Abstract
A method with few markers to determine multicomponents was established and validated to evaluate the quality of Shenfu injection by ultraperformance liquid chromatography coupled with a photodiode array detector. The separations were performed on an ACQUITY UPLC BEH C18 (2.1 × 50 mm2, 1.7 μm) column. Methanol and 0.1% formic acid aqueous solution were used as the mobile phase. The flow rate was 0.3 mL/min. 2 aconitum alkaloids and 12 ginsenosides could be perfectly separated within 15 minutes. Ginsenoside Rg1 and benzoylmesaconine, the easily available active components, were employed as the maker components to calculate the relative correction factors of other components in Shenfu injection, Panax ginseng and Aconitum carmichaeli. The external standard method was also established to validate the feasibility of the method with few markers to determine multicomponents. Parameter p and the principal component analysis method were employed to investigate the disparities among batches for the effective quality control of Shenfu injection. The results demonstrated that the ultraperformance liquid chromatography coupled with a photodiode array detector method with few markers to determine multicomponents could be used as a powerful tool for the quality evaluation of traditional Chinese medicines and their preparations.
Keywords: aconitum alkaloid, few markers, ginsenoside, multicomponents, Shenfu injection, ultraperformance liquid, chromatography
1. Introduction
The health-promoting benefits of traditional Chinese medicines (TCMs) and their preparations have been gaining more and more attention [1]. The simultaneous determination of multicomponents was considered to be one of the key methods to evaluate the quality of TCMs [2,3]. However, the limited availability of reference standards hampers the wide popularization for quality control of TCMs. Therefore, it should be a practical option to use few easily available components to simultaneously determine multicomponents for quality evaluation of TCMs.
Shenfu injection (SFI) was derived from “Shenfu decoction,” which is an ancient herbal medicinal formula in China [4]. SFI is composed of extracts of steamed roots of Panax ginseng and processed lateral roots of Aconitum carmichaeli. SFI has been used for the treatment of cardiovascular diseases such as coronary artery disease, myocardial infarct, cardiac insufficiency, and arrhythmia [5–8]. Aconitum alkaloids and ginsenosides are main active components of SFI. Aconitum alkaloids can exhibit cardiotonic, anti-inflammatory, and analgesic activities [9]. Ginsenosides possess many biological activities, including anticerebral ischemic injury, neuro-protective, and cardiotonic activities [10]. Therefore, aconitum alkaloids and ginsenosides contribute to the curative effects of SFI.
At present, high-performance liquid chromatography (HPLC), ultraperformance liquid chromatography coupled with a photodiode array detector (UPLC-PDA), and liquid chromatography tandem mass spectrometry are used for analyzing a few ginsenosides and/or aconitum alkaloids in SFI [11–13], but the chromatographic analysis time of those methods are more than 60 minutes. The analysis time is so long that these methods are not suitable for the effective quality control of SFI. It was reported that the content of a single or a few marker compounds might not accurately reflect the quality of the complex herbal products [14]. In order to guarantee the clinic safety of SFI, a reasonable method for simultaneous determination of aconitum alkaloids and ginsenosides should be established.
UPLC-PDA has become a powerful analytical tool for its fast separation, sensitive and specific detection, and good chromatographic resolution [15,16]. It can shorten analysis time obviously, compared to the conventional HPLC system with 5 μm particle-packed analytical columns [17–19]. Recently, HPLC with a single marker to determine multicomponents has been used to control the quality of some herbal medicines [20–22]. Based on the above research, a UPLC method with few markers to determine multicomponents that belong to different types of chemicals (UPLC-FMCMC) was proposed to control the quality of TCMs.
To our knowledge, the UPLC-FMCMC method for quality control of SFI has not been reported in the literature. Considering the structural differences of aconitum alkaloids and ginsenosides, SFI was investigated as a typical example to validate the new UPLC-FMCMC method for quality control of TCM preparations. The UPLC-FMCMC method was developed for the simultaneous quantitative analysis of two aconitum alkaloids and 12 ginsenosides in SFI. The feasibility and precision of the UPLC-FMCMC method have been discussed in the present report. UPLC-FMCMC will become an advantageous tool for quality control of TCMs and their preparations.
2. Materials and methods
2.1. Materials, chemicals, and reagents
Twenty-two batches of SFIs (number of batches: 120609, 110804, 131006010, 131005010, 131013010, 131008010, 130902010, 130813010, 130812010, 130904010, 130905010, 130903010, 130505010, 130506010, 130508010, 130606010, 130605010, 130604010, 130713010, 130715010, 130705010, and 130703010) were obtained from YaAn Sanjiu Pharmaceutical Co., Ltd. (Sichuan, China) and deposited at the Academy of Traditional Chinese Medicine in Tianjin. Five samples of P. ginseng (P1–P5) and six samples of A. carmichaeli (S1–S6), gathered from different pharmacies of Tianjin, China were authenticated by Dr Yan-Xu Chang, Tianjin University of Traditional Chinese Medicine, Tianjin, China. Acetonitrile and methanol (Tianjin Concord Science Co. Ltd., Tianjin, China) were of HPLC grade. HPLC-grade formic acid was purchased from Tedia Company Inc (Fairfield, OH, United States of American). Deionized water was purified with a Milli-Q Academic ultrapure water system (Millipore; Milford, MA, USA). Reference standards such as ginsenoside Re (Re), ginsenoside Rg1 (Rg1), ginsenoside Rf (Rf), ginsenoside Rb1 (Rb1), ginsenoside Rc (Rc), ginsenoside Rb2 (Rb2), ginsenoside Rb3 (Rb3), ginsenoside Rd (Rd), ginsenoside S-Rg2 (S-Rg2), ginsenoside S-Rh1 (S-Rh1), ginsenoside S-Rg3 (S-Rg3), and ginsenoside S-Rh2 (S-Rh2) (purity > 98%) were purchased from Chengdu Must Biotechnology Co., Ltd (Chengdu, China). Benzoylhypacoitine and benzoylmesaconine (purity > 98%) were purchased from the National Institute for the Chinese National Institute of Control of Pharmaceutical and Biological Products (Beijing, China). Other reagents were of analytical grade.
2.2. Preparation of sample solutions
The SFI solutions were diluted with methanol. After centrifugation for 10 minutes at 1098×g, the supernatant was transferred into another centrifuge tube. The final solution was filtered through a membrane (0.22 μm) until analysis. Then, 4 μL aliquot of the solution was injected into the UPLC system for analysis.
Dried and powdered P. ginseng (0.300 g) and A. carmichaeli (0.400 g) were ultrasonically extracted with 10 mL methanol for 30 minutes and cooled at room temperature. It was made up to the volume with methanol and subsequently centrifuged for 10 minutes at 1098×g. An aliquot of 4 μL supernatant solution was used for UPLC analysis.
2.3. Preparation of standard solutions
The standard stock solutions of ginsenosides Re (1.03 mg/mL), Rg1 (1.02 mg/mL), Rf (1.00 mg/mL), Rb1 (1.03 mg/mL), Rc (1.01 mg/mL), Rb2 (1.02 mg/mL), Rb3 (0.98 mg/mL), Rd (1.01 mg/mL), S-Rg2 (1.03 mg/mL), S-Rh1 (1.01 mg/mL), S-Rg3 (1.02 mg/mL), and S-Rh2 (1.01 mg/mL); benzoylhypacoitine (1.00 mg/mL); and benzoylmesaconine (1.03 mg/mL) were prepared in methanol. Appropriate volumes of each stock solution were calculated and mixed together. Then, the mixture was diluted serially to achieve the standard working solutions. All solutions were kept at 4°C until use.
