Abstract
Rhodiola is a genus of medicinal plants that originated in Asia and Europe and are used traditionally as adaptogens, antidepressants, and anti-inflammatory remedies. Rhodiola plants are rich in polyphenols, and salidroside and tyrosol are the primary bioactive marker compounds in the standardized extracts of Rhodiola rosea. This review article summarizes the bioactivities, including adaptogenic, antifatigue, antidepressant, antioxidant, anti-inflammatory, antinoception, and anticancer activities, and the modulation of immune function of Rhodiola plants and its two constituents, as well as their potential to prevent cardiovascular, neuronal, liver, and skin disorders.
Keywords: bioactivity, Rhodiola, salidroside, tyrosol
1. Introduction
The genus Rhodiola (Hong Jing Tian; Crassulaceae) consists of more than 200 species, of which approximately 20, including Rhodiola rosea, Rhodiola alterna, Rhodiola brevipetiolata, Rhodiola crenulata, Rhodiola kirilowi, Rhodiola quadrifida, Rhodiola sachalinensis, and Rhodiola sacra, are used as traditional medicines in Asia [1]. These plants grow mainly in the Himalayan belt, Tibet, China, and Mongolia, but are also cultivated in Europe and North America and are available on the market as dietary supplements [2,3]. Rhodiola plants extracts are traditionally used in tonics and adaptogen, antidepressant, and anti-inflammatory drugs [2,4]. The most widely known is Rhodiola rosea, which is also called the “golden root” or “roseroot.” The pharmacological effects of Rhodiola rosea, including its role in increasing longevity, stimulating the central nervous system, and elevating work performance as well as its cardio-, neuro-, and hepatoprotective effects and immunotropic, antiviral, anti-inflammatory, and antibacterial activities, have been studied extensively [1,5]. Rhodiola rosea has been used for a long time in Eastern Europe and Asia to enhance physical and mental performance. It is also used in Eastern Europe and Asia as a traditional medicine to stimulate the nervous system, alleviate depression and fatigue, enhance work performance, and prevent high-altitude sickness, mountain malhypoxia, and anoxia [6], and in Russia and Mongolia for treating long-term illness and weakness caused by infection [7]. In addition, Rhodiola rosea has been proven to have cardiovascular protection effects [8,9]. In recent years, the root extracts of Rhodiola rosea have been used as drinks, food additives, and in commercial pharmaceutical preparations offered worldwide [10].
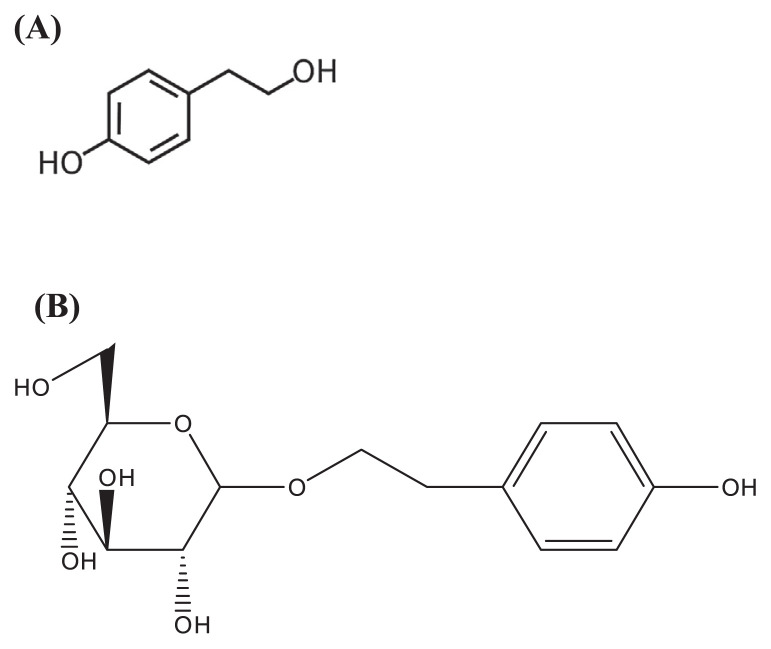
Rhodiola plants contain polyphenols such as flavonoids, proanthocyanidines, tyrosol, and cinnamyl alcohol, as well as glycosides, organic acids, essential oils, sugars, fats, alcohols, and proteins [5]. The polyphenol content of Rhodiola rosea is approximately 41.4 ± 3.41% [11]. Rosavin (such as rosavin, rosarin, rhodionin, rhodiosin, and rosin), cinnamyl alcohol, salidroside (Fig. 1B), and tyrosol (Fig. 1A) are the major components of Rhodiola plants [12–18]. Salidroside and its aglycone, tyrosol, are the major compounds of Rhodiola rosea, and the content of the two compounds are often used as a criterion in evaluating the quality of crude drugs of Rhodiola rosea [19].
Fig. 1.
(A) The structure of tyrosol; (B) the structure of salidroside.
This review article presents an overview of the bioactivities of Rhodiola plants and their major components.
2. Adaptogenic and antifatigue activity
Adaptogens are substances that enable the normalization of physiologic responses to various stressors, enhances work performance, and increases the stress tolerance of the body [20,21]. Researchers have categorized Rhodiola rosea as an adaptogen because of its observed ability to increase resistance to various chemical, biological, and physical stressors [4,6,22,23] and its performance-enhancing effect in humans [24]. A study reported that repeated administration of Rhodiola rosea extract, SHR-5, exerts an antifatigue effect that increases mental performance, particularly the ability to concentrate, and reduces cortisol response to awakening stress in patients with burnout and fatigue syndrome [25]. The adaptogenic and central nervous system activities of Rhodiola rosea may be attributable to its influence on the levels and activity of monoamines and opioid peptides such as beta-endorphins [3].
Administering 100 mg/kg of an aqueous extract of Rhodiola imbricata provided maximum resistance to cold-hypoxia-restraint stress-induced hypothermia and quickened recovery from the stressor [26]. In addition, the aqueous extract of Rhodiola imbricata exhibited dose-dependent adaptogenic activity [27]. Research indicated that Rhodiola extracts have great utility in treating asthenic conditions which develop after intense physical or intellectual strain, including a decline in work performance, sleep difficulties, poor appetite, irritability, hypertension, headaches, and fatigue [27].
Intense exercise increases oxygen consumption and causes oxidative stress as a result of increased reactive oxygen species (ROS) production [28]. Exogenous antioxidants may prevent oxidative damage because they scavenge ROS generated during exercise [29–32]. Salidroside was reported to elevate exercise tolerance and increase the liver glycogen levels of rats after exhaustive exercise such as swimming [29]. In addition, salidroside reduced malondialdehyde (MDA) levels and enhanced the activity of antioxidant enzymes [such as catalase, superoxide dismutase (SOD), and glutathione peroxide] in the liver tissue of Sprague-Dawley (SD) rats [29].
The above mentioned studies suggested that Rhodiola plants elevate work performance and resistance to stress, and that salidroside is effective in preventing oxidative stress following exhaustive exercise.
3. Elongation of lifespan and anti-aging activity
Rhodiola markedly increased the lifespan of Drosophila melanogaster, possibly by increasing the resistance of organs to stress and alleviating oxidative stress [33]. Salidroside considerably reversed senescence-like phenotypes in an oxidant-challenged model, altering the morphology, cell cycle, SA-b-gal staining, and DNA damage as well as the expression of related molecules such as p53, p21, and p16 in the 2BS cell line (human fetal lung fibroblasts). Furthermore, the protection occurred in a dose-dependent manner [34]. In addition, salidroside blocked D-galactose-induced increases in serum advanced glycation endproduct levels in C57BL/6J mice [35]. It reversed D-galactose-induced aging effects in neural and immune systems, improved motor activity, increased memory latency time, and enhanced lymphocyte mitogenesis and interleukin-2 (IL-2) production [35]. Furthermore, salidroside reduced the elevated expression of glial fibrillary acidic protein and neurotrophin-3 in a mouse model of aging [35].
Accordingly, Rhodiola plant extracts and salidroside are potential agents for retarding aging and attenuating age-related diseases in humans and animals.
4. Antioxidative activity
ROS such as superoxide anions ( ), hydroxyl radicals (OH•), and hydrogen peroxide (H2O2) can cause oxidative stress in cellular DNA, protein, and lipids, resulting in numerous disorders and diseases, such as aging-related diseases, cancer, cardiovascular diseases, diabetes mellitus (DM), and various neurodegenerative diseases [32,36,37]. In addition, a Rhodiola plant extract exhibited antioxidant activity and reduced lipid peroxidation in SD rats [38]. Rhodiola rosea showed singlet oxygen scavenging, H2O2 scavenging, hypochlorite scavenging, ferric reducing, ferrous chelating, and protein thiol protection activities [11]. Rhodiola rosea extract reduced glutathione levels, glyceraldehyde-3-phosphate dehydrogenase activity, and thiobarbituric acid reactive substances levels in cultured human keratinocytes exposed to various oxidative insults such as Fe2+/ascorbate, Fe2+/H2O2, and tert-butyl-hydroperoxide insults [39]. In addition, Rhodiola rosea extract inhibited the production of intracellular ROS and increased the activity of antioxidant enzymes such as catalase, SOD, glutathione peroxidase, and glutathione reductase. Rhodiola rosea extract also increased in a time- and dose-dependent manner the activity of transplasma membrane oxidoreductase according to an indirect evaluation of the intracellular redox status in keratinocytes [39]. Rhodiola rosea extract and salidroside protected human cortical neurons from oxidative stress and prevented glutamate-induced cell apoptosis in a human cortical cell line (HCN 1-A) [40].
