Abstract
Ruta graveolens (the common rue) has been used for various therapeutic purposes, including relief of rheumatism and treatment of circulatory disorder. To elucidate the effects of rue on main drug-metabolizing enzymes, effects of an aqueous extract of the aerial part of rue and its ingredients on cytochrome P450 (P450/CYP), uridine diphosphate (UDP)-glucuronosyltransferase, and reduced nicotinamide adenine dinucleotide (phosphate) (NAD(P)H):quinone oxidoreductase were studied in C57BL/6JNarl mice. Oral administration of rue extract to males increased hepatic Cyp1a and Cyp2b activities in a dose-dependent manner. Under a 7-day treatment regimen, rue extract (0.5 g/kg) induced hepatic Cyp1a and Cyp2b activities and protein levels in males and females. This treatment increased hepatic UDP-glucuronosyltransferase activity only in males. However, NAD(P)H:quinone oxidoreductase activity remained unchanged. Based on the contents of rutin and furanocoumarins of mouse dose of rue extract, rutin increased hepatic Cyp1a activity and the mixture of furanocoumarins (Fmix) increased Cyp2b activities in males. The mixture of rutin and Fmix increased Cyp1a and Cyp2b activities. These results revealed that rutin and Fmix contributed at least in part to the P450 induction by rue.
Keywords: cytochrome P450, furanocoumarins, Ruta graveolens, rutin, UDP-glucuronosyltransferase
1. Introduction
Drug-metabolizing enzymes play a crucial role in the biotransformation of xenobiotics, including drugs and chemical carcinogens, as well as physiological substances such as steroids. In Phase I metabolism, microsomal cytochrome P450 (P450/CYP)-dependent monooxygenase is the primary enzyme system that is responsible for oxidations. This monooxygenase system participates in the oxidation of a variety of endogenous and exogenous substrates, such as testosterone, methadone, and codeine [1]. P450 catalyzes the oxidations by transfer of two electrons through NADPH–P450 reductase (CPR), and the second electron can also be transferred by cytochrome b5. Enzymes of the P450 superfamily exhibit broad substrate specificity and functional diversity. Therefore, selective substrates have been used as chemical probes for characterizing functional changes in individual P450 isoforms [1]. In the nomenclature of P450 isoforms, lowercase letters are used only for mouse P450, such as Cyp1a2 (http://drnelson.uthsc.edu/cytochromeP450.html). In Phase II metabolic reactions, uridine diphosphate (UDP)-glucuronosyltransferase (UGT)-catalyzed glucuronidation is quantitatively the most important conjugative reaction and appears to be the main metabolic pathway of a variety of drugs, such as acetaminophen. Reduced nicotinamide adenine dinucleotide (phosphate) (NAD(P)H):quinone oxidoreductase (NQO) catalyzes further oxidation of quinone substrates, including the antineoplastic agent mitomycin C [2]. Functions of drug-metabolizing enzymes can be modulated by several natural products, such as psoralen derivatives [3–5]. Alterations of drug-metabolizing enzymes may result not only in herb–drug interaction, but also in changes in physiological functions of endogenous substrates or in carcinogenicity of xenobiotics.
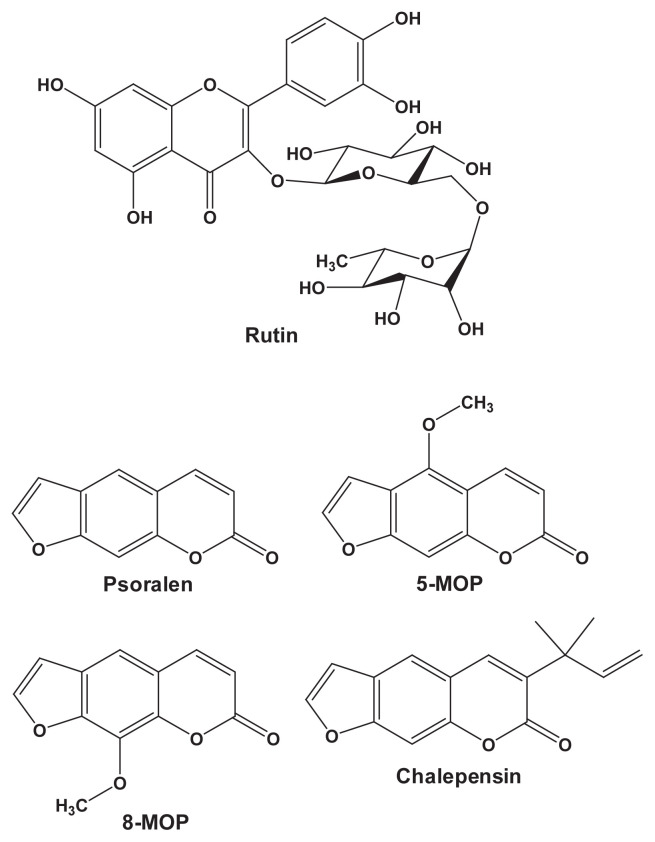
Ruta graveolens L. (Yuin Siang), commonly known as rue, has been used in America, Asia, and Europe for the treatment of heart disease, wounds, and rheumatism [6,7]. In Taiwan, the aerial part of rue has been reputedly used as a folk medicine for the treatment of circulatory disorders. In Europe, this plant has also been used as an abortifacient and emmenagogue [6]. Aqueous extract of rue was found to reduce human sperm motility [8]. According to the 53-day spermatogenic cycle in rats, a study of 60-day treatment of rue was designed and the changes in the rue-treated group were compared to those in the vehicle control group [9]. Oral administration of rue aqueous extract at 0.5 g/kg/d to male adult rats for 60 days decreased their serum testosterone level, suppressed sperm motility and number of mounts, and prolonged ejaculatory latency. Types of chemical compounds identified in rue include coumarins, flavonoids, lignans, and quinoline alkaloids [10]. Furanocoumarins, such as 8-methoxypsoralen (MOP) and chalepensin (Fig. 1), represent one group of biologically active rue constituents and can be linked to the phototoxicity and antifertility of rue [10]. The flavonoid rutin (Fig. 1) has been identified as a water-soluble glycoside in rue [11]. In the evaluation of the uterotonic activities of rue fractionations, rutin was found in the active fraction and had the most potent uterotonic effect [11]. It has been suggested that furanocoumarins and rutin play important roles in the pharmacological/toxicological effects of rue.
Fig. 1.
Structures of rutin and furanocoumarins identified in the rue aqueous extract. MOP = methoxypsoralen.
In the modulation of drug-metabolizing enzymes, intraperitoneal administration of a single dose of 25 mg/kg 8-MOP to rats induced hepatic CYP2B after 24 hours and 48 hours [3]. Chalepensin activated the mouse constitutive androstane receptor (CAR) and induced Cyp2b9/10 in male C57BL/6JNarl mice after 1 week of treatment [4]. By contrast, some of the furanocoumarins in rue, such as 5-MOP, 8-MOP, and chalepensin, are known potent mechanism-based inhibitors of CYP2A and CYP3A in vitro and/or in vivo [5,12]. Rutin is catabolized by human intestinal/fecal bacteria to form deglycosylated products including leucocyanidin and quercetin [13]. Quercetin induced CYP1A1 expression in HepG2 cells, whereas rutin did not [14]. To a lesser extent, quercetin elevated the UGT mRNA level. These reports indicated that rutin and furanocoumarins may affect drug-metabolizing enzymes. However, the effect of rue remained unclear. In this study, to elucidate the effects of rue on hepatic and renal drug-metabolizing enzymes, an aqueous extract of rue was prepared and the contents of rutin and furanocoumarins in the extract were determined. The effects of oral administration of rue on P450 isoforms, UGT, and NQO were studied. Based on the contents of the rue extract, the effects of rutin and the mixture of furanocoumarins (Fmix) were studied to reveal their contribution to the enzyme induction by rue.