2.4. UPLC analysis
All analyses were performed using a Waters Acquity UPLC System (Waters Corp., Milford, MA, USA) consisting of a binary solvent manager, a sampler manager, a column compartment, and a PDA (Waters Acquity model code UPD), all controlled by the Waters Empower 2 data station software (Waters Corp.).
The separations were achieved on an ACQUITY UPLC BEH C18 (1.7 μm, 2.1 mm × 50 mm) column. Formic acid aqueous solution (0.1%, v/v) and methanol (B) were used as mobile phases. The gradient elution was conducted as follows: 30–52% (v/v) B at 0–4 minutes; 52–57% B at 4–6 minutes; 57–65% B at 6–7 minutes; 65–69% B at 7–8 minutes; 69–73% B at 8–9 minutes; 73–75% B at 9–10 minutes; 75–80% B at 10–11 minutes; 80–90% B at 11–13 minutes; and 90–30% B at 13–15 minutes. The flow rate was set at 0.3 mL/min. The column and sample temperatures were maintained at 50°C and 15°C, respectively. The total run time for analysis was 15 minutes. The injection volume was 4 μL. Detection wavelengths were set at 235 nm for alkaloids and 203 nm for ginsenosides.
2.5. Statistical analysis
SPSS version 19.0 (SPSS Inc., Chicago, IL, USA) was used for the data analysis.
3. Results and discussion
3.1. Optimization of chromatographic conditions
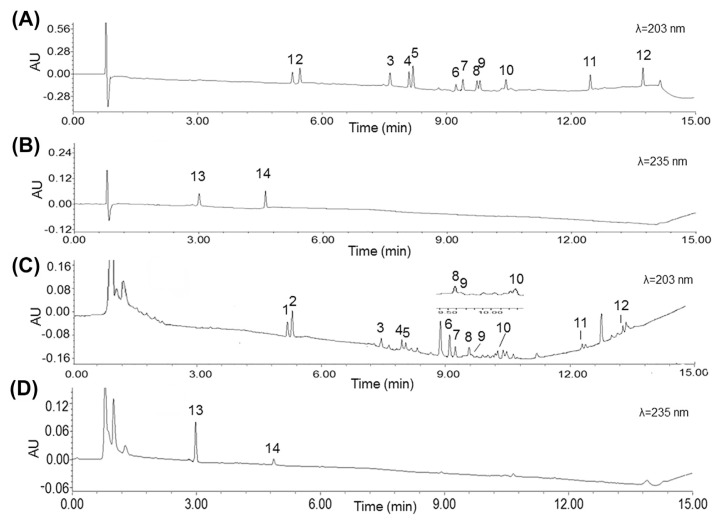
The chromatographic conditions were optimized in the study in order to obtain better resolution of adjacent peaks. The different mobile phases (acetonitrile–water and methanol–-water), column temperatures (30°C, 40°C, and 50°C), flow rates (0.2 mL/min, 0.3 mL/min, and 0.4 mL/min), and concentrations of additive (0.1%, 0.2%, and 0.3% formic acid) were optimized in our studies. The results showed that good resolution and a symmetric peak shape were obtained when methanol–formic acid aqueous solution (0.1%) was selected as a mobile phase, at a temperature of 50°C and a flow rate of 0.3 mL/min under gradient elution modes. On the basis of the absorption maxima of the 14 compounds, dual wavelengths of 203 nm and 235 nm were set to monitor signals of ginsenosides and alkaloids, respectively. The typical chromatogram is illustrated in Fig. 1.
Fig. 1.
Representative chromatograms for simultaneous quantification of the 14 active compounds in Shenfu injection: (A) Mixed standards at 203 nm; (B) mixed standards at 235 nm; (C) Shenfu injection sample at 203 nm; and (D) Shenfu injection sample at 235 nm. Peak 1 represents Re, peak 2 Rg1, peak 3 Rf, peak 4 S-Rg2, peak 5 S-Rh1, peak 6 Rb1, peak 7 Rc, peak 8 Rb2, peak 9 Rb3, peak 10 Rd, peak 11 S-Rg3, peak 12 S-Rh2, peak 13 benzoylmesaconine, and peak 14 benzoylhypacoitine.
3.2. Selection of two markers
Due to the lack of many highly purified chemical references, sometimes it is difficult to obtain multiple components for quantitative analysis of TCM. It may be an alternative approach to select a few chemicals as references for determining other ingredients with similar chemical structures. This approach is very feasible without sufficient reference substances. Among the components in SFI, benzoylmesaconine and ginsenoside Rg1 were chosen as markers for other aconitum alkaloids and ginsenosides in this study, respectively. The relative correction factors for other components were calculated by two different methods in the study. The intercepts of the regression equations of the markers and other components were calibrated to zero (y = ax + b to be y = ax). The relative correction factors were calculated by the ratios of the coefficients (RCF = a1/a2) [23]. The other way was in accordance with the rationale of quantitative analysis of multicomponents by a single marker, that is the relative correction factors:
where fm/x is the relative correction factor, Am and Ax are peak areas, and Cm and Cx are the concentrations of the marker and other components, respectively [24]. In our study, the relative correction factors of aconitum alkaloids and ginsenosides, except for benzoylmesaconine and ginsenoside Rg1, were optimized according to these methods. The results showed that ginsenoside Rb3 and benzoylhypacoitine showed good results with the second method, while the other standards were more favored by the first one. The relative correction factors of the 14 components were 1.20, 1.00, 0.90, 0.86, 0.60, 0.40, 1.35, 1.50, 2.30, 0.90, 0.82, 10.45, 1.00, and 8.80. Based on the relative correction factors, the other 12 components could be determined using the UPLC-PDA method.
3.3. Method validation of UPLC-FMCMC
The method was validated in terms of limit of quantification (LOQ), limit of detection (LOD), linearity, precision, stability, repeatability, and recovery.
3.4. Linearity, linear range, LODs, and LOQs
Stock solutions were diluted to appropriate concentrations in order to construct calibration curves. All calibration curves were calculated based on linear regression analysis of the plots of peak areas (y) versus concentrations (x, μg/mL) for the 14 reference compounds. The relative correction factors, correlation coefficients (R2), linear ranges, and regression equations are listed in Table 1. As a consequence, each coefficient of regression (R2) was > 0.9991, as determined by the least square analysis, which suggests good linearity between peak areas (y) and compound concentrations (x) over a wide concentration range. The lowest concentration of working solution was diluted with methanol to a series of appropriate concentrations and injected into the UPLC system for analysis. The LOD and LOQ for each compound under the optimal chromatographic conditions were determined at signal-to-noise ratios of 3 and 10, respectively. The LODs and LOQs of the 14 compounds are shown in Table 1. The LODs and LOQs were 0.003–0.210 μg/mL and 0.013–0.650 μg/mL, respectively, which indicated high sensitivity under these UPLC conditions.
Table 1.
