oil samples were homogenized and filtered through filter paper and heated at 50°C to demolish all fat crystal remains and cooled again to room temperature. The samples (each about 16 mg) were then put into the hermetically sealed aluminum pans for analysis by the DSC operating in the temperature range of −50°C to 50°C at a heating rate of 5°C/minute, associated with purge liquid nitrogen gas[17].
2.5. Chemical analyses of seed oil
The acid value of oil was determined according to AOAC method No. 940.28 [16]. The oil sample (2 g) was dissolved in 10 mL ethanol and titrated with 0.1M NaOH solution using phenolphthalein indicator until the pink color disappeared. The acid was calculated from the function below:
| (2) |
The percentage of free fatty acids in the oil was determined by using the AOAC method No. 940.28 [16] and were calculated using linoleic acid as the factor. The percentage fatty acid was calculated from the function below:
| [3] |
Saponification value was determined according to the AOAC method No. 920.160 [18]. The unsaponification value was determined according to the AOAC method No. 933.08 [18]. The peroxide value (PV) was determined according to the AOAC method No. 965.33 [16].
Oxidation reaction as indicated by thiobarbituric acid reactive substances (TBARSs) were determined on oil samples as described by Buege and Aust [19]. Oil samples (5 g) were weighed into a 25 mL test tube then homogenized with 25 mL of thiobarbituric acid for 2 minutes at high speed. Samples were boiled for 1 hour, cooled and centrifuged at 5500 × g for 25 minutes. Absorbance of the supernatants was spectrophotometrically measured at 532 nm.
The sterols (esterified and free) were determined as trimethyl silyl ether derivatives by gas chromatography (GC)-flame ionization detector separations using RTX-5TM fused silica capillary columns with a Hewlett Packard series 6890 GC (Waldbronn, Germany) equipped with a split/splitless injector, an autosampler, and a flame ionization detector. Identification of sterols was based on their retention times relative to reference compounds (campesterol, stigmasterol, β-sitosterol, brassicasterol, and stigmastanol; Sigma–Aldrich, St. Louis, MO, USA) [20]. The sterol concentrations were calculated using the area of the internal standard peak. The carrier gas was helium. The GC operating parameters were as follows: Initial oven temperature was 290°C. After an isothermal period of 20 minutes, the oven temperature was increased at 1°C/minute to 300°C and was held at this temperature for 10 minutes. Detection was done by flame ionization with the detector temperature set at 360°C. Peak identification was carried out by comparison of relative retention times of standards.
The tocopherol contents were separated and quantified by high-performance liquid chromatography (HPLC) with a UV detector (set at 292 nm), according to the American Oil Chemists’ Society Ce 8–89 methodology [21]. The TC oil was dissolved in n-hexane and submitted directly to HPLC analysis. The components were separated in a normal phase chromatographic column SI 60 (5 μm, 250 mm × 4.6 mm inner diameter) purchased from SGE Analytical Science (Ringwood, Victoria 3134, Australia). The mobile phase was 1.2 mL/min flow n-hexane/isopropanol (99.5:0.5 V/V). The solvents used were HPLC grade. The mobile phase was filtered through a 0.45 μm membrane and degassed with helium gas for 15 minutes in a degasser on line with the chromatograph. Individual tocopherol isomers were quantified using separate calibration curves for α-, β-, and γ-tocopherols and tocotrienols.
Fatty acids were obtained by saponification of fats according to the AOAC method [22]. Then, free fatty acids were esterified by boron trifluoride catalyst. The methyl esters of the fatty acids were extracted by petroleum ether. The fatty acid methyl esters were analyzed by GC (Agilent 6890N), using a fused silica capillary column, HP-88 (100 m × 0.250 mm × 0.2 μm) capillary column (Agilent Technologies Inc., Palo Alto, CA, USA) equipped with a flame ionization detector. The temperature profiles were as follows: initial temperature, 90°C and held for 5 minutes; final temperature, 240°C and held for 20 minutes; injector temperature, 250°C and detector temperature, 260°C. Total run time was 120 minutes. Nitrogen was used as the carrier gas and the gas flow rates used were 0.3 mL/minutes. The identification of the peaks was achieved by comparing their relative and absolute retention times with authentic standards. The ratio of SFA:-MUFA:PUFA was obtained from the sum of each type of fatty acid and calculated by proportion.
Iron, copper, lead, and arsenic were characterized and quantified by atomic absorption spectrophotometry (Model 2100; Perkin Elmer). Phosphorus was quantified by gravimetric method [22].
2.6. Evaluation of the effect of partially refining on oil quality
The TC seed oil extracted by the Soxhlet extraction method was used for investigation of the effect of partially refining on the physicochemical properties of TC oil. Oil refining processes used included degumming, neutralization, and bleaching [23,24]. For degumming, boiling water (25 mL) was added to crude oil (1 kg) and the mixture was stirred for 30 minutes and centrifuged at 6000 × g for 5 minutes. For the neutralization, degummed oil was poured into a beaker and heated to 80°C, and NaOH (0.1M) solution was added based on the acid value and specific gravity of the oil to obtain a uniform solution [25]. NaCl was then added to help settle out the soap formed. This mixture was transferred into a separatory funnel and allowed to stand for 1 hour; the soap that was formed was separated from the oil. Hot water was added repeatedly to the oil solution until all of the soap in the solution was removed. Bleaching was performed by heating partially refined oil at 90°C and activated clay (15% by weight of oil) was added. The mixture was stirred continuously for 30 minutes. The temperature was allowed to rise to 110°C for another 30 minutes. The content was hot filtered at 70°C. Then the oil was cooled and used for further analysis.