2. Materials and methods
2.1. Chemicals and antibodies
Coumarin, dextromethorphan, dextrophan, glucose-6-phosphate, glucose-6-phosphate dehydrogenase, nicotina mide adenine dinucleotide phosphate (NADP+), 7-ethoxyresorufin, 7-methoxyresorufin, 7-pentoxyresorufin, 5-MOP, 8-MOP, nifedipine, psoralen, and rutin were purchased from Sigma-Aldrich Co. (St Louis, MO, USA). Rutin was further purified by column chromatography using Sephadex LH-20 and the purity was 98% [high-performance liquid chromatography (HPLC) analysis]. Chalepensin was isolated and purified from the ethanolic extract of rue with a purity of ≥ 99% (HPLC analysis), as described previously [12]. The oxidation product [2,6-dimethyl-4-(2-nitrophenyl)-3,5-pyridinedicarboxylic acid dimethyl ester] of nifedipine was generously provided by Dr F. Peter Guengerich (Nashville, TN, USA). Acrylamide, N,N′-methylene-bis-acrylamide and sodium dodecyl sulfate for immunoblot analyses were purchased from Bio-Rad Laboratories (Hercules, CA, USA). Acetonitrile and methanol were purchased from Merck KGaA (Darmstadt, Germany). Monoclonal antibody Mab1-7-1 against rat CYP1A was generously provided by Dr S.S. Park (Seoul, Korea) [15]. Rabbit polyclonal antibody against rat CYP2B1, which was immunoreacted with mouse Cyp2b, was purchased from NOSAN Co. (Yokohama, Japan) [16]. Rabbit polyclonal antibody against rat CPR was prepared following the method of Kaminsky et al [17]. Rabbit polyclonal antibodies against human UGT1A, which was immunoreacted with mouse UGT in the liver lysate shown in the datasheet (Cat. No. GTX114131), was purchased from GeneTex Intl Co. (Hsinchu City, Taiwan). Horseradish peroxidase-conjugated rabbit antimouse IgG and goat antirabbit IgG were purchased from Thermo Scientific Pierce Protein Research Products (Rockford, IL, USA).
2.2. Preparation and HPLC analysis of rue aqueous extract
The fresh aerial part of R. graveolens (rue) was purchased from the herb market in Taipei, and the species was authenticated by Dr I.-J. Lee. Rue (2.46 kg) was blended in 7 L of deionized water and then 3.5 L of water was added to it. After heating at 95°C for 1 hour, the herbal decoction was cooled to room temperature and filtered using a steel sieve. The filtrate was centrifuged at 9000g for 40 minutes at 4°C. The supernatant was collected, filtered through a filter paper (ADVANTEC; Toyo Roshi Kaisha, Ltd, Tokyo, Japan) and lyophilized. A total of 20.54 g of extract was obtained from 1 kg of rue. To determine the rutin and furanocoumarin contents, rue aqueous extract (100 mg) was extracted twice using 2 mL of methanol. After centrifugation and filtration through a 0.45-μm filter, the filtrate was subjected to HPLC analysis. An Agilent 1100 HPLC system (Agilent Technology, Santa Clara, CA, USA) equipped with a photodiode array detector and a C18 column (Cosmosil 5C18-AR-II, 4.6 × 250 mm2, 5 μm; Nacalai Tesque, Kyoto, Japan) was used. Ingredients of the rue extract were separated using acetonitrile/methanol (1:1) (A) and 10% methanol (B), with stepwise changes of linear gradients: 0 minute, 100% B; 45 minutes, 50% A and 50% B; 50 minutes, 50% A and 50% B; 60 minutes, 100% A; 62 minutes, 100% B; 70 minutes, 100% B. Rutin and furanocoumarins were detected by measuring the absorbance at 246 nm. In addition to the retention time and absorbance spectrum, the presence of furanocoumarins was further confirmed by an analysis of rue extract spiked with individual standards (chromatogram not shown). Purified or commercially available compounds were used as the standards for quantification.
2.3. Animal treatments and determination of serum biochemical parameters
C57BL/6JNarl mice (5-week old, weighing 17–20 g) were purchased from the National Laboratory Animal Center (Taipei, Taiwan). All experimental protocols involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the National Research Institute of Chinese Medicine (IACUC 100-10, approved on December 22, 2011). Prior to experimentation, mice were allowed an 1-week acclimation period at the climate-controlled animal quarters with free access to laboratory rodent chow (No. 5P14; PMI Feeds Inc., Richmond, IN, USA) and water. The known Cyp1a and Cyp2b inducers are, respectively, 3-methylcholanthrene (3-MC) and phenobarbital (PB). Mice in the Cyp1a and Cyp2b induction positive control groups were treated by intraperitoneal injection of 3-MC (a single injection of 80 mg/kg and microsomes were prepared after 2 days) and PB (80 mg/kg daily for 4 days), respectively. Rue extract was ground using a mortar and pestle, and suspended in deionized water using a set of Teflon pestle-glass homogenizers. The suspension was mixed by vortexing before administration to each mouse by oral gavage. The control group received the same amount of water only. Based on the contents of rutin and furanocoumarins in the rue extract, 1.2 mg/mL rutin and Fmix (a mixture of 0.13 mg/mL psoralen, 5 μg/mL 8-MOP, 0.14 mg/mL 5-MOP, and 5 μg/mL chalepensin) were dissolved in corn oil in brown vials. Mice were treated with rutin and Fmix at 0.01 mL/g body weight through oral gavage for 7 days. The control group received the same amount of corn oil. Mice were euthanized by CO2 asphyxiation 22 hours after the last treatment. Prior to asphyxiation, the mice were anesthetized using ether (in a chemical safety hood); blood was collected from mice by heart puncture, and the sera were separated by centrifugation at 9600g for 10 minutes at 4°C. Serum aspartate aminotransferase and alanine aminotransferase activities, and blood urea nitrogen and creatinine levels were determined using analysis kits designed for a Fujifilm Dri-Chem 3000 colorimetric analyzer (Fujifilm, Saitama, Japan).
2.4. Microsomal and cytosolic preparations, and enzyme assays
Microsomal and cytosolic fractions of liver and kidney tissues were prepared by differential centrifugation at 4°C [4]. Liver microsomal pellets were further resuspended using 1.15% KCl and centrifuged again at 100,000g for 1 hour at 4°C to prepare washed microsomes. The washed liver microsomes, kidney microsomes, and cytosols of kidney and liver samples were used for enzyme assays. Microsomal contents of P450 and cytochrome b5 were determined using spectrophotometric methods [18]. Cytochrome c was used as a substrate to determine CPR activity, as described by Phillips and Langdon [19]. Activities of 7-ethoxyresorufin O-deethylation (EROD), 7-methoxyresorufin O-demethylation (MROD), and 7-pentoxyresorufin O-dealkylation (PROD) were determined by measuring the fluorescence of resorufin [20,21]. Activities of dextromethorphan O-demethylation and coumarin 7-hydroxylation were determined by HPLC with a fluorescence detector [22,23]. Nitrosodimethylamine N-demethylation activity was determined by measuring formaldehyde formation using Nash’s reagent [24]. Nifedipine oxidation activity was determined by HPLC with a UV detector [25]. Microsomal UGT and cytosolic NQO activities were determined using p-nitrophenol and menadione as substrates, respectively [26,27]. Protein concentrations were determined using bovine serum albumin as a standard for establishing the linear relationship between protein concentration and absorbance, as described by Lowry et al [28].