Calibration curves, LOD, and LOQ of the investigated compounds.
| Analytes | RCFs | Linear regression data | LOD (μg/mL) | LOQ (μg/mL) | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Regressive equation | Test range (μg/mL) | R 2 | ||||
| Re | 1.20 | y = 1743.4x+1131.2 | 5.06~636 | 0.9997 | 0.270 | 0.940 |
| y = 3351.2x+996.54 | 2.20~275 | 0.9995 | 0.210 | 0.650 | ||
| Rg1 | 1.00 | y = 4009.8x+1131.2 | 2.20~275 | 0.9997 | 0.200 | 0.630 |
| Rf | 0.90 | y = 4465.5x+1131.2 | 1.98~372 | 0.9997 | 0.300 | 0.820 |
| y = 4488.9x−1757.4 | 1.20~150 | 0.9991 | 0.120 | 0.350 | ||
| S-Rg2 | 0.86 | y = 4678.5x+1131.2 | 1.89~111 | 0.9997 | 0.170 | 0.250 |
| y = 4735.2x−3296.3 | 0.76~95 | 0.9998 | 0.007 | 0.025 | ||
| S-Rh1 | 0.60 | y = 6717.1x+1131.2 | 1.31~236 | 0.9997 | 0.080 | 0.380 |
| y = 6782.1x−3648.3 | 0.760~95.0 | 0.9997 | 0.004 | 0.013 | ||
| Rb1 | 0.40 | y = 9943.7x+1131.2 | 0.89~111 | 0.9997 | 0.080 | 0.250 |
| y = 10119x−10622 | 0.76~95.0 | 0.9997 | 0.008 | 0.025 | ||
| Rc | 1.35 | y = 2966.1x+1131.2 | 2.97~372 | 0.9997 | 0.270 | 0.850 |
| y = 3007x−3809.8 | 1.20~150 | 0.9996 | 0.125 | 0.320 | ||
| Rb2 | 1.50 | y = 2673.8x+1131.2 | 3.30~412 | 0.9997 | 0.300 | 0.940 |
| y = 2697.2x−2038.9 | 1.20~150 | 0.9991 | 0.115 | 0.300 | ||
| Rb3 | 2.30 | y = 1743.4x+1131.2 | 2.87~359 | 0.9997 | 0.260 | 0.820 |
| y = 2064.02x−1340.7 | 0.76~95.0 | 0.9992 | 0.003 | 0.008 | ||
| Rd | 0.90 | y = 4446.1x+1131.2 | 1.98~248 | 0.9997 | 0.180 | 0.570 |
| y = 4381.2x+4999 | 0.760~95.0 | 0.9993 | 0.008 | 0.025 | ||
| S-Rg3 | 0.82 | y = 4894.5x+1131.2 | 1.80~225 | 0.9997 | 0.160 | 0.520 |
| y = 4969.3x−5026.9 | 0.760~95.0 | 0.9993 | 0.010 | 0.040 | ||
| S-Rh2 | 10.5 | y = 383.66x+1131.2 | 22.9~287 | 0.9997 | 2.09 | 6.580 |
| y = 621.16x−628.36 | 0.760~95.0 | 0.9993 | 0.010 | 0.032 | ||
| Benzoylmesaconine | 1.00 | y = 7478.9x+100.21 | 0.760~95.0 | 1.0000 | 0.030 | 0.080 |
| Benzoylhypacoitine | 8.80 | y = 849.88x+100.21 | 2.50~308 | 0.9997 | 0.180 | 0.480 |
| y = 1065.1x−159.6 | 0.280~35.0 | 0.9995 | 0.010 | 0.032 | ||
In the regression equation, the x value is the concentration of analytes (μg/mL), the y value is the peak area.
LOD = limit of detection; LOQ = limit of quantification; RCF = relative correction factor.
3.5. Precision, repeatability, and stability
The precision was investigated by one sample solution using six replicates. As shown in Table 2, all relative standard deviations (RSDs) of the precision of the method were < 5% indicating that the method was precise enough for quantitative evaluation of the analytes in SFI. The repeatability of the method was assessed by performing replicate analysis (n = 6) of the sample solutions. RSDs of repeatability were < 5%, which demonstrates that the analytical method was reproducible for the components analyzed. Stability of those analytes was assessed by analyzing SFI under the following conditions: 0 hour, 2 hours, 4 hours, 6 hours, 8 hours, 12 hours, and 24 hours. The results indicated that the RSDs of the analytes were < 5%, indicating that the sample solutions were stable for 24 hours.
Table 2.
Precision, repeatability, stability of the investigated compounds (n =6).
| Analytes | Precision (RSD) | Repeatability (RSD) | Stability (RSD) |
|---|---|---|---|
| Re | 3.11 | 1.64 | 3.44 |
| Rg1 | 1.32 | 2.03 | 3.92 |
| Rf | 3.47 | 3.65 | 0.87 |
| S-Rg2 | 1.10 | 2.60 | 2.27 |
| S-Rh1 | 1.36 | 2.27 | 1.72 |
| Rb1 | 2.77 | 1.62 | 1.09 |
| Rc | 1.68 | 2.20 | 3.51 |
| Rb2 | 3.12 | 3.64 | 3.46 |
| Rb3 | 0.91 | 2.13 | 1.10 |
| Rd | 1.92 | 3.32 | 4.54 |
| S-Rg3 | 2.27 | 3.51 | 3.83 |
| S-Rh2 | 1.75 | 4.50 | 1.73 |
| Benzoylmesaconine | 1.06 | 1.11 | 1.55 |
| Benzoylhypacoitine | 4.73 | 4.20 | 3.80 |
3.6. Recovery
The recovery experiment was performed by adding a known amount of reference compound to a certain amount of SFI. The quantity of each analyte was subsequently realized from the corresponding calibration curve and the relative correction factors. The recovery of each compound was calculated using the following formula:
As shown in Table 3, the average recoveries of the investigated targets ranged from 91.4% to 105%, and all RSD values were < 5%. In order to validate the accuracy of the developed UPLC-FMCMC method, the recoveries were evaluated by the traditional methods (external standard method). The results showed that the recovery of each component ranged from 95.4% to 105% (Table 4). These results were consistent with those obtained by the developed UPLC-FMCMC method, which demonstrated that this method was reliable and accurate for the measurement of two aconitum alkaloids and 12 ginsenosides in SFI.
Table 3.
Recoveries of the investigated compounds by UPLC-FMCMC.
| Analytes | Original (μg/mL) | Spiked (μg/mL) | Found (μg/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Re | 16.6 | 15.0 | 32.6 | 105 | 0.50 |
| Rg1 | 25.0 | ||||
| Rf | 8.14 | 10.0 | 17.9 | 98.1 | 1.05 |
| S-Rg2 | 7.42 | 10.0 | 17.4 | 99.3 | 0.78 |
| S-Rh1 | 3.76 | 5.00 | 8.37 | 92.3 | 0.86 |
| Rb1 | 8.56 | 10.0 | 17.7 | 91.4 | 0.23 |
| Rc | 14.8 | 15.0 | 29.7 | 99.6 | 0.41 |
| Rb2 | 10.2 | 10.0 | 20.2 | 99.8 | 0.84 |
| Rb3 | 2.23 | 2.50 | 4.58 | 94.0 | 1.00 |
| Rd | 3.16 | 4.00 | 7.40 | 106 | 4.02 |
| S-Rg3 | 3.65 | 4.00 | 7.55 | 97.6 | 1.55 |
| S-Rh2 | 4.39 | 4.00 | 8.51 | 103 | 1.04 |
| Benzoylmesaconine | 5.00 | ||||
| Benzoylhypacoitine | 0.47 | 1.00 | 1.43 | 96.0 | 3.78 |
Table 4.
