Abstract
Melilotus albus Medic. and Dorycnium herbaceum Vill. (Fabaceae) acetone, ethyl acetate, and ethanol extracts were investigated for their in vitro antimicrobial, antibiofilm, and anti-oxidant activity with quantification of phenolic compound contents. In general, D. herbaceum extracts showed better antibacterial and antioxidant activity than M. albus extracts. Bacteria Bacillus subtilis, Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa, and Proteus mirabilis were the most susceptible with the minimum inhibitory concentrations (MICs), determined by microdilution method, between 1.25–10 mg/mL. Antifungal activity was lower with the detectable MICs at 10 mg/mL and 20 mg/mL. The plant extracts, using the crystal violet assay, inhibit P. aeruginosa biofilm formation in concentration range from 5 mg/mL to 20 mg/mL whereas the effect on mature bacterial biofilm was lower. The antioxidant activity was evaluated using 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radicals scavenging and reducing power model systems. The intensity of DPPH radicals scavenging activity, expressed as half maximal effective concentration (EC50) values, was from 84.33 μg/mL to >1000 μg/mL. The extracts demonstrated reduced power in a concentration-dependent manner, with ethanol extract as the most active. The total phenols, flavonoids, and proanthocyanidins were determined spectrophotometrically while total extractable tannins were obtained by precipitation method. The phenolic compounds showed differences in their total contents depending on solvents polarities and plant species. Although the plants M. albus and D. herbaceum have not yet been fully explored, these results contribute better understanding of their biotic properties and potential application as antimicrobial and antioxidant agents.
Keywords: antimicrobial activity, antioxidant activity, phenolic compounds, plant extracts
1. Introduction
A large number of aromatic, spicy, medicinal, and other plants contain chemical compounds exhibiting different biological and pharmacological activity. These compounds are products of plant secondary metabolism, and most often represent the response on negative biotic and abiotic environmental factors. The main bioactive secondary metabolites are terpenes, phenolics, and alkaloids. Numerous studies were carried out on biological and pharmacological activity of plants, such as antimicrobial, antioxidant, antitumor, anti-inflammatory, hypoglycemic, etc. [1–5]. However, scientific information on biological properties of various plants that are less widely used in culinary and medicine is still rather scarce. Therefore, the assessment of such properties remains an interesting and useful task, particularly for finding new sources for natural antimicrobial and antioxidant agents. Furthermore, there is growing interest in the development of “natural” labeled agents for food, medicine, pharmacy, cosmetic, and other applications.
Melilotus albus Medic. and Dorycnium herbaceum Vill. are plants belonging to the Fabaceae family. They are widely distributed in Europe and Asia. M. albus is an aromatic, herbaceous biennial plant with strong taproot, trifoliate leaves, and racemes of white flowers. It grows in full sun or partial shade, calcareous, loamy soils. M. albus is regarded as an important plant for honey production [6]. D. herbaceum is a perennial herbaceous plant or small shrub. The hairy leaves are composed of five segments. The white flowers are organized in terminal umbels. It prefers semiarid, sunny, and calcareous soils [6].
According to the literature data, biological properties as well as content and composition of bioactive compounds of M. albus and D. herbaceum have been poorly explored. It has been found that M. albus is rich in coumarins [7], which explains their use in traditional medicine as an anticoagulant agent and as ointments for external ulcers [8]. Also, the oleanan-type triterpene saponins have been isolated from the roots of M. albus [9]. D. herbaceum has been investigated for anti-Helicobacter pylori activity [10]. Previous phytochemical study has been performed for D. herbaceum from Greece [11]. This investigation led to the isolation and identification of phenylbutanone glucoside, flavonoids, cyanogenic glucoside, cyclitol, and hydroquinone glucoside.
Considering the fact that biological activities of M. albus and D. herbaceum have been insufficiently investigated and their therapeutic potential has not yet been fully explored, the aims of this study were to investigate and compare antimicrobial, antibiofilm, and antioxidant activity of acetone, ethyl acetate, and ethanol extract of wild-growing M. albus and D. herbaceum as well as to measure the contents of active compounds: total phenols, flavonoids, and extractable condensed tannins.
