Abstract
To avoid or retard the lipid peroxidation of meat products, antioxidants are commonly added. Considering the safety and health of additives in meat products, consumers prefer natural antioxidants rather than synthetic ones. Gentisic acid and epicatechin were identified as the major phenolic acid and flavonoid, respectively, of litchi flowers (LFs). The physicochemical properties of pork meatballs with or without dried LF powders (0.5%, 1.0%, and 1.5%, w/w) and tert-butylhydroquinone (TBHQ; 0.01%, w/w) were analyzed during a 4-week frozen storage period. LF and TBHQ decreased (p < 0.05) thiobarbituric acid reactive substance (TBARS) values but increased (p < 0.05) thiol group contents in meatballs. LF added to meatballs improved (p < 0.05) texture and water-holding capacity (centrifugation/purge losses) more than in the control group upon the storage. Although LF powders made meatballs redder and darker (p < 0.05) than the control and TBHQ groups, they did not affect the preference of panelists. The addition of 0.5% LF powders exhibited the best (p < 0.05) overall sensory panel acceptance. LFs may be an effective natural antioxidant to reduce lipid and protein oxidation for frozen cooked meat products.
Keywords: lipid peroxidation, litchi flower powder, polyphenol profile, pork meatball, protein degradation
1. Introduction
Meat products provide essential nutrients such as protein, fat, and minerals. However, due to the fat content, meat products are easily oxidized during storage. Lipid peroxidation causes the deterioration of food, resulting in off flavors and degradation products. In addition, to negatively affecting physicochemical properties, lipid peroxidation can also affect nutritional value, texture, and water holding capacity of meat products [1]. Hence, understanding the mechanisms of lipid and protein oxidation is a high-priority challenge regarding the meat product industry.
To retard the lipid peroxidation of meat products, antioxidants are commonly added. There are two categories of antioxidants: (1) synthetic antioxidants, such as butylated hydroxytoluene, butylated hydroxyanisole, and tert-butylhydroquinone (TBHQ), and (2) natural antioxidants, such as rosemary, lemon balm, thyme, marjoram, and Western parsley [2,3]. The safety and healthiness of additives in meat products causes consumers to seek natural antioxidants rather than synthetic ones. Litchi (Litchi chinensis Sonn.) is a tropical and subtropical fruit of the Sapindaceae family and also an important fruit in Taiwan’s economics. The litchi fruit is edible and juicy, but the unpollinated LFs are discarded as a waste product. LFs contain rich polyphenols which can scavenge free radicals [4]. Moreover, based on our previous study, LF water extracts show a good protective effect on cardiovascular health via lowering serum lipid and thiobarbituric acid reactive substances (TBARS) values [5].
An emulsified pork meatball, commonly called “Kungwan”, is a popular meat product in Taiwan as well as in other Chinese communities. The emulsified pork meatballs mainly consist of 70–80% pork leg meat and 20–30% pork fat. A stable meat emulsion possesses a solubilized protein network that contributes to an elastic texture that is different from ground meat products. However, the higher fat content in these meatball products promotes rancidity and deterioration of physicochemical properties during refrigerated or frozen storage. Blackcurrants (Ribes nigrum L.) as a natural antioxidant reduced lipid and protein oxidation, and stabilized the red color during storage in pork patties [1]. It was reported that extracts from rosemary (Rosmarinus officinalis L.) and lemon balm (Melissa officinalis L.) can reduce lipid and protein oxidation, preserve color/texture, and add aroma to cooked pork patties [2]. An addition of olive leaf extracts offered inhibitory effects on lipid and protein oxidation in cooked pork patties enriched with omega-3 fatty acids [6]. Based on previously described researches, these plants possess antioxidative properties which are attributed to their phytochemicals, i.e., anthocyanin, carnosic acid, rosmarinic acid, flavones, and ascorbic acid. The objectives of this research were to study effects of ground LF powders on lipid peroxidation and physical properties of meatballs during frozen storage.