2.7. The storage stability test
The stability of the partially refined TC oil without any additives compared to soybean oil as a control was evaluated using an accelerated shelf-life test [26]. Tests were conducted with about 25 mL of sample oil in a test tube size 25 mm × 150 mm tightly closed with a plastic cap and stored in an incubator oven at 35°C, 45°C, and 55°C for 3 months. Every 7 days each individual oil sample was withdrawn from the chamber for chemical property analyses including acidity, peroxide value, and TBARS using a spectrophotometer. The shelf life of the TC oil was also calculated compared with soybean oil.
3. Results and discussion
3.1. The fatty acid profiles of the TC oil extracted by three conditions
Our previous data [10] showed that the fatty acid balance of TC seed for SFA:MUFA:PUFA was closer to the fat dietary guidelines of the current NCEP and the AHA, which are 1:1.1:1 or 1:1.5:1 [11] than fatty acid profile of other vegetable oils including soybean, palm, coconut, and sesame oil. This study investigated the effect of TC oil extraction, the refining process, and the storage stability on physicochemical properties. Solvent extraction of TC seed oil was conducted by Soxhlet extraction and maceration with and without Grant OLS 200 orbital shaker and temperature. Hexane was used as extraction solvent. The oil extraction yield by Soxhlet extraction was higher (57.5%) than both maceration methods with and without the accelerated condition (51% and 49%, respectively). Accelerated condition by temperature at 55°C together with shaking did not affect the yield percentage of oil because there is no significant difference between the yields of both maceration methods. The fatty acid profiles of the TC oil extracted by three conditions are presented in Table 1. The result showed that palmitic acid (C16:0) was the major SFA in all three conditions and its content ranged from 31.38% to 33.08%. Soxhlet extraction significantly gives the highest level of stearic acid (8.46%). The content of oleic acid (C18:1)—a MUFA—in TC oil ranges between 24.65% and 31.72%. The level of oleic acid extracted by three conditions was not significantly different. The appreciable concentration of oleic acid in oils makes them desirable in terms of nutrition [27] since many studies suggest that high intake of MUFA has been associated with protection against coronary heart disease [28]. The abundant PUFA is linoleic acid (C18:2; 31.07 %). The results showed that hexane extraction with temperature including Soxhlet and maceration extraction at 55°C caused the loss of unsaturated fatty acids, because unsaturated fatty acids are more susceptible to oxidation than SFA. The fatty acid profile of TC oil (Table 1) also reveals that unsaturated fatty acids including oleic acid (C18:1) and linoleic acid (C18:2) are the main fatty acids. Compared to regularly consumed oil, the ratio of SFA:MUFA:PUFA of TC oil was found to be what is recommended in the dietary guideline of the American Heart Association [11]. We also found no significant difference in the chemical properties including saponification, unsaponification, acid value, free fatty acid, and peroxide value of TC oil extracted by three different conditions (data not shown). Oil extracted by Soxhlet extraction was chosen for further studies due to the highest percentage yield.
Table 1.
Fatty acid profile of TC oil extracted by hexane extraction in different conditions.
| Fatty acid | Extraction | ||
|---|---|---|---|
|
| |||
| Maceration | Maceration with temperature and shaking | Soxhlet | |
| Saturated fatty acid (S) | |||
| C16:0 | 33.08 ± 0.81a | 33.72 ± 1.36a | 31.38 ± 10.26a |
| C18:0 | 6.06 ± 0.21b | 4.94 ± 0.54b | 8.46 ± 0.41a |
| C20:0 | 0.75 ± 0.03b | 0 | 1.03 ± 0.06a |
| Total | 39.89 | 38.66 | 40.08 |
| Monounsaturated fatty acid (M) | |||
| C18:1 | 24.65 ± 1.97a | 28.42 ± 1.40a | 31.72 ± 13.56a |
| Total | 24.65 | 28.42 | 31.72 |
| Polyunsaturated fatty acid (P) | |||
| C18:2 | 31.07 ± 0.92a | 28.52 ± 1.39a,b | 22.92 ± 3.77b |
| C18:3 | 0 | 0 | 0.11 ± 0.01a |
| Total | 31.07 | 28.52 | 23.03 |
| Unsaturated/saturated | 1.4 | 1.5 | 1.4 |
| S:M:P | 1.6:1:1.3 | 1.4:1:1 | 1.7:1.4:1 |
Data are presented as mean ± SD unless otherwise indicated. Mean values with different letters in row are significantly different (p ≤ 0.05).
