Abstract
A liquid chromatography/tandem mass spectrometry (LC-MS/MS) method is developed to simultaneously determine 20 synthetic dyes (New Coccine, Indigo Carmine, Erythrosine, Tartrazine, Sunset Yellow FCF, Fast Green FCF, Brilliant Blue FCF, Allura Red AC, Amaranth, Dimethyl Yellow, Fast Garnet GBC, Para Red, Sudan I, Sudan II, Sudan III, Sudan IV, Sudan Orange G, Sudan Red 7B, Sudan Red B, and Sudan Red G) in food samples. This method offers high sensitivity and selectivity through the selection of two fragment ion transitions under multiple reaction monitoring mode to satisfy the requirements of both quantitation and qualitation. Using LC-MS/MS, the newly developed extraction protocol used in this study is rapid and simple and does not require the use of solid-phase extraction cartridges. The linearities and recoveries of the method are observed at the concentration range of 0.10–200 μg/kg and more than 90% for all dyes, respectively. The method has been successfully applied to screen 18 commercial chili powders and six commercial syrup-preserved fruits purchased from retail establishments in Taipei City. The results show that three legal food dyes, Tartrazine, and/or Sunset Yellow FCF, and/or New Coccine, are present in some syrup-preserved fruits. Amaranth, an illegal food dye in certain countries but declared illegal in Taiwan, is found in an imported syrup-preserved fruit.
Keywords: chili powder, dyes, LC-MS/MS, Sudan dyes, syrup-preserved fruit
1. Introduction
Food dye additives are defined as dyes, pigments, or substances that impart colors into foods, drugs, or cosmetics. Originally, natural dyes present in foods are unstable and altered rapidly during food processing and storage. Hence, synthetic dyes that outperform natural ones at various aspects such as low price, high effectiveness, and excellent stability are widely used by food companies all over the world [1,2].
The toxicities of synthetic azo type food dyes, such as Sudan I–IV, have been confirmed [3–8] and classified as category 3 carcinogen to humans by the International Agency for Research on Cancer [1]. Although Sudan dyes have been forbidden in recent decades, it can be still found in various imported food products in many countries [4]. In Taiwan, food dyes are strictly regulated by the Standards for Specification, Scope, Application, and Limitation of Food Additives [9], and food additives require governmental approvals for use prior to their inclusion in food. To ensure consumers’ health, a reliable screening method for the determination of synthetic dyes in foods is required. In the literature, thin layer chromatography (TLC) [10–12], spectrophotometry [13,14], high-performance liquid chromatography (HPLC) [2,15], capillary electrophoresis [16], liquid chromatography/tandem mass spectrometry (LC-MS/MS) [17–21], and gas chromatography/mass spectrometry (GC-MS) [22] have been used in the determination of dyes in foods. Among these mentioned methods, TLC and spectrophotometry are widely used for the determination of water-soluble dyes because of their low cost although they often suffer from poor sensitivity and interference from the food matrix. GC or LC methods offer good separation but suffer from time-consuming procedures and complicated instrumentation. In this work, we developed an LC-MS/MS method applied as a single-step extraction protocol offering simple and rapid sample preparation for the determination of dyes in foods.
2. Materials and methods
2.1. Reagents
New Coccine, Indigo Carmine, Erythrosine, Tartrazine, Sunset Yellow FCF, Fast Green FCF, Brilliant Blue FCF, Allura Red AC, Amaranth, Sudan II, Sudan IV, Dimethyl Yellow, Fast Garnet GBC, Para Red, Sudan Orange G, Sudan Red 7B, Sudan Red B and Sudan Red G were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sudan I was purchased from Tokyo Chemical Industry (Tokyo, Japan). Sudan III was purchased from Kanto Chemical (Tokyo, Japan). Methanol of HPLC grade was obtained from Mallinckrodt (Paris, KY, USA). Dimethyl sulfoxide (DMSO), acetonitrile, and acetic acid of HPLC grade were obtained from Merck (Darmstadt, Germany). Ammonia acetate was from Nacalai Tesque (Kyoto, Japan).
2.2. Instrumentation
The HPLC system consists of an UltiMate 3000 Standard LC System (Thermo Fisher Scientific Inc., Waltham, MA, USA) coupled with a triple quadrupole mass spectrometer API 3200 (AB SCIEX, Framingham, MA, USA) with a Turbo V ion source and Analyst software. Gas nitrogen was supplied by a nitrogen generator (Peak Scientific Instruments Ltd., Chicago, IL, USA). Nitrogen was used as curtain gas, nebulizer gas, and collision gas on the MS. Separation was carried out on an Acclaim Polar Advantage C16 (3 μm, 120 Å, 4.6 × 150 mm) column (Thermo Fisher Scientific Inc.). Allegra 25R centrifuge was from Beckman Coulter (Brea, CA, USA).
2.3. Standard solutions
A stock solution of individual dyes was prepared by dissolving 50 mg of the compound in 50 mL DMSO (1 mg/mL). This stock solution was further diluted with methanol to obtain a working solution of 10 μg/mL and stored in the dark at −18 °C.
2.4. Sample preparation and extraction
Each homogenized chili powder or syrup-preserved fruit sample (10 g) obtained from retail stores was extracted with acetonitrile twice (50 mL × 2, 5 minutes). Two extracts were combined and then centrifuged at 4000 rpm (5 minutes, 15°C). Supernatant was filtered through a 0.45-μm nylon membrane filter and then transferred into an amber autosampler vial. Sample vials were stored at 4°C prior to analysis by LC-MS/MS.