2. Methods
2.1. Plant material
The aerial parts of M. albus and D. herbaceum were collected on Mount Goc (Serbia) during the summer of 2010. Identification and classification of the plant material was performed at the Faculty of Science, University of Kragujevac, Kragujevac, Serbia. The voucher samples (200964, 200917) were deposited at the Herbarium of the Department of Biology and Ecology, Faculty of Science, University of Kragujevac. The collected plant materials were air-dried in darkness at ambient temperature.
2.2. Extraction
The dried, ground plant material was extracted by maceration with acetone, ethyl acetate, and ethanol. Briefly, 30 g of plant material was soaked with 150 mL of the solvent for 24 hours at room temperature. Next, the sample was filtered through filter paper. The residue from the filtration was extracted again, twice, using the same procedure. The filtrates obtained were combined and then evaporated to dryness using a rotary evaporator (IKA, Germany) at 40°C. The obtained extracts were stored in sterile sample tubes at −20°C.
2.3. Phytochemical analysis of plant extracts
2.3.1. Determination of total phenol content
The total phenol content of the extracts was quantified according to the Folin-Ciocalteu’s method as described by Wootton-Beard et al [12]. Gallic acid (Sigma Aldrich, St. Louis, USA) was used as the standard and the total phenolic content was expressed as milligram of gallic acid equivalents (GAE) per gram of extract (mg GAE/g of extract).
2.3.2. Determination of total flavonoid content
The total flavonoid content of the extracts was determined using the aluminium chloride method as described by Quettier-Deleu et al [13]. Rutin (Sigma Aldrich, St. Louis, USA) was used as the standard and the concentrations of flavonoids were expressed as milligram of rutin equivalents (RUE) per gram of extract (mg of RUE/g of extract).
2.3.3. Determination of total extractable tannin content
Total extractable tannin (TET) content was estimated indirectly by spectrophotometric measurement of the absorbance of the solution obtained after the precipitation of the tannins with polyvinylpolypyrrolidone (PVPP; Sigma Aldrich, St. Louis, USA) as described by Makkar et al [14]. The TET was expressed as milligram of gallic acid equivalents (GAE) per gram of extract (mg GAE/g of extract).
2.3.4. Determination of proanthocyanidin content
The proanthocyanidin content was measured by the butanol-HCl method with ferric ammonium sulfate as a catalyst as described by Porter et al [15]. Cyanidin chloride (Sigma Aldrich, St. Louis, USA) was used as the standard and the proanthocyanidin content was expressed as milligrams of cyanidin chloride equivalents (CChE) per gram of extract (mg CChE/g of extract).
2.4. Determination of antioxidant activity
2.4.1. DPPH radicals scavenging capacity assay
The ability of M. albus and D. herbaceum extracts to scavenge DPPH free radicals was assessed using the method described by Takao et al [16]. The tested concentrations of plant extracts were from 15.62 μg/mL to 1000 μg/mL. Diluted solutions of extract (2 mL each) were mixed with 2 mL of DPPH methanolic solution (40 μg/mL). Ascorbic acid (Sigma Aldrich, St. Louis, USA) was used as a reference compound. Radical scavenging activity is expressed as EC50 value. The EC50 value is the effective concentration at which 50% of DPPH radicals were scavenged. It was obtained from the nonlinear graph of scavenging activity (%) versus concentration of samples. Low EC50 value indicates strong ability of the extract to act as DPPH scavenger.
2.4.2. Reducing power
The reducing power of the plant extracts was determined according to the method of Oyaizu [17]. The tested concentrations of plant extracts were from 15.62 μg/mL to 1000 μg/mL. The absorbance of the reaction mixture was measured at 700 nm. Increased absorbance of the reaction mixture indicated increased reducing power. Ascorbic acid was used as a reference compound.