2. Materials and methods
2.1. Preparation of LFs, and analyses of polyphenol compounds and ascorbic acid in LFs
Three batches of fresh LFs (about 3 kg each) were collected in different local fruit farms (Taichung City, Taiwan). Flowers were dried in a hot-air dryer (Chi-Yeh Electric and Machinery Co., Taipei, Taiwan) at 40°C for 16 hours, and ground to powders (moisture < 1% by using an oven method). Polyphenolic compounds in LF powders were analyzed according to a previous report [7]. Dried LF powders (1 g) were steeped with 100 mL boiled distilled water for 30 minutes, and then filtered through a No. 1 filter paper (Advantec, Tokyo Roshi Kaisha Ltd., Japan). The high performance liquid chromatography (HPLC) system consisted of a PrimeLine Gradient Model 500G HPLC pump system (Analytical Scientific Instruments, Inc., El Sobrante, CA, USA) and an S-3210 photodiode-array detector (Schambeck SFD GmbH, Bad Honnef, Germany). A Hypersil GOLD C18 column (250 mm × 4.6 mm, 5 μm; Thermo Fisher Scientific Inc., Runcorn, Cheshire, UK) and a gradient solvent system consisting of methanol (MeOH; solvent A; Sigma Co., St. Louis, MO, USA) and distilled and deionized water (dd H2O) with 9% glacial acetic acid (solvent B) (Sigma Co.; conditions: 5–17% A from 0 minutes to 5 minutes and kept at 17% A from 5 minutes to 25 minutes; 17–31% A from 25 minutes to 40 minutes and kept at 31% A from 40 minutes to 76 minutes; 31–40% A from 76 minutes to 80 minutes and kept at 40% A from 80 minutes to 120 minutes; flow rate = 0.8 mL/min) were employed for separation of flavonoids and phenolic acids. UV spectra were recorded from 220 nm to 450 nm. Contents of flavonoids and phenolic acids in 1% (w/v) LF powder water extracts (LFPWEs) were quantified using standard curves of authentic compounds. Authentic compounds were purchased from Sigma Co. and included phenolic acid compounds: gallic acid, gentisic acid, chlorogenic acid, p-hydroxybenzoic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid, sinapic acid, syringic acid, p-anisic acid, and rosmarinic acid, as well as flavonoid compounds: catechin, epicatechin, rutin, naringin, myricetin, hesperidin, quercitrin, neohesperidin, eriodictyol, diosmin, morin, daidzein, quercetin, glycitein, naringenin, luteolin, genistein, hesperetin, kaempferol, apigenin, and isorhamnetin. Ascorbic acid contents in 1% LF water extracts were quantified according to the method, and expressed to mg/100 mL [8].
2.2. Preparation of emulsified pork meatballs
Pork back-leg muscle tissues and back fat were purchased from a meat packer (Shang Lee Food Co., Ltd, Nantou County, Taiwan). The preparation of emulsified meatballs based on a commercial production scale is described as following: first, pork leg meat (7.5 kg) was ground by a meat slicer (K3 Slicer, Kinn Shang Hoo Iron Works Co., Kaohsiung City, Taiwan), and the meat paste was always kept below 10°C; it was then mixed with NaCl (138 g; Taiyen Biotech Co., Ltd., Tainan City, Taiwan) and polyphosphate (23 g; Gemfont Co., Taipei, Taiwan) for 5 minutes. The meat paste was blended with sugar (213 g; Taiwan Sugar Co., Tainan City, Taiwan), garlic powder (20 g), and pepper (25 g). Then, pork back fat (2.5 kg) was added and blended with the meat paste. The same emulsified meat pastes were assigned to five treatments: (1) Control group: a basal formulation; (2) TBHQ group: a basal formulation with an addition of 0.01% (w/w) TBHQ (Wellwiz Enterprise Co., Ltd., Changhua County, Taiwan); (3) LF0.5 group: a basal formulation with an addition of 0.5% (w/w) dried LF powders; (4) LF1.0 group: a basal formulation with an addition of 1.0% (w/w) dried LF powders; and (5) LF1.5 group: a basal formulation with an addition of 1.5% (w/w) dried LF powders. After 5 minutes, the emulsified meat paste was cooled at 4°C for 30 minutes which is responsible for the “setting (suwari)” phenomenon, resulting in more elastic gel, and then handmade meatballs (approximately 4 cm, diameter; 15 g/meatball) were cooked in 90°C water for 10 minutes (internal endpoint temperature: 75°C). The cooked emulsified meatballs were water-cooled (0°C) for 10 minutes and then dried via a fan. Finally, the meatballs were vacuum-packed (−760 mmHg) in high-density polyethylene bags (Taipei Pack Industries Co., Taipei, Taiwan) and stored at −20°C for a 4 week storage period. The following analyzing parameters of meatballs in week 0 were measured after 1 day of meatballs being boiled and kept at 4°C.
2.3. Hardness, springiness, and penetration force
Frozen meatballs were thawed in a cooler (4°C) for approximately 24 hours. The texture profile analysis (TPA) indices of meatballs were determined using a texture analyzer (Model TA.XTplus Texture Analyzer, Stable Micro Systems, Godalming, UK). The conditions of the texture analyzer were as follows: pretest speed, 5.0 mm/s; posttest speed, 5.0 mm/s; distance, 5.0 mm; trigger type, auto; and trigger force, 10 g. For TPA measurement, the meatballs were cut to 1 cm3 size. The calculation of TPA values was obtained by graphing a curve using force and time plots. Values for hardness (peak force of the first compression cycle) and springiness (ratio of the time duration of the second compression to that of the first compression) were measured by using a P/50 probe (50 mm diameter cylinder aluminum, Stable Micro Systems). Penetration values (peak force of the compression cycle) of meatballs were measured by using a P/5S probe (5 mm spherical stainless, Stable Micro Systems).
2.4. Color measurement
Color measurements were taken in the surface of the meatballs immediately after opening the package. The following color coordinates were determined: lightness (L*), redness (a*), and yellowness (b*). CIE-L*, a*, and b* values were measured by a color difference meter (Nippon Denshoku Industries Co., Ltd., Model NR-11, Tokyo, Japan). L*, a*, and b* values were also used to calculate whiteness of samples [9]:
Eight cubic gels (each length: 2.0 cm, diameter, 2.0 cm) per treatment were used for color measurement.