2.5. LC-MS/MS analysis
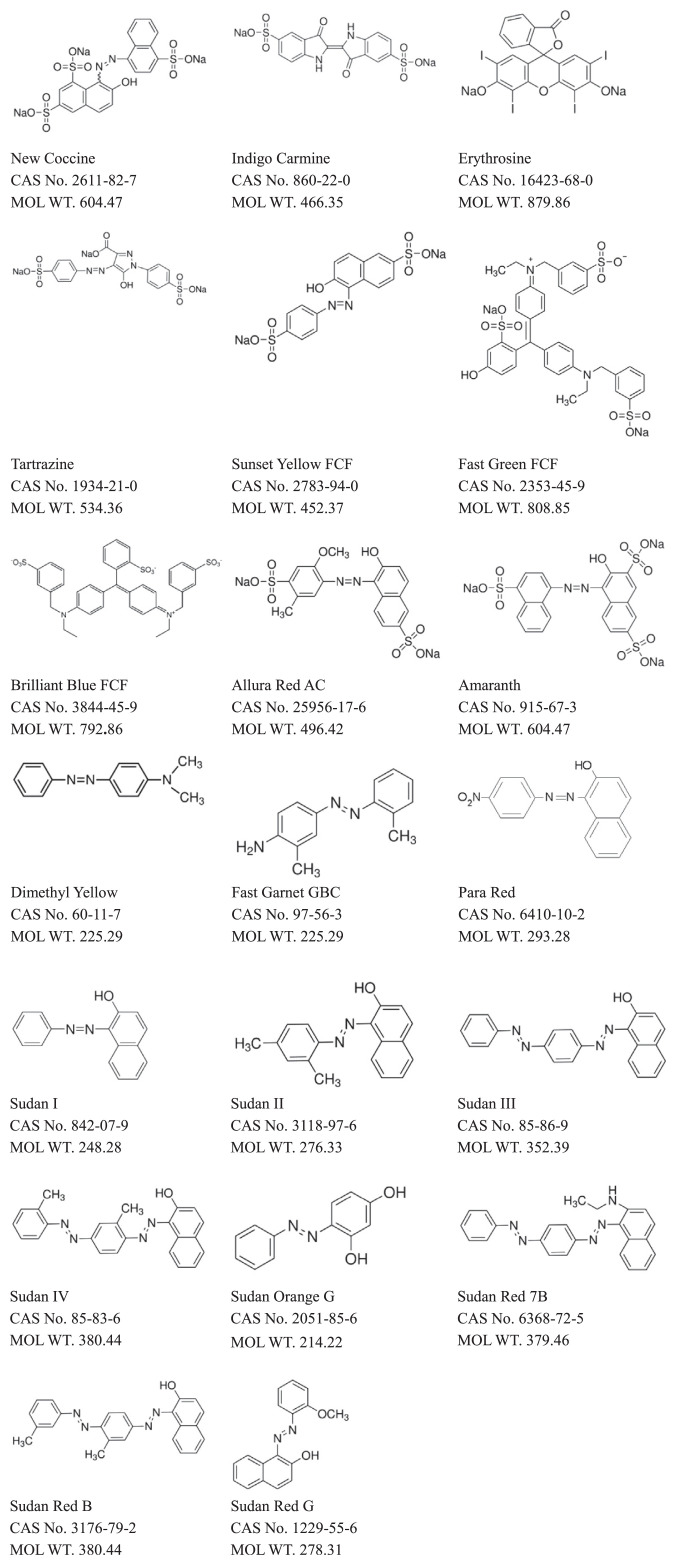
The mobile phase of the HPLC system consisted of acetonitrile (A) and 20 mM ammonia acetate buffer with 1% acetic acid (B). Linear gradient elution was programmed as follows: 0–3 minutes, 98–50% B; 3–8 minutes, 50–0% B; 8–15 minutes, 0% B; 15–15.5 minutes, 0–98% B; 15.5–20 minutes, 98% B. The flow rate was set at 1 mL/min. Electrospray ionization mass spectra (ESI-MS) were acquired in the positive (ESI +) and then negative (ESI –) ion mode. Other MS parameters were as follows: turbo gas, 50 psi; curtain gas, 10 psi; collision gas, 5 psi; source temperature, 300°C; nebulizer gas, 60 psi; interface heater, on; needle current, 4000 V; entrance potential, 10 V. A dwell time of 200 milliseconds was set between transitions of the ions. A total of 11 dyes—Dimethyl Yellow, Fast Garnet GBC, Para Red, Sudan I, Sudan II, Sudan III, Sudan IV, Sudan Orange G, Sudan Red 7B, Sudan Red B, and Sudan Red G—were detected by positive ESI, and nine other dyes—New Coccine, Indigo Carmine, Erythrosine, Tartrazine, Sunset Yellow FCF, Fast Green FCF, Brilliant Blue FCF, Allura Red AC, and Amaranth (Fig. 1)—were detected by negative ion ESI. Target dyes were identified by their retention times (Rt) and selected ions in multiple reaction monitoring (MRM) mode summarized in Table 1. Quantification was conducted with the MRM transition by selecting two characteristic ions that gave the most intense signal/noise ratio.
Fig. 1.
Structures of dyes.
Table 1.