2.5. Immunoblot analyses
Microsomal proteins (12–50 μg) were resolved by electrophoresis on a 7.5% (for UGT) or 10% (for P450) (w/v) polyacrylamide gel (sodium dodecyl sulfate-polyacrylamide gel electrophoresis, 14 × 20 × 0.15 cm3) and electrotransferred from the slab gel to a nitrocellulose membrane, as described previously [4]. Immunoblot analysis of microsomal Cyp1a1/2 was performed using Mab 1-7-1 [15]. Immunoblot analyses of microsomal Cyp2b9/10 and CPR were performed using rabbit polyclonal antibodies against rat CYP2B1 and CPR, respectively. Cytosolic proteins (20 μg) were resolved by electrophoresis on a 12% (w/v) polyacrylamide gel. Immunoreactive proteins were detected using a goat antirabbit IgG conjugated with horseradish peroxidase and visualized using an enhanced chemiluminescence detection kit (PerkinElmer Life and Analytical Sciences, Inc., Shelton, CA, USA). Relative band intensity was analyzed with the Multi Gauge software (version 2.2; Fujifilm Co., Tokyo, Japan).
2.6. Data analyses
The differences in the experiments with more than two sets of data from mice treated with different doses of rue extract were analyzed by one-way analysis of variance, followed by Dunnett’s test for multiple comparisons (SigmaStat version 2.03; SPSS Inc., Chicago, IL, USA). Statistical significance of the difference between the control and individual treatment groups was evaluated with Student t test. A p value <0.05 was considered statistically significant.
3. Results
3.1. Quantitation of rutin and the furanocoumarins in the aqueous extract of the aerial part of rue
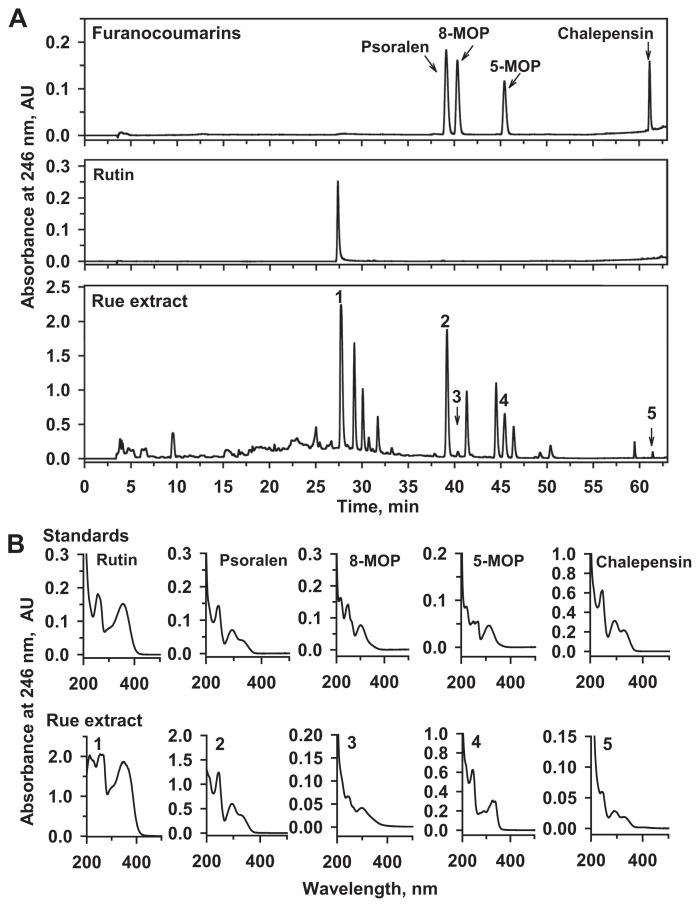
The chromatogram in Fig. 2A shows the absorbance of standards and rue extract at 246 nm under the HPLC conditions described in the Materials and method section. The retention time (Fig. 2A) and absorbance spectra (Fig. 2B) of the peaks in the chromatogram indicated that the identified peaks were rutin (1), psoralen (2), 8-MOP (3), 5-MOP (4), and chalepensin (5). There were 23.7 ± 0.3 mg rutin, 2.6 ± 0.1 mg psoralen, 81 ± 6 μg 8-MOP, 2.7 ± 0.1 mg 5-MOP, and 87 ± 1 μg chalepensin (mean ± SE of triple determinations) in 1 g of rue extract.
Fig. 2.
(A) Chromatograms and (B) spectra of peaks in HPLC analyses of a mixture of furanocoumarin standards, rutin, and Ruta graveolens (rue) extract. A standard furanocoumarin mixture (50 μl) containing chalepensin (0.25 μg), 5-MOP (1.08 μg), 8-MOP (0.54 μg), and psoralen (0.47 μg) were prepared in methanol and injected into HPLC. Rutin (3.05 μg) was dissolved in methanol and subjected to HPLC analysis. Rue extract was mixed vigorously in methanol, and 15 μl of the filtrate was subjected to HPLC analysis. The spectra of peaks appearing in the chromatogram of standards and the rue extract are shown. HPLC = high-performance liquid chromatography; MOP = methoxypsoralen.
3.2. Dose and time dependence of rue extract-mediated induction of Cyp1a and Cyp2b
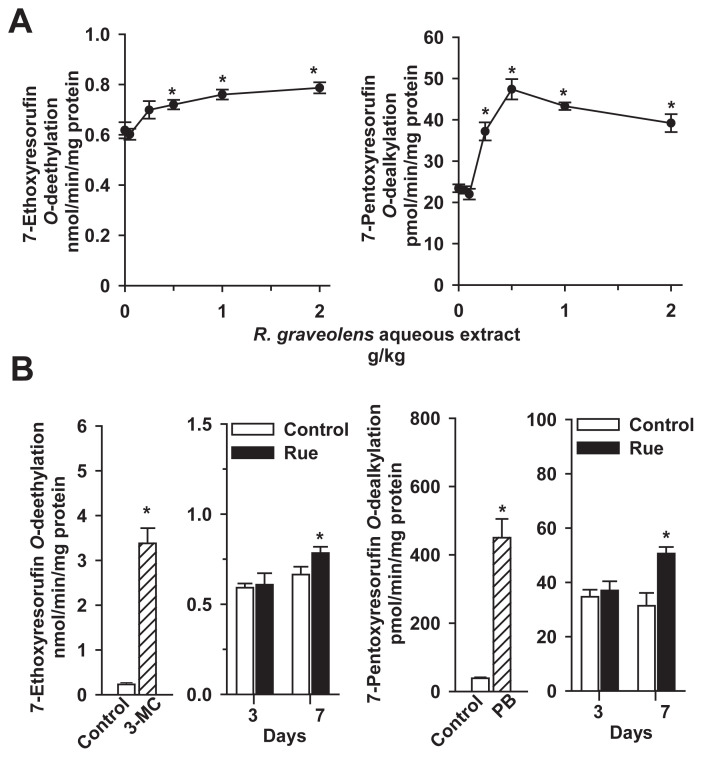
To determine the dose–response of the effects of rue extract on P450s, male mice were treated with rue extract at a daily dose of 0.05–2 g/kg for 7 days. In the determination of liver microsomal activities toward CYP1A substrate 7-ethoxyresorufin, treatment of mice with rue extract at a dose lower than 0.25 g/kg did not affect liver microsomal EROD activity (Fig. 3A, left panel). Treatment with 0.5 g/kg, 1 g/kg, and 2 g/kg extract significantly increased EROD activity by 17%, 23%, and 27%, respectively. In the determination of P450 activity toward CYP2B substrate 7-pentoxyresorufin, rue extract at a dose lower than 0.1 g/kg could not affect liver microsomal PROD activity (Fig. 3A, right panel). Rue extract at 0.25–2 g/kg significantly increased PROD by 59–102%. Thus, male mice were treated with 0.5 g/kg rue extract for 3 days and 7 days to examine the time dependence of P450 induction.
Fig. 3.