Aqueous and alcoholic extracts of Rhodiola rhizomes were observed to inhibit apoptosis and tert-butyl hydroperoxide (tert-BHP)-induced free radical production and to restore the antioxidant levels of U-937 human macrophages [41]. They also inhibited tert-BHP-induced decreases in mitochondrial transmembrane potential, significantly lowered the percentage of early and late apoptotic cells, and inhibited DNA single-strand breaks induced by tert-BHP [41].
Rhodiola rosea supplementation can protect human osteosarcoma-derived 143B cells or human fibroblast cell line IMR-90 from ultraviolet light, paraquat, and H2O2 [42]. However, Rhodiola rosea did not alter the levels of the major antioxidant defenses, nor did it markedly activate the antioxidant response element or modulate hemeoxygenase-1 expression levels at relevant concentrations [42].
Water and methanol extracts of Rhodiola sacra exhibit active oxygen scavenging activities such as radical scavenging [43]. Oligomeric proanthocyanidin from Rhodiola rosea (OPCRR) exhibited free-radical-scavenging activities such as the scavenging of 1,1-diphenyl-2-picrylhydrazyl (DPPH), OH•, and [44]. In addition, OPCRR significantly enhanced the oxidative stress defense system, including SOD and glutathione peroxidase activities, and reduced the MDA content in the serum, heart, liver, and brain tissue of mice [44]. These results suggest that OPCRR has great potential to be a natural antioxidant because of its antioxidant activities in vitro and in vivo [44].
Salidroside reduced hydrogen-peroxide-induced intracellular ROS production in human erythrocytes. In addition, salidroside increased cell survival significantly and prevented human erythrocytes from undergoing eryptosis or erythroptosis mediated by H2O2 [45]. The protection activity of salidroside may rise in a dose-dependent manner through its antioxidative activity and the inhibition of caspase-3 activation and stress-induced intracellular Ca2+ production [45]. Salidroside is a protective agent against oxidative stress in human erythrocytes and may be a suitable adaptogen for enhancing the resistance of the body to stress and fatigue.
Like salidroside, Rhodiola rosea’s aglycone, tyrosol, has various biological properties, including antioxidative, cancer preventive, and anti-inflammatory properties [46]. Tyrosol exhibits antioxidant activity, scavenges DPPH free radicals, and has an IC50 of 4.7 μg/mL [47].
Rhodiola extract, salidroside, and tyrosol may prevent oxidative-stress-related disorders.
5. Anti-inflammatory activity
Studies have reported that the tincture extract of Rhodiola rosea exhibits anti-inflammatory activity. In the plethysmometer test, orally administering Rhodiola rosea extract (50 mg/kg body weight and 100 mg/kg body weight) significantly reduced carrageenan-induced paw edema in Wistar rats [48]. The extract (250 mg/kg body weight) inhibited carrageenan-induced paw edema, formaldehyde-induced arthritis, and nystatin-induced paw edema in a rat model in a dose-dependent manner [1].
Rhodiola aqueous extract treatment of human peripheral blood mononuclear cells (PBMCs) increased the production of IL-6 and tumor necrosis factor-alpha (TNF-α) through phosphorylated IκB and the transcription factor NF-κB, showing that this treatment has immunostimulatory potential [49]. The treatment also increased the synergistic production of nitric oxide (NO) and lipopolysaccharide (LPS) in RAW 264.7 cells. Rhodiola extract at 250 μg/mL increased p-IκB expression and activated the nuclear translocation of NF-κB in human PBMCs. The results of the mentioned study suggest that Rhodiola activated proinflammatory mediators through the phosphorylation of inhibitory κB and the transcription factor NF-κB [49].
The tincture extract from Rhodiola rosea inhibited the activities of enzymes relating to inflammation, including cyclooxygenase-1 (COX-1), COX-2, and phospholipase A2 (PLA2) in an in vitro study [1]. The inhibition of nystatin-induced edema and PLA2 suggested that membrane stabilization is the most probable mechanism of the extract’s anti-inflammatory action [1].
A Rhodiola rosea extract inhibited inflammatory C-reactive protein and creatinine kinase expression in the blood levels of healthy untrained volunteers after exhausting exercise [50], indicating that the extract has an anti-inflammatory effect and protects muscle tissue during exercise.
Rhodiola crenulata increased the survival rates of adult flies and the expression of antimicrobial peptide genes after pathogen or toxic compound ingestion. Moreover, decreased levels of ROS and epithelial cell death were associated with improvements in intestinal morphology in Drosophila melanogaster [51]. Rhodiola crenulata extract may prevent inflammatory diseases of the intestine [51].
Salidroside attenuated D-galactosamine (700 mg/kg)-induced and LPS (10 mg/kg)-induced increases in the levels of TNF-α and serum nitric oxide in mouse liver tissue in a dose-dependent manner [52]. Salidroside significantly attenuated TNF-α, IL-1β, and IL-6 production in serum from mice challenged with LPS [53]. In a murine model of endotoxemia, mice were treated with salidroside before or after LPS challenge, and salidroside significantly increased the likelihood of survival [53]. Salidroside downregulated LPS-induced nuclear transcription factor-B (NF-B) DNA-binding activation and extracellular signal-related kinase (ERK)/Mitogen-activated protein kinases (MAPK) signal transduction pathway production in RAW 264.7 macrophages [53]. The results of this study indicated that salidroside modulated early cytokine responses by blocking NF-B and ERK/MAPK activation and increased mouse survival. These effects of salidroside may be useful in treating inflammation-mediated endotoxemia [53]. In addition, salidroside protected LPS-induced acute lung injury in mice [54]. Pretreatment with a single 120 mg/kg dose of salidroside prior to the administration of intratracheal LPS induced mouse myeloperoxidase activity in lung tissue and reduced the protein concentration and the total number of cells, neutrophils, and macrophages in the bronchoalveolar lavage fluid in BALB/c mice [54].
Salidroside administered 1 hour before LPS infusion significantly attenuated inflammatory cell infiltration, reduced the activity of myeloperoxidase in mammary tissue, and reduced the concentration of TNF-α, IL-1β, and IL-6 in a dose-dependent manner [55]. Salidroside also inhibited the production of several inflammatory cytokines, including TNF-α, IL-6, and IL-1β, and NF-κB DNA-binding activation after LPS challenge [54]. These results indicated that salidroside exerts a protective effect on LPS-induced acute lung injury in mice. Another study reported that salidroside downregulated the phosphorylation of LPS-induced NF-κB p65 and an inhibitor of NF-κB α (IκBα) in the NF-κB signal pathway and suppressed the phosphorylation of p38, ERK, and c-jun NH(2)-terminal kinase (JNK) in MAPK signaling pathways [55,56]. Salidroside is an effective suppressor of inflammation and may be a candidate for the prophylaxis of mastitis.
Salidroside pretreatment reduced the ratio of concanavalin-A-induced aspartate transaminase to alanine transaminase (also called the aspartate-aminotransferase-to-alanine-aminotransferase ratio) markedly and suppressed the secretion of proinflammatory cytokines by downregulating the activity of NF-κB [57]. Salidroside altered the distribution of CD4+ and CD8+ T lymphocytes in the liver and spleen by regulating CXCL-10 and reduced the severity of liver injuries [57].
6. Antinociceptive effect
Studies have reported that Rhodiola extract exhibits analgesic activity. The antinociceptive effect of Rhodiola extract was evaluated using the hot-plate test, Randall-Selitto test, and formalin test in male Wistar rats. In the hot-plate test, orally administering Rhodiola rosea at 50 mg/kg and 100 mg/kg body weight increased the latency reaction [48]. In the Randall-Selitto test, Rhodiola rosea caused a significant increase in pressure reaction at 50 mg/kg [48]. In the formalin test, Rhodiola rosea significantly reduced the paw-licking time during the first phase at 100 mg/kg [48]. The studies indicated that Rhodiola rosea extract exhibited significant analgesic activity in different pain models, inhibiting thermal pain, mechanical hyperalgesia, and formalin-induced pain behavior.