LC-MS/MS acquisition parameters for the target synthetic dyes.
| Compound | Precursor ion (m/z) | Product ion (m/z) | Declustering potential (V) | Entrance potential (V) | CEP (V) | Collision energy (V) | CXP (V) |
|---|---|---|---|---|---|---|---|
| Sudan Orange G | 215.1 | 93.1 | 31 | 8 | 30 | 31 | 4 |
| 215.1 | 122.1 | 31 | 8 | 30 | 21 | 4 | |
| Dimethyl Yellow | 226.1 | 120.1 | 41 | 8.5 | 30 | 43 | 4 |
| 226.1 | 105.1 | 41 | 8.5 | 30 | 25 | 4 | |
| Sudan I | 249.1 | 93 | 26 | 4.5 | 30 | 33 | 4 |
| 249.1 | 156.1 | 26 | 4.5 | 30 | 21 | 4 | |
| Sudan II | 277.1 | 121.1 | 26 | 4.5 | 30 | 25 | 4 |
| 277.1 | 106.1 | 26 | 4.5 | 30 | 55 | 4 | |
| Sudan Red G | 279.1 | 123.1 | 26 | 4.5 | 30 | 23 | 4 |
| 279.1 | 108.1 | 26 | 4.5 | 30 | 47 | 4 | |
| Para Red | 294.1 | 156.1 | 31 | 5 | 30 | 21 | 4 |
| 294.1 | 128.1 | 31 | 5 | 30 | 35 | 4 | |
| Sudan III | 353.1 | 197.1 | 51 | 10.5 | 30 | 27 | 4 |
| 353.1 | 128.1 | 51 | 10.5 | 30 | 51 | 4 | |
| Sudan Red 7B | 380.2 | 183.1 | 31 | 9 | 30 | 21 | 4 |
| 380.2 | 115.1 | 31 | 9 | 30 | 65 | 4 | |
| Sudan IV | 381.1 | 224.1 | 51 | 9 | 30 | 31 | 4 |
| 381.1 | 225.1 | 51 | 9 | 30 | 25 | 4 | |
| 381.1 | 143.1 | 51 | 9 | 30 | 37 | 4 | |
| 381.1 | 104.1 | 51 | 9 | 30 | 83 | 4 | |
| Sudan Red B | 381.2 | 224.1 | 56 | 10 | 30 | 29 | 4 |
| 381.2 | 225.1 | 56 | 10 | 30 | 29 | 4 | |
| 381.2 | 156.1 | 56 | 10 | 30 | 27 | 4 | |
| 381.2 | 134.1 | 56 | 10 | 30 | 27 | 4 | |
| Fast Garnet GBC | 226.1 | 91 | 46 | 9.5 | 30 | 29 | 4 |
| 226.1 | 107 | 46 | 9.5 | 30 | 31 | 4 | |
| Fast Green FCF | 381 | 80 | −40 | −10 | −33 | −98 | −8 |
| 381 | 170 | −40 | −10 | −33 | −38 | −8 | |
| Tartrazine | 244 | 80 | −21 | −10 | −29.5 | −62 | −8 |
| 244 | 198 | −21 | −10 | −29.5 | −20 | −8 | |
| New Coccine | 206 | 80 | −35 | −10 | −28.4 | −40 | −8 |
| 268 | 206 | −25 | −10 | −30 | −18 | −8 | |
| Indigo Carmine | 226 | 198 | −42 | −10 | −29 | −27 | −8 |
| 226 | 105 | −42 | −10 | −29 | −53 | −8 | |
| Brilliant Blue | 373 | 80 | −45 | −10 | −33 | −92 | −8 |
| 373 | 170 | −45 | −10 | −33 | −42 | −8 | |
| Sunset Yellow | 203 | 171 | −27 | −10 | −28 | −20 | −8 |
| 203 | 207 | −27 | −10 | −28 | −20 | −8 | |
| Erythrosine | 834 | 227 | −22 | −10 | −45 | −91 | −8 |
| 834 | 127 | −80 | −10 | −45 | −84 | −8 | |
| Allura Red | 225 | 80 | −32 | −10 | −29 | −59 | −8 |
| 225 | 136 | −32 | −10 | −29 | −34 | −8 | |
| Amaranth | 279 | 206 | −32 | −10 | −30 | −30 | −8 |
| 279 | 221 | −32 | −10 | −30 | −22 | −8 |
CEP = collision cell entrance potential; CXP = collision cell exit potential; LC-MS/MS = liquid chromatography/tandem mass spectrometry.
2.6. Analytical performance parameters
Three replicates of chili powder and syrup-preserved fruit samples spiked with dyes at different concentrations—5, 10, and 50 mg/kg for Indigo Carmine, Sunset Yellow FCF, Tartrazine, Brilliant Blue FCF, Fast Green FCF, New Coccine, Erythrosine, Allura Red AC, and Amaranth (group A), and 10, 100, and 200 μg/kg for Sudan Red G, Sudan Red 7B, Dimethyl Yellow, Sudan II, Sudan Orange G, Sudan I, Para Red, Fast Garnet GBC, Sudan Red B, Sudan III, and Sudan IV (group B)— were extracted and detected as described above.
3. Results and discussion
The aim of this work was to develop and validate a simple and rapid method for the detection of dyes in foods.