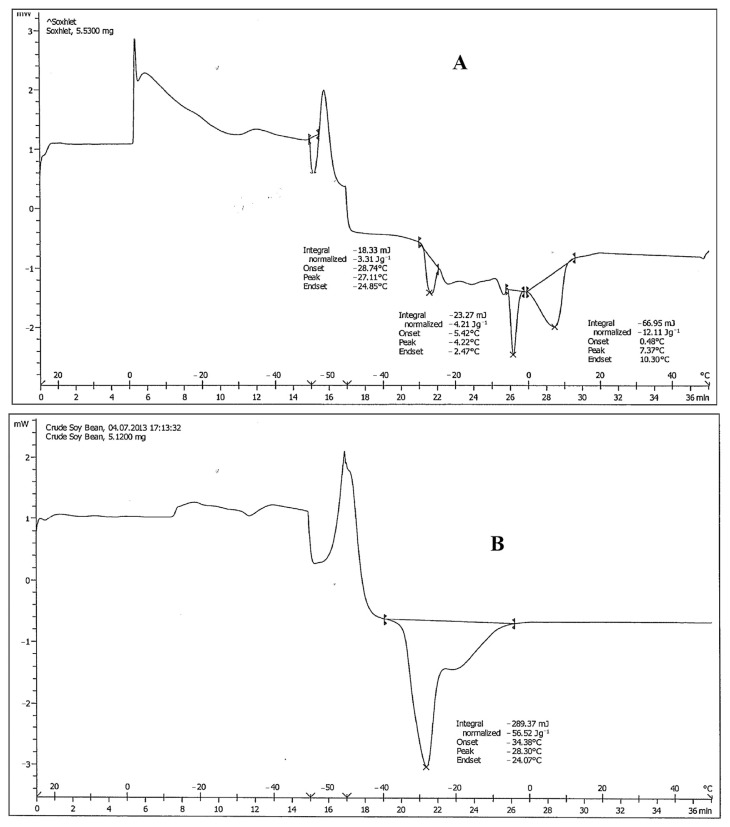
Table 2 shows the physicochemical properties of TC oil compared with soybean oil. TC oil contains a relatively high percentage of total lipid content (58.90 ± 0.71%) compared to the soybean oil, which is 20.59 ± 0.72%. DSC offers a simple method of investigating characteristics of melting points of fats [17]. In the oil industry, the DSC cooling/melting thermograms give valuable information on the thermal properties of fat products [29]. Each oil has its own unique characteristics of fatty acids and triacylglycerol profiles. In the DSC melting curves from the heating rate studies, the melting curve for TC oil consisted of three peaks (Fig. 1 and Table 3). In the TC oil and soybean oil samples, one major melting peak was seen as a single, tall endotherm peak shown at −27.11°C and −28.30°C, respectively (Table 3). For the TC oil thermogram, there was a sharp peak (peak 2) that might be a melting point of freezable water close to 0°C, due to part of the water during extraction of oil remaining in the crude oil. Peak 3 was not sharp, indicating impurities such as aldehydes, ketones, and pigments. The data from DSC indicate that the melting point of TC is quite similar to soybean oil. In addition, specific gravity of TC oil was 0.89 similar to soybean oil (0.91).
Table 2.
Physicochemical properties of TC and soybean crude oil and Codex Alimentarius Commission for edible oil.
| Properties | Soybean crude oil | TC crude oil | Standards of edible oil |
|---|---|---|---|
| Total lipid content (g/100g) | 20.59 ± 0.72b | 57.50 ± 0.71a | |
| Specific gravity | 0.91 ± 0.00a | 0.89 ± 0.00b | |
| Melting point (°C) | −28.30 | −27.11 | — |
| Viscosity (mPa.s) | 25.4 ± 0.21b | 33.50 ± 1.06a | |
| Acid value (mg KOH/g oil) | 2.62 ± 1.21a | 2.34 ± 0.26a | < 4 |
| % free fatty acid as oleic acid | 1.31 ± 0.61a | 1.17 ± 0.13a | |
| Saponification value (mg KOH/g oil) | 172.02 ± 11.36a | 178.7 ± 8.91a | 189–198 |
| Unsaponification value (% by weight) | 2.15 ± 0.29a | 1.93 ± 0.33b | < 13 |
| Peroxide value (mEq/kg) | 1.13 ± 0.25a | 0.65 ± 0.10b | < 10 |
| γ-tocopherol (mg/kg) | — | 431.80 ± 13.66 | |
| Sterol (mg/kg) | — | 3402.2 ± 78.29 | |
| β-sitosterol | — | 1995 ± 49.44 | |
| Stigmasterol | — | 907.68 ± 25.14 | |
| Campesterol | — | 296.77 ± 3.46 | |
| Stigmastanol | — | 202.53 ± 0.25 | |
| Color | |||
| a* | 1.69 ± 0.69a | −0.56 ± 0.13b | |
| b* | 2.57 ± 0.31a | 1.03 ± 0.11b | |
| L* | 5.36 ± 0.02b | 6.33 ± 0.10a | |
| Metal (mg/kg) | |||
| Fe | < 0.1 | < 1.5 | |
| Cu | < 0.1 | < 0.1 | |
| Pb | < 0.1 | < 0.1 | |
| As | < 0.1 | < 0.1 | |
Data are presented as mean ± SD unless otherwise indicated. Mean values with different letters (a and b) in row are significantly different (p ≤ 0.05).
Fig. 1.
The differential scanning calorimetry thermogram of (A) Terminalia catappa oil and (B) soybean oil.