2.5. Measurements of lipid and protein oxidation
Lipid oxidation was evaluated by a TBARS assay according to previously described procedures [10]. The TBARS value, expressed as nmole of malondialdehyde/g meatball, was calculated using a molar extinction coefficient (156,000 M−1cm−1). Protein oxidation was evaluated by a sulfhydryl content assay according to previously described procedures [1]. The sulfhydryl concentration in meatballs was calculated using a molar extinction coefficient of 13,600 M−1cm−1, and then expressed to nmole/g meatball.
2.6. Water-holding capacity measurement
Centrifugation and purge losses of stored meatballs were applied to understand the water-holding capacities of meatballs. A measurement of centrifugation loss of meatballs was modified based on a previous method [11]. The samples were cut into 1.0–1.5 cm long sections weighting approximately 0.15–0.20 g. The samples were placed in 1.5 mL tubes with a filter paper (Toyo Roshi Kaisha, Ltd., Japan) in the bottom of tubes to separate the meatballs from the expelled liquid. The samples were then centrifuged at 1000g for 1 hour at 4°C. After centrifugation the samples were weighed again, and the centrifugation loss was calculated as the difference in weight before and after centrifugation.
A measurement of purge loss of meatballs was according to a previous method with a slight modification [12]. Purge loss was measured in a 7 day storage interval at 4°C. Prior to storage, samples were dried with Kimwipes (Kimberly-Clark Global Sales, Inc., Roswell, GA, USA) to remove excess surface moisture, weighed to determine initial weight, and packaged in Ziploc (S. C. Johnson & Son, Inc., Racine, WI, USA). After storage, samples were removed from their storage bags, dried with Kimwipes, and weighed again. Purge loss was calculated as a percentage of the weight of each sample at each storage period compared to their initial weights.
2.7. Sodium dodecyl sulfate polyacrylamide gel electrophoresis
Protein patterns and amounts in meatballs were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 8% (w/v) polyacrylamide gels. Briefly, 1 g of meatball was homogenized in 4 mL 1X phosphate buffered saline, centrifuged at 800g for 15 minutes, and then filtered. Filtrate (30 μL) was mixed with 10 μL loading dye [2% (w/v) SDS (Sigma Co.), 5% (v/v) β-mercaptoethanol (Sigma Co.), 10% (v/v) glycerol (Sigma Co.), and 0.05% (w/v) bromophenol blue (Sigma Co.) in 0.5 mmol/L Tris-HCl (Sigma Co.) at pH 6.8] and heated at 95°C for 5 minutes. Mixed filtrate (16 μL) was applied onto the gels, and 5 μL sharp protein marker III was used as molecular weight marker and to identify the myofibrillar proteins, respectively. Electrophoresis was carried out in a Mini-PROTEAN Tetra Cell (Bio-Rad Laboratories, Informatic Division, Philadelphia, PA, USA) at 110 V until the bromophenol blue marker reached the bottom of the gel. Gels were subsequently stained for 12 hours in 0.06% (w/v) Coomassie Brilliant Blue G-250 in 10% acetic acid and then destained for 4 hours in 10% (v/v) acetic acid.
2.8. Sensory evaluation
The sensory evaluation consisted of a preference test (flavor, surface color, and mouthfeel), sensorial test (juiciness and springiness), and scores of overall acceptance. Twenty panelists (20–40 years old, 10 female and 10 male) from the staff, faculty, and students at National Taiwan University were recruited for this panel assessment. The evaluation was done using a 5-point scale (5 = very good and 1 = very bad). Samples (2 meatballs/treatment) were prepared in the usual manner in Chinese society by warming the meatballs in hot water (100°C) for 10 minutes. The meatballs of each treatment were distributed on white plate for evaluation, and distilled water was provided for cleaning the palate. All sensory evaluation was conducted at room temperature (25°C).
2.9. Statistical analysis
The experiment was conducted using a completely random design. Data were analyzed using analysis of variance. The significant differences were determined at the 0.05 probability level, and differences between treatments were tested using the least significant difference test. All statistical analyses of data were performed using SAS 9.0 (SAS Institute Inc., Cary, NC, USA).
3. Results and discussion
3.1. Phenolic acid, flavonoid, and ascorbic acid contents in 1% LFPWEs
In 1% LFPWEs, gallic acid, gentisic acid, chlorogenic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid, sinapic acid, syringic acid, and p-anisic acid were identified as phenolic acids, while identified flavonoids included catechin, epicatechin, rutin, quercitrin, and neohesperidin (Table 1). Ascorbic acid was measured in the LFPWEs as well (Table 1). An amount of phenolic acids were approximately equal to that of flavonoids (14.60 mg/100 mL and 15.14 mg/100 mL LFPWEs, respectively), and the ascorbic-acid content was 0.50 mg/100 mL. Gentisic acid and epicatechin were the major phenolic acid and flavonoid in LFPWEs, respectively (10.95 mg/100 mL and 9.65 mg/100 mL LFPWEs, respectively; Table 1).
Table 1.