Recoveries of the investigated compounds by traditional UPLC method.
| Analytes | Original (μg/mL) | Spiked (μg/mL) | Found (μg/mL) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Re | 16.6 | 15.0 | 32.1 | 103 | 1.06 |
| Rg1 | 25.4 | 25.0 | 50.9 | 102 | 1.82 |
| Rf | 8.42 | 10.0 | 18.3 | 99.2 | 0.83 |
| S-Rg2 | 7.80 | 10.0 | 17.9 | 101 | 1.55 |
| S-Rh1 | 4.07 | 5.00 | 8.96 | 97.8 | 2.08 |
| Rb1 | 8.99 | 10.0 | 18.5 | 95.4 | 0.46 |
| Rc | 15.4 | 15.0 | 31.0 | 104 | 1.93 |
| Rb2 | 10.7 | 10.0 | 21.1 | 104 | 1.35 |
| Rb3 | 2.17 | 2.50 | 4.57 | 96.0 | 2.49 |
| Rd | 3.26 | 4.00 | 7.41 | 104 | 3.61 |
| S-Rg3 | 3.74 | 4.00 | 7.83 | 102 | 2.67 |
| S-Rh2 | 4.13 | 4.00 | 8.01 | 96.9 | 1.11 |
| Benzoylmesaconine | 4.68 | 5.00 | 9.61 | 98.5 | 2.73 |
| Benzoylhypacoitine | 0.74 | 1.00 | 1.79 | 105 | 2.89 |
3.7. Application of UPLC-FMCMC
The newly developed UPLC-FMCMC method was used to simultaneously determine the two types of components in SFI. Twenty-two batches of SFIs obtained from the same manufacturer were tested. The contents of 14 investigated components are listed in Table 5. The results indicated that among the 14 compounds analyzed, ginsenoside Rg1 was present in the highest concentrations (ranging from 71.8 μg/mL to 105 μg/mL), followed by Rc (52.0–92.0 μg/mL), Re (47.2–66.4 μg/mL), and Rb2 (41.0–59.0 μg/mL). All the concentrations of the four components were > 40.0 μg/mL. The maximum concentrations of Rb1 (28.2–49.6 μg/mL), S-Rg2 (20.6–29.6 μg/mL), Rf (8.48–32.6 μg/mL), S-Rg3 (11.7–27.1 μg/mL), benzoylmesaconine (5.52–26.2 μg/mL), S-Rh1 (8.32–15.6 μg/mL), Rb3 (7.44–11.8 μg/mL), Rd (5.50–17.1 μg/mL), and S-Rh2 (14.4–18.2 μg/mL) were in the range of 5.00–40.0 μg/mL. Benzoylhypacoitine was present in the lowest concentrations (ranging from 1.74 μg/mL to 4.14 μg/mL). In order to investigate the difference between the newly developed UPLC-FMCMC method and the traditional methods, simultaneous determination of the 14 investigated components present in these samples was also carried out at the same time by the traditional method. As shown in Table 5, no remarkable differences (RDs < 5%) were observed between the two methods (the traditional method and the UPLC-FMCMC method).
Table 5.
Contents of 12 ginsenosides and two aconitum alkaloides in 22 batches of Shenfu injection (μg/mL).
| No. | Batch | Method | Re | Rg1 | Rf | S-Rg2 | S-Rh1 | Rb1 | Rc | Rb2 | Rb3 | Rd | S-Rg3 | S-Rh2 | Benzoylmesaconine | Benzoylhypacoitine |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 120609 | E.M | 66.6 | 102 | 33.6 | 31.2 | 16.3 | 36.0 | 61.6 | 43.0 | 8.68 | 13.0 | 14.9 | 16.5 | 18.7 | 2.72 |
| N.M | 66.4 | 32.6 | 29.6 | 15.6 | 34.2 | 59.2 | 41.0 | 8.94 | 12.7 | 14.6 | 16.8 | 2.78 | ||||
| R.D | 0.13 | 3.32 | 4.86 | 4.08 | 4.81 | 4.03 | 4.65 | 2.97 | 3.03 | 2.30 | 1.55 | 2.77 | ||||
| 2 | 110804 | E.M | 60.0 | 105 | 36.6 | 28.6 | 15.9 | 47.6 | 67.6 | 53.6 | 8.60 | 17.6 | 22.2 | 14.5 | 26.2 | 2.24 |
| N.M | 59.8 | 35.4 | 27.8 | 15.4 | 46.2 | 65.2 | 51.6 | 8.82 | 17.1 | 22.0 | 14.4 | 2.18 | ||||
| R.D | 0.14 | 3.02 | 2.60 | 2.95 | 3.19 | 3.56 | 3.55 | 2.61 | 2.93 | 0.80 | 1.20 | 2.06 | ||||
| 3 | 131006010 | E.M | 56.8 | 90.2 | 11.4 | 21.2 | 11.5 | 38.0 | 64.8 | 46.2 | 8.88 | 7.82 | 17.7 | 15.7 | 17.6 | 3.14 |
| N.M | 57.0 | 11.1 | 20.0 | 11.0 | 36.0 | 62.6 | 44.2 | 8.68 | 8.02 | 17.6 | 16.4 | 3.34 | ||||
| R.D | 0.150 | 1.86 | 5.53 | 3.99 | 5.22 | 3.89 | 4.24 | 2.29 | 2.63 | 0.54 | 4.11 | 5.90 | ||||
| 4 | 131005010 | E.M | 58.2 | 88.0 | 11.9 | 22.2 | 10.7 | 34.4 | 66.2 | 44.8 | 7.96 | 7.80 | 15.9 | 15.8 | 16.5 | 2.38 |
| N.M | 58.2 | 11.3 | 21.2 | 10.5 | 32.2 | 63.8 | 42.8 | 7.80 | 8.00 | 16.3 | 16.5 | 2.38 | ||||
| R.D | 0.15 | 5.08 | 4.29 | 2.01 | 5.89 | 3.79 | 4.41 | 2.14 | 2.65 | 2.31 | 4.51 | 0.29 | ||||
| 5 | 131013010 | E.M | 61.8 | 87.0 | 12.5 | 20.2 | 11.3 | 41.6 | 75.0 | 50.2 | 9.44 | 6.56 | 20.2 | 18.1 | 17.6 | 2.84 |
| N.M | 61.8 | 12.1 | 19.3 | 11.1 | 39.8 | 72.6 | 48.4 | 9.54 | 6.78 | 20.1 | 18.2 | 2.94 | ||||
| R.D | 0.16 | 3.22 | 3.90 | 1.81 | 4.68 | 3.19 | 3.82 | 1.07 | 3.46 | 0.25 | 0.56 | 3.74 | ||||
| 6 | 131008010 | E.M | 55.4 | 84.8 | 11.5 | 22.0 | 13.2 | 37.6 | 72.4 | 45.4 | 10.2 | 7.26 | 17.1 | 16.9 | 21.2 | 2.62 |
| N.M | 55.4 | 11.3 | 21.8 | 12.6 | 35.6 | 70.0 | 43.6 | 9.82 | 7.08 | 17.5 | 16.7 | 2.66 | ||||
| R.D | 0.14 | 1.82 | 0.70 | 4.78 | 5.29 | 3.35 | 4.32 | 3.56 | 2.50 | 2.35 | 1.39 | 1.90 | ||||
| 7 | 130902010 | E.M | 51.6 | 75.4 | 10.4 | 21.2 | 10.9 | 30.2 | 54.6 | 37.2 | 7.50 | 5.62 | 14.3 | 16.2 | 15.7 | 1.74 |
| N.M | 51.6 | 10.0 | 21.6 | 10.3 | 28.2 | 52.0 | 35.2 | 7.44 | 5.56 | 14.0 | 16.0 | 1.74 | ||||
| R.D | 0.13 | 3.48 | 2.04 | 5.38 | 6.79 | 4.85 | 5.47 | 0.75 | 0.95 | 2.04 | 0.70 | 0.60 | ||||
| 8 | 130813010 | E.M | 47.4 | 72.2 | 10.5 | 21.6 | 10.0 | 33.6 | 63.2 | 42.6 | 7.70 | 5.90 | 13.9 | 15.9 | 14.6 | 1.80 |