2.5. Determination of antimicrobial activity
2.5.1. Test microorganisms
Antimicrobial activity was tested against 10 strains of bacteria (Bacillus subtilis PMFKgB18, Klebsiella pneumoniae PMFKgB13, Staphylococcus aureus PMFKgB12, S. aureus ATCC 25923, Enterococcus faecalis PMFKgB11, Pseudomonas aeruginosa PMFKgB15, P. aeruginosa ATCC 27853, Proteus mirabilis PMFKgB10, Escherichia coli PMFKgB14, E. coli ATCC 25922), and five strains of fungi (Candida albicans PMFKgF2, C. albicans ATCC 10231, Aspergillus niger PMFKgF7, Penicillium italicum PMFKgF8, Penicillium digitatum PMFKgF9, Penicillium verrucosum PMFKgF10). All bacterial isolates were a generous gift from the Institute of Public Health, Kragujevac. The other microorganisms (the fungi and the ATCC strains) were provided from a collection held by the Microbiology Laboratory, Faculty of Science, University of Kragujevac. The bacterial strains were kept in glycerol stock at −80°C and the fungal strains paraffin oil stock at 4°C. For experiments, the bacterial strains were grown on Nutrient agar (Liofilchem, Roseto, Italy) at 37°C for 18 hours whereas Potato dextrose agar (Liofilchem, Roseto, Italy) at 28°C for 4 days was used for fungi.
2.5.2. Microdilution method
Antimicrobial activity was tested by determining the minimum inhibitory concentration (MIC) using microdilution method with resazurin [18]. Twofold serial dilutions of the plant extracts were made in sterile 96-well microtiter plates containing 0.1 mL of Mueller-Hinton broth (Liofilchem) per well for bacteria and 0.1 mL of Sabouraud dextrose broth (Liofilchem) per well for fungi. The tested concentration range was from 0.156 mg/mL to 20 mg/mL. The microtiter plates were inoculated with the suspensions to give a final concentration of 5 × 105 colony forming units (CFU)/mL for bacteria and 5 × 103 CFU/mL for fungi. The growth of the bacteria and the yeasts was monitored by adding resazurin, an indicator of microbial growth. Resazurin is a blue nonfluorescent dye that becomes pink and fluorescent when reduced to resorufin by oxidoreductases within viable cells. The inoculated microtiter plates were incubated at 37°C for 24 hours for bacteria, at 28°C for 48 hours for yeasts, and at 28°C for 72 hours for molds. MIC was defined as the lowest concentration of tested plant extracts that prevented resazurin color change from blue to pink. For molds, MIC values of the tested plant extracts were determined as the lowest concentration that inhibited visible mycelia growth.
Cephalexin (Galenika, Belgrade, Serbia) and fluconazole (Pfizer Inc., New York, USA), dissolved in nutrient liquid medium, were used as reference compounds. Stock solutions of crude extracts were obtained by dissolving in 10% dimethylsulfoxide (DMSO), which was used as a control. Each test included growth control and sterility control. All tests were performed in duplicate and MICs were constant.
2.6. Determination of antibiofilm activity
2.6.1. Biofilm production assay
The bacteria chosen for antibiofilm assay were: Pseudomonas aeruginosa ATCC 27853 and P. aeruginosa (clinical isolate). The ability of bacteria to in vitro adhere to the abiotic surface was checked by the crystal violet staining assay [19]. The biofilm biomass was quantified by measuring the intensity of crystal violet at optical density (OD)630 nm using a microplate reader. All tests were performed in triplicate. The cutoff optical density (ODc) was defined as three standard deviations above the mean OD of the negative control (culture medium), and bacterial strains were classified as nonadherent (OD ≤ ODc), weakly adherent (ODc < OD ≤ 2 × ODc), moderately adherent (2 × ODc < OD ≤ 4 × ODc), or strongly adherent (OD > 4 × ODc) [19].
2.6.2. Effect on biofilm formation
The effect of M. albus and D. herbaceum extracts on biofilm formation was evaluated as described by Nostro et al [20] with some modifications. Twofold serial dilutions of the plant extracts were made in sterile 96-well tissue culture microtitre plates containing 0.1 mL of Mueller–Hinton broth per well. The tested concentration range was from 0.156 mg/mL to 20 mg/mL. In addition, 10 μL of fresh bacterial suspension (1.0 McFarland) was added to each well. Growth control wells (cells + broth) and negative controls (only broth) were included. The inoculated plates were incubated at 37°C for 24 hours. After incubation, the biofilm biomass was assayed using the crystal violet staining assay [14]. Biofilm positive was considered to be those wells of which the OD630 was higher than the ODc. The results were expressed as biofilm inhibitory concentration (BIC).
2.6.3. Effect on established biofilms
The biofilms of tested bacterial strains were initially allowed to develop in sterile 96-well tissue microtiter plates during 24 hours of incubation at 37°C. The effects of plant extracts on established biofilms was assayed using the crystal violet staining assay as described previously. The tested concentration range was from 0.156 mg/mL to 20 mg/mL.
