Abstract
Cosmos caudatus, which is a commonly consumed vegetable in Malaysia, is locally known as “Ulam Raja”. It is a local Malaysian herb traditionally used as a food and medicinal herb to treat several maladies. Its bioactive or nutritional constituents consist of a wide range of metabolites, including glucosinolates, phenolics, amino acids, organic acids, and sugars. However, many of these metabolites are not stable and easily degraded or modified during storage. In order to investigate the metabolomics changes occurring during post-harvest storage, C. caudatus samples were subjected to seven different storage times (0 hours, 2 hours, 4 hours, 6 hours, 8 hours, 10 hours, and 12 hours) at room temperature. As the model experiment, the metabolites identified by gas chromatography-mass spectrometry (GC-MS) were correlated with α-glucosidase inhibitory activity analyzed with multivariate data analysis (MVDA) to find out the variation among samples and metabolites contributing to the activity. Orthogonal partial least squares (OPLS) analysis was applied to investigate the metabolomics changes. A profound chemical alteration, both in primary and secondary metabolites, was observed. The α-tocopherol, catechin, cyclohexen-1-carboxylic acid, benzoic acid, myo-inositol, stigmasterol, and lycopenecompoundswerefoundtobethediscriminatingmetabolitesatearlystorage;however, sugars such as sucrose, α-d-galactopyranose, and turanose were detected, which was attributed to the discriminating metabolites for late storage. The result shows that the MVDA method is a promising technique to identify biomarker compounds relative to storage at different times.
Keywords: Cosmos caudatus, GC-MS, MVDA, OPLS, storage
1. Introduction
The importance of plants to human life can be seen in their diverse utilization, such as medicine and food. Recently, researchers have been trying to profile their active compounds to scrutinize their beneficial effects. Plants form a major part of the diet of many consumers worldwide, because of the diverse health benefits that can be derived from them [1]. These health benefits have been confirmed by different studies which indicate a sharp decline in the occurrences of certain diseases along with increased consumption of plant-based foods [2,3].
Diabetes mellitus is a disease which is characterized by the increased level of blood sugar (hyperglycemia) caused by the inability of the body to produce or use insulin, a hormone responsible for the regulation of blood glucose. Diabetes mellitus, indeed, takes the largest proportion in terms of prevalence among the endocrine disorders globally. An estimated 382 million people were reported to be living with this disease in 2013 and this figure is projected to increase to about 592 million by 2035 [4]. This disease can be divided into two kinds, namely type 1 (insulin-dependent) and type 2 (noninsulin-dependent). Although some antidiabetic drugs are effectively used as medicine [5,6], they have some serious side effects, which obligate finding alternative ones. For example, miglitol and voglibose [7], which act as inhibitors of α-glucosidase activity [8], are available commercially. The prolonged usage of these medications is frequently associated with undesirable side effects, including liver toxicity and other gastrointestinal symptoms [7,9]. Therefore, the search for effective and safe α-glucosidase inhibitors from natural sources in order to develop a physiologically functional food, or to design drugs for use against diabetes, is of great interest. Several medicinal plants are used in different parts of the world for the treatment of diabetes. Numerous studies have shown the potency of the crude extracts of different plants as well as specific bioactive compounds in lowering blood glucose levels [10]. The ability of polyphenolic compounds in plants to inhibit the activities of digestive enzymes due to their potential to form complexes with protein has been reported [11]. Cosmos caudatus is originally from Meso-america, and is a well-known herb and vegetable in Malaysia and other tropical countries [12]. In the Malay society, it is called “Ulam Raja”, and is consumed raw or cooked, which adds a pleasant aroma and taste to food. It belongs to the Asteraceae family and has several varieties [12,13]. All of its parts are traditionally used for several purposes, including food additives, medicines, and perfumes [12,14]. Profiling data for the investigation of the role of metabolites is necessary to generate reliable metabolites. Thus, further research is required to evaluate the metabolites content and their efficiencies. Many approaches have been successfully used in analyzing and evaluating the metabolites variation of organism, such as metabolomics.
The metabolomics approach can be defined as qualitatively and quantitatively comprehension assessment of all existing metabolites and their variation in biological systems of organisms [15–17]. Metabolomics has also been used for monitoring plant metabolic changes [18]. In many physiological circumstances, a wide range of metabolites were found to be changed, including phenolic compounds, amino acids, fatty acids, organic acids, carbohydrates, and sterol based compounds [19]. Changes in environmental patterns and processing methods can alter metabolic contents [20]. Medicinal herbs are usually subjected to long time storage in the chain of production of various products [21–23]. Studies to determine the effect of storage on medicinal plants provide important information, not only for traditional healers and consumers, but also in designing sustainable harvesting methods for these plants.
With improved knowledge of the “shelf-life” of plants used in traditional medicine, better sustainable harvesting measures can be implemented [24]. This differentiation can be measured via the metabolomics approach based on several techniques and the implementation of sophisticated statistical analysis. Gas chromatography-mass spectrometry is one of the most utilized techniques for analyzing and identifying the constituents of biological samples [25,26]. However, there is no published data currently available on the effect of storage time on antidiabetic activity of this herb. Therefore, the aim of the present study is to determine the effect of storage time on α-glucosidase inhibitory activity of C. caudatus samples by using the GC-MS based metabolomics approach. This information acquired will be useful as the basis for recommendation regarding the suitability of the storage time in order to preserve the metabolic features and beneficial values of the herbal material.