| N.M | 47.4 | 9.90 | 20.8 | 9.74 | 31.6 | 60.8 | 40.6 | 7.28 | 6.02 | 14.0 | 16.7 | 1.78 | ||||
| R.D | 0.12 | 5.37 | 3.52 | 2.82 | 6.02 | 4.02 | 4.67 | 5.45 | 1.99 | 0.85 | 4.84 | 0.80 | ||||
| 9 | 130812010 | E.M | 47.0 | 71.8 | 10.8 | 21.2 | 8.54 | 32.8 | 62.8 | 41.6 | 8.28 | 6.40 | 11.4 | 16.2 | 14.4 | 1.84 |
| N.M | 47.2 | 10.6 | 21.8 | 8.32 | 30.8 | 60.2 | 39.8 | 8.36 | 6.24 | 11.7 | 16.0 | 1.78 | ||||
| R.D | 0.12 | 1.51 | 2.03 | 2.53 | 6.20 | 4.06 | 4.79 | 1.03 | 2.49 | 2.38 | 0.71 | 3.44 | ||||
| 10 | 130904010 | E.M | 47.8 | 74.4 | 8.80 | 20.4 | 10.1 | 31.6 | 59.0 | 39.0 | 8.68 | 5.90 | 14.2 | 17.2 | 15.0 | 1.90 |
| N.M | 47.8 | 8.48 | 20.6 | 10.0 | 29.6 | 56.4 | 41.0 | 8.46 | 5.98 | 14.1 | 16.7 | 1.78 | ||||
| R.D | 0.12 | 3.60 | 2.06 | 0.62 | 6.48 | 4.40 | 5.10 | 2.61 | 1.19 | 1.14 | 2.61 | 5.83 | ||||
| 11 | 130905010 | E.M | 49.4 | 79.6 | 11.8 | 25.2 | 12.0 | 35.0 | 67.2 | 42.8 | 9.40 | 5.68 | 19.8 | 16.5 | 15.6 | 1.78 |
| N.M | 49.6 | 11.2 | 24.6 | 11.7 | 33.0 | 64.8 | 40.8 | 8.90 | 5.96 | 19.3 | 17.4 | 1.74 | ||||
| R.D | 0.13 | 5.13 | 2.02 | 2.52 | 5.76 | 3.71 | 4.65 | 5.37 | 4.92 | 2.15 | 5.48 | 1.30 | ||||
| 12 | 130903010 | E.M | 63.0 | 103 | 12.3 | 22.8 | 11.5 | 37.3 | 73.3 | 45.8 | 10.6 | 7.70 | 18.1 | 15.7 | 16.7 | 3.46 |
| N.M | 63.2 | 11.9 | 22.2 | 11.3 | 35.3 | 70.8 | 43.8 | 10.7 | 7.80 | 18.0 | 16.3 | 3.52 | ||||
| R.D | 0.16 | 3.28 | 2.40 | 2.25 | 5.35 | 3.3 | 4.29 | 1.02 | 1.41 | 0.28 | 3.88 | 1.89 | ||||
| 13 | 130505010 | E.M | 62.6 | 97.8 | 12.4 | 25.6 | 10.7 | 40.2 | 76.6 | 49.8 | 12.4 | 6.52 | 16.2 | 17.2 | 11.56 | 2.30 |
| N.M | 62.8 | 12.0 | 25.0 | 10.9 | 38.2 | 74.2 | 47.8 | 11.9 | 6.72 | 16.0 | 16.8 | 2.26 | ||||
| R.D | 0.16 | 3.26 | 1.96 | 1.70 | 4.89 | 3.11 | 3.86 | 4.45 | 2.95 | 1.16 | 2.50 | 1.36 | ||||
| 14 | 130506010 | E.M | 63.0 | 104. | 17.1 | 27.4 | 14.4 | 51.4 | 94.2 | 61.0 | 12.4 | 8.42 | 26.9 | 16.8 | 11.78 | 2.74 |
| N.M | 63.2 | 16.7 | 25.8 | 14.0 | 49.6 | 92.0 | 59.0 | 11.8 | 8.64 | 27.1 | 16.7 | 2.82 | ||||
| R.D | 0.16 | 2.43 | 5.37 | 2.52 | 3.61 | 2.29 | 3.00 | 4.56 | 2.62 | 0.59 | 0.67 | 3.00 | ||||
| 15 | 130508010 | E.M | 59.0 | 97.6 | 13.4 | 25.8 | 11.0 | 41.2 | 75.8 | 49.2 | 10.1 | 7.58 | 21.0 | 16.8 | 6.04 | 2.60 |
| N.M | 59.2 | 12.8 | 25.2 | 10.8 | 39.4 | 73.4 | 47.2 | 9.94 | 7.78 | 21.4 | 17.0 | 2.64 | ||||
| R.D | 0.15 | 4.44 | 1.94 | 2.12 | 4.74 | 3.15 | 3.93 | 1.75 | 2.73 | 2.01 | 1.67 | 1.82 | ||||
| 16 | 130606010 | E.M | 58.8 | 102 | 13.4 | 24.4 | 13.5 | 43.4 | 80.0 | 53.0 | 10.0 | 6.80 | 21.8 | 15.2 | 5.52 | 1.92 |
| N.M | 59.0 | 12.8 | 24.0 | 13.3 | 41.4 | 77.6 | 51.0 | 9.84 | 7.08 | 21.4 | 15.6 | 1.80 | ||||
| R.D | 0.15 | 4.44 | 1.31 | 1.69 | 4.47 | 2.92 | 3.58 | 1.91 | 4.21 | 1.69 | 2.17 | 6.56 | ||||
| 17 | 130605010 | E.M | 59.2 | 103 | 10.8 | 23.8 | 12.6 | 43 | 80.0 | 52.2 | 9.12 | 6.66 | 19.9 | 16.3 | 5.64 | 2.22 |
| N.M | 59.4 | 10.6 | 22.8 | 12.0 | 41.2 | 77.6 | 50.2 | 8.76 | 6.68 | 19.6 | 17.2 | 2.18 | ||||
| R.D | 0.15 | 2.07 | 3.91 | 5.10 | 4.49 | 2.92 | 3.65 | 3.95 | 0.17 | 1.52 | 6.01 | 2.22 | ||||
| 18 | 130604010 | E.M | 62.2 | 101 | 12.4 | 25.4 | 11.4 | 43.2 | 71.0 | 53.0 | 11.4 | 6.76 | 16.8 | 17.3 | 6.94 | 1.86 |
| N.M | 62.2 | 12.0 | 24.4 | 11.0 | 41.2 | 68.6 | 51.0 | 11.0 | 6.80 | 16.9 | 18.1 | 1.78 | ||||
| R.D | 0.16 | 3.21 | 3.56 | 4.04 | 4.48 | 3.45 | 3.58 | 3.04 | 0.50 | 0.36 | 4.64 | 4.21 | ||||
| 19 | 130713010 | E.M | 57.4 | 95.6 | 12.5 | 21.8 | 13.2 | 42.6 | 80.8 | 57.2 | 9.46 | 7.52 | 25.3 | 16.5 | 14.9 | 3.96 |
| N.M | 57.6 | 12.1 | 21.2 | 12.6 | 40.8 | 78.4 | 55.4 | 9.36 | 7.74 | 25.1 | 17.2 | 4.14 | ||||
| R.D | 0.15 | 3.33 | 2.57 | 4.79 | 4.50 | 2.88 | 3.24 | 1.03 | 2.92 | 0.60 | 4.41 | 4.79 | ||||
| 20 | 130715010 | E.M | 63.0 | 109 | 14.7 | 23.4 | 12.6 | 38.6 | 74.2 | 48.4 | 11.0 | 5.22 | 19.9 | 17.2 | 20.2 | 3.10 |
| N.M | 63.2 | 14.3 | 23.8 | 11.92 | 36.6 | 71.8 | 46.4 | 10.8 | 5.50 | 20.0 | 17.7 | 3.26 | ||||
| R.D | 0.16 | 2.65 | 1.99 | 5.12 | 5.12 | 3.24 | 4.01 | 1.99 | 5.28 | 0.57 | 3.23 | 5.57 | ||||
| 21 | 130705010 | E.M | 55.2 | 95.4 | 12.4 | 23.2 | 11.9 | 40.2 | 78.4 | 51.8 | 9.96 | 7.40 | 20.6 | 15.6 | 15.9 | 1.88 |
| N.M | 55.4 | 12.2 | 23.6 | 11.2 | 38.2 | 76.0 | 50.0 | 9.96 | 7.54 | 20.5 | 16.2 | 1.94 | ||||
| R.D | 0.14 | 1.63 | 1.99 | 5.51 | 4.88 | 3.00 | 3.67 | 0.05 | 1.98 | 0.64 | 3.71 | 3.37 | ||||
| 22 | 130703010 | E.M | 60.6 | 100 | 13.0 | 21.0 | 12.1 | 42.6 | 82 | 53.2 | 10.2 | 6.54 | 19.4 | 16.2 | 9.46 | 2.36 |
| N.M | 60.8 | 12.6 | 21.4 | 11.8 | 40.6 | 79.6 | 51.4 | 9.98 | 6.84 | 19.3 | 17.1 | 2.34 | ||||
| R.D | 0.16 | 2.61 | 2.04 | 2.08 | 4.56 | 2.82 | 3.55 | 1.66 | 4.48 | 0.48 | 5.72 | 0.68 |
E.M = concentration calculated by traditional method; N.M = concentration calculated by new developed method; R.D = relative deviation.