Increases of hepatic Cyp1a and Cyp2b activities in male mice treated with (A) rue extract at increasing doses (0.05–2.0 g/kg/d) for 7 days and (B) 0.5 g extract/kg/d rue extract for 3 days and 7 days. Mice were intraperitoneally treated with a single injection of 80 mg/kg 3-MC for 48 hours and a daily dose of 80 mg/kg PB for 4 days for the induction of Cyp1a and Cyp2b, respectively. Control mice received the same volume of vehicle. Liver microsomal 7-ethoxyresorufin O-deethylation and 7-pentoxyresorufin O-dealkylation activities were determined as described in the Materials and methods section. Data represent the mean ± standard error of the mean of four or five in each group of mice in the dose–response study and six mice in each group in the time-course study. *p < 0.05, compared with the respective control group. 3-MC = 3-methylcholanthrene; PB = phenobarbital.
The 3-day treatment did not affect hepatic EROD and PROD activities (Fig. 3B). However, rue extract increased EROD and PROD activities by 18% and 61%, respectively, after 7 consecutive days. To examine the effects of rue on the activities of other P450s and Phase II enzymes, male and female mice were treated with 0.5 g/kg rue extract for 7 days in the following studies. Treatment of mice with a prototype CYP1A inducer, 3-MC, caused a 15-fold increase in liver microsomal EROD activity. PB, a potent CYP2B inducer, caused an 11-fold increase of PROD activity. Microsomes of 3-MC- and PB-treated mice were used as markers for the immunoblot analyses of microsomal Cyp1a and Cyp2b.
3.3. Effects of rue extract on hepatic P450, UGT, and NQO activities in male and female mice
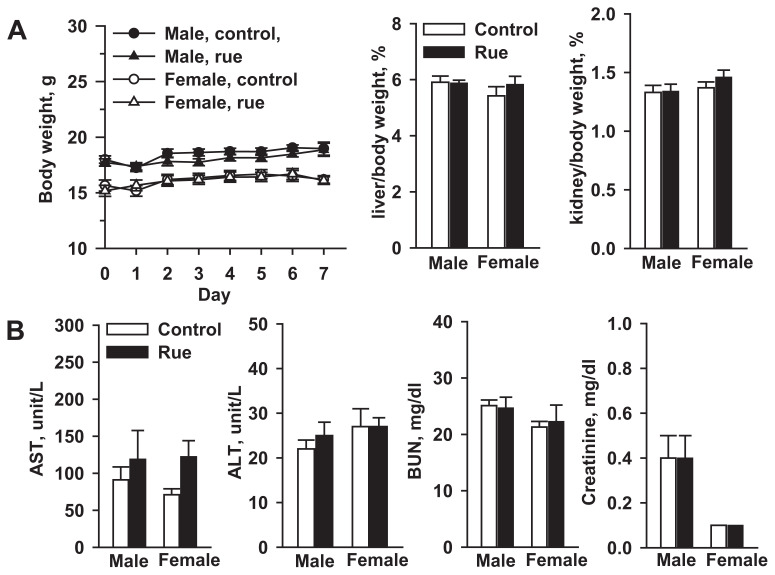
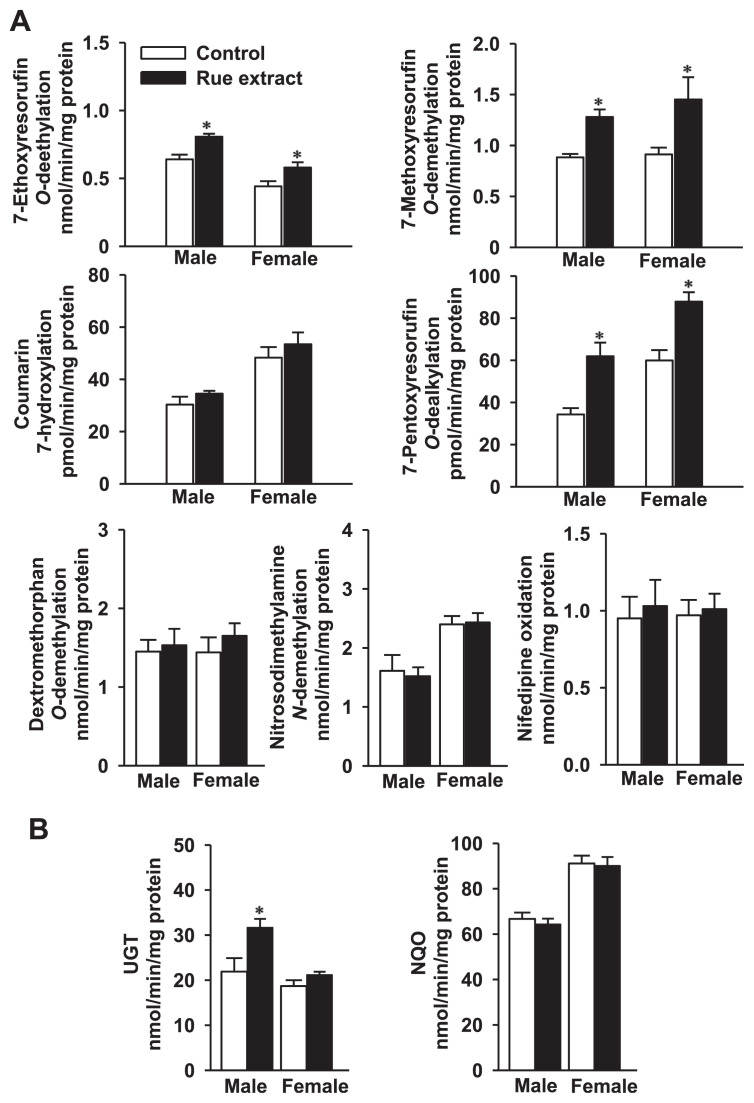
Mouse body and tissue weights were not significantly affected after a 1-week treatment with 0.5 g/kg rue extract (Fig. 4A). This treatment did not elevate male and female mouse serum alanine aminotransferase and aspartate aminotransferase levels (Fig. 4B). Treatment of male mice with 0.5 g/kg/d rue extract for 1 week did not affect liver microsomal P450 and cytochrome b5 contents, and CPR activity (Table 1). This rue treatment caused 27%, 47%, and 80% increases of hepatic EROD (CYP1A), MROD (CYP1A), and PROD (CYP2B) activities, respectively (Fig. 5A). However, under this treatment regimen, rue extract could not affect hepatic coumarin 7-hydroxylation, dextromethorphan O-demethylation, nitrosodimethylamine N-demethylation, and nifedipine oxidation activities, which were the marker activities of CYP2A, CYP2D, CYP2E1, and CYP3A, respectively. Microsomal UGT activity was induced by 45% (Fig. 5B), whereas rue extract did not affect cytosolic NQO activity. At a dose lower than 0.1 g/kg, rue extract did not affect UGT activity (data not shown).
Fig. 4.
Effects of (A) rue extract on the body weight and tissue/body weight ratio and (B) serum biochemical markers in male and female mice. Serum activities of AST and ALT, and contents of BUN and creatinine were determined. Data represent the mean ± standard error of the mean of seven mice. ALT = alanine aminotransferase; AST = aspartate aminotransferase; BUN = blood urea nitrogen.
Table 1.
Effects of R. graveolens extract on hepatic content and reduction activity of cytochrome P450-dependent monooxygenase components in male and female mice.
| Monooxygenase component | Male | Female | ||
|---|---|---|---|---|
|
|
|
|||
| Control | Rue extract | Control | Rue extract | |
| Cytochrome P450 (nmol/mg protein) | 0.64 ± 0.05 | 0.63 ± 0.05 | 0.41 ± 0.02 | 0.41 ± 0.01 |
| Cytochrome b5 (nmol/mg protein) | 0.30 ± 0.03 | 0.34 ± 0.03 | 0.39 ± 0.02 | 0.43 ± 0.01 |
| NADPH-P450reductase (nmol/min/mg protein) | 189 ± 23 | 168 ± 16 | 246 ± 7 | 249 ± 6 |
Mice were treated with water or 0.5 g rue extract/kg/d for 7 days. The control group received the same volume of water. Data represent the mean ± standard error of the mean of seven male and eight female mice.