2.7. Statistical analysis
The experiments, except for antimicrobial activity, were performed in triplicate. One-way analysis of variance (ANOVA) and Pearson correlation coefficients was done using SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL, USA). Statistically significant difference was defined as p < 0.05.
3. Results
3.1. Phytochemical analysis
The total phenol contents and the contents of various classes of polyphenols in M. albus and D. herbaceum extracts are presented in Table 1. The phenolic compounds showed differences in their total contents depending on solvents polarities and plant species. D. herbaceum extracts contained higher level of phenolic compounds than M. albus extracts.
Table 1.
Total phenolic, flavonoid, and tannin contents and DPPH scavenging activity expressed as EC50 values.
| Plant extracts | Total phenolic content (mg GAE/g) | Total flavonoid content (mg RUE/g) | Total condensed tannin content | EC50 (μg/mL) | |
|---|---|---|---|---|---|
|
| |||||
| PVPP method (mg GAE/g) | Butanol-HCl method (mg CChE/g) | ||||
| Dorycnium herbaceum | |||||
| Acetone | 31.34 ± 0.10a | 231.75 ± 1.23a | 29.68 ± 0.83a | 0.44 ± 0.14a | 255.67 ± 3.05a |
| Ethyl acetate | 50.33 ± 0.27b | 207.61 ± 1.12b | 26.81 ± 0.03a | 5.13 ± 0.46b | 550.67 ± 9.02b |
| Ethanol | 75.77 ± 0.42c | 110.07 ± 0.58c | 65.99 ± 0.85b | 4.91 ± 0.04b | 84.33 ± 1.15c |
| Melilotus albus | |||||
| Acetone | 28.80 ± 0.38a | 132.76 ± 0.41a | 9.06 ± 0.72a | 2.24 ± 0.32a | >1000 |
| Ethyl acetate | 27.97 ± 0.37a | 74.17 ± 0.16b | 13.89 ± 0.05b | 1.16 ± 0.27a | >1000 |
| Ethanol | 14.80 ± 0.41b | 36.96 ± 0.11c | 6.41 ± 0.40a | n.d. | >1000 |
| Ascorbic acid | 5.23 ± 0.23 | ||||
Data are presented as mean ± standard deviation. Means with different letters in the same column indicate significant (p < 0.05) differences among the solvents used for both plants separately.
CChE = cyanidin chloride equivalents; DPPH = 2,2-diphenyl-1-picrylhydrazyl; EC50 = effective concentration at which 50% of DPPH radicals were scavenged; GAE = gallic acid equivalents; n.d. = not detected; PVPP = polyvinylpolypyrrolidone; RUE = rutin equivalents.
The highest content of total phenolics was found in ethanol extract of D. herbaceum (75.77 mg GAE/g) followed by ethyl acetate extract (50.33 mg GAE/g) and acetone extract (31.34 mg GAE/g). The content of total phenolics in M. albus extracts was lower; the results varied from 14.80 mg GAE/g (ethanol extract) to 28.80 mg GAE/g (acetone extract).
It is evident from the analysis that D. herbaceum and M. albus are rich in flavonoids. The total flavonoid content was ranged from 110.07 mg RUE/g to 231.75 mg RUE/g in extracts of D. herbaceum and from 36.96 mg RUE/g to 132.76 mg RUE/g in M. albus extracts. Acetone extracts of both plant species contained the highest levels of flavonoids.
The content of condensed tannins was higher in D. herbaceum extracts than in M. albus extracts. D. herbaceum ethanol extract and M. albus ethyl acetate extract had the highest level of tannins, 65.99 mg GAE/g and 13.89 mg GAE/g, respectively. The concentrations of proanthocyanidins were up to 5.13 mg CChE/g. In D. herbaceum ethyl acetate extract, the highest content of proanthocyanidins was measured. According to literature data this is the first report of total condensed tannin contents from these plants.