3.1. LC-MS/MS determination and quantitation
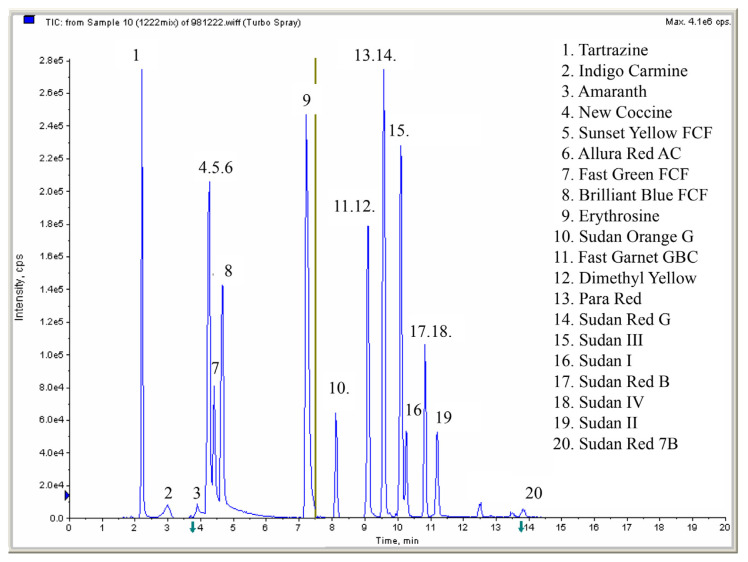
Each target analyte (10 μg/mL, in DMSO/acetate buffer solution/acetonitrile = 1:50:49, v/v/v) was tuned individually in order to obtain stable precursor and product ion abundance. These solutions were introduced into the mass spectrometer via a syringe pump at a flow rate of 5–20 μL/min. The analyte-dependent parameters, such as declustering potential, entrance potential, and collision energy, were optimized to increase sensitivity (summarized in Table 1). According to the European guidelines EC/657/2002[8],each analyte will earn 4 identification points(IPs) in this study based on the determination of one precursor (1 IP) and two product ions (1.5 × 2 = 3 IPs) using the LC-MS/MS technique. The developed mass spectrometric condition in this study met the EU confirmation requirement [23]. Satisfactory separation and response for all analytes were obtained under the gradient elution described above. The LC-MS/MS chromatograms of all dyes are shown in Fig. 2.
Fig. 2.
Liquid chromatography/tandem mass spectrometry (LC-MS/MS) chromatogram of 20 dyes.
3.2. Method validation
The selectivity of the LC method was investigated by observing the potential interferences between the analytes and impurities/food matrix in the sample extracts. Chili powder and syrup-preserved fruit samples spiked with various concentrations of dyes were analyzed, and the results showed that there was no significant interference observed at the corresponding retention time of each target analyte.
3.2.1. Linearity
Standard curves were made in triplicate for each concentration of all dyes. Good linearities were achieved at the concentrations of 0.1–200 ng/mL for Sudan Orange G, Dimethyl Yellow, Sudan I, Sudan II, Sudan Red G, Para Red, Sudan III, Sudan Red 7B, Sudan IV, Sudan Red B, Fast Garnet GBC (group A dyes), and 0.1–50 μg/mL for Fast Green FCF, Tartrazine, New Coccine, Indigo Carmine, Brilliant Blue FCF, Sunset Yellow FCF, Erythrosine, Allura Red AC, and Amaranth (group B dyes) (Table 2). The regression equation and determination of the coefficient (R2) of each dye are shown in Table 2. All R2 values exceeded 0.994, indicating good linearities.
Table 2.
Linear ranges, equations, and determination of coefficients (R2) of 20 dyes in chili powder and syrup-preserved fruit samples.
| Compound | Linear range | Chili powder | Raisins | ||
|---|---|---|---|---|---|
|
|
|
||||
| Linear equation | R 2 | Linear equation | R 2 | ||
| Sudan Orange G | 0.1–200 ng/mL | y = 314.460x + 449.8 | 0.9985 | y = 313.574x + 1190.2 | 0.9985 |
| Dimethyl Yellow | 0.1–200 ng/mL | y = 582.447x + 794.7 | 0.9965 | y = 813.147x + 855.1 | 0.9965 |
| Sudan I | 0.1–200 ng/mL | y = 290.223x + 129.3 | 0.9961 | y = 211.347x + 127.5 | 0.9961 |
| Sudan II | 0.1–200 ng/mL | y = 466.798x + 548.3 | 0.9956 | y = 430.291x + 711.5 | 0.9956 |
| Sudan Red G | 0.1–200 ng/mL | y = 633.668x + 1262.5 | 0.9986 | y = 614.833x + 802.5 | 0.9986 |
| Para Red | 0.1–200 ng/mL | y = 112.067x + 427.6 | 0.9985 | y = 1030.80x + 136.9 | 0.9985 |
| Sudan III | 0.1–200 ng/mL | y = 394.643x + 297.7 | 0.9968 | y = 335.834x + 376.1 | 0.9968 |
| Sudan Red 7B | 0.1–200 ng/mL | y = 205.369x + 540.8 | 0.9978 | y = 192.426x + 289.2 | 0.9978 |
| Sudan IV | 0.1–200 ng/mL | y = 637.006x + 318.4 | 0.9975 | y = 603.386x + 105.7 | 0.9975 |
| Sudan Red B | 0.1–200 ng/mL | y = 871.714x + 442.1 | 0.9943 | y = 861.649x + 531.0 | 0.9943 |
| Fast Garnet GBC | 0.1–200 ng/mL | y = 771.906x + 356.4 | 0.9979 | y = 680.185x + 289.2 | 0.9979 |
| Fast Green FCF | 0.1–50 μg/mL | y = 33,738x + 15,134 | 0.9972 | y = 35,358x + 19,933 | 0.9972 |
| Tartrazine | 0.1–50 μg/mL | y = 61,987x + 24,647 | 0.9991 | y = 63,975x + 25,357 | 0.9991 |
| New Coccine | 0.1–50 μg/mL | y = 12,869x + 20,973 | 0.9983 | y = 12,943x + 28,456 | 0.9983 |
| Indigo Carmine | 0.1–50 μg/mL | y = 23,832x + 45,322 | 0.9968 | y = 21,445x + 48,732 | 0.9968 |
| Brilliant Blue FCF | 0.1–50 μg/mL | y = 30,091x + 33,557 | 0.9954 | y = 29,630x + 31,787 | 0.9954 |
| Sunset Yellow FCF | 0.1–50 μg/mL | y = 12,162x + 44,064 | 0.9947 | y = 12,936x + 44,523 | 0.9947 |
| Erythrosine | 0.1–50 μg/mL | y = 79,847x + 16,843 | 0.9949 | y = 73,580x + 14,818 | 0.9949 |
| Allura Red AC | 0.1–50 μg/mL | y = 34,718x + 20,513 | 0.9987 | y = 36,704x + 20,688 | 0.9987 |
| Amaranth | 0.1–50 μg/mL | y = 14,927x + 37,365 | 0.9966 | y = 17,963x + 32,978 | 0.9966 |
3.2.2. Sample extraction procedure and recovery
Based on the chemical structures and polarities of all dyes, acetonitrile was chosen as an extraction solvent for chili powder and syrup-preserved fruit samples. Furthermore, acetonitrile offered other advantages such as good extraction yield, less fat solubility, precipitation of carbohydrates, and precipitation of proteins. Satisfactory recovery rates were obtained using this rapid and simple sample preparation procedure (Table 3).