2. Materials and methods
2.1. Plant material
Seeds of C. caudatus were obtained from the nursery at the Institute of Bioscience, Universiti Putra Malaysia, Serdang, Malaysia and identified by voucher specimens (SK 1934/11), before being deposited to herbarium of the Laboratory of Natural Products, Universiti Putra Malaysia. The planting was implemented in an open plot, which was divided into seven sections, each one containing seven plants. The necessary needs and care for good germination were well provided. For sampling, seven replicated leaf samples of the 8-week-old plants were randomly collected in the early morning from each section. Any damaged leaves were discarding by using laboratory scissors [27]. For consistency in the resulting data due to possible technical differences in the sampling and sample preparation, these processes were done in groups and in a parallel manner.
2.2. Preparation of plants
The collected leaves were washed and dried with tissue paper to remove any debris prior to immediate grinding with a mortar and pestle under liquid nitrogen. C. caudatus samples were divided into seven parts; one part was immediately used for the preparation of the extract and the other parts were kept at room temperature for different times (2 hours, 4 hours, 6 hours, 8 hours, 10 hours, and 12 hours) before extraction. The samples were then subjected to freezing in a deep freezer (−80°C) and freeze drying for 3 days. The dried matrix was ground and sieved through a sieve of 0.70 mm particle size and then the obtained fine powder was kept in a chiller at 4°C before further analysis. The grouping was made on the basis of storage time (7 groups) resulting in a grand total of 49 samples for analysis. The samples were weighed to 5.5 g, labelled in separate plastic bags, and subjected to further analysis.
2.3. Preparation of plant extracts
In this study, approximately 5.5 g of powdered samples from each group (0 hours, 2 hours, 4 hours, 6 hours, 8 hours, 10 hours, and 12 hours) was used and subjected to extraction with sonication for 30 minutes by immersing them in 150 mL of EtOH. The extracts were then filtered using Whatman paper No. 1 and concentrated using a rotary evaporator at 40°C before freeze-drying to remove any remaining moisture. All of the extracts were prepared in seven replicates and then stored in chiller at −4°C prior to GC-MS and enzyme analyses.
2.4. α-Glucosidase inhibitory activity
The α-glucosidase inhibitory analyses of the EtOH extracts were determined in accordance with the procedure specified by Collins et al [28]. The respective microplate assay provides an acceptable range of speed, convenience, and reproducibility. The release of p-nitrophenol from p-nitrophenyl-α-d-gluco-pyranose was measured to determine α-glucosidase inhibitory activity. The released p-nitrophenol indicated a yellow color once the stopping reagent glycine (pH 10) was added. To prepare the stock solution, 4 mg of the different groups of plant extracts were dissolved in 1 mL ethanol. In addition, 5 mg quercetin and acarbose were dissolved in 1 mL ethanol and labelled as positive controls [29]. The prepared plant extracts as the stock solution were afterwards diluted with incorporation of phosphate buffer (30 mM; pH 6.5) [30] to obtain a final concentration of 160 μg/mL. The enzyme (α-glucosidase from Saccharomyces cerevisiae; Sigma-Aldrich (St. Louis, USA)) was prepared and used at the highest concentration (0.02 U/well) for 5 minutes at room temperatures The substrate consisting of 0.06 g p-nitrophenyl-α-d-glucopyranose (Sigma-Aldrich (St. Louis, USA)) was dissolved in 20 mL buffer 50 Mm (pH 6.5) and incubated at room temperature for 15 minutes. The plant extract, positive control, enzyme, buffer 30 mM, and substrate were placed in the 96-well microplate according to the layout to a volume of 200 μL. After an incubation period of 15 minutes at room temperature, the reaction was stopped by adding 50 μL glycine (pH 10). The absorbance was read at 405 nM in a microplate reader (SPECTRAmax PLUS, Sunnyvale, CA, USA) [30]. Finally, the results were described as IC50 inhibition.
| (1) |
2.5. Derivatization
The samples were derivatized for GC-MS analysis as described by Robinson et al [31]. The sample extract (25 mg) was immersed with 50 μL pyridine in 2 mL centrifuge tubes and sonicated for 10 minutes at 30°C. The sample solutions were vortexed after adding 100 μL of methoxyamine HCl (20 mg/mL in pyridine). The mixture was incubated for 2 hours at 60°C in shaking incubator (VorTemp 56, Labnet International, Inc., Woodbridge, NJ, USA). Following that, the tube was incubated for 30 minutes at 60°C after the addition of 300 μL of N-Methyl-N-(trimethylsilyl)tri-fluoroacetamide (MSTFA). Finally, the sample solutions were filtered, injected, covered with aluminum foil, and left to stand overnight at room temperature.