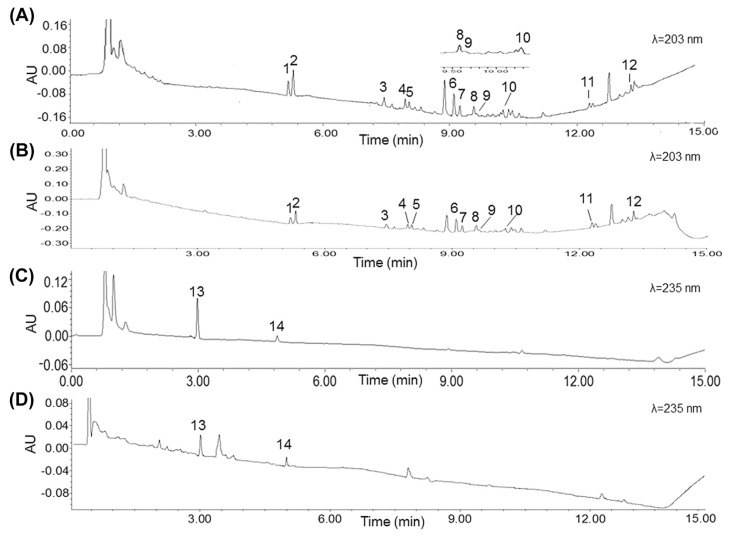
In order to further verify the developed UPLC-FMCMC method, the two crude herb components of SFI, roots of P. ginseng and processed lateral roots of A. carmichaeli, were analyzed under the UPLC system. The chromatograms were compared with the chromatograms of SFI, which are shown in Fig. 2. The results demonstrated that the main components in SFI were from P. ginseng, and the chromatograms of SFI and P. ginseng at 203 nm were nearly the same. Contents of the 14 investigated components in P. ginseng and A. carmichaeli are listed in Tables 6 and 7. The results indicated that among the 14 analyzed compounds, contents of ginsenoside Rg1 were highest (ranging from 0.85 mg/g to 1.11 mg/g), followed by those of Rc (0.52–1.12 mg/g), Re (0.55–0.74 mg/g), and Rb2 (0.43–1.02 mg/g). The contents of Rf (0.27–0.54 mg/g), Rb1 (0.29–0.41 mg/g), S-Rh2 (0.23–0.31 mg/g), S-Rg3 (0.11–0.22 mg/g), Rd (0.09–0.30 mg/g), S-Rg2 (0.03–0.23 mg/g), Rb3 (0.09–0.12 mg/g), and S-Rh1 (0.03–0.08 mg/g) were in the range of 0.03–0.54 mg/g in P. ginseng extract. The content of benzoylmesaconine was in the range of 0.68–0.90 mg/g and that of benzoylhypacoitine 0.21–0.31 mg/g in A. carmichaeli extract. As shown in Tables 6 and 7, no remarkable differences (RDs < 5%) were observed between the two methods (the traditional method and the UPLC-FMCMC method). As a consequence, the developed UPLC-FMCMC method could be applied for simultaneous determination of active components for the quality evaluation of SFI, P. ginseng, and A. carmichaeli.
Fig. 2.
Representative chromatograms for simultaneous quantification of the 14 active compounds: (A) Shenfu injection at 203 nm; (B) Panax ginseng extract; (C) Shenfu injection at 235 nm; and (D) Aconitum carmichaeli extract. Peak 1 represents Re, peak 2 Rg1, peak 3 Rf, peak 4 S-Rg2, peak 5 S-Rh1, peak 6 Rb1, peak 7 Rc, peak 8 Rb2, peak 9 Rb3, peak 10 Rd, peak 11 S-Rg3, peak 12 S-Rh2, peak 13 benzoylmesaconine, and peak 14 benzoylhypacoitine.
Table 6.
Contents of 12 ginsenosides in Panax ginseng (mg/g).
| No. | Method | Re | Rg1 | Rf | S-Rg2 | S-Rh1 | Rb1 | Rc | Rb2 | Rb3 | Rd | S-Rg3 | S-Rh2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | E.M | 0.56 | 0.87 | 0.35 | 0.03 | 0.08 | 0.29 | 0.98 | 1.02 | 0.12 | 0.14 | 0.17 | 0.24 |
| N.M | 0.55 | 0.34 | 0.03 | 0.09 | 0.28 | 0.95 | 1.00 | 0.12 | 0.14 | 0.17 | 0.23 | ||
| R.D | 1.66 | 3.84 | 3.57 | 4.04 | 2.28 | 3.00 | 2.02 | 2.39 | 0.42 | 0.73 | 2.53 | ||
| P2 | E.M | 0.63 | 1.06 | 0.45 | 0.08 | 0.03 | 0.35 | 0.52 | 0.44 | 0.12 | 0.09 | 0.22 | 0.31 |
| N.M | 0.62 | 0.43 | 0.07 | 0.03 | 0.33 | 0.50 | 0.44 | 0.11 | 0.09 | 0.22 | 0.32 | ||
| R.D | 1.46 | 2.88 | 3.35 | 1.93 | 4.60 | 3.80 | 1.28 | 4.43 | 0.18 | 0.95 | 2.91 | ||
| P3 | E.M | 0.63 | 1.11 | 0.54 | 0.09 | 0.03 | 0.36 | 0.55 | 0.64 | 0.15 | 0.12 | 0.11 | 0.29 |
| N.M | 0.62 | 0.52 | 0.08 | 0.03 | 0.34 | 0.54 | 0.62 | 0.16 | 0.11 | 0.11 | 0.29 | ||
| R.D | 1.46 | 2.27 | 2.74 | 1.91 | 4.44 | 2.66 | 3.74 | 3.66 | 2.00 | 0.30 | 1.97 | ||
| P4 | E.M | 0.68 | 0.88 | 0.28 | 0.23 | 0.06 | 0.41 | 0.70 | 0.75 | 0.05 | 0.11 | 0.17 | 0.23 |
| N.M | 0.67 | 0.27 | 0.23 | 0.06 | 0.41 | 0.67 | 0.72 | 0.05 | 0.11 | 0.17 | 0.24 | ||
| R.D | 1.32 | 1.50 | 0.06 | 5.00 | 1.32 | 4.70 | 3.08 | 1.10 | 0.41 | 0.73 | 4.11 | ||
| P5 | E.M | 0.74 | 0.85 | 0.27 | 0.07 | 0.04 | 0.38 | 1.12 | 0.43 | 0.10 | 0.30 | 0.13 | 0.30 |
| N.M | 0.75 | 0.27 | 0.07 | 0.04 | 0.37 | 1.09 | 0.41 | 0.10 | 0.31 | 0.13 | 0.31 | ||
| R.D | 0.56 | 1.51 | 3.93 | 1.80 | 1.53 | 2.47 | 3.71 | 4.61 | 2.21 | 0.45 | 3.40 |
E.M = concentration calculated by traditional method; N.M = concentration calculated by new developed method; R.D = relative deviation.