Contents of identified flavonoids, phenolic acids, and ascorbic acid in 1% (w/v) litchi-flower water extract determined by high performance liquid chromatography (HPLC).
| Retention time (min) | Compound | Contents (mg/100 mL) |
|---|---|---|
| 7.59 | Gallic acid | 0.21 ± 0.01 |
| 14.35 | Catechin | 4.60 ± 0.02 |
| 15.65 | Gentisic acid | 10.95 ± 0.05 |
| 16.81 | Chlorogenic acid | 0.51 ± 0.01 |
| 21.25 | Vanillic acid | 0.31 ± 0.02 |
| 22.70 | Caffeic acid | 0.36 ± 0.01 |
| 24.54 | Epicatechin | 9.65 ± 0.09 |
| 36.63 | p-Coumaric acid | 0.19 ± 0.01 |
| 41.64 | Ferulic acid | 1.30 ± 0.01 |
| 43.04 | Sinapic acid | 0.45 ± 0.02 |
| 45.19 | Syringic acid | 0.15 ± 0.01 |
| 53.28 | Rutin | 0.47 ± 0.02 |
| 53.88 | p-Anisic acid | 0.17 ± 0.01 |
| 64.44 | Quercitrin | 0.28 ± 0.02 |
| 66.11 | Neohesperidin | 0.15 ± 0.01 |
| Identified phenolic acid amount | 14.60 ± 0.06 | |
| Identified flavonoid amount | 15.14 ± 0.07 | |
| Ascorbic acid | 0.50 ± 0.01 | |
Data are presented as mean ± standard error of the mean (n = 3).
Some natural extracts from plant kingdoms exhibit antioxidative effects due to their rich phenolic acids and flavonoids. It was indicated that phenolic acids such as carnosic acid and rosmarinic acid in rosemary (Rosmarinus officinalis L.) and lemon balm (Melissa officinalis L.) extracts, respectively, retarded lipid and protein oxidation in pork patties [2]. Blackcurrant (Ribes nigrum L.) extracts contain anthocyanin and also delayed lipid and protein oxidation in pork patties during the chilled storage [1]. Especially, epicatechin-rich grape seed flour prolonged the storage of frankfurters by reducing lipid oxidation [13]. It was reported that sinapic, caffeic, ferulic, gentisic, syringic, and cinnamic acids are the major phenolic acids in canola extracts [14]. Canola extracts showed antioxidative abilities in precooked ground meat. It was also indicated that ascorbic acid can retard lipid oxidation in cultured blue mussels (Mytilus edulis) during storage [15]. Our previous studies indicated that phytochemical-rich LF demonstrated cardiovascular and hepatic protection against a high fat diet via lowering serum/hepatic lipid contents and inflammatory responses, and increasing antioxidative capacities in a rodent model [5,16]. Hence, it is supposed that LFs can be a natural antioxidant to reduce the lipid peroxidation in emulsified pork meatballs.
3.2. Effects of LF powders on textural, and color changes as well as sensory evaluation of emulsified pork meatballs
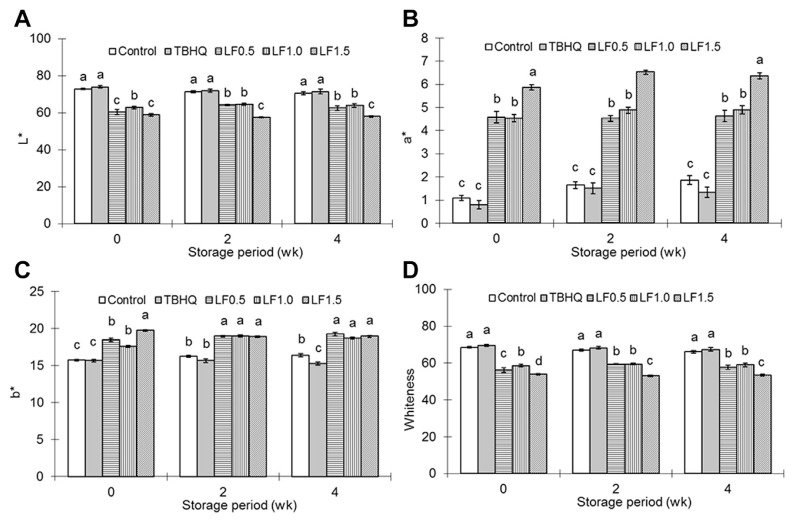
Table 2 shows the textural parameters of emulsified meatballs during 4 weeks of storage. Higher (p < 0.05) values of hardness and penetration of emulsified meatballs immediately after being cooked (Week 0) were measured in the LF1.0 and LF1.5 groups than those in the control and TBHQ groups. During each storage period, meatballs with LFs (LF0.5, LF1.0, and LF1.5) still showed higher (p < 0.05) values of hardness and penetration compared to the control and TBHQ treatments. There were no (p > 0.05) differences among groups for springiness, except higher (p < 0.05) values in the LF1.5 group at the 2nd week of storage. The color parameters of emulsified meatballs during 4 weeks of storage are illustrated in Fig. 1. Control and TBHQ groups showed higher (p < 0.05) L* value (Fig. 1A) and whiteness (Fig. 1D) than ones with LFs. LF0.5, LF1.0, and LF1.5 groups also had higher (p < 0.05) a* and b* values (Figs. 1B and 1C, respectively), than the control and TBHQ groups, while the LF1.5 group showed the highest (p < 0.05) a* value during storage. According to the results of sensory evaluation on meatballs immediately after being cooked (Table 3), the only difference noted in the flavor of meatballs where LF0.5, LF1.0, and LF1.5 groups had higher (p < 0.05) scores of flavor than the Control and TBHQ groups. Addition of TBHQ and LFs did not (p > 0.05) affect panelists’ evaluations of surface color and mouthfeel of meatballs. In the sensory test, LF0.5, LF1.0, and LF1.5 groups demonstrated higher (p < 0.05) scores of juiciness than the Control and TBHQ groups, but there were no (p > 0.05) differences in springiness among groups. Overall, the LF0.5 group had the highest (p < 0.05) scores of overall acceptance followed by LF1.5, TBHQ, LF1.0, and Control groups.