Table 3.
Recoveries and coefficient of variation (CV, n = 3) of 20 dyes in spiked chili powder and syrup-preserved fruit samples.
| Compound | Spiked levels (μg/g) | Chili powder | Raisins | ||
|---|---|---|---|---|---|
|
|
|
||||
| Recoveries (%) | CV (%) | Recoveries (%) | CV (%) | ||
| Sudan Orange G | 0.01 | 99.9 | 2.2 | 100.9 | 2.9 |
| 0.1 | 99.7 | 3.2 | 99.8 | 2.1 | |
| 0.2 | 100.2 | 3.6 | 100.6 | 6.5 | |
| Dimethyl Yellow | 0.01 | 98.0 | 1.4 | 99.3 | 13.4 |
| 0.1 | 97.0 | 2.3 | 98.3 | 4.3 | |
| 0.2 | 98.4 | 11.8 | 98.3 | 7.1 | |
| Sudan I | 0.01 | 94.4 | 8.3 | 94.7 | 5.9 |
| 0.1 | 94.2 | 1.7 | 93.1 | 5.2 | |
| 0.2 | 94.7 | 2.3 | 93.3 | 3.0 | |
| Sudan II | 0.01 | 91.4 | 13.6 | 97.4 | 14.2 |
| 0.1 | 91.2 | 8.5 | 96.7 | 9.2 | |
| 0.2 | 91.9 | 9.5 | 97.0 | 8.8 | |
| Sudan Red G | 0.01 | 92.3 | 13.0 | 94.0 | 9.6 |
| 0.1 | 91.8 | 13.3 | 93.2 | 4.9 | |
| 0.2 | 92.7 | 6.8 | 93.8 | 4.6 | |
| Para Red | 0.01 | 91.2 | 15.2 | 100.5 | 7.3 |
| 0.1 | 91.1 | 13.5 | 99.4 | 2.4 | |
| 0.2 | 91.2 | 10.6 | 99.7 | 9.3 | |
| Sudan III | 0.01 | 92.2 | 11.6 | 94.5 | 4.6 |
| 0.1 | 92.0 | 12.0 | 93.1 | 12.1 | |
| 0.2 | 92.7 | 2.5 | 93.8 | 8.3 | |
| Sudan Red 7B | 0.01 | 100.0 | 3.4 | 95.2 | 8.2 |
| 0.1 | 99.9 | 12.8 | 94.2 | 13.4 | |
| 0.2 | 100.4 | 9.0 | 94.4 | 5.4 | |
| Sudan IV | 0.01 | 99.2 | 1.7 | 97.5 | 8.4 |
| 0.1 | 98.7 | 3.3 | 96.2 | 4.7 | |
| 0.2 | 99.3 | 8.8 | 96.8 | 4.8 | |
| Sudan Red B | 0.01 | 99.2 | 1.5 | 96.0 | 15.7 |
| 0.1 | 98.8 | 1.8 | 94.9 | 4.9 | |
| 0.2 | 99.6 | 7.1 | 95.6 | 1.5 | |
| Fast Garnet GBC | 0.01 | 95.3 | 8.3 | 92.4 | 6.1 |
| 0.1 | 94.4 | 1.1 | 91.3 | 10.9 | |
| 0.2 | 95.8 | 3.2 | 92.2 | 2.8 | |
| Fast Green FCF | 5 | 92.7 | 13.7 | 100.7 | 2.8 |
| 10 | 91.8 | 7.0 | 99.2 | 6.3 | |
| 50 | 93.2 | 4.9 | 99.4 | 7.8 | |
| Tartrazine | 5 | 91.9 | 13.9 | 96.1 | 3.4 |
| 10 | 91.2 | 13.1 | 94.9 | 5.4 | |
| 50 | 92.5 | 12.8 | 95.3 | 9.3 | |
| New Coccine | 5 | 99.2 | 1.2 | 98.4 | 9.4 |
| 10 | 98.8 | 14.2 | 97.7 | 2.3 | |
| 50 | 99.7 | 8.3 | 98.2 | 2.8 | |
| Indigo Carmine | 5 | 93.2 | 10.4 | 91.1 | 4.8 |
| 10 | 93.0 | 2.7 | 90.1 | 5.6 | |
| 50 | 93.4 | 8.6 | 90.7 | 7.9 | |
| Brilliant Blue FCF | 5 | 99.0 | 5.2 | 92.5 | 5.5 |
| 10 | 98.5 | 9.8 | 91.4 | 5.2 | |
| 50 | 99.2 | 5.5 | 91.6 | 4.4 | |