Fig. 5.
(A) Effects of rue extract on hepatic marker activities of cytochrome P450 isoforms and (B) activities of UGT and NQO in male and female mice. Mice were treated with 0.5 g extract/kg/d for 7 days. The control group received the same amount of water. Data represent the mean ± standard error of the mean of seven male and eight female mice. *p < 0.05, compared with the control group. NQO = NAD(P)H:quinone oxidoreductase; UGT = UDP-glucuronosyltransferase.
In female mice, 7-day treatment with 0.5 g/kg rue extract did not affect liver microsomal P450 and cytochrome b5 contents and CPR activity (Table 1). Microsomal EROD, MROD, and PROD activities were increased by 31%, 59%, and 47%, respectively (Fig. 5A). Hepatic marker activities of CYP2A, CYP2D, CYP2E1, and CYP3A were not affected by this treatment regimen. The activities of liver microsomal UGT and cytosolic NQO remained unchanged (Fig. 5B).
3.4. Effects of rue extract on renal P450, UGT, and NQO activities in male and female mice
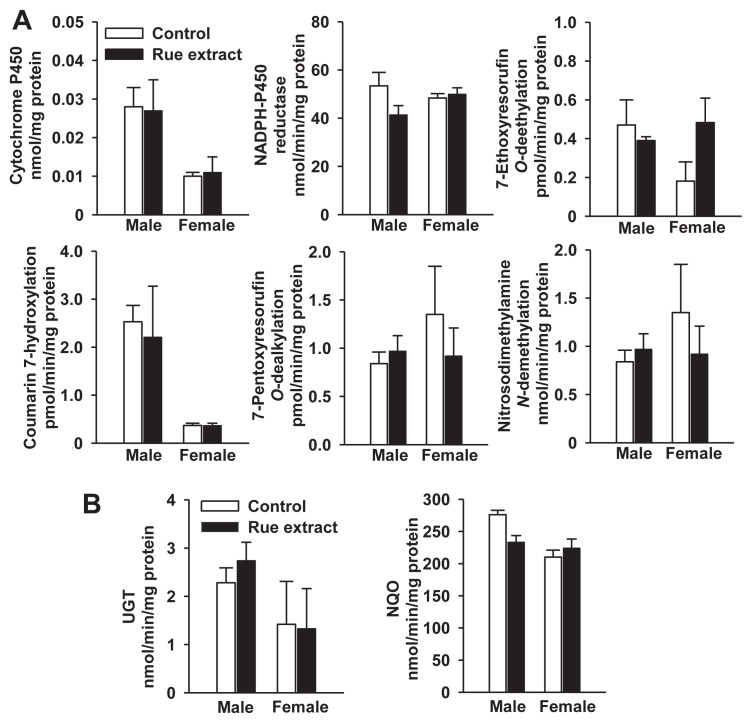
With the exception of renal cytosolic NQO activity being higher than hepatic activity, renal P450 and UGT activities were lower than hepatic activities (Figs. 5 and 6). Rue did not change kidney microsomal P450 content and CPR activity in male and female mice (Fig. 6A). Due to the elevation of hepatic Cyp1a and Cyp2b activities by rue, kidney microsomal P450 activities toward 7-ethoxyresorufin and 7-pentoxyresorufin were determined. In contrast to the inductive response in the liver, 7-day treatment with 0.5 g/kg rue extract did not affect the renal EROD and PROD activities. Under this treatment regimen, kidney microsomal coumarin 7-hydroxylation activity was not altered by rue extract (Fig. 6A). The rue extract did not affect renal UGT and NQO activities either (Fig. 6B).
Fig. 6.
(A) Effects of rue extract on renal content and activities of cytochrome P450-dependent monooxygenase system and (B) activities of UGT and NQO in male and female mice. Mice were treated with 0.5 g rue extract/kg/d for 7 days. The control group received the same amount of water. Data represent the mean ± standard error of the mean of samples from seven male and eight female mice, in which tissues of two mice were randomly pooled as one sample. NQO = NAD(P)H:quinone oxidoreductase; UGT = UDP-glucuronosyltransferase.
3.5. Elevation of liver microsomal Cyp1a, Cyp2b9/10, and UGT proteins by rue extract
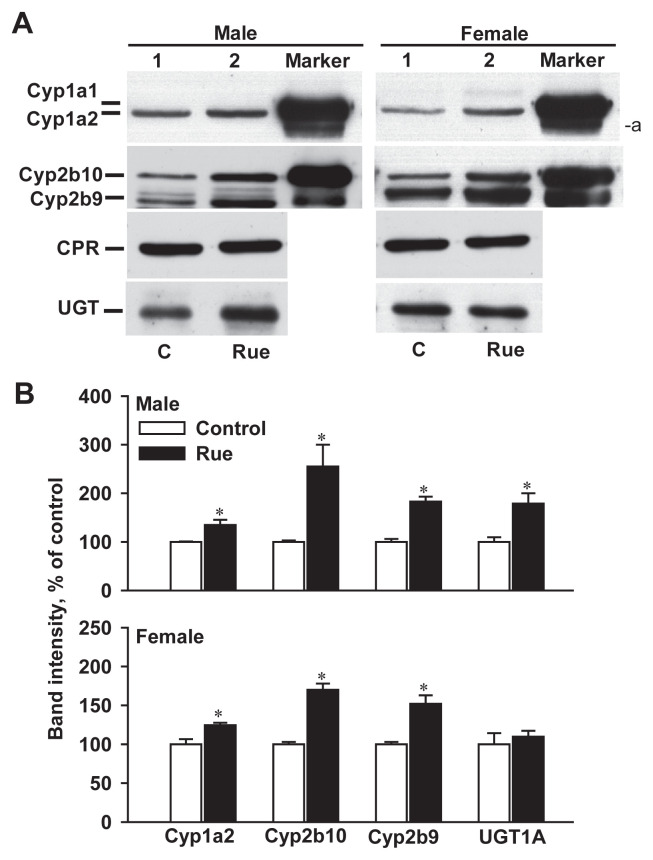
Since rue extract at 0.5 g/kg induced hepatic Cyp1a and Cyp2b activities in male and female mice, protein levels of Cyp1a1/2, Cyp2b9/10, and CPR were determined by immunoblot analysis (Fig. 7). Consistent with the unchanged activity of CPR, there was no significant difference in the protein levels of microsomal CPR in control and rue extract-treated mice. Cyp1a2 was the primary CYP1A member in the mouse liver [1] and was detected in the blot of liver microsomes of the control group (Fig. 7A). Treatment with 3-MC increased the mouse Cyp1a2 protein level and strongly induced the Cyp1a1 protein with an apparent molecular weight greater than that of Cyp1a2 [1]. Therefore, 3-MC-treated mouse liver microsomes were used as a positive control for Cyp1a1 and Cyp1a2 induction (Fig. 7A). Due to the potent induction of Cyp1a1/2 by 3-MC, the luminescence-visualized bands of Cyp1a1- and Cyp1a2-immunoreacted proteins were very close in the blot. An unidentified protein band with an apparent molecular weight lower than that of Cyp1a2 was immunodetected in the 3-MC-treated, but not in the vehicle- and rue-treated mouse liver microsomes. The results of intensity scanning showed that rue treatment resulted in a 34% and 24% increase in the Cyp1a2 protein level in male and female mice, respectively (Fig. 7B). This rue treatment did not elevate Cyp1a1 expression to a detectable level. PB-treated mouse liver microsomes were used as markers of Cyp2b9 and Cyp2b10 induction. Immunoblot analysis with an antirat CYP2B1 antibody, which cross-reacts with mouse Cyp2b9 and Cyp2b10, showed that rue extract increased the Cyp2b10 protein levels by 155% and 70% in male and female mice, respectively. Similarly, rue extract increased Cyp2b9 protein levels by 83% and 52% in male and female mice, respectively. However, the blot of male mouse liver microsomes showed some light immunoreacted bands with mobility between Cyp2b10 and Cyp2b9; these were mainly and markedly induced by PB (Fig. 7A). Rue extract increased the UGT1a protein level by 79% in males, but not in females.