7. Immune system
Rhodiola aqueous extract significantly enhanced tetanus toxoid-specific immunoglobulin levels in rats [58] and the level of ovalbumin-induced antibody responses. Tetanus toxoid and ovalbumin in combination with complete Freund's adjuvant or Rhodiola aqueous extract were observed to evoke a significant delayed-type hypersensitivity response [58]. Rhodiola aqueous extract did not suppress the swelling response in an adjuvant-induced arthritis model [58]. Rhodiola aqueous extract exhibits adjuvant and immunopotentiating activity.
Rhodiola imbricata rhizome extract inhibited the proliferation of the human T-cell lymphoma cell line EL-4 and erythroleukemic cell line HL-60 [59]. Furthermore, treating human peripheral blood mononuclear cells (hPBMCs) with LPS and Rhodiola imbricata extract suppressed regulated upon activation, normal T cell expressed and secreted production [59]. However, the number of TNF-α spots in Rhodiola imbricata-rhizome-extract-treated hPBMCs increased. Rhodiola imbricata extract upregulated TLR-4 mRNA expression in the splenocytes of rats [59].
Aqueous and 50% hydroalcoholic extracts of Rhodiola quadrifida stimulated granulocyte activity in vitro and increased the response of lymphocytes to mitogens in mice and rats [60]. The ability of parental strain mouse lymphocytes to induce a local cutaneous graft-versus-host reaction in F1 hybrids was stimulated by the 50% hydroalcoholic extract [60].
In vitro aqueous and 50% hydroalcoholic Rhodiola kirilowii extracts stimulated granulocyte activity and increased lymphocyte response to mitogens, and, in vivo, they enhanced the ability of lymphocytes derived from parental strain mice fed Rhodiola kirilowii aqueous and hydroalcoholic extracts to induce a local cutaneous graft-versus-host reaction in F1 hybrids [61]. The results of this study suggested that Rhodiola kirilowii extract enhances cellular immunity [61].
In another study, the dietary intake of salidroside increased the total number of T cells (CD3+) and T helper cells (CD4+) in aged Wistar male rats (21 months old), and salidroside supplementation significantly increased the delayed-type hypersensitivity response in aged rats and substantially increased the production of anti-keyhole limpet hemocyanin (anti-KLH) IgG, anti-KLH IgG 1, and anti-KLH IgG 2 α in aged rats without disturbing immune homeostasis [62].
Intraperitoneally administering salidroside before an ovalbumin challenge resulted in the significant inhibition of asthmatic reactions [63]. Moreover, ovalbumin significantly increased the activation of NFκB and p38 in lung tissue, whereas salidroside markedly suppressed NF-κB translocation and reduced the phosphorylation of p38 [63]. Salidroside attenuated the expression of intercellular adhesion molecule 1 and IL-6 by modulating the activities of p38 and NF-κB in BEAS-2B cells stimulated by proinflammatory cytokines [63].
These results indicate that Rhodiola plant extracts influence immune modulation and salidroside prevents ovalbumin-induced airway inflammation and airway hyper responsiveness, at least in part by downregulating NF-κB and p38 activities [63].
8. Antidepression
Extracts of SHR-5 from Rhodiola rosea rhizomes alleviated depressive symptoms in patients with mild or moderate depression, yielding no severe side effects [20]. Orally administering salidroside (20 mg/kg and 40 mg/kg) for 2 weeks notably alleviated olfactory-bulbectomy-induced hyperactivity in an open-field test and reduced immobility time in a forced swimming test [64]. Chronic treatment with salidroside greatly reduced TNF-α and IL-1β levels in the hippocampus [64]. Salidroside increased glucocorticoid receptor and brain-derived neurotrophic factor expression in the hippocampus of rats. In addition, salidroside attenuated corticotropin-releasing hormone expression in the hypothalamus and the levels of serum corticosterone [64]. Salidroside significantly reduced depression-like behavior in olfactory bulbectomized rats; the mechanisms of this reduction might be associated with the anti-inflammatory effects of and regulation of hypothalamic–pituitary–adrenal axis activity by salidroside.
9. Skin care and skin whitening
Studies have reported the potential for using Rhodiola rosea extract in skin care and wound healing, and that Rhodiola rosea extract modulates skin melanogenesis. Rhodiola rosea extract/ L-carnosine-associated compound (RCAC) treatment for sensitive skin reduced transepidermal water loss in humans, thereby improving skin barrier function [65]. RCAC treatment also exhibited a positive subjective response in patients with sensitive skin, promoted the release of proopiomelanocortin peptides, and returned to normal levels the increased number of neuropeptides and cytokines produced by keratinocytes exposed to ultraviolet radiation [65].
Rhodiola imbricata-treated wounds healed more rapidly than control group, and the plant extract promoted cellular proliferation and collagen synthesis at the wound site in SD rats [38]. Rhodiola imbricata extract increased DNA, protein, hydroxyproline, and hexosamine expression in granulation cells more than providone–iodine ointment [38]. The extract also exhibited antioxidant activity and reduced lipid peroxidation. Thus, it exhibits wound healing activity.
Propionibacterium acnes, a Gram-positive bacterium, is critical in the pathogenesis of acne vulgaris [66]. Propionibacterium acnes is capable of biofilm formation, and the decreased antimicrobial susceptibility of biofilm-associated cells may hamper the effective treatment of Propionibacterium acnes infection. Rhodiola crenulata extract exhibited antibiofilm activity against Propionibacterium acnes [66].
Melanin is responsible for skin color and plays a major role in the defense against harmful external factors such as ultraviolet irradiation [67–70]. Tyrosinase is the rate-limiting step of tyrosine hydroxylation and is responsible for the critical steps of melanogenesis [70,71]. The mechanisms of the action of skin hypopigmenting agents are thought to be based on the ability of an agent to inhibit the activity of tyrosinase and, thus, down-regulate melanin synthesis [70,72,73]. Studies have shown that the acetone extract of Rhodiola rosea exhibits antityrosinase activity [74] and that the hydroalcoholic extract of Rhodiola rosea extract and its hydrolysate inhibited melanin synthesis and tyrosinase activity in B16F0 cells (mouse melanoma cells) [75]. Rhodiola rosea extract also inhibited the gene and protein expression of melanocortin 1 receptor (MC1R), inhibited c-AMP response element binding protein phosphorylation, suppressed the activation of AKT and glycogen synthase kinase-3 beta (GSK3b), and inhibited the expression of microphthalmia-associated transcription factor and tyrosinase-related protein 1 (TRP-1) [70,76,77]. The hydrolysate of Rhodiola rosea inhibited the phosphorylation of CREB, activation of AKT and GSK3β, and expression of MITF and tyrosinase.
In one study, tyrosol and its glycoside, salidroside, exhibited antimelanogenesis activity [78]. Treating B16F0 cells with tyrosol and salidroside reduced melanin content and the inhibition oftyrosinase activity and its expression[78]. Tyrosolsuppressed α-MSH-induced MC1R and TRP-1 expression, but salidroside did not. Neither tyrosol nor salidroside affected MITF or TRP-2 expression. The compounds exhibited metal-coordinating interactions with copper ions in molecular docking with tyrosinase [78]. Salidroside significantly inhibited tyrosinase activity in B16F0 cells at 1000 μM and melanin content at 100–1000 μM in a dose-dependent manner [79]. Salidroside inhibited UVB-induced hyperpigmentation in brown guinea pig skin by reducing the number of DOPA-positive melanocytes in the basal layer of the epidermis and reducing tyrosinase activity and melanin synthesis in melanocytes [79]. Rhodiola rosea extract, salidroside, and tyrosol may be effective skin-whitening agents; Table 1 summarizes their effects on skin.
Table 1.
The activities of Rhodiola plants and its active constituents on skin disorders.
| Plants/constituents | Models | Results | References |
|---|---|---|---|
| Rhodiola rosea | B16F0 cells |
|
[74,75] |
| Rhodiola rosea extract/L-carnosine-associated compound | Human skin |
|
[65] |
| Rhodiola rosea extract/L-carnosine-associated compound | Keratinocytes |
|
[65] |
| Rhodiola imbricata | SD rats |
|
[38] |
| Rhodiola crenulata extract | Propionibacterium acnes | antibiofilm activity against Propionibacterium acnes | [66] |
| Salidroside | B16F0 cells | Suppressed melanin synthesis and tyrosinase activity, but no effect on the expression of MC1R, MITF, TRP-1, or TRP-2 | [74,78,79] |
| Tyrosol | B16F0 cells |
|
[78] |
10. Protection against neuron and central neuron system disorders
A titolated extract from Rhodiola rosea and salidroside protected human cortical cells (HCN 1-A) from oxidative stressors such as H2O2 and glutamate-induced cell apoptosis [40]. Pre-treatment with Rhodiola rosea extract significantly increased cell survival and prevented plasma membrane damage and morphological disruption caused by glutamate or H2O2, indicating that Rhodiola rosea extract protects neurons from oxidative-stress-induced disorders [40]. In addition, Rhodiola rosea extract significantly reduced oxidative-stress-induced elevation of intracellular free Ca2+ concentrations [40].