Table 3.
Comparison of differential scanning calorimetry transition temperatures for melting point of Terminalia catappa oil with soybean oil.
| Sample | Transition temperature (°C) | ||
|---|---|---|---|
|
| |||
| 1 | 2 | 3 | |
| Terminalia catappa oil | −27.11 | −4.22 | 7.37 |
| Soybean oil | −28.30 | ||
The acid value of oil is dependent on the amount of free fatty acids present or on the degree of hydrolysis of the oil. Acid value of oil suitable for edible purposes should not exceed 4 mg/g [30]. The acid value of TC seed oil shows a comparatively low value (2.6 mg KOH/g oil) due to its low content of free fatty acid (1.3%). The results indicated that acid, free fatty acid, and the saponification value of TC crude oil compared to soybean crude oil were not significantly different and they were acceptable levels based on the standard for edible oils. Saponification value is inversely proportional to the mean molecular weight of the fatty acid in the glyceride present in the lipid [31]. The saponification number of TC seed oil showed a significantly high number of 178.7 mg KOH/g oil compared to soybean oil, which has a saponification number of 172 mg KOH/g oil, suggesting that TC oil contains a high proportion of fatty acids of low molecular weight. The saponification values of TC oil was comparable to that of safflower, sunflower, and corn oil, which have average saponification numbers ranging between 191 mg KOH/g oil and 250 mg KOH/g oil [32]. Nonetheless, the TC seed oil showed a low unsaponifiable matter of 1.93%. Unsaponifiable matter includes the higher aliphatic alcohols, sterols, pigments, and hydrocarbons. These are substances frequently found dissolved in fatty acids. The phytosterol contents of some seed oils including soybean, cottonseed, safflower, and corn oil were reported at 2500 mg/kg, 3240 mg/kg, 4440 mg/kg, and 9680 mg/kg, respectively [33]. We found that β-sitosterol was the major form and total sterol contents of TC crude oil is 3402 mg/kg, which is close to cottonseed oil and higher than soybean oil. Generally, vegetable oils are an important source of tocopherols, so called vitamin E, which have free radical-scavenging properties [34]. TC crude oil contains a low content of tocopherols compared with soybean oil as (271 mg/kg) [35] and the only isoform found was γ-tocopherol (431.8 mg/kg). Plant sterols have been proposed to have potential positive health effects especially a cholesterol-lowering effect [36] and γ-tocopherol has been reported to be more potent than α-tocopherol in decreasing platelet aggregation and LDL oxidation, and delaying intra-arterial thrombus formation [37]. However, the concentration of both sterols and tocopherols are normally lost because of the chemicals used for refining (sodium hydroxide and activated bleaching clays) or the extreme physical conditions (high temperature and low pressure) on the edible oil industry.
Peroxide value is a measure of the reaction rate of lipid oxidation, which causes rancidity. Normally, oils become rancid when the peroxide value ranges from 20.0 mg/g oil to 40.0 mg/g oil [32]. The peroxide value of soybean crude oil (1.13 mEq/kg) was significantly higher than TC crude oil (0.65 mEq/kg); however, the peroxide values of both oils are below the maximum acceptable value of 10 mEq KOH/g set by the Codex Alimentarius Commission for oil seeds [38]. The high oxidative stability of TC seed oil compared to soybean oil shows that TC seed oil may possess good qualities as edible oil or can be stored for a long period without deterioration. The color of TC oil was lighter than soybean oil as indicated by Hunter L*, a*, and b* values. According to the food standard regulation of edible oil based on Ministry of Industry rules, the metals of concern in edible oil have to be < 2.5 mg/kg for iron and 0.1 mg/kg oil for arsenic, copper, and lead. These metal levels of TC oil extracted by Soxhlet extraction are < 0.1 mg/kg. The results here show that the physicochemical properties of TC crude oil are compatible with soybean oil, the major consumed oil worldwide, suggesting their application as a new edible oil. By the Soxhlet extraction method, the peroxide value (0.65 mEq/kg), viscosity (33.5 mPa.s), and oil extraction yield (57.5%) found here are consistent with the earlier study of Matos et al [39] (0.51 mEq/kg, 32.92 mPa.s, and 56.3% respectively). In addition, we found the same pattern of the fatty acid profile and the fatty acid ratio as results of Matos et al’s study [39].
3.2. Effect of partially refining process on TC oil properties
The crude oil extracted by Soxhlet was selected for studying the effect of partially refining on oil properties. After partially refining crude oil by degumming, neutralization, and bleaching, the physicochemical properties of TC oil compared with soybean oils were tested and the results are shown in Table 4. As targeted in the refining process, the results show the reduction of acid value (88%) and phosphorus content (66%) and these decreases would result in an improvement in the quality of the oil. Acid value is used as an indicator for edibility of oil. The partial refining did not significantly affect on the saponification and unsaponification values. The peroxide value of the TC oil increased from 0.65 to 1.45 after refining due to the oxidative rancidity of the oil in an open system process and the process of bleaching at 100°C but the value is still under the standard acceptable value. Acid and peroxide values of the TC oil were not significantly different from soybean oil. Both the saponification and unsaponification values of TC oil were significantly less than soybean oil. The results show that chemical properties of TC oil after partially refining by degumming, neutralization, and bleaching are under the standards of Codex Alimentarius Commission for edible oil [38]. Therefore, TC oil could be purified into edible oil.