Fig. 7.
Immunoblot analyses of liver microsomal Cyp1a1/2, Cyp2b9/10, CPR, and UGT in control and rue extract-treated mice: (A) representative blots and (B) relative band intensity compared to that of control mice. Male and female mice were treated with a daily dose of 0.5 g/kg rue extract for 7 days. Microsomes of mice treated with inducers 3-MC and PB were loaded as markers for Cyp1a1/2 and Cyp2b9/10, respectively. For the analysis of Cyp1a enzymes, microsomal proteins of control (50 μg/well), rue-treated (50 μg/well), and 3-MC-treated (25 μg/well) mice were loaded on a polyacrylamide gel. For the analysis of Cyp2b, microsomal proteins of control (30 μg/well), rue-treated (30 μg/well), and PB-treated (12 μg/well) mice were loaded. In each well, 30 μg and 50 μg microsomal proteins were loaded for the analyses of CPR and UGT levels, respectively. Data of band intensity represent the mean ± standard error of the mean of three mice. *p < 0.05, compared with the control group. a Unidentified protein band. CPR = NADPH–cytochrome P450 reductase; 3-MC = 3-methylcholanthrene; PB = phenobarbital; UGT = UDP-glucuronosyltransferase.
3.6. Effects of rutin and Fmix on hepatic P450, UGT, and NQO activities
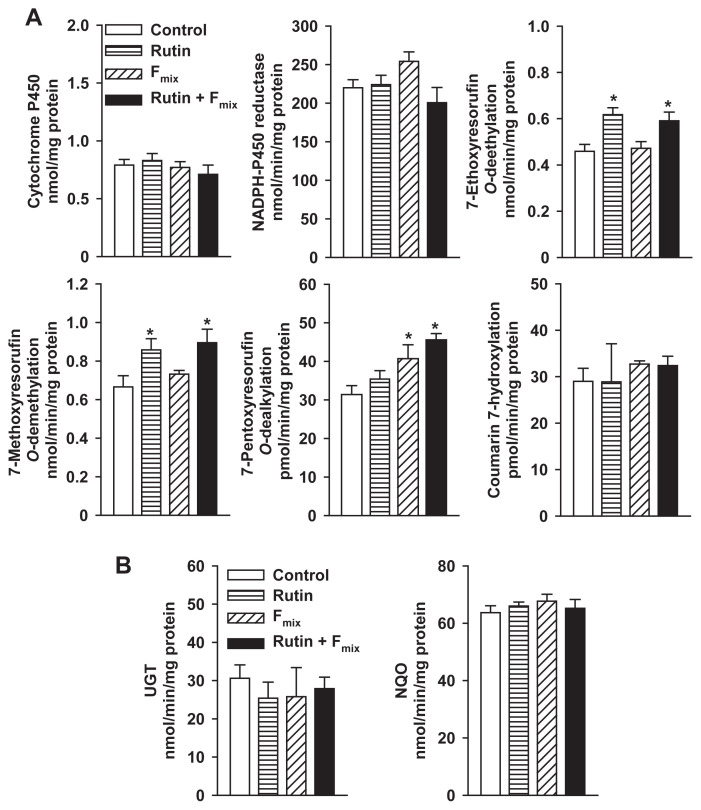
Based on the contents of furanocoumarins in rue extract, the administration of 0.5 g/kg rue extract resulted in a concurrent exposure to 12 mg/kg rutin, 1.3 mg/kg psoralen, 0.05 mg/kg 8-MOP, 1.4 mg/kg 5-MOP, and 0.05 mg/kg chalepensin. Due to its solubility, corn oil was used as a vehicle. There were no significant differences in activities between the water and corn-oil control groups. To examine the contribution of rutin and furanocoumarins to the enzyme induction by rue extract, male mice were treated with rutin, Fmix, and the mixture of rutin and Fmix for 7 days. Oral administration of rutin in mice increased liver microsomal EROD and MROD activities by 35% and 29%, respectively (Fig. 8A). However, rutin treatment had no effect on PROD activity. Fmix treatment increased PROD activity by 30%. Treatment with the mixture of rutin and Fmix resulted in 29%, 35%, and 45% increases of EROD, MROD, and PROD activities, respectively (Fig. 8A). However, microsomal activities toward P450 substrates, coumarin (7-hydroxylation), dextromethorphan, nifedipine, and nitrosodimethylamine were not affected by rutin and Fmix (results not shown). These treatments did not affect microsomal UGT and cytosolic NQO activities (Fig. 8B).
Fig. 8.
(A) Effects of rutin and Fmix on hepatic activities of cytochrome P450 isoforms and (B) activities of UGT and NQO in male mice. Mice were treated with rutin (12 mg/kg), Fmix (0.05 mg/kg chalepensin, 1.4 mg/kg 5-MOP, 0.05 mg/kg 8-MOP, and 1.3 mg/kg psoralen), and the mixture of rutin and Fmix daily for 1 week. Control group received the same amount of corn oil. Data represent the mean ± standard error of the mean of five mice. *p < 0.05, compared with the control group. Fmix = furanocoumarin mixture; MOP = methoxypsoralen; NQO = NAD(P)H:quinone oxidoreductase; UGT = UDP-glucuronosyltransferase.
4. Discussion
Our findings revealed that rue extract at 0.5 g/kg significantly elevated hepatic Cyp1a and Cyp2b protein levels and activities in both male and female mice, whereas activities toward the marker substrates of CYP2A, CYP2E1, CYP2D, and CYP3A remained the same. Induction of Cyp1a and Cyp2b occurred after 7-day treatment, but not after 3-day treatment. This reveals that the induction requires a lag-time period, which may be attributed to the time-consuming processes including absorption, tissue accumulation, receptor activation, and protein synthesis. The unchanged activity and protein level of CPR indicate that the increases in Cyp1a and Cyp2b activities were not due to the induction of CPR. The induction of mouse Cyp1a2 and Cyp2b9/10 by rue extract was mild to moderate in comparison with the potent inducers 3-MC and PB. Under this treatment regimen, rue-mediated increases in UGT activity were seen in males but not in females. The increase of UGT activity was consistent with the elevated protein levels. The actual cause of the predominantly male induction of UGT by rue needs further investigation. However, a high-fat diet significantly induced UGT1A1/6 in male rats, but not in females [29]. Nuclear levels of the CAR and peroxisome proliferator-activated receptor α were stimulated only in males. The elevated CAR and peroxisome proliferator-activated receptor α levels were suggested to be involved in the male-predominant UGT induction. Testosterone can be metabolized by glucuronide conjugation and P450-mediated hydroxylations, in which CYP2B is one of the key isoforms [30]. Epileptic patients taking a typical CYP2B inducer, PB, had a reduced level of free testosterone [31]. It was suggested that P450 induction was one of the potential factors of sexual dysfunction. It is of interest to clarify the contribution of CYP2b and UGT induction in the rue-mediated reduction of testosterone and sexual behavior in experimental animals [9] and sperm motility in humans [8]. CYP2B and UGT are also important enzymes for the metabolism of drugs, such as methadone [32]. Alterations of CYP2B and UGT function can be associated with the methadone dose and the incidence of withdrawal symptoms. The potential interaction of rue extract and CYP2B/UGT drug substrates needs further evaluation.