2.6. GC-MS analysis
GC-MS analysis was performed following the method described Canini et al [32]. The samples were analyzed using the TSQ Quantum XLS Ultra system (Thermo Finnigan San Jose, CA, USA). A sample volume of 1 μL was injected with a splitless mode into the GC-MS system connected with an MS/MS triple quad detector. The GC column used for the analysis was DB-5MS 5% phenyl methyl siloxane with an inner diameter of 250 μm and 0.25 μm film thickness. The initial oven temperature was set to 50°C for 3 minutes, and then increased to a target temperature of 315°C for 10 minutes at a rate 10°C /min. Helium was used as the carrier gas at a rate of 1 mL/min. The injector and ion source temperatures were set at 330°C and 250°C, respectively. Mass spectra were acquired using a full scan, monitoring mode with a mass scan range of 50–550 m/z after a solvent delay of 7 minutes. The spectra for each of the chromatogram peaks were compared with the National Institute of Standards and Technology (NIST) database library and the retention time (RT) index of common primary and secondary metabolites. The chromatogram and mass spectra were processed using the Xcalibur software (Thermo Finnigan San Jose, CA, USA). After GC-MS analysis, the integration and normalization for the entire peak areas were conducted by performing XCMS.
2.7. XCMS
The achievement of biomarker recognition and metabolite profiling were carried out by using data preprocessing methods, such as XCMS. XCMS is a package developed in R (www.r-project.org) and made available by the Bioconductor Project (http://www.bioconductor.org/), for the treatment of (hyphenated) MS data. It is a sophisticated data analysis tool that includes many options for data handling and visualization and includes novel algorithms for data analysis. This approach is also useful to correlate particular metabolites to their biological activities. The introduction to GC/MS-based metabolomics and data analysis approach requires accurate methods that assign RT, peak, and matched peak without the need for internal standards [33,34]. The XCMS package in R version 3.0.1 was applied to align the GC-MS chromatograms with the following values: XCMS (full width at half maximum (fwhm) = 30, step = 0.1, method = bin), group (band width = 10).
2.8. Data processing
The processed data was fed to the SIMCA P+ 13 software (Umetrics, Umeå, Sweden) for multivariate data analysis (MVDA) using orthogonal partial least squares (OPLS) following the UV scaling method, with 49 observations, 1002 independent variables, and one dependent variable [35]. There are two variables in OPLS, including IC50 value as Y and RT as X. OPLS was performed to discriminate between C. caudatus extracts and the compounds contributing to their variation and to determine the correlation between the chemical constituents and biological activity. The results are reported as the mean of seven measurements and the standard deviation. One-way analysis of variance (ANOVA) with a Tukey comparison test was used to evaluate the major difference between the samples with a confidence interval of 95%. The correlation between the α-glucosidase inhibition activity and metabolites composition by GC-MS was also performed using a Pearson test. Significant differences were determined using Minitab 14 (Minitab Inc., State College, PA, USA) through performing an ANOVA test, and then identified via GC-MS assay utilizing commercial compounds based on the NIST library.
3. Results and discussion
3.1. The effect of different storage time on α-glucosidase inhibitory activity of C. caudatus
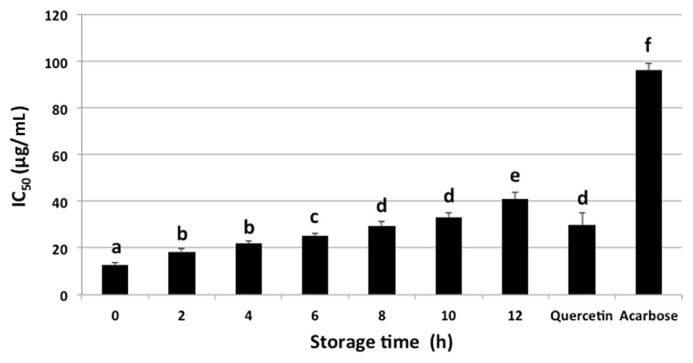
Fig. 1 shows the α-glucosidase inhibitory activity and IC50 values of ethanolic extract of C. caudatus at different storage times (0 hours, 2 hours, 4 hours, 6 hours, 8 hours, 10 hours, and 12 hours). The results showed that by increasing the time of storage, the α-glucosidase inhibitory activity of C. caudatus dropped significantly. The trend of α-glucosidase inhibitory activity at different time was 0 hours > 2 hours > 4 hours > 6 hours > 8 hours > 10 hours > 12 hours (Fig. 1). This might be due to the nonavailability of nutrients or degradation of compounds responsible for the activity by the microbial or enzymatic activities. The temperature and water activity might provide appropriate conditions for the enzymatic reactions which might cause a decrease in the bioactivity [24,36]. A lower IC50 value is desirable for better α-glucosidase inhibition activity. The IC50 values ranged between 12.6 μg/mL and 40.9 μg/mL. The highest α-glucosidase inhibitory activity was for a fresh sample taken from the field (0 hour) with an IC50 value of 12.6 μg/mL, which was comparable to quercetin and higher than acarbose, whereas the sample stored for 12 hours showed the lowest inhibitory activity. This may be explained by the instability of compounds that resulted in lowering α-glucosidase inhibitory activity along with the increase of storage time [37]. Another reason for such a trend may be attributed to the variations in the reaction between the present inhibitors and reagents at different times of storage [38].
Fig. 1.