Table 4.
Physicochemical properties of Terminalia catappa (TC) oil extracted by Soxhlet extraction after degumming neutralization and bleaching.
| Properties | Standards of edible oila | TC crude oil | Partially refined TC oil | Partially refined soybean oil |
|---|---|---|---|---|
| Acid value (mg KOH/g oil) | < 0.6 | 2.34 ± 0.26a | 0.27 ± 0.00b | 0.28 ± 0.00b |
| Saponification value (mg KOH/g oil) | 189–198 | 178.71 ± 8.91b | 188.21 ± 1.61b | 195.25 ± 2.22a |
| Unsaponification value (% by weight) | < 13 | 0.99 ± 0.33b | 0.82 ± 0.13b | 1.66 ± 0.17a |
| Peroxide value (mEq/kg) | < 10 | 0.65 ± 0.10b | 1.45 ± 0.02a | 1.77 ± 0.26a |
| % Phosphorus | — | 1.03 ± 0.29a | 0.36 ± 0.26b | — |
| Metal (mg/kg) | ||||
| Fe | < 1.5 | < 0.1 | < 0.1 | — |
| Cu | < 0.1 | < 0.1 | < 0.1 | — |
| Pb | < 0.1 | < 0.1 | < 0.1 | — |
| As | < 0.1 | < 0.1 | < 0.1 | — |
Data are presented as mean ± SD unless otherwise indicated. Mean values with different letters in row are significantly different (p ≤ 0.05).
3.3. The storage stability test of partially refined TC oil
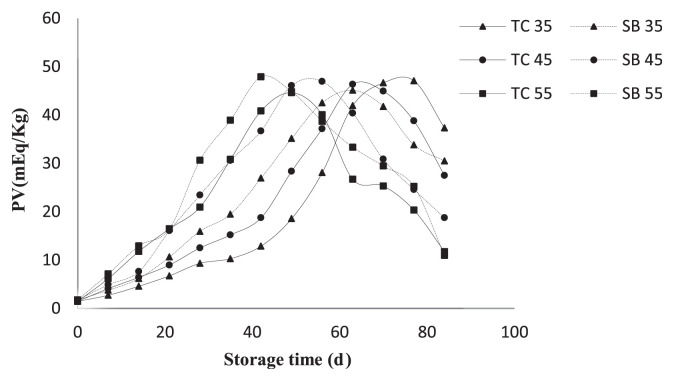
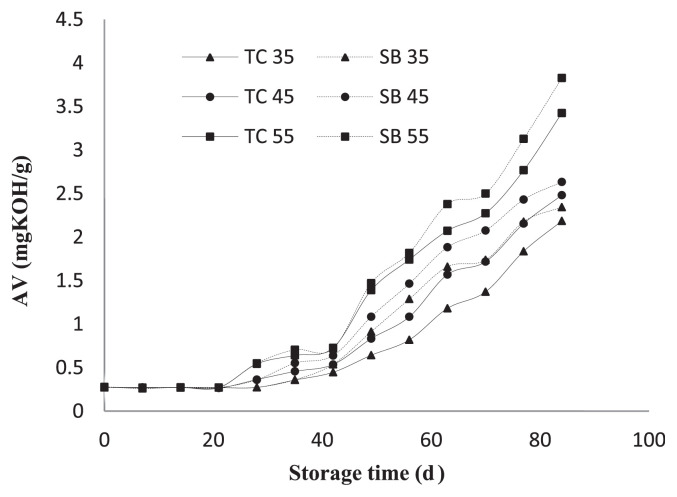
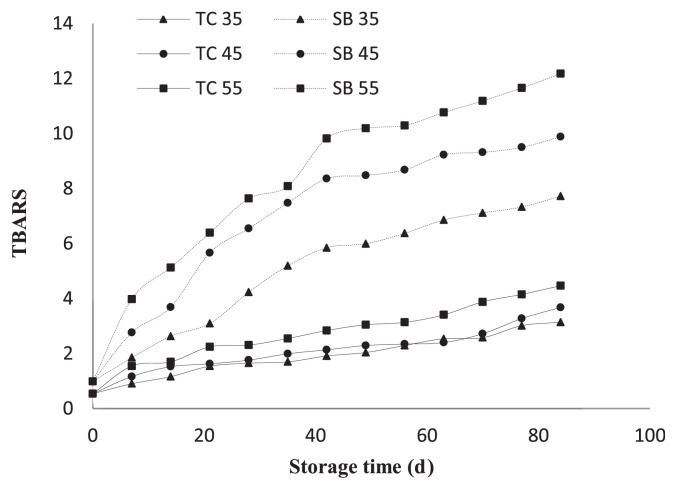
In order to evaluate oil stability and a quick estimation of shelf-life storage, accelerated conditions were used to facilitate the oxidation process. The oil’s resistance to oxidative deterioration on evolution of peroxide, acid, and TBARS value of partially refined TC oils is shown in Figs. 2 and 3, respectively. Apparently, the higher storage temperatures will accelerate the oxidation reaction than lower temperatures. Using an accelerated shelf-life test at 35°C, 45°C, and 55°C for 3 months, the PV increased progressively especially during the storage at 55°C, which presented a maximum in PV and thereafter decreased as the decomposition reactions became prevailing. Initial peroxide value of TC oil and soybean oil were 1.45 mEq/kg and 1.77 mEq/kg, respectively and are likely to increase as the storage period increases, as shown in Fig. 2. The rate of peroxide formation of TC oil was slower than that of soybean oil as indicated by the longer time it takes to reach the maximum peroxide values at all storage temperatures tested. AV, an indicator of the aldehyde content, remained practically constant at the earlier stages of oxidation, but then increased sharply following the decomposition of peroxides (Fig. 3). TBARS values of soybean oil are higher than TC oil. The initial TBARS values of TC oil and soybean oil were 0.54 mg malondialdehyde/kg and 0.98 mg malondialdehyde/kg, respectively and tended to increase in the longer-term storage (Fig. 4). The main TBARS is malondialdehyde, which is a secondary product formed as a result of lipid peroxidation of PUFAs [40]. Therefore, the presence of TBARS indicates that lipid peroxidation has taken place and the level of TBARS shows the amount of peroxidation that has already occurred [41]. TBARS values tend to have a good correlation with sensory testing in terms of food rancidity [42].