Significant improvement in long-term memory was observed after 10 days of treatment with 0.1 mL of Rhodiola rosea extract [6]. However, in another study, Rhodiola rosea exhibited cytotoxic effects at 100 μg/mL in cultured primary cortical neurons [80]. Rhodiola rosea extracts exhibited anti-oxidant capacity but did not exhibit neuroprotective effects in primary cortical neurons [80].
Salidroside suppressed the LPS-induced expression of iNOS and cytokines in BV2 cells in a concentration-dependent manner [81]. Orally administering Rhodiola rosea crude extract (500 mg/kg) suppressed the expression of the proin-flammatory factors iNOS, IL-1β, and TNF-α in the kidney and prefrontal cortex of the brain in mice [81]. L-Glutamate treatment increased the levels of phosphorylated MAPK, p-JNK, and p-p38 [81]. These results indicate that Rhodiola rosea may have therapeutic potential for treating inflammation and neurodegenerative disease [81].
Oxidative stress plays a crucial role in Parkinson's disease and other neurodegenerative disorders. Salidroside has a neuroprotective effect in cortical neurons because of its antioxidant activity and ability to stabilize cellular Ca2+ ho-meostasis [40]. Incubating PC12 cells with salidroside prior to MPP+ exposure significantly reduced cell apoptosis and attenuated the collapse of the mitochondrial membrane potential [82]. Furthermore, salidroside inhibited MPP+-induced NO increases, the overexpression of nNOS and iNOS, and the accumulation of ROS and intracellular free Ca2+[82]. Salidroside inhibited ROS and NO production, protecting PC12 cells from oxidative stress. The protective effects of salidroside on PC12 cells are mediated by the inhibition of ROS generation and the NO pathway [82]. In addition, salidroside pretreatment protected dopaminergic neurons against MPTP/MPP+-induced toxicity in a dose-dependent manner [83]. The mechanisms of this protection included salidroside reducing the production of ROS and NO, regulating the ratio of Bcl-2/ Bax, reducing cytochrome-c and Smac release, inhibiting caspase-3, caspas-6, and caspas-9 activation, and reducing α-synuclein aggregation [83].
Pretreatment with salidroside markedly attenuated H2O2-induced cell viability loss and apoptotic cell death in a dose-dependent manner in human neuroblastoma SH-SY5Y cells [84]. Salidroside protected neuron cells from oxidative stress by inducing several antioxidant enzymes such as thioredoxin, heme oxygenase-1, and peroxiredoxin-I and downregulating the proapoptotic gene Bax and upregulating the antiapoptotic genes Bcl-2 and Bcl-XL [84]. Furthermore, salidroside dose-dependently restored H2O2-induced loss of mitochondrial membrane potential and elevated intracellular calcium levels [84]. Salidroside alleviated hydroxyl-peroxide-induced cell viability loss and apoptotic cell death in a dose-dependent manner in cultured nerve-growth-factor-differentiated PC12 cells [85]. The neuroprotective effects of salidroside might be modulated by the ERK signaling pathway, particularly at the level of or upstream from caspase-3 [85]. In addition, salidroside (100 μM) significantly reduced hydroxyl-peroxide-induced apoptosis and necrosis and markedly attenuated oxidative insult caused by hydroxyl peroxide exposure in cultured rat cortical neurons [86]. Salidroside prevented cerebral ischemic injury induced by middle cerebral artery occlusion and reperfusion in SD rats. Furthermore, there were more normal neurons and cells in the hippocampus after salidroside treatment [86]. These results suggest that salidroside has protective effects against oxidative-stress-induced cell apoptosis and is thus a potential therapeutic agent for treating or preventing neurodegenerative diseases involving oxidative stress.
Salidroside reduced neuronal death and behavioral dysfunction mediated by polyglutamine expressed in ASH neurons in transgenic Caenorhabditis elegans [87]. Salidroside’s antioxidative capability, but not its direct inhibition of polyglutamine aggregation, may contribute to neuron protection [87].
Rhodiola plant extracts and salidroside prevented neural disorders such as Parkinson's disease and other neurodegenerative disorders. Table 2 lists studies of the effects of these agents on neurons.
Table 2.
The activities of Rhodiola plants and its active constituents on neuron system.
| Plants/constituents | Models | Results | References |
|---|---|---|---|
| Rhodiola rosea | Human cortical cell line (HCN 1-A) |
|
[40] |
| Rhodiola rosea | Rats | improvement of the long-term memory | [6] |
| Salidroside | Transgenic Caenorhabditis elegans models |
|
[87] |
| Salidroside | PC12 cells |
|
[82] |
| Salidroside | PC12 cells |
|
[85] |
| Salidroside | PC12 cells C57BL/6 mice |
|
[83] |
| Salidroside | Human neuroblastoma SH-SY5Y cells |
|
[84] |
11. Liver protection
The methanolic extract from the roots of Rhodiola sachalinensis exhibited a protective effect on D-galactosamine-induced cytotoxicity in primary cultured mouse hepatocytes [88]. In addition, the principal constituents, sachalosides III and IV, rhodiosin, and transcaffeic acid, exhibited hepatoprotective effects [88].
Salidroside attenuated D-galactosamine- and LPS-induced acute increases in serum aspartate aminotransferase and alanine aminotransferase activities and in levels of TNF-α and serum NO in a dose-dependent manner [52]. It restored depleted hepatic glutathione, SOD, catalase, and glutathione peroxidase activities, reduced MDA levels, and considerably reduced histopathological changes in liver tissue [52]. In addition, salidroside reduced the size of necrotic regions, caspase-3 expression, and hypoxia-inducible factor (HIF)-1α in liver tissue in mice [52]. The hepatoprotective mechanism of salidroside may be related to antioxidant activity and the inhibition of HIF-1α [52]. Rhodiola sachalinensis and salidroside protected liver tissue from oxidative-stress-induced damage.
12. Cardiovascular disease
Rhodiola rosea was observed to prevent stress-induced cardiac damage [9]. The cardioprotective effects of Rhodiola rosea, including a pronounced antiarrhythmic effect [9], the prevention of reduced coronary flow, and an increase in contractility in the postischemic period, were observed in animals [89]. Rhodiola rosea was ascertained to prevent both stress-induced catecholamine release and elevation of cAMP levels in the myocardium [9]. Moreover, it lowered blood pressure [90] and prevented stress-induced cardiac damage, indicating its critical role as a cardioprotective agent in animals [8].
The rhizome of Rhodiola kirilowii significantly increased the expression of von Willebrand factor in the infarct border zone and noninfarct zone in the myocardium of rats [91]. The expression of HIF-1α, HIF-1β, and vascular endothelial growth factor (VEGF) mRNAs as well as HIF-1α and VEGF proteins was significantly increased in a Rhodiola kirilowii group [91].
Salidroside exhibited activity similar to that of Rhodiola kirilowii, increasing the expression of HIF-1α, HIF-1β, and VEGF during ischemia and hypoxia [91]. In addition, salidroside protected human endothelial cells (EVC-304) from H2O2-induced oxidative damage in a dose-dependent manner [92]. Salidroside inhibited the activation of caspase-3, caspase-9, cleavage of poly(ADP-ribose)polymerase, and Bax induced by endogenous H2O2 [92].
Salidroside and tyrosol dose-dependently inhibited nuclear condensation in H9c2 cells [93]. Furthermore, salidroside and tyrosol, separately and in combination, significantly reduced caspase-3 activity, cytochrome c release, and JNK activation. The antiapoptotic effect of the combination was markedly higher than that of salidroside and tyrosol alone [93]. The inhibition of the JNK-signaling pathway is the key mechanism for the cytoprotective effects of salidroside and tyrosol in ischemia-reperfusion-induced apoptosis in H9c2 cells [93]. Rhodiola plants, salidroside, and tyrosol may facilitate preventing and treating oxidative stress in cardiovascular and cerebrovascular diseases.
13. DM
DM is associated with increased oxidative stress. Rats with streptozotocin (STZ)-induced DM experienced heart failure caused by increased PPARδ expression. The ethanol extract of Rhodiola increased PPARδ expression and cardiac output in STZ-diabetic rats [94]. Salidroside administered to mice daily (50 mg/kg, 100 mg/kg, and 200 mg/kg for 28 days) was demonstrated to cause hypoglycemic activity and have protective effects against DM-induced oxidative stress, including significantly reduced fasting blood glucose, total cholesterol, triglyceride, and MDA levels [95]. In addition, it increased serum insulin, SOD, and glutathione peroxidase levels as well as catalase activity in the liver in mice [95]. Therefore, Rhodiola extracts and salidroside should be considered for use in treating DM [95].