Table 2.
Effects of litchi flower powders on hardness, springiness, and penetration of pork meatballs during the storage period.
| Storage period (wk) | Treatment | Hardness (g force) | Springiness | Penetration (g force) |
|---|---|---|---|---|
| 0 | Control | 37.92 ± 1.40c | 0.89 ± 0.00a | 4.11 ± 0.20c |
| TBHQ | 40.96 ± 1.27bc | 0.90 ± 0.00a | 4.18 ± 0.14bc | |
| LF0.5 | 38.46 ± 1.41bc | 0.90 ± 0.01a | 4.17 ± 0.25c | |
| LF1.0 | 42.03 ± 1.56b | 0.88 ± 0.01a | 4.85 ± 0.18ab | |
| LF1.5 | 46.41 ± 1.27a | 0.89 ± 0.01a | 5.04 ± 0.35a | |
| 2 | Control | 24.64 ± 0.84a | 0.88 ± 0.00b | 4.26 ± 0.21c |
| TBHQ | 27.62 ± 0.89a | 0.86 ± 0.01b | 4.69 ± 0.15b | |
| LF0.5 | 24.56 ± 0.47a | 0.88 ± 0.01b | 4.99 ± 0.13ab | |
| LF1.0 | 25.76 ± 0.91a | 0.88 ± 0.01b | 5.08 ± 0.07ab | |
| LF1.5 | 24.91 ± 1.16a | 0.91 ± 0.01a | 5.19 ± 0.12a | |
| 4 | Control | 23.24 ± 0.82b | 0.89 ± 0.00a | 4.12 ± 0.20c |
| TBHQ | 22.53 ± 0.69b | 0.91 ± 0.00a | 4.63 ± 0.17b | |
| LF0.5 | 26.76 ± 0.63a | 0.90 ± 0.01a | 4.98 ± 0.04ab | |
| LF1.0 | 27.92 ± 0.80a | 0.89 ± 0.01a | 5.11 ± 0.15a | |
| LF1.5 | 27.60 ± 0.44a | 0.90 ± 0.01a | 5.39 ± 0.19a |
Data are presented as mean ± standard error of the mean (n = 8).
Mean values within a column in each storage period that are not followed by a common letter are significantly different (p < 0.05).
LF = litchi flower; TBHQ = tert-butylhydroquinone.
Fig. 1.
Effects of litchi flower powders on color parameters of pork meatballs during the storage period. Data are presented as mean ± standard error of the mean (n = 8). A–D Columns with unlike letters within each storage period differ significantly (p < 0.05).
Table 3.
Effects of litchi flower powders on sensory analyses of cooked fresh pork meatballs.
| Preference test | Sensorial test | Overall acceptance | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Flavor | Surface color | Mouthfeel | Juiciness | Springiness | ||
| Control | 2.8 ± 0.2b | 3.2 ± 0.1a | 3.1 ± 0.2a | 2.9 ± 0.2b | 3.3 ± 0.2a | 3.0 ± 0.2b |
| TBHQ | 2.8 ± 0.1b | 3.1 ± 0.2a | 3.4 ± 0.1a | 3.0 ± 0.1b | 3.3 ± 0.1a | 3.4 ± 0.1ab |
| LF0.5 | 3.5 ± 0.1a | 3.6 ± 0.1a | 3.6 ± 0.1a | 3.5 ± 0.1a | 3.5 ± 0.1a | 3.7 ± 0.1a |
| LF1.0 | 3.4 ± 0.2a | 3.3 ± 0.1a | 3.4 ± 0.1a | 3.3 ± 0.2ab | 3.6 ± 0.1a | 3.3 ± 0.1ab |
| LF1.5 | 3.2 ± 0.1ab | 3.3 ± 0.1a | 3.4 ± 0.1a | 3.3 ± 0.2ab | 3.6 ± 0.1a | 3.4 ± 0.1ab |
Data are presented as mean ± standard error of the mean (n = 20). The evaluation was done using a 5-point scale (5 = very good and 1 = very bad).
Mean values within a column that are not followed by a common letter are significantly different (p < 0.05).
LF = litchi flower; TBHQ = tert-butylhydroquinone.