Fig. 2.
Peroxide value (PV) changes in Terminalia catappa oil (TC: –) compared to soybean oil (SB: –) stored at 35°C, 45°C, and 55°C.
Fig. 3.
Acid value (AV) changes in Terminalia catappa oil (TC: –) compared to soybean oil (SB: –) stored at 35°C, 45°C, and 55°C.
Fig. 4.
Thiobarbituric acid reactive substances (TBARS) value changes in Terminalia catappa oil (TC: –) compared to soybean (SB: –) stored at 35°C, 45°C, and 55°C.
These results strongly suggest that the oxidative stability of TC oil is greater than soybean. The reason might be that, compared with soybean, TC oil has a lower percentage of the PUFAs linoleic and linolenic acid, but has a high percentage of the MUFA oleic acid. The ratio of oleic acid to linoleic acid is an indicator of oil stability and quality. Linoleic is more susceptible to oxidation than oleic acid; therefore increasing oleic acid levels improves the oxidative stability of oil [43].
An evaluation of the kinetic study of the autoxidation reaction following the estimation of shelf-life storage of TC oil was performed. Since the data show that PV, but not AV and TBARS, show a good linear relationship (r2 = 0.99) with storage time, a kinetic study of the autoxidation reaction was evaluated as a function of PV and is reported in Table 5. As indicated by the regression coefficient (r2), the behaviors of the autoxidation reaction of TC and soybean oil were apparently zero-order, whereas the rate of the reaction does not depend on the concentration of the oxidizing substrate and the rate of the reaction is equal to the rate constant, k, of that reaction. The slope of the plot of the PV versus the storage times for each sample at various storage temperatures provides the reaction rate constant (k) shown in Table 6. As the temperature of storage increased, reaction rate constant (k) of both TC and soybean oil increased due to higher temperatures, resulting in the catalytic oxidation of oil. The reaction rate constant (k) of TC oil is lower than soybean oil, suggesting the better resistance to oxidation of TC oil.
Table 5.
Relationship (r2) of each kinetic model based on peroxide value.
| Kinetic model | r2 | ||
|---|---|---|---|
|
| |||
| 35°C | 45°C | 55°C | |
| Zero-order | |||
| Terminalia catappa oil | 0.983 | 0.991 | 0.966 |
| Soybean oil | 0.955 | 0.970 | 0.966 |
| First-order | |||
| Terminalia catappa oil | 0.965 | 0.955 | 0.924 |
| Soybean oil | 0.984 | 0.966 | 0.947 |
| Second-order | |||
| Terminalia catappa oil | 0.869 | 0.763 | 0.628 |
| Soybean oil | 0.882 | 0.810 | 0.682 |
Table 6.
Values of the oxidation rate constants (k) for peroxide value and the predicted shelf life storage of partially refined oil compared with soybean oil.
| Sample | Reaction rate constant | Predicted shelf life (d) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 35°C | 45°C | 55°C | 35°C | 45°C | 55°C | |
| Terminalia catappa oil | 0.276 | 0.408 | 0.900 | 30 | 20 | 9 |
| Soybean oil | 0.595 | 0.877 | 1.208 | 13 | 9 | 6 |
Since the reaction of the study is better represented by a kinetic model composed of a zero-order autocatalytic reaction, the reaction rate constant (k) were used to predict the shelf life at each temperature test of TC compared with soybean oil using the following equation:
| (4) |
where C = maximum value of peroxide value for edible oil which is 10; C0 = initial peroxide value; k = reaction rate constant; and t = time (days).
The data show that the predicted shelf life of TC oil based on the maximum standard peroxide value of edible oil allowed at 10, was 30 days, 20 days, and 9 days at the temperatures of 35°C, 45°C, and 55°C, respectively, and they were longer than soybean oil at all temperatures. From a practical point of view, the predicted shelf-life in normal storage conditions of TC oil tend to be longer than soybean oil, suggesting the potential use as edible oil.