Table 7.
Contents of two aconitum alkaloides in Aconitum carmichaeli (mg/g).
| No. | Method | Benzoylmesaconine | Benzoylhypacoitine |
|---|---|---|---|
| S1 | E.M | 0.90 | 0.30 |
| N.M | 0.31 | ||
| R.D | 3.68 | ||
| S2 | E.M | 0.68 | 0.21 |
| N.M | 0.22 | ||
| R.D | 2.78 | ||
| S3 | E.M | 0.97 | 0.28 |
| N.M | 0.27 | ||
| R.D | 0.84 | ||
| S4 | E.M | 0.78 | 0.25 |
| N.M | 0.24 | ||
| R.D | 2.14 | ||
| S5 | E.M | 0.75 | 0.21 |
| N.M | 0.22 | ||
| R.D | 2.35 | ||
| S6 | E.M | 0.75 | 0.31 |
| N.M | 0.33 | ||
| R.D | 3.77 |
E.M = concentration calculated by traditional method; N.M = concentration calculated by new developed method; R.D = relative deviation.
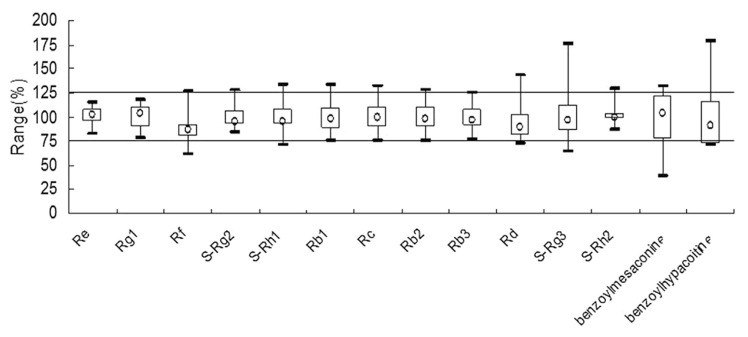
3.8. Parameter p analysis
To investigate the quality fluctuations among batches, a parameter p was employed. In general, a value in the range of 75–125% was considered acceptable [25]. The value of parameter p was calculated using the following formula:
(Ch represents the measured concentration of the components in each batch and Ci denotes the average concentration of the components in the 22 batches). As illustrated in Fig. 3, only the p values of Re, Rg1, and Rb3 were in the range of 75–125%, while for others the values were all beyond this range, with the highest p values exceeding 175% (recorded for S-Rg3 and benzoylhypacoitine), indicating that great fluctuations may exist among batches. Moreover, the results demonstrated that both S-Rg3 and benzoylhypacoitine were mainly responsible for the fluctuation among batches.
Fig. 3.
Box chart of 14 components from 22 batches of Shenfu injection.
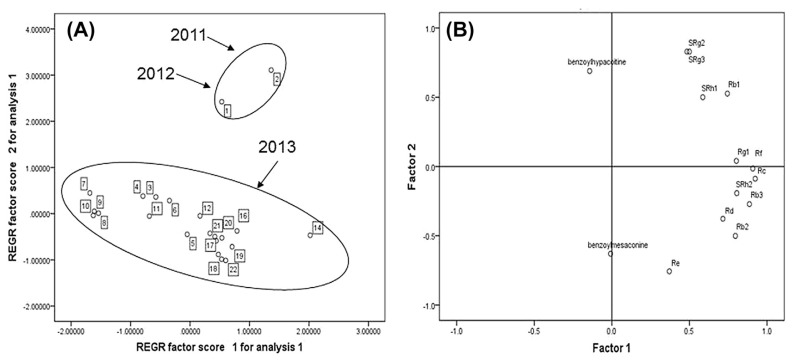
3.9. Principal component analysis
To investigate further disparities among batches, principal component analysis was employed. The sum of PC1 and PC2 were above 75.6% of the total variance, which meant that the two PCs were sufficient to describe the variability. The score plot and the loading plot for active components generated from a comparison of the two principal components are depicted in Fig. 4. As shown in Fig. 4A, 22 batches were classified into two groups, which showed the disparities between different batches. A clear classification of two clusters, based on the production date, could be observed: PC2 values of samples made in 2013 were comparatively clustered into one group, while those made in 2011 and 2012 were clustered into other groups. The loading plot highlights the importance of the contribution of each variable to the sample classification in the principal component analysis. As can be seen in the loading plot in Fig. 4B, the component regions that most strongly contributed to the separation of the samples corresponded to benzoylhypacoitine, S-Rg2, S-Rg3, S-Rh1, and Rb1, based on PC2 values, which indicates that the fluctuation of components had a strong influence on the disparity among batches. From Table 5, the average contents of benzoylhypacoitine, S-Rg2, S-Rg3, S-Rh1, and Rb1 in the 20 batches of samples made in 2013 were 2.42 ± 0.63 μg/mL, 22.99 ± 2.06 μg/mL, 18.52 ± 3.79 μg/mL, 11.66 ± 1.37 μg/mL, and 38.93 ± 5.08 μg/mL, respectively. Among these marker components of the samples made in 2011 and 2012, the contents of S-Rg2 (31.2 μg/mL and 28.6 μg/mL, respectively) and S-Rh1 (16.3 μg/mL and 15.9 μg/mL, respectively) were higher than the average contents of samples made in 2013. This may be the reason why PC2 values of batches made in 2011 and 2012 were clustered into other groups. Therefore, more attention should be paid to study and normalize the contents of benzoylhypacoitine, S-Rg2, S-Rg3, S-Rh1, and Rb1 for ensuring the quality of SFI. These components have been selected as makers in the method developed for the quality control of SFI.
Fig. 4.
PCA analysis of 22 batches of Shenfu injection samples: (A) score plot and (B) loading plot. PCA =principal component analysis; REGR (Regression). The number “1 to 22” represent different batches of Shenfu injection. 1 was produced in 2012, 2 was 2011 and 3 to 22 was made in 2013.
4. Conclusion
A simple and rapid UPLC-PDA method with few markers to determine multicomponents were developed and validated to simultaneously determine two aconitum alkaloids and 12 ginsenosides in SFI. Method validation revealed that the method was acceptable as a practical technique and fulfilled the routine quality control requirements of SFI. The results showed that it was promising to improve the quality control of SFI with few markers to determine the multicomponents. In summary, the proposed UPLC-PDA method with few markers to determine multicomponents can be employed as a useful tool to evaluate the quality of TCMs. Although the method recommended here is considered to be an alternative technique when there is a shortage of chemical references, it should not be neglected of the potential fluctuation in relative correction factors in different laboratories for more precise results. Interlab cross validation is needed in further research.
Acknowledgments
This research was financially supported by the Ministry of Science and Technology of China (No. 973: 2012CB518404 and 2012CB723504), Doctoral Fund of Ministry of Education of China (20131210120015), Program for Innovative Research Team in Universities of Tianjin (TD12-5033), and Tianjin Research Program of Application Foundation and Advanced Technology (12JCQNJC08800).
Funding Statement
This research was financially supported by the Ministry of Science and Technology of China (No. 973: 2012CB518404 and 2012CB723504), Doctoral Fund of Ministry of Education of China (20131210120015), Program for Innovative Research Team in Universities of Tianjin (TD12-5033), and Tianjin Research Program of Application Foundation and Advanced Technology (12JCQNJC08800).