Alpha glucosidase inhibitory activity and IC50 value of Cosmos caudatus samples at different storage times. Data are presented as mean ± SD, n = 7. Data in each column with different subscript letters are significantly different (p < 0.05).
Phenolic compounds have a decisive role in evaluation of the α-glucosidase inhibitory activity [37,39]. In the present study, phenolic compounds were only found in ethanolic extract at 0 hours, 2 hours, 4 hours, 6 hours, 8 hours, and 10 hours, which might support the consistent α-glucosidase inhibitory activity of this extract before 12 hours. Moreover, catechin, which was previously reported to possess α-glucosidase inhibition activity [40], might be degraded to other metabolites during the storage time [41]. However, the contribution of other metabolites in α-glucosidase inhibitory activity has not been reported yet. This can also be explained through degradation of compounds such as α-tocopherol, catechin, cyclohexen-1-carboxylic acid, stigmasterol, benzoic acid, lycopene, and myo-inositol, which were observed in samples until 10 hours, into free sugars such as sucrose, α-d-galactopyranose, and turanose, which was identified after 12 hours. This may hence justify the reducing trend of α-glucosidase inhibitory activity of this plant in the course of time, which is in agreement with Jahangir [42].
Other studies have also revealed that the metabolite changes still continue even after harvesting the plants. In this study, no significant difference (p > 0.05) was observed among the stored materials before 12 hours. After 12 hours of storage at room temperature, it was observed that leaves of C. caudatus turned into a yellow color, indicating the loss of pigments. Other studies have reported the same trend; however, the loss of pigments happens in different periods of time for certain plants. For example, yellowing of rocket salad [41] and radish [42] leaves occurred after 3 days of storage at room temperature. In general, this study showed that C. caudatus extract during the first 10 hours of storage time possessed a potential α-glucosidase inhibition activity due to the availability of several bioactive metabolites.
3.2. Identification of compounds by GC-MS
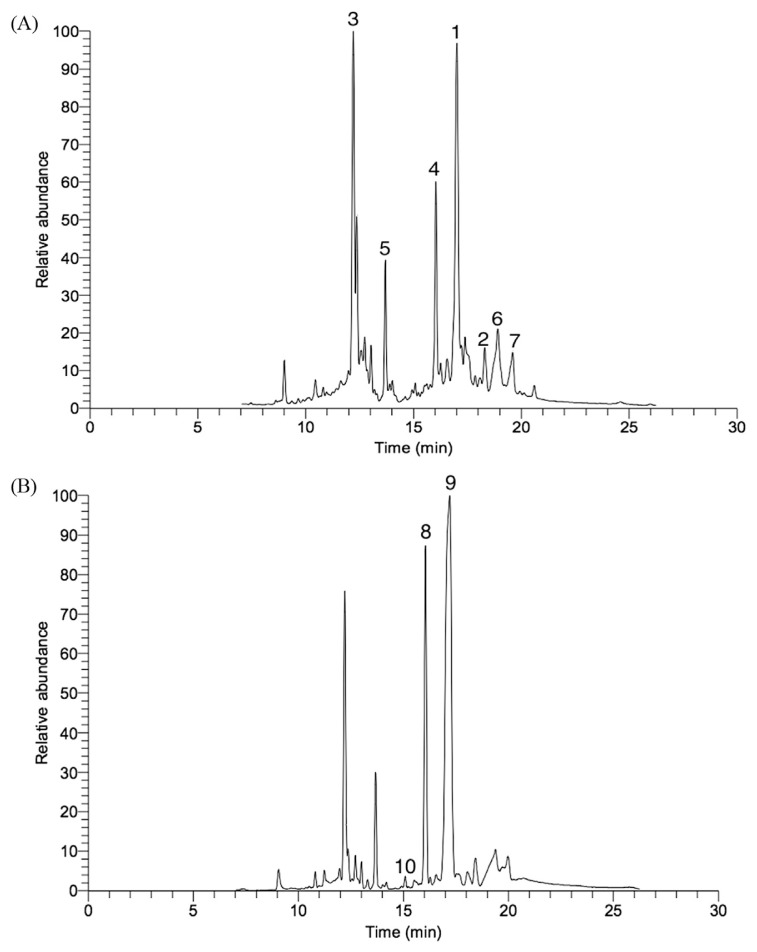
GC-MS spectra of the plant materials processed with the seven different storage times displayed both quantitative and qualitative variations of some metabolites (Figs. 2A and 2B). Metabolites from this plant, including catechin, cyclohexen-1-carboxylic acid, vitamin E, stigmasterol, benzoic acid, lycopene, sucrose, α-d-galactopyranose, and turanose, were well identified. The GC-MS results of different ratios of EtOH extracts for the healthy aerial parts (leaves) of C. caudatus were studied previously [43].
Fig. 2.
Gas chromatography-mass spectrometry (GC-MS) total ion chromatogram of Cosmos caudatus (A) in fresh sample and (B) after 12 hours of storage. 1 = catechin; 2 = α-tocopherol; 3 = cyclohexen-1-carboxylic acid; 4 = benzoic acid; 5 = myo-inositol; 6 = stigmasterol; 7 = lycopene; 8 = sucrose; 9 = α-d-galactopyranose; 10 = turanose.