| Sunset Yellow FCF | 5 | 92.1 | 14.0 | 100.2 | 3.9 |
| 10 | 91.7 | 4.6 | 98.9 | 6.3 | |
| 50 | 92.5 | 5.8 | 99.4 | 4.5 | |
| Erythrosine | 5 | 95.1 | 6.6 | 91.7 | 6.8 |
| 10 | 95.0 | 13.2 | 90.6 | 3.7 | |
| 50 | 95.7 | 9.0 | 91.4 | 7.4 | |
| Allura red AC | 5 | 93.4 | 9.2 | 96.1 | 5.5 |
| 10 | 93.2 | 4.7 | 95.6 | 3.9 | |
| 50 | 93.7 | 8.8 | 95.9 | 6.4 | |
| Amaranth | 5 | 94.6 | 9.1 | 91.9 | 4.0 |
| 10 | 93.6 | 9.1 | 91.4 | 4.4 | |
| 50 | 95.2 | 3.8 | 92.1 | 8.3 | |
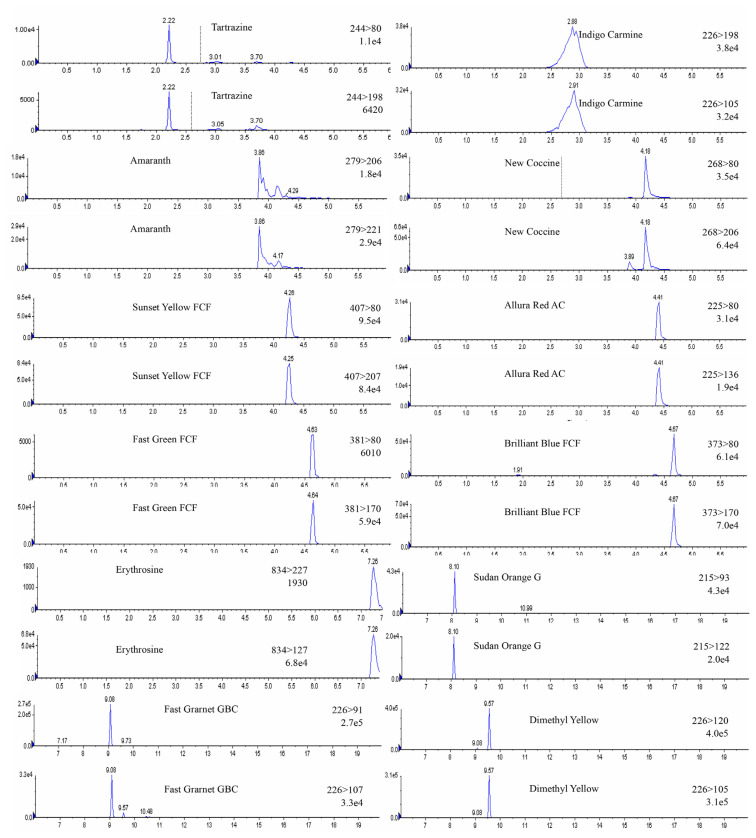
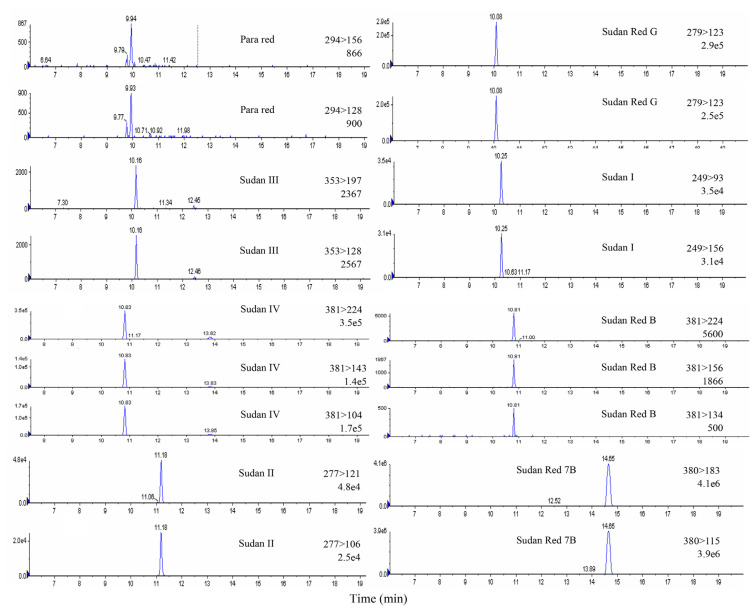
This LC-MS/MS method was validated in different matrices, including chili powder and syrup-preserved fruit. To determine the recovery rates of the developed method for 20 dyes, standard dye solutions in three different concentrations (0.01, 0.1, and 0.2 μg/g in group A dyes, and 5, 10, and 50 μg/g in group B dyes) were spiked individually into blank chili powder and syrup-preserved fruit samples in triplicate. The results showed that the recoveries ranged from 90.1% to 100.4 % for group A dyes, and from 91.1% to 100.9% for group B dyes (Table 3). The coefficient of variation of most data was below 10% and below 15.7% for some owing to the effects of interference in low-concentration samples. Overall, the recovery results were satisfactory. This developed LC-MS/MS method has many advantages over traditional TLC or HPLC methods [24] such as high sensitivity, no extra cleanup procedure, multidye analysis, and simultaneous quantitation and confirmation. In the traditional TLC method, 20 dyes required four developing conditions, and each took 60 minutes developing time. In the traditional HPLC method, 20 dyes required different LC conditions in order to obtain good separation, and each run took 1–2 hours to complete. In this work, the LC-MS/MS analysis time was significantly reduced from hours to 20 minutes. Moreover, the sample extraction time was shortened by a simple procedure and simultaneous treatment. The amount of solvent required in the analysis was also substantially decreased. This method, which offers rapid and high-throughput dye analysis, is suitable to be applied in routine tests for screening of banned dyes in foods (Fig. 3).