The British Herbal Pharmacopoeia (1990 ed.) recommends a daily dose of 1.5–3.0 g dried rue [7]. In Taiwan, a female patient with nonobstructive-type hypertrophic cardiomyo pathy was taking bisoprolol, diltiazem, and amiloride/hydrochlorothiazide, the oxidation of which was influenced by CYP2D6 and CYP3A4 [7]. After the patient took a daily dose of a decoction prepared from 100 g of the aerial parts of rue (~1.7 g/kg body weight) for 3 days, the symptoms of cardiotoxicity, nephrotoxicity, hepatotoxicity, and coagulopathy were observed. This patient apparently received a high daily dose compared to the dose ranges described in the pharmacopeias or on different websites. The toxicity event in Taiwan emphasizes the problem of overdosing with rue and the importance of the regulation of medicinal herbs. Using the Guidance for Industry (specifically a section on estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers) issued by the Center for Drug Evaluation and Research, a division of the United States Department of Health and Human Services Food and Drug Administration, the human equivalent dose for mice was calculated using a standardized factor of 12.3 based on the human to mouse surface area ratio. Watanabe et al [33] reported that the estimate of human equivalent dose is equivalent to the animal dose (g/kg) (Wanimal/Whuman)1–b, in which a value of b of 0.75 or 0.67 was considered for use. Since the aqueous extract yield was 20.54 g aqueous extract/kg aerial part of rue, the administration of 3 g rue to a 60 kg patient was roughly estimated to be equivalent to 0.007–0.014 g rue extract/kg in mice (20 g). Our results revealed that rue extract at a dose lower than 0.1 g/kg did not alter Cyp1a, Cyp2b, and UGT activities in mice. Cyp2d and Cyp3a activities were not affected by rue extract at a dose of up to 0.5 g/kg. However, species difference in the P450 induction is one of the key factors of the difficulty of direct extrapolation from mouse study to human response. To facilitate the assessment of rue-mediated P450 induction in humans, the effects of rue extract and its ingredients on human receptor activation and P450 expression shall be studied in cultured human hepatocytes.
Rutin and the rue furanocoumarins, chalepensin, 5-MOP, 8-MOP, and psoralen were identified in our preparation of rue extract. A single intraperitoneal injection of 1 mg/kg 8-MOP stimulated rat CYP1A and CYP2B activities after 24 hours [3]. Furthermore, rat CYP1A1/2 and UGT were induced by 8-MOP at an oral dose of 150 mg/kg [34]. Our previous study demonstrated that chalepensin (10μM) could activate mouse CAR in HepG2 cells. Oral administration of chalepensin at 10 mg/kg induced Cyp2b9/10 [4], whereas chalepensin did not affect Cyp1a and UGT (unpublished results) activities in mice. Considering the amounts of individual furanocoumarins in rue extract, the mouse dose of a single furanocoumarin seems to be not enough to induce P450 or UGT. Our current studies demonstrated that oral administration of Fmix (based on the contents in the rue extract) to mice induced hepatic Cyp2b activity. Rutin significantly induced hepatic Cyp1a activity. The induction of Cyp1a and Cyp2b by the mixture of rutin and Fmix provided further evidence for the significant roles of rutin and Fmix in the enzyme induction by rue extract (Table 2). The ingredient(s) of rue, which stimulates microsomal UGT activity in males, remains unclear.
Table 2.
Summary of effects of the components present in R. graveolens aqueous extract on hepatic cytochrome P450, UGT, and NQO activities in male mice.a
| Treatment | Cyp1a | Cyp2a | Cyp2b | UGT | NQO |
|---|---|---|---|---|---|
| Rue aqueous extract | + | − | + | + | − |
| Rutin | + | − | – | − | − |
| Fmix | − | − | + | − | − |
| Rutin + Fmix | + | − | + | − | − |
Fmix = mixture of furanocoumarins; NQO = NAD(P)H-quinone oxidoreductase; UGT = UDP-glucuronosyltransferase; + = induction; – = unchanged by treatments.
Mice were treated with 0.5 g/kg rue extract for 7 days. Rutin and the mixture of furanocoumarin were administered to mice in a dose according to their respective amount in the mouse dose of rue extract. Cyp1a activities toward 7-ethoxyresorufin and 7-methoxyresorufin were determined. Coumarin and 7-pentoxyresorufin were used as substrates for Cyp2a and Cyp2b, respectively. The UGT and NQO activities were determined using p-nitrophenol and menadione as substrates, respectively.
In summary, our findings first demonstrate that the repeated administration of rue extract induces hepatic Cyp1a and Cyp2b activities in a dose-dependent manner in mice. Compared with females, male mice were more responsive to the rue-mediated induction of UGT. The elevation of P450 and UGT activities was consistent with the elevation of protein levels. Although the effects of the other types of rue ingredients cannot be excluded, rutin and Fmix contribute at least in part to the P450 induction by rue.
Acknowledgments
We sincerely appreciate the technical assistance of Mr Cin-Haw Yang and Ms Yu-Ping Chang (National Research Institute of Chinese Medicine, Taipei, Taiwan). We thank Professor Ann-Ping Tsou (National Yang-Ming University, Taipei, Taiwan) for kindly providing us with the Fujifilm Dri-Chem 3000 colorimetric analyzer for the determination of serum biomarkers. This work was supported mainly by grants from the National Science Council, Taipei, Taiwan (grant numbers: NSC97-2320-B-077-009-MY3 and NSC101-2320-B-077-001-MY3) and partially by the National Research Institute of Chinese Medicine.