Hard and elastic textures contribute to the characteristics of emulsified pork balls. The increased hardness and penetration of meatballs in Week 0 may be partially due to the addition of TBHQ or LF powders. In addition, the textural properties of meat or meat products are also mainly related to myofibrillar proteins, where myosin and actin can produce three dimensional heat set gels. Myofibrillar proteins are also responsible for the cohesive structure and firm texture of meat products. It was reported that adding rosemary extracts into pork patties produced better textures [2]. Kiam wood extracts, which contain rich tannic acid, also enhanced the hardness, chewiness, and gumminess of fish emulsion sausages during storage [17]. Similar results were observed in this study where the addition of LFs improved the textural properties of emulsified meatballs (hardness and penetration values) and increased panelists’ preferences due to its good mouthfeel and juiciness. Color also plays an important role in both the quality and consumer’s preference of meat products. The formation of metmyoglobin and lipid oxidation products contributes to discoloration. Several natural antioxidants stabilize meat color, such as Pu-Erh tea on fresh pork sausages [18]. Due to hot-air drying, LFs were brown in color. Treatment groups with LFs have higher redness and yellowness but lower lightness and whiteness (Fig. 1). However, according to the sensorial evaluation, redder and darker meatballs with LFs did not affect panelists’ preferences (Fig. 1 and Table 3).
3.3. Effects of LF powders on lipid and protein oxidation values, water-holding capacity, and protein patterns of emulsified pork meatballs
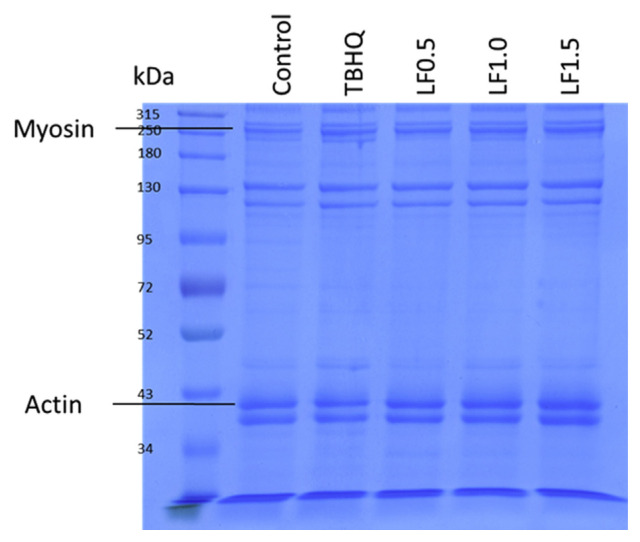
TBARS values, thiol group contents, centrifugation loss, and purge loss of emulsified meatballs during storage are shown in Table 4. The control group had the highest (p < 0.05) TBARS value, followed by TBHQ, LF1.0, LF1.5, and LF0.5 groups immediately after being cooked (Week 0). However, during 4 weeks of storage, the TBARS value in the control group obviously increased, but treatments containing LFs did not. The Control group still exhibited the highest (p < 0.05) TBARS value compared to other groups upon storage, while the LF0.5 group had the lowest (p < 0.05) TBARS value among groups. In contrast with TBARS values, the lowest (p < 0.05) thiol group content was measured in the Control group during the storage period. The thiol-group contents in all groups decreased upon storage. Generally, the LF0.5 group remained the most (p < 0.05) thiol-group content during the storage period. With regards to water-holding capacity, the Control group had the highest (p < 0.05) centrifugation loss during a 4-week storage than other groups. Regarding purge loss of emulsified meatballs, the TBHQ group had the lowest (p < 0.05) purge loss after 2 weeks of storage, followed by the LF1.0, LF0.5, LF1.5, and Control groups. Fig. 2 showed the protein patterns of emulsified pork meatballs immediately after being cooked, where two main myofibrillar proteins (myosin and actin) were marked. TBHQ, LF0.5, LF1.0, and LF1.5 groups had higher myosin contents than the control group. However, no differences on actin contents were detected among the control, TBHQ, and LF0.5 groups, but there was a tendency towards higher actin contents in the LF1.0 and LF1.5 groups than other groups.
Table 4.
Effects of litchi flower powders on thiobarbituric acid reactive substance (TBARS), thiol-group contents, centrifugation loss (%), and purge loss (%) of pork meatballs during the storage period.