Fig. 3.
Multiple reaction monitoring (MRM) chromatograms of 20 dyes (10 μg/mL).
3.2.3. Limit of quantitation
The limit of quantitation (LOQ) in this study was determined by the sample concentration, which produced a peak area 10 times greater than noise. The LOQ was significantly improved by the LC-MS/MS method in comparison with the traditional HPLC. The obtained LOQ of dyes ranged from approximately 0.001 to 1 mg/kg (Table 4). Some dyes, such as Fast Green FCF, New Coccine, Erythrosine, Allura Red AC, and Amarath, had higher LOQs at 1 mg/kg because of their weak ionization.
Table 4.
Limit of quantitation (LOQ) of 20 dyes.
| Compound | LOQ (mg/kg) |
|---|---|
| Sudan Orange G | 0.01 |
| Dimethyl Yellow | 0.005 |
| Sudan I | 0.01 |
| Sudan II | 0.005 |
| Sudan Red G | 0.001 |
| Para Red | 0.01 |
| Sudan III | 0.025 |
| Sudan Red 7B | 0.001 |
| Sudan IV | 0.05 |
| Sudan Red B | 0.02 |
| Fast Garnet GBC | 0.01 |
| Fast Green FCF | 1 |
| Tartrazine | 0.5 |
| New Coccine | 1 |
| Indigo Carmine | 0.25 |
| Brilliant Blue FCF | 0.5 |
| Sunset Yellow FCF | 0.25 |
| Erythrosine | 1 |
| Allura Red AC | 1 |
| Amaranth | 1 |
3.3. Application to real samples
The developed method in this study was applied for the determination of 20 dyes in six commercial syrup-preserved fruit and 18 commercial chili powder products collected from supermarkets in Taipei City, Taiwan. The results are shown in Table 5. Tartrazine, Sunset Yellow, and New Coccine were detected in two, three, and two of six syrup-preserved fruit samples, respectively, and they are labeled on the packages. However, Amaranth, which is an illegal food dye in certain countries but declared illegal in Taiwan, was detected in one of six syrup-preserved fruit samples. This sample was imported and the use of Amaranth is not indicated on its package. No dyes were detected in 18 chili powder samples.
Table 5.
Quantitation results for dyes in positive chili powders analyzed by LC-MS/MS.
| Sample | Dyes | Concentration (mg/kg) |
|---|---|---|
| Syrup-preserved fruit (S1) | Tartrazine | 250.8 |
| Sunset Yellow | 22.8 | |
| Syrup-preserved fruit (S2) | Tartrazine | 597.8 |
| Sunset Yellow | 15.2 | |
| Syrup-preserved fruit (S3) | Amaranth | 169.5 |
| Sunset Yellow | 29.3 | |
| New Coccine | 214.6 | |
| Syrup-preserved fruit (S3) | New Coccine | 43.6 |
LC-MS/MS = liquid chromatography/tandem mass spectrometry.
4. Conclusion
Monitoring of synthetic dyes in foods is very important in both domestic and imported foods. This study presents a suitable analysis method for the extraction, detection, and quantitation of 20 dyes using LC-MS/MS in chili powder and syrup-preserved fruit products. The newly developed preparation procedure, through the extraction of acetonitrile, is rapid and simple and offers very good recovery and precise results. The 20-minute LC-MS/MS method under MRM mode is able to detect all target compounds in a single run with an LOQ between 0.001 and 1 mg/kg. Overall, the LC-MS/MS method can be applied in routine dye testing and surveillance programs for the control of the presence of dyes in chili powders and syrup-preserved fruits.
Acknowledgments
The authors disclosed that there is no conflict of financial interest, and are grateful to the Food and Drug Administration, Ministry of Health and Welfare of Taiwan for the financial support.