Funding Statement
This work was supported mainly by grants from the National Science Council, Taipei, Taiwan (grant numbers: NSC97-2320-B-077-009-MY3 and NSC101-2320-B-077-001-MY3) and partially by the National Research Institute of Chinese Medicine.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
REFERENCES
- 1. Hrycay EG, Bandiera SM. Expression, function and regulation of mouse cytochrome P450 enzymes: comparison with human cytochrome P450 enzymes. Curr Drug Metab. 2009;10:1151–83. doi: 10.2174/138920009790820138. [DOI] [PubMed] [Google Scholar]
- 2. Danson S, Ward TH, Butler J, Ranson M. DT-diaphorase: a target for new anticancer drugs. Cancer Treat Rev. 2004;30:437–49. doi: 10.1016/j.ctrv.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 3. Gwang JH. Induction of rat hepatic cytochrome P4501A and P4502B by the methoxsalen. Cancer Lett. 1996;109:115–20. doi: 10.1016/s0304-3835(97)82727-9. [DOI] [PubMed] [Google Scholar]
- 4. Lo WS, Lim YP, Chen CC, Hsu CC, Soucek P, Yun CH, Xie W, Ueng YF. A dual function of the furanocoumarin chalepensin in inhibiting Cyp2a and inducing Cyp2b in mice: the protein stabilization and receptor-mediated activation. Arch Toxicol. 2012;86:1927–38. doi: 10.1007/s00204-012-0902-7. [DOI] [PubMed] [Google Scholar]
- 5. Koenigs LL, Trager WF. Mechanism-based inactivation of P450 2A6 by furanocoumarins. Biochemistry. 1998;37:10047–61. doi: 10.1021/bi980003c. [DOI] [PubMed] [Google Scholar]
- 6. San Miguel E. Rue (Ruta L., Rutaceae) in traditional Spain: frequency and distribution of its medicinal and symbolic applications. Econ Bot. 2003;57:231–44. [Google Scholar]
- 7. Seak CJ, Lin CC. Ruta graveolens intoxication. Clin Toxicol. 2007;45:173–5. doi: 10.1080/15563650600956667. [DOI] [PubMed] [Google Scholar]
- 8. Harat ZN, Sadeghi MR, Sadeghipour HR, Kamalinejad M, Eshraghian MR. Immobilization effect of Ruta graveolens L. on human sperm: a new hope for male contraception. J Ethnopharmacol. 2008;115:36–41. doi: 10.1016/j.jep.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 9. Khouri NA, El-Akawi Z. Antiandrogenic activity of Ruta graveolens L in male Albino rats with emphasis on sexual and aggressive behavior. Neuroendocrinol Lett. 2005;26:823–9. [PubMed] [Google Scholar]
- 10. Kong YC, Lau CP, Wat KH, Ng KH, But PPH, Cheng KF, Waterman PG. Antifertility principle of Ruta graveolens. Planta Med. 1989;55:176–8. doi: 10.1055/s-2006-961917. [DOI] [PubMed] [Google Scholar]
- 11. Salib JY, El-Toumy SA, Hassan EM, Shafik NH, Abdel-Latif SM, Brouard I. New quinoline alkaloid from Ruta graveolens aerial parts and evaluation of the antifertility activity. Nat Product Res. 2014;28:1335–42. doi: 10.1080/14786419.2014.903395. [DOI] [PubMed] [Google Scholar]
- 12. Ueng YF, Chen CC, Yamazaki H, Kiyotani K, Chang YP, Lo WS, Li DT, Tsai PL. Mechanism-based inhibition of CYP1A1 and CYP3A4 by the furanocoumarin chalepensin. Drug Metab Pharmacokinet. 2013;28:229–38. doi: 10.2133/dmpk.dmpk-12-rg-113. [DOI] [PubMed] [Google Scholar]
- 13. Yang J, Qian D, Jiang S, Shang E-X, Guo J, Duan J-A. Identification of rutin deglycosylated metabolites produced by human intestinal bacteria using UPLC-Q-TOF/MS. J Chromatogr B. 2012;898:95–100. doi: 10.1016/j.jchromb.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 14. Sugatani J, Yamakawa K, Tonda E, Nishitani S, Yoshinari K, Degawa M, Abe I, Noguchi H, Miwa M. The induction of human UDP-glucuronosyltransferase 1A1 mediated through a distal enhancer module by flavonoids and xenobiotics. Biochem Pharmacol. 2004;67:989–1000. doi: 10.1016/j.bcp.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 15. Gelboin HV. Cytochrome P450 and monoclonal antibodies. Pharmacol Rev. 1993;45:413–53. [PubMed] [Google Scholar]
- 16. Sugawara M, Okamoto K, Kadowaki T, Kusano K, Fukamizu A, Yoshimura T. Inoculation of human tumor cells alters the basal expression but not the inducibility of cytochrome P450 enzymes in tumor-bearing mouse liver. Drug Metab Dispos. 2009;37:2244–54. doi: 10.1124/dmd.109.028571. [DOI] [PubMed] [Google Scholar]
- 17. Kaminsky LS, Fasco MJ, Guengerich FP. Production and application of antibodies to rat liver cytochrome P450. Methods Enzymol. 1981;74:262–72. doi: 10.1016/0076-6879(81)74018-7. [DOI] [PubMed] [Google Scholar]
- 18. Omura T, Sato R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem. 1964;239:2370–9. [PubMed] [Google Scholar]
- 19. Phillips AH, Langdon RG. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962;237:2652–60. [PubMed] [Google Scholar]
- 20. Pohl RJ, Fouts JR. A rapid method for assaying the metabolism of 7-ethoxyresorufin by microsomal subcellular fractions. Anal Biochem. 1980;107:150–5. doi: 10.1016/0003-2697(80)90505-9. [DOI] [PubMed] [Google Scholar]
- 21. Lubet RA, Mayer RT, Cameron JW, Nims RW, Burke MD, Wolff T, Guengerich FP. Dealkylation of pentoxyresorufin: a rapid and sensitive assay for measuring induction of cytochrome(s) P-450 by phenobarbital and other xenobiotics in the rat. Arch Biochem Biophys. 1985;238:43–8. doi: 10.1016/0003-9861(85)90138-9. [DOI] [PubMed] [Google Scholar]
- 22. Kerry NL, Somogyi AA, Mikus G, Bochner F. Primary and secondary oxidative metabolism of dextromethorphan: in vitro studies with female Sprague-Dawley and Dark Agouti rat liver microsomes. Biochem Pharmacol. 1993;45:833–9. doi: 10.1016/0006-2952(93)90166-t. [DOI] [PubMed] [Google Scholar]
- 23. Souček P. Novel sensitive high-performance liquid chromatographic method for assay of coumarin 7-hydroxylation. J Chromatogr B. 1999;734:23–9. [PubMed] [Google Scholar]
- 24. Nash T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953;55:416–21. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guengerich FP, Martin MV, Beaune PH, Kremers P, Wolff T, Waxman DJ. Characterization of rat and human liver microsomal cytochrome P-450 forms involved in nifedipine oxidation, a prototype for genetic polymorphism in oxidative drug metabolism. J Biol Chem. 1986;261:5051–60. [PubMed] [Google Scholar]
- 26. Bock KW, Burchell B, Ditton GJ, Hanninen O, Mulder GJ, Owens IS, Siest G, Tephyl TR. UDP-glucuronosyltransferase activities: guidelines for consistent interim terminology and assay condition. Biochem Pharmacol. 1983;32:953–5. doi: 10.1016/0006-2952(83)90610-x. [DOI] [PubMed] [Google Scholar]
- 27. Liu XF, Yuan H, Haniu M, Iyanagi T, Shively JE, Chen S. Reaction of rat liver dt-diaphorase (NAD(P)H: quinone acceptor reductase) with 5′-[p-(fluorosulfonyl)benzoyl]-adenosine. Mol Pharmacol. 1989;35:818–22. [PubMed] [Google Scholar]
- 28. Lowry OH, Roseborough NJ, Farr AL, Randall RL. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 29. Osabe M, Sugatan J, Fukuyama T, Ikushiro S-I, Ikari A, Miwa M. Expression of hepatic UDP-glucuronosyltransferase 1A1 and 1A6 correlated with increased expression of the nuclear constitutive androstane receptor and peroxisome proliferator-activated receptor a in male rats fed a high-fat and high-sucrose diet. Drug Metab Dispos. 2008;36:294–302. doi: 10.1124/dmd.107.017731. [DOI] [PubMed] [Google Scholar]
- 30. Zhang YY, Yang L. Interactions between human cytochrome P450 enzymes and steroids: physiological and pharmacological implications. Expert Opin Drug Metab Toxicol. 2009;5:621–9. doi: 10.1517/17425250902967648. [DOI] [PubMed] [Google Scholar]
- 31. Verrotti A, Loiacono G, Laus M, Coppola G, Chiarelli F, Tiboni GM. Hormonal and reproductive disturbances in epileptic male patients: emerging issues. Reprod Toxicol. 2011;31:519–27. doi: 10.1016/j.reprotox.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 32. Wang SC, Tsou HH, Ho IK, Lin KM, Liu YL. Pharmacogenomics study in a Taiwanese methadone maintenance cohort. J Food Drug Anal. 2013;21:s62–8. doi: 10.1016/j.jfda.2013.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Watanabe K, Bois FY, Zeise L. Interspecies extrapolation: a reexamination of acute toxicity data. Risk Anal. 1992;12:301–10. doi: 10.1111/j.1539-6924.1992.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 34. Diawara MM, Chavez KJ, Simpleman D, Williams DE, Franklin MR, Hoyer PB. The psoralens adversely affect reproductive function in male Wistar rats. Reprod Toxicol. 2001;15:137–44. doi: 10.1016/s0890-6238(01)00118-6. [DOI] [PubMed] [Google Scholar]