| Storage period (wk) | Treatment | TBARS (nmole MDA eq. g−1 meatball) | Thiol group (nmole g−1 meatball) | Storage period (wk) | Centrifugation loss (%) | Storage period (wk) | Purge loss (%) |
|---|---|---|---|---|---|---|---|
| 0 | Control | 252.09 ± 2.58a | 34.44 ± 0.50c | 0 | 9.16 ± 0.37a | 1 | 0.95 ± 0.10a |
| TBHQ | 92.99 ± 2.90c | 36.39 ± 0.23b | 6.32 ± 0.19b | 0.32 ± 0.07b | |||
| LF0.5 | 81.68 ± 3.01d | 38.06 ± 0.17a | 5.04 ± 0.47c | 0.36 ± 0.04b | |||
| LF1.0 | 103.14 ± 2.35b | 36.31 ± 0.24b | 4.63 ± 0.70c | 0.35 ± 0.06b | |||
| LF1.5 | 89.91 ± 1.88c | 36.08 ± 0.18b | 3.92 ± 0.28c | 0.48 ± 0.05b | |||
| 2 | Control | 296.16 ± 2.46a | 30.57 ± 0.36d | 2 | 7.80 ± 0.55a | 2 | 3.03 ± 0.34a |
| TBHQ | 90.26 ± 2.00c | 33.33 ± 0.35b | 4.60 ± 0.35b | 0.60 ± 0.13c | |||
| LF0.5 | 75.74 ± 1.63e | 35.51 ± 0.51a | 5.06 ± 0.19b | 0.85 ± 0.13bc | |||
| LF1.0 | 113.06 ± 2.42b | 32.19 ± 0.17c | 5.30 ± 0.36b | 0.71 ± 0.08bc | |||
| LF1.5 | 83.68 ± 1.11d | 33.57 ± 0.34b | 4.48 ± 0.24b | 1.21 ± 0.10b | |||
| 4 | Control | 299.45 ± 4.45a | 25.57 ± 0.22c | 4 | 6.44 ± 0.73a | ||
| TBHQ | 100.60 ± 1.85c | 29.81 ± 0.31a | 4.58 ± 0.38bc | ||||
| LF0.5 | 73.10 ± 1.26e | 30.28 ± 0.13a | 5.74 ± 0.40ab | ||||
| LF1.0 | 108.12 ± 1.87b | 26.63 ± 0.43b | 4.96 ± 0.39bc | ||||
| LF1.5 | 86.97 ± 1.32d | 27.18 ± 0.26b | 3.75 ± 0.22c |
Data are presented as mean ± standard error of the mean (n = 8).
Mean values within a column in each storage period that are not followed by a common letter are significantly different (p < 0.05).
Fig. 2.
Effects of litchi flower powders on protein patterns of fresh pork meatballs.
Oxidation causes rancidity and deterioration in meat products. Many studies have been available for the retardation effects of plant extracts on lipid peroxidation in meat products, such as blackcurrant (Ribes nigrum L.) extract, rosemary, and lemon balm extracts in pork patties or packaged beef, tea catechins in pork sausages, and grape seed flour in frankfurters [1,2,13,18–20]. It was also reported that gentisic acid was an effective antioxidant against lipid oxidation [14]. These studies indicate that polyphenol contents of certain plants can provide an antioxidative effect in meat products. Hence, it is speculative that lower TBARS values in emulsified pork meatballs with LFs result from their rich polyphenol content, especially gentisic acid and epicatechin (Table 1). Moreover, it was reported that lipid peroxidation is always coupled with protein oxidation [6]. It was reported that disulfide bonds were formed if protein was oxidized; hence, the thiol-group contents of protein were reduced [1]. Therefore, a higher TBARS value indicated a reduction of the thiol-group content simultaneously [6]. Protein oxidation destroys the structure of proteins and affects protein properties, such as water-holding capacity in meat products, while centrifugation and purge losses are common indicators for water-holding capacity [12]. According to the results of this study, meatballs with TBHQ and LFs had a lower centrifugation and purge loss, which might be related to their lower degree of protein oxidation (Table 4). Moreover, the lower myofibrillar protein (myosin and actin) content of control meatballs resulted in less firmness of emulsified pork meatballs, thus contributing to poor textural characteristics and water-holding capacity (Fig. 2, Tables 2 and 4). Commonly, the water-holding capacity of meat products gets worse with time of storage. The same results were observed for purge loss, but the opposite was observed for centrifugation loss (Table 4). It is speculated that the moisture of meatballs exudates on the package during storage, so the moisture content of meatballs is cut back and results in centrifugation loss. Similar results were reported by Kezban and Nuray [21]. Overall, LFs containing high levels of polyphenols demonstrated antioxidative effects, as well as acceptable texture and water-holding capacity of emulsified pork meatballs during storage.
In conclusion, an addition of polyphenol-rich LFs in emulsified pork meatballs delayed lipid and protein oxidation. Moreover, due to higher myofibrillar protein contents which remained in LF emulsified pork meatballs, better textural profile and water-holding capacity of meatballs were obtained. In addition, although an addition of LFs made emulsified pork meatballs redder and darker, ones with 0.5% LFs exhibit the best overall sensory panel acceptance. Hence, LFs may be an effective natural antioxidant to reduce lipid and protein oxidation for frozen cooked meat products.
Acknowledgments
We acknowledge the funding of this research by the Council of Agriculture, Executive Yuan, Taiwan (project number: 101AS-3.1.4-AD-U1(2)).
Funding Statement
We acknowledge the funding of this research by the Council of Agriculture, Executive Yuan, Taiwan (project number: 101AS-3.1.4-AD-U1(2)).