Tables 1 and 2 show the metabolites present in the fresh and after 12 hours, respectively. It shows that the phenolic peak was high in fresh samples whereas it disappeared after 12 hours of storage, indicating the reduction of bioactivity of stored C. caudatus samples in the course of time. Such a trend was further observed in the GC-MS results (Figs. 2A and 2B), which confirm the results of the bioactivity for the different stored C. caudatus samples. α-Tocopherol, catechin, cyclohexen-1-carboxylic acid, stigmasterol, benzoic acid, lycopene, and myo-inositol were identified and exhibited at high amounts in fresh samples and after storage of 2 hours, 4 hours, 6 hours, 8 hours, and 10 hours. However, sucrose, α-d-galactopyranose, and turanose were found to be the discriminating metabolites for C. caudatus stored for 12 hours at room temperature. This was possibly due to degradation of some of the aforementioned metabolites with increasing storage time, particularly after 12 hours, as discussed earlier. This decrease may be accompanied by the release of degraded products of plant metabolites [41]. This may account for the presence of high amounts of primary metabolites, including sugars, which were observed in long stored samples (Tables 1 and 2).
Table 1.
Metabolites identified from Cosmos caudatus fresh samples.
| Number | RT | Probability | Molecular weight (M+) | Tentative metabolites |
|---|---|---|---|---|
| 1 | 17.01 | 82.0 | 290.26 | Catechin |
| 2 | 18.29 | 81.6 | 430.71 | α-Tocopherol (vitamin E) |
| 3 | 12.25 | 91.0 | 126.15 | Cyclohexen-1-carboxylic acid |
| 4 | 16.90 | 92.26 | 122.12 | Benzoic acid |
| 5 | 13.68 | 65.0 | 180.16 | Myo-inositol |
| 6 | 19.00 | 20.0 | 412.69 | Stigmasterol |
| 7 | 19.62 | 20.0 | 536.87 | Lycopene |
RT = retention time.
Table 2.
Metabolites identified from Cosmos caudatus after 12 hours of storage.
| Number | RT | Probability | Molecular weight (M+) | Tentative metabolites |
|---|---|---|---|---|
| 8 | 16.03 | 91.0 | 342.29 | Sucrose |
| 9 | 17.51 | 35.0 | 180.06 | α-d-Galactopyranose |
| 10 | 15.99 | 55.0 | 342.11 | Turanose |
RT = retention time.
3.3. Classification of stored C. caudatus samples
The use of MVDA in this study was helpful in reducing the huge data sets, which were obtained from GC-MS analysis and biological activity, to a more manageable size [44]. The bioactive metabolites of stored C. caudatus extracts were then evaluated and classified using MVDA. In this study, a supervised MVDA, OPLS was used. OPLS was therefore performed to discriminate C. caudatus extracts and the compounds contributing to their variations, and to correlate between the chemical constituents and α-glucosidase inhibitory activity.
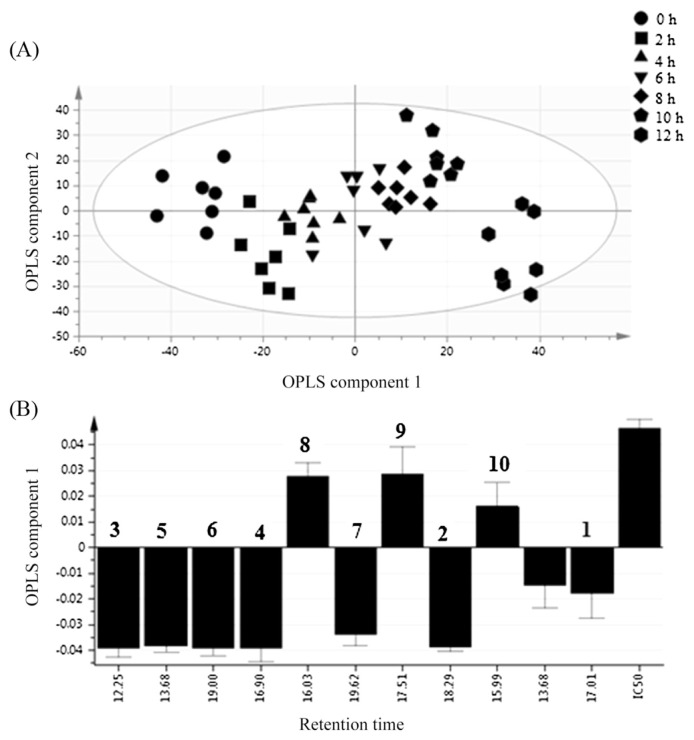
The first two OPLS components showed the maximum variation with a value of 40% and 22.5% for OPLS components 1 and 2, respectively. The processed data exhibited that the C. caudatus extracts were noticeably distinguished from each other (Fig. 3A). The most active extract (1st group, in farm) is placed in the left side along OPLS component 1 while the nonactive extract (Group 7, after 12 hours) is in the right side. A gradient shifting of the extract along OPLS 1 to the left side represents the increment of bioactivity.
Fig. 3.