4. Conclusion
The results of the oil content, chemical and physical analyses, and the storage stability test of the oil extracted from the TC seeds were favorably comparable with soybean oil, the most popular edible oil globally. The data from the refining process suggest that TC oil offers the possibility of further industrial extraction and refining process successfully into edible oil. The result of this study shows that TC oil possesses a good quality making it suitable as a novel edible oil.
Acknowledgments
This project was supported by the Thailand Research Fund (TRF), project No. 5480015 and the National Research Council of Thailand (NRCT), project No. R 2557B111.
Funding Statement
This project was supported by the Thailand Research Fund (TRF), project No. 5480015 and the National Research Council of Thailand (NRCT), project No. R 2557B111.
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1. Lin CC, Chen YL, Lin JM, et al. Evaluation of the antioxidant and hepatoprotective activity of Terminalia catappa. Am J Chin Med. 1997;25:153–61. doi: 10.1142/S0192415X97000172. [DOI] [PubMed] [Google Scholar]
- 2. Masuda T, Yonemori S, Oyama Y, et al. Evaluation of the antioxidant activity of environmental plants: activity of the leaf extracts from seashore plants. J Agric Food Chem. 1999;47:1749–54. doi: 10.1021/jf980864s. [DOI] [PubMed] [Google Scholar]
- 3. Lin CC, Hsu YF, Lin TC. Effects of punicalagin and punicalin on carrageenan-induced inflammation in rats. Am J Chin Med. 1999;27:371–6. doi: 10.1142/S0192415X99000422. [DOI] [PubMed] [Google Scholar]
- 4. Chen PS, Li JH, Liu TY, et al. Folk medicine Terminalia catappa and its major tannin component, punicalagin, are effective against bleomycin-induced genotoxicity in Chinese hamster ovary cells. Cancer Lett. 2000;152:115–22. doi: 10.1016/s0304-3835(99)00395-x. [DOI] [PubMed] [Google Scholar]
- 5. Fan YM, Xu LZ, Gao J, et al. Phytochemical and anti-inflammatory studies on Terminalia catappa. Fitoterapia. 2004;75:253–60. doi: 10.1016/j.fitote.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 6. Nagappa AN, Thakurdesai PA, Venkat Rao N, et al. Antidiabetic activity of Terminalia catappa Linn fruits. J Ethnopharmacol. 2003;88:45–50. doi: 10.1016/s0378-8741(03)00208-3. [DOI] [PubMed] [Google Scholar]
- 7. Ratnasoosiri WD, Dharmasiri MG. Effects of Terminalia catappa seeds on sexual behavior and fertility of males rats. Asian J Androl. 2000;2:213–9. [PubMed] [Google Scholar]
- 8. Ajayi IA, Oderinde RA, Taiwo VO, et al. Short-term toxicological evaluation of Terminalia catappa, Pentaclethra macrophylla and Calophyllum inophyllum seed oils in rats. Food Chem. 2008;106:458–65. [Google Scholar]
- 9. Oliveria JTA, Vasconcelos IM, Bezerra LCNM, et al. Composition and nutritional properties of seeds from Pachira aquatica Aubl, Sterculia striata St Hil et Naud and Terminalia catappa Linn. Food Chem. 2000;70:185–91. [Google Scholar]
- 10.Weerawatanakorn M.Terminalia catappa seeds oil as a new dietary healthy oil source. The annual meeting of the International Society for Nutraceuticals and Functional Foods (ISNFF); Taipei, Taiwan. November 5–9, 2013; [Google Scholar]
- 11. Hayes KC. Dietary fat and heart health: in search of the ideal fat. Asia Pac J Clin Nutr. 2002;11:394–400. doi: 10.1046/j.1440-6047.11.s.7.13.x. [DOI] [PubMed] [Google Scholar]
- 12. Pestana VR, Zambiazi RC, Mendonca CRB, et al. Quality changes and tocopherols and γ-orizanol concentrations in RBO during the refining process. J Am Oil Chem Soc. 2008;85:113–9. [Google Scholar]
- 13.Zambiazi RC. The role of endogenous lipid components on vegetable oil stability. Tese (Doutorado em Fisiologia), foods and nutritional sciences interdepartmental program. Manitoba: Canada University; 1997. p. 304. [Google Scholar]
- 14. Pestana VR, Zambiazi RC, Mendonca CRB, et al. γ-Oryzanol and tocopherol contents in residues of rice bran oil refining. Food Chem. 2012;134:1479–83. doi: 10.1016/j.foodchem.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 15. Yong OY, Salimon J. Characteristics of Elateriospermum tapos seed oil as a new source of oilseed. Ind Crop and Prod. 2006;24:146–51. [Google Scholar]
- 16.AOAC. Official methods of analysis. 15th ed. Washington D.C: Association of Official Analytical Chemists; 1990. [Google Scholar]
- 17. Tan CP, Che Man YB. Differential scanning calorimetric analysis of edible oils: comparison of thermal properties and chemical composition. J Am Oil Chem Soc. 2000;77:142–55. [Google Scholar]
- 18.AOAC. Official methods of analysis. 16th ed. Washington DC, USA: Association of Official Analytical Chemists; 1995. [Google Scholar]