References
- 1. Ma M, Luo X, Chen B, Su S, Yao S. Simultaneous determination of water-soluble and fat-soluble synthetic colorants in foodstuff by high-performance liquid chromatography-diode array detection-electrospray mass spectrometry. J Chromatogr A. 2006;1103:170–6. doi: 10.1016/j.chroma.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2. Vachirapatama N, Mahajaroensiri J, Visessanguan W. Identification and determination of seven synthetic dyes in foodstuffs and soft drinks on monolithic C18 column by high performance liquid chromatography. J Food Drug Anal. 2008;16:77–82. [Google Scholar]
- 3.IARC monographs on the evaluation of the carcinogenic risk of chemicals to man. Vol. 8. Lyon: IARC; 1975. p. 125. [PubMed] [Google Scholar]
- 4. Abdelmigid HM. Risk assessment of food coloring agents on DNA damage using RAPD markers. Open Biotechnol J. 2009;3:96–102. [Google Scholar]
- 5. Das A, Mukherjee A. Genotoxicity testing of the food colours amaranth and tartrazine. Int Hum Genet. 2004;4:277–80. [Google Scholar]
- 6. Prival MJ, Davis VM, Peiperl MD, Bell SJ. Evolution of azo food dyes for mutagenicity and inhibition of mutagenicity by methods using S. typhimurium. Mutat Res. 1988;206:247–59. doi: 10.1016/0165-1218(88)90168-1. [DOI] [PubMed] [Google Scholar]
- 7.FAO/WHO. Specifications and toxicological evaluation of some food additives. Geneva: World Health Organization; 1975. (FAO Nutrition Meeting Report, Series 55A). [Google Scholar]
- 8. Garner RC, Nutman CA. Testing of some azo dyes and their reduction products for mutagenicity using Salmonella typhimurium TA 1538. Mutat Res. 1977;44:9–19. doi: 10.1016/0027-5107(77)90110-5. [DOI] [PubMed] [Google Scholar]
- 9.Ministry of Health and Welfare. Standards for specification, scope, application and limitation of food additives. Food and Drug Administration, Ministry of Health and Welfare MOHW Food no 1021351259; Amended. November 2013; [Google Scholar]
- 10. Oka H, Ikai Y, Ohno T, Kawamure N, Hayakawa J, Harada K, Suzuki M. Identification of unlawful food dyes by thin-layer chromatography-fast atom bombardment mass spectrometry. J Chromatogr A. 1994;674:301–7. doi: 10.1016/0021-9673(94)85235-9. [DOI] [PubMed] [Google Scholar]
- 11. Dugar SM, Leibowitz JN, Dyer RH. Synthetic colorant chromatography for beverages and wine. J Assoc Off Anal Chem. 1994;77:1335–7. [Google Scholar]
- 12. Hoodless RA, Pitman KG, Stewart TE, Thomson J, Amold JE. Separation and identification of food colours: I. Identification of synthetic water soluble food colours using thin-layer chromatography. J Chromatogr A. 1971;54:393–404. doi: 10.1016/s0021-9673(01)80295-8. [DOI] [PubMed] [Google Scholar]
- 13. Zalacain A, Ordoudi SA, Blázquez I, Diaz-plaza EM, Carmona M, Tsimidou MZ, Alons GL. Screening method for the detection of artificial colours in saffron using derivative UV–Vis spectrometry after precipitation of crocetin. Food Addit Contam. 2005;22:607–15. doi: 10.1080/02652030500150051. [DOI] [PubMed] [Google Scholar]
- 14. Üstün Özgür M. A rapid spectrophotometric method to resolve a binary mixture of food colorants (riboflavine and sunset yellow) Turk J Chem. 2004;28:325–33. [Google Scholar]
- 15. Daood HG, Biacs PA. Simultaneous determination of Sudan dyes and carotenoids in red pepper and tomato products by HPLC. J Chromatogr Sci. 2005;43:461–5. doi: 10.1093/chromsci/43.9.461. [DOI] [PubMed] [Google Scholar]
- 16. Huang HY, Chiu CW, Sue SL, Cheng CF. Analysis of food colorants by capillary electrophoresis with large-volume sample stacking. J Chromatogr A. 2003;995:29–36. doi: 10.1016/s0021-9673(03)00530-2. [DOI] [PubMed] [Google Scholar]
- 17. Calbiani F, Careri M, Elviri L, Mangia A, Pistara L, Zangnoni I. Development and in-house validation of a liquid chromatography–electrospray–tandem mass spectrometry method for the simultaneous determination of Sudan I, Sudan II, Sudan III and Sudan IV in hot chilli products. J Chromatogr A. 2004;1042:123–30. doi: 10.1016/j.chroma.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 18. Calbiani F, Careri M, Elviri L, Mangia A, Zagnoni I. Accurate mass measurements for the confirmation of Sudan azo-dyes in hot chilli products by capillary liquid chromatography–electrospray tandem quadrupole orthogonal-acceleration time of flight mass spectrometry. J Chromatogr A. 2004;1058:127–35. [PubMed] [Google Scholar]
- 19. Donna LD, Maiuolo L, Mazzotti F, Luca DD, Sindona G. Assay of Sudan I contamination of foodstuff by atmospheric pressure chemical ionization tandem mass spectrometry and isotope dilution. Anal Chem. 2004;76:5104–8. doi: 10.1021/ac0498821. [DOI] [PubMed] [Google Scholar]
- 20. Mazzetti M, Fascioli R, Mazzoncini I, Spinelli G, Morelli I, Bertoli A. Determination of 1-phenylazo-2-naphthol (Sudan I) in chilli powder and in chilli-containing food products by GPC clean-up and HPLC with LC/MS confirmation. Food Addit Contam. 2004;21:935–41. doi: 10.1080/02652030400007252. [DOI] [PubMed] [Google Scholar]
- 21. Reyns T, Fraselle S, Laza D, Loco JV. Rapid method for the confirmatory analysis of chrysoidine in aquaculture products by ultraperformance liquid chromatography–tandem mass spectrometry. Biomed Chromatogr. 2010;24:982–9. doi: 10.1002/bmc.1396. [DOI] [PubMed] [Google Scholar]
- 22. Wang X, Song G, Wu W, Zhao J, Hu Y. Determination of the food colorant, chrysoidine, in fish by GC-MS. Chromatographia. 2008;68:659–62. [Google Scholar]
- 23. Commission Decision 2002/65 7/EC. Implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off J Eur Comm No L221/8. 2002 [Google Scholar]
- 24.Ministry of Health and Welfare. Method of Test for Colors in Foods. Food and Drug Administration, Ministry of Health and Welfare, MOHW Food no 1021950329; Amended. September 2013; [Google Scholar]