Orthogonal partial least squares (OPLS) score (A) and loading column plots (B) at different storage times (0 hours, 2 hours, 4 hours, 6 hours, 8 hours, 10 hours, and 12 hours) of Cosmos caudatus extracts. 1 = catechin; 2 = α-tocopherol; 3 = cyclohexen-1-carboxylic acid; 4 = benzoic acid; 5 = myo-inositol; 6 = stigmasterol; 7 = lycopene; 8 = sucrose; 9 = α-d-galactopyranose; 10 = turanose.
Fig. 3 illustrates the loading plot of the C. caudatus extracts. From the loading plot, the farther the compounds from the origin, the more the contribution to the variation [45]. RT at 16.98, 18.29, 12.25, 15, 19, and 19.62 were the ones inhibiting α-glucosidase activity, because they are located opposite to the IC50, while the other RTs induced α-glucosidase activity, since they are located at the same space as that of IC50 in the loading column plot.
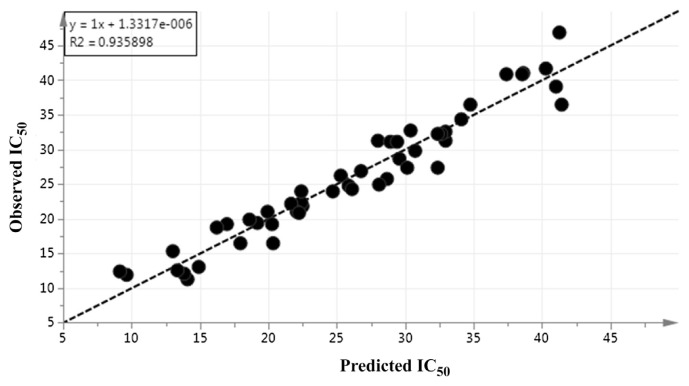
The model diagnostic of OPLS was also confirmed by the Q2Y and R2Y accumulative. The goodness of fit is mainly based on R2Y. The predictive quality of the model can be determined by Q2Y. The model fitness and predictability are considered good with Q2 and R2 accumulative values > 0.5. In the current study, all Q2Y and R2Y values ranged between 0.8 and 0.93. Hence, the model had notable fitness and predictive features (Fig. 4).
Fig. 4.
Prediction versus observation of IC50 values from all samples. The R2 of the regression line indicates the goodness of fit between experimental observations and predicted model. R2 in this correlation was 0.935898.
4. Conclusion
In this study, α-glucosidase inhibitory activity of C. caudatus leaves at different storage times (0 hours, 2 hours, 4 hours, 6 hours, 8 hours, 10 hours, and 12 hours) was correlated with GC-MS results. The results revealed that C. caudatus showed high potential α-glucosidase inhibitory activity. This inhibitory activity was nonsignificantly varied for samples processed between 0 hours and 10 hours. However, it was significantly higher from those samples processed at 12 hours. In fact, it was observed that the inhibitory activity of C. caudatus reduced proportionally with the increase of storage time. Metabolites including α-tocopherol, catechin, cyclohexen-1-carboxylic acid, benzoic acid, myo-inositol, stigmasterol, and lycopene were identified from stored C. caudatus samples. All these aforementioned metabolites were strongly attributed to the high α-glucosidase inhibitory activity. In general, the results suggest that C. caudatus will decrease the activity if the samples were stored for > 10 hours after harvest. This is the first step which will lead to innovation in the food industry for controlling the technical and environmental parameters and hence, the optimization of the end product. This study may be beneficial for the development of a C. caudatus product as an antidiabetic medicine or functional food.
Acknowledgments
This study was supported by the Agro-Biotechnology Institute, Ministry of Science, Technology and Innovation (MOSTI; grant no: 6370007).
Funding Statement
This study was supported by the Agro-Biotechnology Institute, Ministry of Science, Technology and Innovation (MOSTI; grant no: 6370007).
Footnotes
Conflicts of interest
All authors declare no conflicts of interest.