14. Obesity and hyperlipidemia
Rhodiola rosea inhibited the activity of lipase in isolated mouse plasma in vitro and in the mouse gastrointestinal tube in vivo and can be used in treating or preventing lifestyle-related diseases such as hyperlipidemia and exogenous obesity [14]. Rhodiola extracts dose-dependently increased SOD activity, resulting in a reduced ROS level during adipogenesis [96]. Treatment with Rhodiola extract inhibited the activities of proline dehydrogenase (PDH) and glucose-6-phosphate dehydrogenase (G6PDH) as well as lipid accumulation and ROS production in 3T3-L1 preadipocytes [96]. In addition, SOD activity in cells treated with Rhodiola extract increased significantly during the differentiation of 3T3-L1 preadipocytes [96]. The inhibition of PDH and G6PDH prevented the proline oxidation required for critical ATP generation that is coupled with the antioxidant enzyme response via the proline-mediated pentose phosphate pathway, leading to the inhibition of adipogenesis [96]. The antiadipogenic effects of Rhodiola extract may disrupt proline-mediated energy generation and antioxidant enzyme response via the proline-mediated pentose phosphate pathway, resulting in the suppression of adipogenesis and lipid accumulation [96].
Tyrosol at 1.0 mg/mL significantly increased SOD activity during the differentiation of 3T3-L1 preadipocytes [96]. In addition, tyrosol inhibited the activities of PDH and G6PDH and lipid accumulation [96]. Rhodiola extract and tyrosol may be used to prevent obesity.
15. Anticancer
Incubation with Rhodiola imbricata aqueous extract at 100 mg/ mL and 200 mg/mL for 72 hours significantly reduced the proliferation of K-562 human erythroleukemic cells in a dose-dependent manner but not the proliferation of normal human peripheral blood lymphocytes or RAW-264.7 mouse macrophage cells [97]. In addition, Rhodiola imbricata aqueous extract was observed to induce intracellular ROS in K-562 cells at 200 mg/mL and, thus, induce apoptosis [97]. Rhodiola imbricata aqueous extract arrested the cell cycle progression of K562 and NK cells in the G2/M phase in early and late periods of exposure [97].
Salidroside significantly reduced the proliferation of A549 human alveolar adenocarcinoma cells, inhibited cell cycle arrest in the G0/G1 phase, and induced apoptosis in A549 cells by reducing pp38 protein expression [98]. Salidroside inhibited transforming growth factor-β-induced tumor invasion and suppressed protein expression [98]. Salidroside inhibited intracellular ROS formation in a dose-dependent manner in A549 cells [98]. Another study reported that salidroside significantly reduced tumor-induced angiogenesis in mice [2].
16. Antivirus activity
Salidroside provided protection against coxsackievirus B3, which causes viral myocarditis, in both in vitro and in vivo experiments [99]. The IC50 of salidroside for coxsackievirus B3 is 39.0 ± 1.2 mg/L. Salidroside can modulate the mRNA expression of interferon-g, IL-10, TNF-α, and IL-2 in coxsackievirus B3. Salidroside increased lactic dehydrogenase, aspartate transaminase, and creatine kinase activities in infected BALB/c mouse serum [100]. Salidroside is a potential agent for treating viral myocarditis [99].
17. Conclusion
Rhodiola plants are commonly used in traditional medicines in Asia and Europe. Studies have shown that the plants and their two major constituents, salidroside and tyrosol, exhibit adaptogenic, antifatigue, antidepressant, antioxidant, anti-inflammatory, antinoception, and anticancer bioactivities, modulate immune function, and prevent cardiovascular, neuronal, liver, and skin disorders.
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest related to this work.
References
- 1. Bawa AS, Khanum F. Anti-inflammatory activity of Rhodiola rosea–”a second-generation adaptogen. Phytother Res. 2009;23:1099–102. doi: 10.1002/ptr.2749. [DOI] [PubMed] [Google Scholar]
- 2. Skopinska-Rozewska E, Malinowski M, Wasiutynski A, Sommer E, Furmanowa M, Mazurkiewicz M, Siwicki AK. The influence of Rhodiola quadrifida 50% hydro-alcoholic extract and salidroside on tumor-induced angiogenesis in mice. Pol J Vet Sci. 2008;11:97–104. [PubMed] [Google Scholar]
- 3. Kelly GS. Rhodiola rosea: a possible plant adaptogen. Altern Med. 2001;6:293–302. [PubMed] [Google Scholar]
- 4. Chan SW. Panax ginseng, Rhodiola rosea and Schisandra chinensis . Int J Food Sci Nutr. 2012;63:S75–81. doi: 10.3109/09637486.2011.627840. [DOI] [PubMed] [Google Scholar]
- 5. Panossian A, Wikman G, Sarris J. Rosenroot (Rhodiola rosea): traditional use, chemical composition, pharmacology and clinical efficacy. Phytomedicine. 2010;17:481–93. doi: 10.1016/j.phymed.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 6. Petkov VD, Yonkov D, Mosharoff A, Kambourova T, Alova L, Petkov VV, Todorov I. Effects of alcohol aqueous extract from Rhodiola rosea L. roots on learning and memory. Acta Physiol Pharmacol Bulg. 1986;12:3–16. [PubMed] [Google Scholar]
- 7. Rohloff J. Volatiles from rhizomes of Rhodiola rosea L. Phytochemistry. 2002;59:655–61. doi: 10.1016/s0031-9422(02)00004-3. [DOI] [PubMed] [Google Scholar]
- 8. Li DJ, Lu B, Mo SR. Effects of rhodiola on hemodynamics in acute myocardial ischemia and heart failure in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2006;22:291–2. [PubMed] [Google Scholar]
- 9. Maslova LV, Kondrat'ev B, Maslov LN, Lishmanov Iu B. The cardioprotective and antiadrenergic activity of an extract of Rhodiola rosea in stress. Eksp Klin Farmakol. 1994;57:61–3. [PubMed] [Google Scholar]
- 10. Evstatieva L, Todorova M, Antonova D, Staneva J. Chemical composition of the essential oils of Rhodiola rosea L. of three different origins. Pharmacogn Mag. 2010;6:256–8. doi: 10.4103/0973-1296.71782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen TS, Liou SY, Chang YL. Antioxidant evaluation of three adaptogen extracts. Am J Chin Med. 2008;36:1209–17. doi: 10.1142/S0192415X08006533. [DOI] [PubMed] [Google Scholar]
- 12. Elameen A, Dragland S, Klemsdal SS. Bioactive compounds produced by clones of Rhodiola rosea maintained in the Norwegian germplasm collection. Pharmazie. 2010;65:618–23. [PubMed] [Google Scholar]
- 13. Ioset KN, Nyberg NT, Van Diermen D, Malnoe P, Hostettmann K, Shikov AN, Jaroszewski JW. Metabolic profiling of Rhodiola rosea rhizomes by (1)H NMR spectroscopy. Phytochem Anal. 2011;22:158–65. doi: 10.1002/pca.1262. [DOI] [PubMed] [Google Scholar]
- 14. Kobayashi K, Yamada K, Murata T, Hasegawa T, Takano F, Koga K, Fushiya S, Batkhuu J, Yoshizaki F. Constituents of Rhodiola rosea showing inhibitory effect on lipase activity in mouse plasma and alimentary canal. Planta Med. 2008;74:1716–9. doi: 10.1055/s-0028-1088318. [DOI] [PubMed] [Google Scholar]
- 15. Kucinskaite A, Poblocka-Olech L, Krauze-Baranowska M, Sznitowska M, Savickas A, Briedis V. Evaluation of biologically active compounds in roots and rhizomes of Rhodiola rosea L. cultivated in Lithuania. Medicina (Kaunas) 2007;43:487–94. [PubMed] [Google Scholar]
- 16. Gupta M, Agrawal U, Vyas SP. Nanocarrier-based topical drug delivery for the treatment of skin diseases. Expert Opin Drug Deliv. 2012;9:783–804. doi: 10.1517/17425247.2012.686490. [DOI] [PubMed] [Google Scholar]
- 17. Ma YC, Wang XQ, Hou F, Ma J, Luo M, Lu S, Jin P, Chen A, Xu I, Patel AV, Gorecki D. Simultaneous quantification of polyherbal formulations containing Rhodiola rosea L. and Eleutherococcus senticosus Maxim. using rapid resolution liquid chromatography (RRLC) J Pharm Biomed Anal. 2011;55:908–15. doi: 10.1016/j.jpba.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 18. Ma YC, Wang XQ, Hou FF, Ma J, Luo M, Lu S, Jin P, Terevsky N, Chen A, Xu I, Pate AV, Gorecki D. Rapid resolution liquid chromatography (RRLC) analysis for quality control of Rhodiola rosea roots and commercial standardized products. Nat Prod Commun. 2011;6:645–50. [PubMed] [Google Scholar]