Footnotes
Conflict of interest
There was no conflict of interest in the study.
REFERENCES
- 1. Wen YL, Yan LP, Chen CS. Effects of fermentation treatment on antioxidant and antimicrobial activities of four common Chinese herbal medicinal residues by Aspergillus oryzae. J Food Drug Anal. 2013;21:219–26. [Google Scholar]
- 2. Gao XY, Jiang Y, Lu J, Tu PF. One single standard substance for the determination of multiple anthraquinone derivatives in rhubarb using high-performance liquid chromatography-diode array detection. J Chromatogr A. 2009;1216:2118–23. doi: 10.1016/j.chroma.2008.11.104. [DOI] [PubMed] [Google Scholar]
- 3. Chuang YK, Chen SM, Martin Lo YM, Yang I-C, Cheng Y-F, Wang C-Y, Tsai C-Y, Hsieh R-M, Wang K-H, Lai C-C, Chen W-C. Quantification of bioactive gentiopicroside in the medicinal plant Gentiana scabra Bunge using near infrared spectroscopy. J Food Drug Anal. 2013;21:317–24. [Google Scholar]
- 4. Hong FF, He CS, Liu XJ, Tu G, Guo F, Yang S. Protective effect of Shenfu injection on thromboangiitis obliterans model rats. J Ethnopharmacol. 2011;138:458–62. doi: 10.1016/j.jep.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 5. Dong H, Xiong LZ, Chen M. Study on protective effect of Shenfu injection on cardiac function of patients undergoing valve replacement. Chin J Integr Trad West Med. 2004;24:32–5. [PubMed] [Google Scholar]
- 6. Su GH, Liu L, Meng QH. Effect of Shenfu injection on brain natriuretic polypeptide and aminoterminal peptide of precollagen type III in patients with acute myocardial infarction during intervention treatment. Chin J Integr Trad West Med. 2005;25:422–4. [PubMed] [Google Scholar]
- 7. Li ZE. Clinical research on effects of Shenfu injection in different dosage in preventing heart failure occurred in patients of acute myocardial infarction with elevated ST segment. Chin J Integr Trad West Med. 2006;26:555–7. [PubMed] [Google Scholar]
- 8. Li DZ, Ye M, Xu Y. Effects of Shenfu injection on inflammatory cytokines during cardiopulmonary bypass in infants. Chin J Integr Trad West Med. 2007;27:211–3. [PubMed] [Google Scholar]
- 9. Tang L, Gong Y, Lv C, Ye L, Liu L, Liu Z. Pharmacokinetics of aconitine as the targeted marker of Fuzi (Aconitum carmichaeli) following single and multiple oral administrations of Fuzi extracts in rat by UPLC/MS/MS. J Ethnopharmacol. 2012;141:736–41. doi: 10.1016/j.jep.2011.08.070. [DOI] [PubMed] [Google Scholar]
- 10. Xia ZY, Liu XY, Zhan LY, He YH, Luo T, Xia Z. Ginsenosides compound (Shenfu injection) attenuates gastrointestinal injury and inhibits inflammatory response after cardiopulmonary bypass in patients with congenital heart disease. J Thorac Cardiovasc Surg. 2005;130:258–64. doi: 10.1016/j.jtcvs.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 11. Yang RJ, Li XW, Zhang PX, Yao H, Yu A-M, Zhao X-Z, Jin Y-R. Determination of four kinds of monoester-diterpenoid aconines in Shenfu injection by homogeneous ionic liquid microextraction-high performance liquid chromatography. Chem J Chinese Univ. 2011;32:2752–6. [Google Scholar]
- 12. Xie H, Wang Y, Wang GL, Sheng L-S, Liu Z-Y. Content determination of ginsenoside Rg1 and ginsenoside Re in Shenfu injection. West China J Pharmaceut Sci. 2006;21:208–9. [Google Scholar]
- 13. Yang H, Liu L, Gao W, Liu K, Qi LW, Li P. Direct and comprehensive analysis of ginsenosides and diterpene alkaloids in Shenfu injection by combinatory liquid chromatography-mass spectrometric techniques. J Pharm Biomed Anal. 2014;92:13–21. doi: 10.1016/j.jpba.2013.12.041. [DOI] [PubMed] [Google Scholar]
- 14. Xue T, Roy R. Studying traditional Chinese medicine. Science. 2003;300:740–1. doi: 10.1126/science.300.5620.740. [DOI] [PubMed] [Google Scholar]
- 15. Nováková L, Matysová L, Solich P. Advantages of application of UPLC in pharmaceutical analysis. Talanta. 2006;68:908–18. doi: 10.1016/j.talanta.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 16. Liu Y, Song X, Yan R, Li T, Chai X, Qi A, Wang Y, Jiang Z. Development and validation of a UPLC-DAD-MS method for characterization and quantification of alkaloids in Menispermi Rhizoma and its preparations. J Food Drug Anal. 2013;21:206–18. [Google Scholar]
- 17. Ming DS, Heathcote J. A rapid and accurate UPLC/MS/MS method for the determination of benzodiazepines in human urine. J Chromatogr B. 2011;879:421–8. doi: 10.1016/j.jchromb.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 18. Wu TY, Lay HL. Effect of growth stages, culture media, and processing methods on the component variations of Bletilla formosana and comparison of its component contents to commercial Rhizoma Bletillae crude drugs. J Food Drug Anal. 2013;21:404–13. [Google Scholar]
- 19. Chen RC, Wei KJ, Wang TM, Yu Y-M, Li J-Y, Lee S-H, Wang W-H, Ren T-J, Tsai C-W. Simultaneous quantification of antibiotic dyes in aquatic products and feeds by liquid chromatography–tandem mass spectrometry. J Food Drug Anal. 2013;21:339–46. [Google Scholar]
- 20. Zhu JJ, Wang ZM, Kuang YH, Zhang QW, Gao QP, Ma N. A quantitative method using one marker for simultaneous assay of ginsenosides in Panax ginseng and P. notoginseng. Acta Pharm Sin. 2008;43:1211–6. [PubMed] [Google Scholar]
- 21. Lu W, Niu Y, Yang H, Sheng Y, Shi H, Yu LL. Simultaneous HPLC quantification of five major triterpene alcohol and sterol ferulates in rice bran oil using a single reference standard. Food Chem. 2014;148:329–34. doi: 10.1016/j.foodchem.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 22. Hou JJ, Wu WY, Da J, Yao S, Long HL, Yang Z, Cai LY, Yang M, Liu X, Jiang BH, Guo DA. Ruggedness and robustness of conversion factors in method of simultaneous determination of multi-components with single reference standard. J Chromatogr A. 2011;1218:5618–27. doi: 10.1016/j.chroma.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 23. Sun P, Wang X, Alquier L, Maryanoff CA. Determination of relative response factors of impurities in paclitaxel with high performance liquid chromatography equipped with ultraviolet and charged aerosol detectors. J Chromatogr A. 2008;1177:87–91. doi: 10.1016/j.chroma.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 24. Zhu JJ, Wang ZM, Ma XY, Feng W-H, Zhang Q-W. A quantitative method for simultaneous determination of four anthraquinones with one marker in Rhei Radix Rhizoma. Chin Herb Med. 2012;4:157–63. [Google Scholar]
- 25. Liu XS, Wu ZZ, Yang K, Ding H, Wu Y. Quantitative analysis combined with chromatographic fingerprint for comprehensive evaluation of Danhong injection using HPLC-DAD. J Pharm Biomed Anal. 2013;76:70–4. doi: 10.1016/j.jpba.2012.12.013. [DOI] [PubMed] [Google Scholar]