References
- 1. Grusak MA, Dellapenna D. Improving the nutrient composition of plants to enhance human nutrition and health 1. Annu Rev Plant Biol. 1999;50:133–61. doi: 10.1146/annurev.arplant.50.1.133. [DOI] [PubMed] [Google Scholar]
- 2. Chiu YW, Lo HJ, Huang HY, Chao PY, Hwang JM, Huang PJ, Huang SJ, Liu JY, Lai TJ. The antioxidant and cytoprotective activity of Ocimum gratissimum extracts against hydrogen peroxide-induced toxicity in human HepG2 cells. J Food Drug Anal. 2013;21:253–60. [Google Scholar]
- 3. Ho CT, Pan MH, Lai CS, Li S. Polymethoxy flavones as food factors for the management of inflammatory diseases. J Food Drug Anal. 2012;20:337–41. [Google Scholar]
- 4.Aguiree F, Brown A, Cho NH, Dahlquist G, Dodd S, Dunning T, Hirst M, Hwang C, Magliano D, Patterson C. IDF Diabetes Atlas. 6th ed. International Diabetes Federation; Basel, Switzerland: 2013. [Google Scholar]
- 5. Standl E, Baumgartl HJ, Füchtenbusch M, Stemplinger J. Effect of acarbose on additional insulin therapy in type 2 diabetic patients with late failure of sulphonylurea therapy. Diabetes Obes Metab. 1999;1:215–20. doi: 10.1046/j.1463-1326.1999.00021.x. [DOI] [PubMed] [Google Scholar]
- 6. Rani MP, Padmakumari KP, Sankarikutty B, Cherian OL, Nisha VM, Raghu KG. Inhibitory potential of ginger extracts against enzymes linked to type 2 diabetes, inflammation and induced oxidative stress. Int J Food Sci Nutr. 2011;62:106–10. doi: 10.3109/09637486.2010.515565. [DOI] [PubMed] [Google Scholar]
- 7. Etxeberria U, de la Garza AL, Campión J, Martínez JA, Milagro FI. Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin Ther Targets. 2012;16:269–97. doi: 10.1517/14728222.2012.664134. [DOI] [PubMed] [Google Scholar]
- 8. Asano N, Tomioka E, Kizu H, Matsui K. Sugars with nitrogen in the ring isolated from the leaves of Morus bombycis. Carbohydr Res. 1994;253:235–45. doi: 10.1016/0008-6215(94)80068-5. [DOI] [PubMed] [Google Scholar]
- 9. Spadiene A, Savickiene N, Ivanauskas L, Jakstas V, Skesters A, Silova A, Rodovicius H. Antioxidant effects of Camellia sinensis L. extract in patients with type 2 diabetes. J Food Drug Anal. 2014;22:505–11. doi: 10.1016/j.jfda.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaushik G, Satya S, Khandelwal RK, Naik SN. Commonly consumed Indian plant food materials in the management of diabetes mellitus. Diabetes Metab Syndr Clin Res Rev. 2010;4:21–40. [Google Scholar]
- 11. Griffiths DW, Moseley G. The effect of diets containing field beans of high or low polyphenolic content on the activity of digestive enzymes in the intestines of rats. J Sci Food Agric. 1980;31:255–9. doi: 10.1002/jsfa.2740310307. [DOI] [PubMed] [Google Scholar]
- 12. Shui G, Leong LP, Wong SP. Rapid screening and characterisation of antioxidants of Cosmos caudatus using liquid chromatography coupled with mass spectrometry. J Chromatogr B. 2005;827:127–38. doi: 10.1016/j.jchromb.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 13. Abas F, Lajis NH, Kalsom YU. Antioxidative and radical scavenging properties of the constituents isolated from Cosmos caudatus Kunth. Nat Prod Sci. 2003;9:245–8. [Google Scholar]
- 14. Abas F, Lajis NH, Israf DA, Khozirah S, Umi Kalsom Y. Antioxidant and nitric oxide inhibition activities of selected Malay traditional vegetables. Food Chem. 2006;95:566–73. [Google Scholar]
- 15. Fiehn O. Metabolomics-the link between genotypes and phenotypes. Plant Mol Biol. 2002;48:155–71. [PubMed] [Google Scholar]
- 16. Kim HK, Choi YH, Verpoorte R. NMR-based metabolomic analysis of plants. Nat Protoc. 2010;5:536–49. doi: 10.1038/nprot.2009.237. [DOI] [PubMed] [Google Scholar]
- 17. Wishart DS. Metabolomics: applications to food science and nutrition research. Trends Food Sci Technol. 2008;19:482–93. [Google Scholar]
- 18. Shuib NH, Shaari K, Khatib A, Kneer R, Zareen S, Raof SM, Lajis NHJ, Neto V. Discrimination of young and mature leaves of Melicope ptelefolia using 1H NMR and multivariate data analysis. Food Chem. 2011;126:640–5. [Google Scholar]
- 19. Sumner LW, Mendes P, Dixon RA. Plant metabolomics: large-scale phytochemistry in the functional genomics era. Phytochemistry. 2003;62:817–36. doi: 10.1016/s0031-9422(02)00708-2. [DOI] [PubMed] [Google Scholar]
- 20. Hsieh P, Huang G, Ho Y, Lin Y, Huang S, Chiang Y, Tseng M, Chang Y. Activities of antioxidants, alpha-Glucosidase inhibitors and aldose reductase inhibitors of the aqueous extracts of four Flemingia species in Taiwan. Bot Stud. 2010;51:293–302. [Google Scholar]
- 21. Chan EWC, Lim YY, Wong SK, Lim KK, Tan SP, Lianto FS, Yong MY. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009;113:166–72. [Google Scholar]
- 22. Lim YY, Murtijaya J. Antioxidant properties of Phyllanthus amarus extracts as affected by different drying methods. LWT-Food Sci Technol. 2007;40:1664–9. [Google Scholar]
- 23. Suvarnakuta P, Chaweerungrat C, Devahastin S. Effects of drying methods on assay and antioxidant activity of xanthones in mangosteen rind. Food Chem. 2011;125:240–7. [Google Scholar]