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
REFERENCES
- 1. Jia N, Kong B, Liu Q, Diao X, Xia X. Antioxidant activity of black currant (Ribes nigrum L.) extract and its inhibitory effect on lipid and protein oxidation of pork patties during chilled storage. Meat Sci. 2012;91:533–9. doi: 10.1016/j.meatsci.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 2. Lara MS, Gutierrez JI, Timón M, Andrés AI. Evaluation of two natural extracts (Rosmarinus officinalis L. and Melissa officinalis L.) as antioxidants in cooked pork patties packed in MAP. Meat Sci. 2011;88:481–8. doi: 10.1016/j.meatsci.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 3. Gramza-Michalowska A, Sidor A, Hes M. Herb extract influence on the oxidative stability of selected lipids. J Food Biochem. 2011;35:1723–36. [Google Scholar]
- 4. Liu SC, Lin JT, Wang CK, Chen HY, Yang DJ. Antioxidant properties of various solvent extracts from lychee (Litchi chinenesis Sonn.) flowers. Food Chem. 2009;114:577–81. [Google Scholar]
- 5. Yang DJ, Chang YY, Hsu CL, Liu CW, Wang Y, Chen YC. Protective effect of a litchi (Litchi chinensis Sonn.)-flower-water-extract on cardiovascular health in a high-fat/cholesterol-dietary hamsters. Food Chem. 2010;119:1457–64. [Google Scholar]
- 6. Botsoglou E, Govaris A, Ambrosiadis I, Fletouris D, Papageorgiou G. Effect of olive leaf (Olea europea L.) extracts on protein and lipid oxidation in cooked pork meat patties enriched with n-3 fatty acids. J Sci Food Agr. 2014;94:227–34. doi: 10.1002/jsfa.6236. [DOI] [PubMed] [Google Scholar]
- 7. Yang DJ, Chang YY, Hsu CL, Lin CW, Lin YL, Lin YH, Liu KC, Chen YC. Antiobesity and hypolipidemic effects of polyphenol-rich longan (Dimocarpus longans Lour.) flower water extract in hypercaloric-dietary rats. J Agr Food Chem. 2010;58:2020–7. doi: 10.1021/jf903355q. [DOI] [PubMed] [Google Scholar]
- 8. Klein BP, Perry AK. Ascorbic acid and vitamin A activity in selected vegetables from different geographical areas of the United States. J Food Sci. 1982;47:941–5. [Google Scholar]
- 9. Kristinsson HG, Theodore AE, Demir N, Ingadottir B. A comparative study between acid- and alkali-aided processing and surimi processing for the recovery of proteins from channel catfish muscle. J Food Sci. 2005;70:C298–306. [Google Scholar]
- 10. Chen YC, Nguyen J, Semmens K, Beamer S, Jaczynski J. Chemical changes in omega-3-enhanced farmed rainbow trout (Oncorhynchus mykiss) fillets during abusive-temperature storage. Food Control. 2008;19:599–608. [Google Scholar]
- 11. Hanne CB, Henrik JA, Anders HK. Comparative study of low-field NMR relaxation measurements and two traditional methods in the determination of water holding capacity of pork. Meat Sci. 2001;57:125–32. doi: 10.1016/s0309-1740(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 12. Li C, Li J, Li X, Hviid M, Lundström K. Effect of low-voltage electrical stimulation after dressing on color stability and water holding capacity of bovine longissimus muscle. Meat Sci. 2011;88:559–65. doi: 10.1016/j.meatsci.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 13. Özvural EB, Vural H. Grape seed flour is a viable ingredient to improve the nutritional profile and reduce lipid oxidation of frankfurters. Meat Sci. 2011;88:179–83. doi: 10.1016/j.meatsci.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 14. Brettonnet A, Hewavitarana A, Dejong S, Lanari MC. Phenolic acids composition and antioxidant activity of canola extracts in cooked beef, chicken and pork. Food Chem. 2010;121:927–33. [Google Scholar]
- 15. Khan MA, Parrish CC, Shahidi F. Effects of mechanical handling, storage on ice and ascorbic acid treatment on lipid oxidation in cultured Newfoundland blue mussel (Mytilus edulis) Food Chem. 2006;99:605–14. [Google Scholar]
- 16. Chang YY, Yang DJ, Chiu CH, Lin YL, Chen JW, Chen YC. Antioxidative and anti-inflammatory effects of polyphenol-rich litchi (Litchi chinensis Sonn.)-flower-water-extract on livers of high-fat-diet fed hamsters. J Funct Foods. 2013;5:44–52. [Google Scholar]
- 17. Maqsood S, Benjakul S, Balange AK. Effect of tannic acid and kiam wood extract on lipid oxidation and textural properties of fish emulsion sausages during refrigerated storage. Food Chem. 2012;130:408–16. [Google Scholar]
- 18. Martínez L, Cilla I, Beltrán JA, Roncalés P. Antioxidant effect of rosemary, borage, green tea, Pu-Erh tea and ascorbic acid on fresh pork sausages packaged in a modified atmosphere: influence of the presence of sodium chloride. J Sci Food Agr. 2006;86:1298–307. [Google Scholar]
- 19. McBride NTM, Hogan SA, Kerry JP. Comparative addition of rosemary extract and additives on sensory and antioxidant properties of retail packaged beef. Int J Food Sci Tech. 2007;42:1201–7. [Google Scholar]
- 20. Barnnan RG. Effect of grape seed extract on physicochemical properties of ground, salted, chicken thigh meat during refrigerated storage at different relative humidity levels. J Food Sci. 2008;73:C36–40. doi: 10.1111/j.1750-3841.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- 21. Kezban C, Nuray K. The effects of carrageenan and pectin on some quality characteristics of low-fat beef frankfurters. Meat Sci. 2003;64:199–206. doi: 10.1016/s0309-1740(02)00181-x. [DOI] [PubMed] [Google Scholar]