- 19. Linh PT, Kim YH, Hong SP, Jian JJ, Kang JS. Quantitative determination of salidroside and tyrosol from the underground part of Rhodiola rosea by high performance liquid chromatography. Arch Pharm Res. 2000;23:349–52. doi: 10.1007/BF02975446. [DOI] [PubMed] [Google Scholar]
- 20. Darbinyan V, Aslanyan G, Amroyan E, Gabrielyan E, Malmstrom C, Panossian A. Clinical trial of Rhodiola rosea L. extract SHR-5 in the treatment of mild to moderate depression. Nord J Psychiatry. 2007;61:343–8. doi: 10.1080/08039480701643290. [DOI] [PubMed] [Google Scholar]
- 21. Grace MH, Yousef GG, Kurmukov AG, Raskin I, Lila MA. Phytochemical characterization of an adaptogenic preparation from Rhodiola heterodonta. Nat Prod Commun. 2009;4:1053–8. [PMC free article] [PubMed] [Google Scholar]
- 22. Spasov AA, Wikman GK, Mandrikov VB, Mironova IA, Neumoin VV. A double-blind, placebo-controlled pilot study of the stimulating and adaptogenic effect of Rhodiola rosea SHR-5 extract on the fatigue of students caused by stress during an examination period with a repeated low-dose regimen. Phytomedicine. 2000;7:85–9. doi: 10.1016/S0944-7113(00)80078-1. [DOI] [PubMed] [Google Scholar]
- 23. Mattioli L, Perfumi M. Rhodiola rosea L. extract reduces stress- and CRF-induced anorexia in rats. J Psychopharmacol. 2007;21:742–50. doi: 10.1177/0269881106074064. [DOI] [PubMed] [Google Scholar]
- 24. Perfumi M, Mattioli L. Adaptogenic and central nervous system effects of single doses of 3% rosavin and 1% salidroside Rhodiola rosea L. extract in mice. Phytother Res. 2007;21:37–43. doi: 10.1002/ptr.2013. [DOI] [PubMed] [Google Scholar]
- 25. Olsson EM, von Scheele B, Panossian AG. A randomised, double-blind, placebo-controlled, parallel-group study of the standardised extract shr-5 of the roots of Rhodiola rosea in the treatment of subjects with stress-related fatigue. Planta Med. 2009;75:105–12. doi: 10.1055/s-0028-1088346. [DOI] [PubMed] [Google Scholar]
- 26. Gupta V, Saggu S, Tulsawani RK, Sawhney RC, Kumar R. A dose dependent adaptogenic and safety evaluation of Rhodiola imbricata Edgew, a high altitude rhizome. Food Chem Toxicol. 2008;46:1645–52. doi: 10.1016/j.fct.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 27. Gupta V, Lahiri SS, Sultana S, Tulsawani RK, Kumar R. Anti-oxidative effect of Rhodiola imbricata root extract in rats during cold, hypoxia and restraint (C-H-R) exposure and post-stress recovery. Food Chemical Toxicol. 2010;48:1019–25. doi: 10.1016/j.fct.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 28. Kan NW, Huang WC, Lin WT, Huang CY, Wen KC, Chiang HM, Huang CC, Hsu MC. Hepatoprotective effects of Ixora parviflora extract against exhaustive exercise-induced oxidative stress in mice. Molecules. 2013;18:10721–32. doi: 10.3390/molecules180910721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu J, Li Y. Effects of salidroside on exhaustive exercise induced oxidative stress in rats. Mol Medicine Rep. 2012;6:1195–8. doi: 10.3892/mmr.2012.1060. [DOI] [PubMed] [Google Scholar]
- 30. Chiang HM, Chen HC, Chiu HH, Chen CW, Wang S-M, Wen K-C. Neonauclea reticulata (Havil.) Merr stimulates skin regeneration after UVB exposure via ROS scavenging and modulation of the MAPK/MMPs/collagen pathway. Evid Based Complement Alternat Med. 2013;2013:9. doi: 10.1155/2013/324864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chiang HM, Chiu HH, Liao ST, Chen YT, Chang HC, Wen K-C. Isoflavonoid-rich Femingia macrophylla extract attenuates UVB-induced skin damage by scavenging reactive oxygen species and inhibiting MAP kinase and MMP expression. Evid Based Complement Alternat Med. 2013;2013:12. doi: 10.1155/2013/696879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wen KC, Chiu HH, Fan PC, Chen CW, Wu SM, Chang JH, Chiang HM. Antioxidant activity of Ixora parviflora in a cell/ cell-free system and in UV-exposed human fibroblasts. Molecules. 2011;16:5735–52. doi: 10.3390/molecules16075735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jafari M, Felgner JS, Bussel II, Hutchili T, Khodayari B, Rose MR, Vince-Cruz C, Mueller LD. Rhodiola: a promising anti-aging Chinese herb. Rejuvenation Res. 2007;10:587–602. doi: 10.1089/rej.2007.0560. [DOI] [PubMed] [Google Scholar]
- 34. Mao GX, Wang Y, Qiu Q, Deng HB, Yuan LG, Li RG, Song DQ, Li YY, Li DD, Wang Z. Salidroside protects human fibroblast cells from premature senescence induced by H(2)O(2) partly through modulating oxidative status. Mech Ageing Dev. 2010;131:723–31. doi: 10.1016/j.mad.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 35. Mao GX, Deng HB, Yuan LG, Li DD, Li YY, Wang Z. Protective role of salidroside against aging in a mouse model induced by D-galactose. Biomed Environ Sci. 2010;23:161–6. doi: 10.1016/s0895-3988(10)60047-5. [DOI] [PubMed] [Google Scholar]
- 36. Wen KC, Fan PC, Tsai SY, Shih IC, Chiang HM. Ixora parviflora protects against UVB-induced photoaging by inhibiting the expression of MMPs, MAP kinases, and COX-2 and by promoting type I procollagen synthesis. Evid Based Complement Alternat Med. 2012;2012:417346. doi: 10.1155/2012/417346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wen KC, Shih IC, Hu JC, Liao ST, Su TW, Chiang HM. Inhibitory effects of Terminalia catappa on UVB-induced photodamage in fibroblast cell line. Evid Based Complement Altern Med. 2011;2011:904532. doi: 10.1155/2011/904532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gupta A, Kumar R, Upadhyay NK, Pal K, Kumar R, Sawhney RC. Effects of Rhodiola imbricata on dermal wound healing. Planta Med. 2007:73774–7. doi: 10.1055/s-2007-981546. [DOI] [PubMed] [Google Scholar]
- 39. Calcabrini C, De Bellis R, Mancini U, Cucchiarini L, Potenza L, De Sanctis R, Patrone V, Scesa C, Dachà M. Rhodiola rosea ability to enrich cellular antioxidant defences of cultured human keratinocytes. Arch Dermatol Res. 2010;302:191–200. doi: 10.1007/s00403-009-0985-z. [DOI] [PubMed] [Google Scholar]
- 40. Palumbo DR, Occhiuto F, Spadaro F, Circosta C. Rhodiola rosea extract protects human cortical neurons against glutamate and hydrogen peroxide-induced cell death through reduction in the accumulation of intracellular calcium. Phytother Res. 2012;26:878–83. doi: 10.1002/ptr.3662. [DOI] [PubMed] [Google Scholar]
- 41. Kanupriya, Prasad D, Sai Ram M, Kumar R, Sawhney RC, Sharma SK, Ilavazhagan G, Kumar D, Banerjee PK. Cytoprotective and antioxidant activity of Rhodiola imbricata against tert-butyl hydroperoxide induced oxidative injury in U-937 human macrophages. Mol Cell Biochem. 2005;275:1–6. doi: 10.1007/s11010-005-7637-1. [DOI] [PubMed] [Google Scholar]
- 42. Schriner SE, Avanesian A, Liu Y, Luesch H, Jafari M. Protection of human cultured cells against oxidative stress by Rhodiola rosea without activation of antioxidant defenses. Free Radic Biol Med. 2009;47:577–84. doi: 10.1016/j.freeradbiomed.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 43. Ohsugi M, Fan W, Hase K, Xiong Q, Tezuka Y, Komatsu K, Namba T, Saitoh T, Tazawa K, Kadota S. Active-oxygen scavenging activity of traditional nourishing-tonic herbal medicines and active constituents of Rhodiola sacra. J Ethnopharmacol. 1999;67:111–9. doi: 10.1016/s0378-8741(98)00245-1. [DOI] [PubMed] [Google Scholar]