- 19. Buege JA, Aust SD. Microsomal lipid peroxidation methods. Enzymol. 1978;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 20. Laakso P. Analysis of sterols from various food matrices. Eur J Lipid Sci Technol. 2005;107:402–10. [Google Scholar]
- 21.American Oil AOCS Chemists’ Society. Method Ce. Champaign, IL, USA: 1990. Official methods and recommended practices of the American Oil Chemists’ Society; pp. 8–89. [Google Scholar]
- 22.AOAC. Official methods of analysis. 18th ed. Gaithersburg, MD, USA: Association of Official Analytical Chemists; 2005. [Google Scholar]
- 23. Carr RA. Refining and degumming system for edible fat and oils. J Am Oil Chem Soc. 1976;55:766–70. [Google Scholar]
- 24. Nkpa NN, Arowolo TA, Akpan HJ. Quality of Nigerian palm oil after bleaching with local treated clays. J Am Oil Chem Soc. 1989;66:218–22. [Google Scholar]
- 25.Mowlah G, Sheik NM, Kamal ASM. A Handbook on edible oils and fats (with special reference to Bangladesh) 1st ed. The City Press; 1990. p. 524. [Google Scholar]
- 26.Kiratinart P, Anuvat J, Kamolwan J. Shelf-life evaluation of anti-sticking agent by using ASLT method, Kasetsart Conference. Thailand: Kasetsart University; 2010. pp. 452–9. [Google Scholar]
- 27. Corbett P. It is time for an oil change! Opportunities for high oleic vegetables oils. Inform. 2003;14:480–1. [Google Scholar]
- 28. Reaven PD, Grasses BJ, Tribble DL. Effects of linoleate-enriched and oleate enriched diets in combination with alpha-tocopherol on the susceptibility of LDL and LDL subfractions to oxidative modification in humans. Arterioscler Thromb. 1994;14:557–66. doi: 10.1161/01.atv.14.4.557. [DOI] [PubMed] [Google Scholar]
- 29. Biliaderis CG. Differential scanning calorimetry in food research – A review. Food Chem. 1983;10:239–65. [Google Scholar]
- 30. Esuoso KO, Odetokun SM. Proximate chemical composition and possible industrial utilization of Blighia sapida seed oils. La Rivista Italiana Delle Sostanze Grasse. 1995;72:311–3. [Google Scholar]
- 31.Egan H. Pearson’s chemical analysis of Foods. 7th ed. Edinburgh London: Churchill Livingstone; 1976. pp. 487–96. [Google Scholar]
- 32. Babalola TOO, Apata DF. Chemical and quality evaluation of some alternative lipid sources for aqua feed production. Agric Biol J N Am. 2011;2:935–43. [Google Scholar]
- 33. Abidi SL. Chromatographic analysis of plant sterols in foods and vegetable oils. J Chromatogr A. 2001;935:173–201. doi: 10.1016/s0021-9673(01)00946-3. [DOI] [PubMed] [Google Scholar]
- 34. Huang YK, Chang TC, Sheu JR, et al. Comparison of free radical formation induced by baicalein and pentamethyl-hydroxychromane in human promyelocytic leukemia cells using electron spin resonance. J Food Drug Anal. 2014;22:379–90. doi: 10.1016/j.jfda.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lima H, Woo S, Kim HS, et al. Comparison of extraction methods for determining tocopherols in soybeans. Eur J Lipid Sci Technol. 2007;109:1124–7. [Google Scholar]
- 36. De JA, Plat J, Mensink RP. Metabolic effects of plant sterols and stanols. J Nutr Biochem. 2003;14:362–9. doi: 10.1016/s0955-2863(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 37. Saldeen T, Li D, Mehta JL. Differential effects of alpha and gamma-tocopherol on low-density lipoprotein oxidation, superoxide activity, platelet aggregation and arterial thrombogenesis. J Am Coll Cardiol. 1999;34:1208–15. doi: 10.1016/s0735-1097(99)00333-2. [DOI] [PubMed] [Google Scholar]
- 38.Food and Agriculture Organization of the United Nation (FAO) The codex alimentarius. Italy: Codex Publication; 2001. p. 65. [Google Scholar]
- 39. Matos L, Nzikou JM, Kimbonguila A, et al. Composition and nutritional properties of seeds and oil from Terminalia catappa L. Adv J Food Sci Technol. 2009;1:72–7. [Google Scholar]
- 40. Ulu H. Evaluation of three 2-thiobarbituric acid methods for the measurement of lipid oxidation in various meats and meat products. Meat Sci. 2004;67:683–7. doi: 10.1016/j.meatsci.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 41. Lukaszewicz M, Szopa J, Krasowska A. Susceptibility of lipids from different flax cultivars to peroxidation and its lowering by added antioxidants. Food Chem. 2004;88:225–31. [Google Scholar]
- 42. Campo MM, Nute GR, Hughes SI, et al. Flavour perception of oxidation in beef. Meat Sci. 2006;72:303–11. doi: 10.1016/j.meatsci.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 43. Warner K, Orr P, Glynn M. Effect of fatty acid composition of oils on flavor and stability of fired foods. J Am Oil Chem Soc. 1997;74:347–56. [Google Scholar]