- 24. Fennell CW, Light ME, Sparg SG, Stafford GI, van Staden J. Assessing African medicinal plants for efficacy and safety: agricultural and storage practices. J Ethnopharmacol. 2004;95:113–21. doi: 10.1016/j.jep.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 25. Chen HC, Peng LW, Sheu MJ, Lin LY, Chiang HM, Wu CT, Wu CS, Chen YC. Effects of hot water treatment effects on the essential oils of calamondin. J Food Drug Anal. 2013;21:363–8. [Google Scholar]
- 26. Ma C, Wang H, Lu X, Xu G, Liu B. Metabolic fingerprinting investigation of Artemisia annua L. in different stages of development by gas chromatography and gas chromatography-mass spectrometry. J Chromatogr A. 2008;1186:412–9. doi: 10.1016/j.chroma.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 27. Mediani A, Abas F, Ping TC, Khatib A, Lajis NH. Influence of growth stage and season on the antioxidant constituents of Cosmos caudatus. Plant Foods Hum Nutr. 2012;67:344–50. doi: 10.1007/s11130-012-0317-x. [DOI] [PubMed] [Google Scholar]
- 28. Collins RA, Ng TB, Fong WP, Wan CC, Yeung HW. Inhibition of glycohydrolase enzymes by aqueous extracts of Chinese medicinal herbs in a microplate format. IUBMB Life. 1997;42:1163–9. doi: 10.1080/15216549700203631. [DOI] [PubMed] [Google Scholar]
- 29. Subramanian R, Asmawi MZ, Sadikun A. In vitro alpha-glucosidase and alpha-amylase enzyme inhibitory effects of Andrographis paniculata extract and andrographolide. Acta Biochim Pol. 2008;55:391–8. [PubMed] [Google Scholar]
- 30. Deutschlander MS, van de Venter M, Roux S, Louw J, Lall N. Hypoglycaemic activity of four plant extracts traditionally used in South Africa for diabetes. J Ethnopharmacol. 2009;124:619–24. doi: 10.1016/j.jep.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 31. Robinson AR, Gheneim R, Kozak RA, Ellis DD, Mansfield SD. The potential of metabolite profiling as a selection tool for genotype discrimination in Populus. J Exp Bot. 2005;56:2807–19. doi: 10.1093/jxb/eri273. [DOI] [PubMed] [Google Scholar]
- 32. Canini A, Alesiani D, D’Arcangelo G, Tagliatesta P. Gas chromatography–mass spectrometry analysis of phenolic compounds from Carica papaya L. leaf. J Food Compos Anal. 2007;20:584–90. [Google Scholar]
- 33. Smith CA, Want EJ, O’Maille G, Abagyan R, Siuzdak G. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal Chem. 2006;78:779–87. doi: 10.1021/ac051437y. [DOI] [PubMed] [Google Scholar]
- 34. Benton HP, Wong DM, Trauger SA, Siuzdak G. XCMS2: processing tandem mass spectrometry data for metabolite identification and structural characterization. Anal Chem. 2008;80:6382–9. doi: 10.1021/ac800795f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. T’Kindt R, Morreel K, Deforce D, Boerjan W, Van Bocxlaer J. Joint GC–MS and LC–MS platforms for comprehensive plant metabolomics: repeatability and sample pre-treatment. J Chromatogr B. 2009;877:3572–80. doi: 10.1016/j.jchromb.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 36. Nicoli MC, Anese M, Parpinel M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci Technol. 1999;10:94–100. [Google Scholar]
- 37. Ryu HW, Cho JK, Curtis-Long MJ, Yuk HJ, Kim YS, Jung S, Kim YS, Lee BW, Park KH. α-Glucosidase inhibition and antihyperglycemic activity of prenylated xanthones from Garcinia mangostana. Phytochemistry. 2011;72:2148–54. doi: 10.1016/j.phytochem.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 38. Sultana B, Anwar F, Ashraf M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167–80. doi: 10.3390/molecules14062167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim J, Yang J, Kim M. Alpha glucosidase inhibitory effect, anti-microbial activity and UPLC analysis of Rhus verniciflua under various extract conditions. J Med Plants Res. 2011;5:778–83. [Google Scholar]
- 40. Shobana S, Sreerama YN, Malleshi NG. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: Mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009;115:1268–73. [Google Scholar]
- 41. King GA, Morris SC. Early compositional changes during post harvest senescence of broccoli. J Am Soc Hortic Sci. 1994;119:1000–5. [Google Scholar]
- 42.Jahangir M. Stress response and health affecting compounds in Brassicaceae. Department of Pharmacognosy and Metabolomics, Institute of Biology (IBL), Faculty of Science, Leiden University; 2010. [Google Scholar]
- 43. Javadi N, Abas F, Hamid AA, Simoh S, Shaari K, Ismail IS, Mediani A, Khatib A. GC-MS-based metabolite profiling of Cosmos caudatus leaves possessing alpha-glucosidase inhibitory activity. J Food Sci. 2014;79:C1130–6. doi: 10.1111/1750-3841.12491. [DOI] [PubMed] [Google Scholar]
- 44. Cozzolino D, Cynkar WU, Shah N, Smith P. Multivariate data analysis applied to spectroscopy: potential application to juice and fruit quality. Food Res Int. 2011;44:1888–96. [Google Scholar]
- 45. Tian J, Shi C, Gao P, Yuan K, Yang D, Lu X, Xu G. Phenotype differentiation of three E. coli strains by GC-FID and GC-MS based metabolomics. J Chromatogr B. 2008;871:220–6. doi: 10.1016/j.jchromb.2008.06.031. [DOI] [PubMed] [Google Scholar]