- 44. Zhou Q, Yin ZP, Ma L, Zhao W, Hao HW, Li HL. Free radical-scavenging activities of oligomeric proanthocyanidin from Rhodiola rosea L. and its antioxidant effects in vivo. Nat Prod Res. 2014:1–3. doi: 10.1080/14786419.2014.921786. [DOI] [PubMed] [Google Scholar]
- 45. Qian EW, Ge DT, Kong SK. Salidroside protects human erythrocytes against hydrogen peroxide-induced apoptosis. J Nat Prod. 2012;75:531–7. doi: 10.1021/np200555s. [DOI] [PubMed] [Google Scholar]
- 46. Tuck KL, Hayball PJ. Major phenolic compounds in olive oil: metabolism and health effects. J Nutr Biochem. 2002;13:636–44. doi: 10.1016/s0955-2863(02)00229-2. [DOI] [PubMed] [Google Scholar]
- 47. Ko RK, Kim GO, Hyun CG, Jung DS, Lee NH. Compounds with tyrosinase inhibition, elastase inhibition and DPPH radical scavenging activities from the branches of Distylium racemosum Sieb. et Zucc. Phytother Res. 2011;25:1451–6. doi: 10.1002/ptr.3439. [DOI] [PubMed] [Google Scholar]
- 48. Doncheva ND, Mihaylova AS, Getova DP. Antinociceptive and anti-inflammatory effects of Rhodiola rosea L. extract in rats. Folia Med. 2013;55:70–5. doi: 10.2478/folmed-2013-0030. [DOI] [PubMed] [Google Scholar]
- 49. Mishra KP, Padwad YS, Jain M, Karan D, Ganju L, Sawhney RC. Aqueous extract of Rhodiola imbricata rhizome stimulates proinflammatory mediators via phosphorylated IkappaB and transcription factor nuclear factor-kappaB. Immunopharmacol Immunotoxicol. 2006;28:201–12. doi: 10.1080/08923970600815139. [DOI] [PubMed] [Google Scholar]
- 50. Abidov M, Grachev S, Seifulla RD, Ziegenfuss TN. Extract of Rhodiola rosea radix reduces the level of C-reactive protein and creatinine kinase in the blood. Bull Exp Biol Med. 2004;138:63–4. doi: 10.1023/b:bebm.0000046940.45382.53. [DOI] [PubMed] [Google Scholar]
- 51. Zhu C, Guan F, Wang C, Jin LH. The protective effects of Rhodiola crenulata extracts on Drosophila melanogaster gut immunity induced by bacteria and SDS toxicity. Phytother Res. 2014;28:1861–6. doi: 10.1002/ptr.5215. [DOI] [PubMed] [Google Scholar]
- 52. Wu YL, Lian LH, Jiang YZ, Nan JX. Hepatoprotective effects of salidroside on fulminant hepatic failure induced by D-galactosamine and lipopolysaccharide in mice. J Pharm Pharmacol. 2009;61:1375–82. doi: 10.1211/jpp/61.10.0015. [DOI] [PubMed] [Google Scholar]
- 53. Guan S, Feng H, Song B, Guo W, Xiong Y, Huang G, Zhong W, Huo M, Chen N, Lu J, Deng X. Salidroside attenuates LPS-induced pro-inflammatory cytokine responses and improves survival in murine endotoxemia. Int Immunopharmacol. 2011;11:2194–9. doi: 10.1016/j.intimp.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 54. Guan S, Xiong Y, Song B, Song Y, Wang D, Chu X, Chen N, Huo M, Deng X, Lu J. Protective effects of salidroside from Rhodiola rosea on LPS-induced acute lung injury in mice. Immunopharmacol Immunotoxicol. 2012;34:667–72. doi: 10.3109/08923973.2011.650175. [DOI] [PubMed] [Google Scholar]
- 55. Li D, Fu Y, Zhang W, Su G, Liu B, Guo M, Li F, Liang D, Liu Z, Zhang X, Cao Y, Zhang N, Yang Z. Salidroside attenuates inflammatory responses by suppressing nuclear factor-kappaB and mitogen activated protein kinases activation in lipopolysaccharide-induced mastitis in mice. Inflamm Research. 2013;62:9–15. doi: 10.1007/s00011-012-0545-4. [DOI] [PubMed] [Google Scholar]
- 56. Choi SY, Kim S, Kim H, Suk K, Hwang JS, Lee BG, Kim AJ, Kim SY. (4-Methoxy-benzylidene)-(3-methoxy-phenyl)-amine, a nitrogen analog of stilbene as a potent inhibitor of melanin production. Chem Pharm Bull (Tokyo) 2002;50:450–2. doi: 10.1248/cpb.50.450. [DOI] [PubMed] [Google Scholar]
- 57. Hu B, Zou Y, Liu S, Wang J, Zhu J, Li J, Bo L, Deng X. Salidroside attenuates concanavalin A-induced hepatitis via modulating cytokines secretion and lymphocyte migration in mice. Mediators Inflamm. 2014;2014:314081. doi: 10.1155/2014/314081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mishra KP, Chanda S, Shukla K, Ganju L. Adjuvant effect of aqueous extract of Rhodiola imbricata rhizome on the immune responses to tetanus toxoid and ovalbumin in rats. Immunopharmacol Immunotoxicol. 2010;32:141–6. doi: 10.3109/08923970903503668. [DOI] [PubMed] [Google Scholar]
- 59. Mishra KP, Ganju L, Singh SB. Anti-cellular and immunomodulatory potential of aqueous extract of Rhodiola imbricata rhizome. Immunopharmacol Immunotoxicol. 2012;34:513–8. doi: 10.3109/08923973.2011.638307. [DOI] [PubMed] [Google Scholar]
- 60. Skopnska-Rozewska E, Wojcik R, Siwicki AK, Sommer E, Wasiutynski A, Furmanowa M, Malinowski M, Mazurkiewicz M. The effect of Rhodiola quadrifida extracts on cellular immunity in mice and rats. Pol J Vet Sci. 2008;11:105–11. [PubMed] [Google Scholar]
- 61. Wojcik R, Siwicki AK, Skopinska-Rozewska E, Wasiutynski A, Sommer E, Furmanowa M. The effect of Chinese medicinal herb Rhodiola kirilowii extracts on cellular immunity in mice and rats. Pol J Vet Sci. 2009;12:399–405. [PubMed] [Google Scholar]
- 62. Lu L, Yuan J, Zhang S. Rejuvenating activity of salidroside (SDS): dietary intake of SDS enhances the immune response of aged rats. Age (Dordr) 2013;35:637–46. doi: 10.1007/s11357-012-9394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yan GH, Choi YH. Salidroside attenuates allergic airway inflammation through negative regulation of nuclear factor-kappa B and p38 mitogen-activated protein kinase. J Pharmacol Sc. 2014;126:126–35. doi: 10.1254/jphs.14037fp. [DOI] [PubMed] [Google Scholar]
- 64. Yang SJ, Yu HY, Kang DY, Ma ZQ, Qu R, Fu Q, Ma SP. Antidepressant-like effects of salidroside on olfactory bulbectomy-induced pro-inflammatory cytokine production and hyperactivity of HPA axis in rats. Pharmacol Biochemi Behav. 2014;124:451–7. doi: 10.1016/j.pbb.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 65. de Dieamant GC, del Velazquez Pereda MC, Eberlin S, Nogueira C, Werka RM, Queiroz ML. Neuroimmunomodulatory compound for sensitive skin care: in vitro and clinical assessment. J Cosmet Dermatol. 2008;7:112–9. doi: 10.1111/j.1473-2165.2008.00373.x. [DOI] [PubMed] [Google Scholar]
- 66. Coenye T, Brackman G, Rigole P, De Witte E, Honraet K, Rossel B, Nelis HJ. Eradication of Propionibacterium acnes acnes biofilms by plant extracts and putative identification of icariin, resveratrol and salidroside as active compounds. Phytomedicine. 2012;19:409–12. doi: 10.1016/j.phymed.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 67. Lin JW, Chiang HM, Lin YC, Wen KC. Natural products with skin - whitening effects. J Food Drug Anal. 2008;16:1–10. [Google Scholar]
- 68. Brenner M, Hearing VJ. The protective role of melanin against UV damage in human skin. Photochem Photobiol. 2008;84:539–49. doi: 10.1111/j.1751-1097.2007.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Costin GE, Hearing VJ. Human skin pigmentation: melanocytes modulate skin color in response to stress. FASEB J. 2007;21:976–94. doi: 10.1096/fj.06-6649rev. [DOI] [PubMed] [Google Scholar]
- 70. Chiang HM, Chen HW, Huang YH, Chan SY, Chen CC, Wu WC, Wen KC. Melanogenesis and natural hypopigmentation agents. Int J Med Biol Front. 2012;18:1–76. [Google Scholar]
- 71. Chiang HM, Lin JW, Hsiao PL, Tsai SY, Wen KC. Hydrolysates of citrus plants stimulate melanogenesis protecting against UV-induced dermal damage. Phytother Res. 2011;25:569–76. doi: 10.1002/ptr.3302. [DOI] [PubMed] [Google Scholar]
- 72.Kuo YH, Chen CC, Lin P, You YJ, Chiang HM.N-(4-bromophenethyl) caffeamide inhibits melanogenesis by regulating AKT/ glycogen synthase kinase 3 beta/ microphthalmia-associated transcription factor and tyrosinase-related protein 1/tyrosinase. Curr Pharm Biotechnol. 2015. [accepted for publication] [DOI] [PubMed]
- 73. Chiang HM, Ko Y, Shih I, Wen K. Development of wine cake as a skin-whitening agent and humectant. J Food Drug Anal. 2011;19:223–9. [Google Scholar]
- 74. Chen CH, Chan HC, Chu YT, Ho HY, Chen PY, Lee TH, Lee CK. Antioxidant activity of some plant extracts towards xanthine oxidase, lipoxygenase and tyrosinase. Molecules. 2009;14:2947–58. doi: 10.3390/molecules14082947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chiang HM, Chien YC, Wu CH, Kuo YH, Wu WC, Pan YY, Su YH, Wen KC. Hydroalcoholic extract of Rhodiola rosea L. (Crassulaceae) and its hydrolysate inhibit melanogenesis in B16F0 cells by regulating the CREB/MITF/tyrosinase pathway. Food Chem Toxicol. 2014;65:129–39. doi: 10.1016/j.fct.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 76. Hearing VJ. Determination of melanin synthetic pathways. J Invest Dermatol. 2011;131:e8–11. doi: 10.1038/skinbio.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kondo T, Hearing VJ. Update on the regulation of mammalian melanocyte function and skin pigmentation. Expert Rev Dermatol. 2011;6:97–108. doi: 10.1586/edm.10.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wen KC, Chang CS, Chien YC, Wang HW, Wu WC, Wu CS, Chiang HM. Tyrosol and its analogues inhibit alpha-melanocyte-stimulating hormone induced melanogenesis. Int J Mol Sci. 2013;14:23420–40. doi: 10.3390/ijms141223420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Peng LH, Liu S, Xu SY, Chen L, Shan YH, Wei W, Liang WQ, Gao JQ. Inhibitory effects of salidroside and paeonol on tyrosinase activity and melanin synthesis in mouse B16F10 melanoma cells and ultraviolet B-induced pigmentation in guinea pig skin. Phytomedicine. 2013;20:1082–7. doi: 10.1016/j.phymed.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 80. Shen B, Truong J, Helliwell R, Govindaraghavan S, Sucher NJ. An in vitro study of neuroprotective properties of traditional Chinese herbal medicines thought to promote healthy ageing and longevity. BMC Complement Altern Med. 2013;13:373. doi: 10.1186/1472-6882-13-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Lee Y, Jung JC, Jang S, Kim J, Ali Z, Khan IA, Oh S. Anti-inflammatory and neuroprotective effects of constituents isolated from Rhodiola rosea. Evid Based Complement Altern Med. 2013;2013:514049. doi: 10.1155/2013/514049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Li X, Ye X, Li X, Sun X, Liang Q, Tao L, Kang X, Chen J. Salidroside protects against MPP(+)-induced apoptosis in PC12 cells by inhibiting the NO pathway. Brain Res. 2011;1382:9–18. doi: 10.1016/j.brainres.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 83. Wang S, He H, Chen L, Zhang W, Zhang X, Chen J. Protective effects of salidroside in the MPTP/MPP-induced model of Parkinson's disease through ROS-NO-related mitochondrion pathway. Mol Neurobiol. 2015;51:18–28. doi: 10.1007/s12035-014-8755-0. [DOI] [PubMed] [Google Scholar]
- 84. Zhang L, Yu H, Sun Y, Lin X, Chen B, Tan C, Cao G, Wang Z. Protective effects of salidroside on hydrogen peroxide-induced apoptosis in SH-SY5Y human neuroblastoma cells. Eur J Pharmacol. 2007;564:18–25. doi: 10.1016/j.ejphar.2007.01.089. [DOI] [PubMed] [Google Scholar]
- 85. Yu S, Shen Y, Liu J, Ding F. Involvement of ERK1/2 pathway in neuroprotection by salidroside against hydrogen peroxide-induced apoptotic cell death. J Mol Neurosci. 2010;40:321–31. doi: 10.1007/s12031-009-9292-6. [DOI] [PubMed] [Google Scholar]
- 86. Shi TY, Feng SF, Xing JH, Wu YM, Li XQ, Zhang N, Tian Z, Liu SB, Zhao MG. Neuroprotective effects of salidroside and its analogue tyrosol galactoside against focal cerebral ischemia in vivo and H2O2-induced neurotoxicity in vitro. Neurotox Res. 2012;21:358–67. doi: 10.1007/s12640-011-9290-7. [DOI] [PubMed] [Google Scholar]
- 87. Xiao L, Li H, Zhang J, Yang F, Huang A, Deng J, Liang M, Ma F, Hu M, Huang Z. Salidroside protects Caenorhabditis elegans neurons from polyglutamine-mediated toxicity by reducing oxidative stress. Molecules. 2014;19:7757–69. doi: 10.3390/molecules19067757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nakamura S, Li X, Matsuda H, Ninomiya K, Morikawa T, Yamaguti K, Yoshikawa M. Bioactive constituents from Chinese natural medicines. XXVI. Chemical structures and hepatoprotective effects of constituents from roots of Rhodiola sachalinensis. Chem Pharm Bull (Tokyo) 2007;55:1505–11. doi: 10.1248/cpb.55.1505. [DOI] [PubMed] [Google Scholar]
- 89. Zhang Z, Liu J, Shang X, Yang J, Chu J, Wang Z, Yao Z, Ma H, Li Q, Wang Y. The effect of Rhodiola capsules on oxygen consumption of myocardium and coronary artery blood flow in dogs. Zhongguo Zhong Yao Za Zhi. 1998;23:104–6. inside back cover. [PubMed] [Google Scholar]
- 90. LishmanovIu B, Naumova AV, Afanas'ev SA, Maslov LN. Contribution of the opioid system to realization of inotropic effects of Rhodiola rosea extracts in ischemic and reperfusion heart damage in vitro. Eksp Klin Farmakol. 1997;60:34–6. [PubMed] [Google Scholar]
- 91. Gao XF, Shi HM, Sun T, Ao H. Effects of Radix et Rhizoma Rhodiolae Kirilowii on expressions of von Willebrand factor, hypoxia-inducible factor 1 and vascular endothelial growth factor in myocardium of rats with acute myocardial infarction. Zhongguo Zhong Yao Za. 2009;7:434–40. doi: 10.3736/jcim20090507. [DOI] [PubMed] [Google Scholar]
- 92. Zhao X, Jin L, Shen N, Xu B, Zhang W, Zhu H, Luo Z. Salidroside inhibits endogenous hydrogen peroxide induced cytotoxicity of endothelial cells. Biol Pharm Bull. 2013;36:1773–8. doi: 10.1248/bpb.b13-00406. [DOI] [PubMed] [Google Scholar]
- 93. Sun L, Isaak CK, Zhou Y, Petkau JC, OK, Liu Y, Siow YL. Salidroside and tyrosol from Rhodiola protect H9c2 cells from ischemia/reperfusion-induced apoptosis. Life Sci. 2012;91:151–8. doi: 10.1016/j.lfs.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 94. Cheng YZ, Chen LJ, Lee WJ, Chen MF, Jung Lin H, Cheng JT. Increase of myocardial performance by Rhodiola-ethanol extract in diabetic rats. J Ethnopharmacol. 2012;144:234–9. doi: 10.1016/j.jep.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 95. Li F, Tang H, Xiao F, Gong J, Peng Y, Meng X. Protective effect of salidroside from Rhodiolae Radix on diabetes-induced oxidative stress in mice. Molecules. 2011;16:9912–24. doi: 10.3390/molecules16129912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lee OH, Kwon YI, Apostolidis E, Shetty K, Kim YC. Rhodiola-induced inhibition of adipogenesis involves antioxidant enzyme response associated with pentose phosphate pathway. Phytother Res. 2011;25:106–15. doi: 10.1002/ptr.3236. [DOI] [PubMed] [Google Scholar]
- 97. Mishra KP, Padwad YS, Dutta A, Ganju L, Sairam M, Banerjee PK, Sawhney RC. Aqueous extract of Rhodiola imbricata rhizome inhibits proliferation of an erythroleukemic cell line K-562 by inducing apoptosis and cell cycle arrest at G2/M phase. Immunobiol. 2008;213:125–31. doi: 10.1016/j.imbio.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 98. Wang J, Li JZ, Lu AX, Zhang KF, Li BJ. Anticancer effect of salidroside on A549 lung cancer cells through inhibition of oxidative stress and phospho-p38 expression. Oncol Lett. 2014;7:1159–64. doi: 10.3892/ol.2014.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wang H, Ding Y, Zhou J, Sun X, Wang S. The in vitro and in vivo antiviral effects of salidroside from Rhodiola rosea L. against coxsackievirus B3. Phytomedicine. 2009;16:146–55. doi: 10.1016/j.phymed.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 100. Anggakusuma, Yanti, Hwang JK. Effects of macelignan isolated from Myristica fragrans Houtt. on UVB-induced matrix metalloproteinase-9 and cyclooxygenase-2 in HaCaT cells. J Dermatol Sci. 2010;57:114–22. doi: 10.1016/j.jdermsci.2009.10.005. [DOI] [PubMed] [Google Scholar]