3.2. Antioxidant activity
3.2.1. DPPH radical scavenging activity
The DPPH radicals scavenging activity demonstrate the effect of D. herbaceum and M. albus extracts as antioxidants through their hydrogen donating ability, which reduces the stable violet DPPH radical to the yellow DPPH-H. A high percentage of radical scavenging indicated a strong antioxidant activity in the tested sample. The extracts showed concentration-dependent antiradical activity. Furthermore, the extracts of D. herbaceum were more active than M. albus extracts (p = 0.000). The EC50 values for tested plant extracts were in the range from 84.33 to >1000 μg/mL and they were higher than the EC50 obtained for ascorbic acid (5.23 μg/mL) used as reference compound (Table 1). The ethanol extract of D. herbaceum had the lowest EC50 value (84.33 μg/mL) and due to high phenolic content its high antioxidant activity was expected.
Whereas the phenolic compounds contribute significantly to the antioxidant capacity of plants, a linear correlation, determined by Pearson correlation coefficient (r), between total phenolic, flavonoid, tannin, and proanthocyanidin content and DPPH radical scavenging activity was observed (r = 0.84, 0.65, 0.88, and 0.64, respectively).
3.2.2. Reducing power
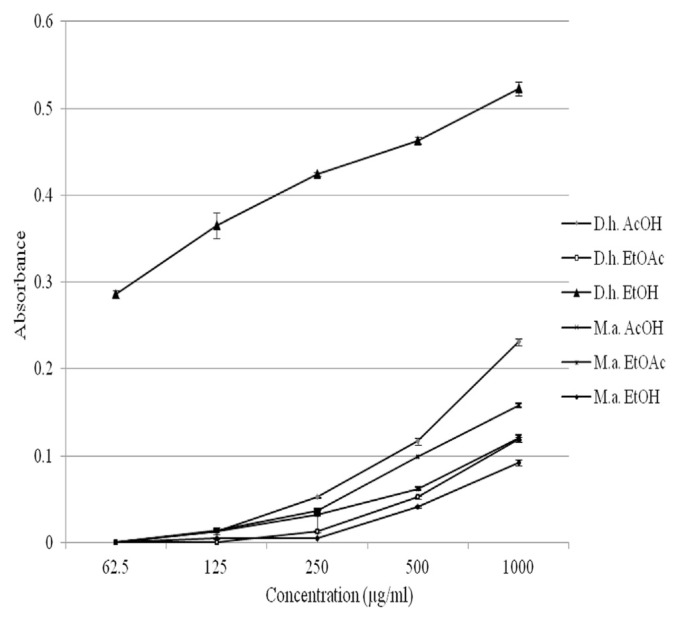
The reducing power of plant extracts is related to their electron transfer ability and may serve as a significant indicator of potential antioxidant activity. As shown in Fig. 1, all the extracts demonstrated reducing power in concentration-dependant manner. The activity of D. herbaceum extracts was not significantly higher than that of M. albus extracts (p = 0.234). The extracts showed activity with absorbance values from 0.004 to 0.523. For comparison, the absorbance value for reference compound, ascorbic acid, was 2.943. The strongest activity was exhibited by the ethanol extract of D. herbaceum; the extract was two or more times superior in regard to the other tested extracts.
Fig. 1.
Reducing power of D. herbaceum and M. albus extracts. AcOH = acetone extract; D. h. = Dorycnium herbaceum; EtOAc = ethyl acetate extract; EtOH = ethanol extract; M.a = Melilotus albus.
The correlation between phenolic compounds contents and reducing power showed linear correlation in relation to the total phenolic, tannin, and proanthocyanidin content (r = 0.84, 0.94, and 0.50, respectively) and no significant correlation in relation to the total flavonoid content (r = 0.08).
3.3. Antibacterial and antifungal activity
In vitro antibacterial and antifungal activity of acetone, ethyl acetate, and ethanol extracts of D. herbaceum and M. albus was tested against a panel of microorganisms including human pathogenic bacteria, yeasts, and molds in order to evaluate broad-spectrum antimicrobial activity (Tables 2 and 3). It was observed that 10% DMSO did not inhibit the growth of microorganisms. The antibacterial activity of D. herbaceum extracts was statistically significant stronger than activity of M. albus extracts (p = 0.01), whereas antifungal activities were similar. In addition, the tested plants exhibit better antibacterial then antifungal activity (p = 0.001).
Table 2.
Antibacterial activity of Dorycnium herbaceum and Melilotus albus extracts.
| Species | Dorycnium herbaceum | Melilotus albus | S (μg/mL) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Acetone | Ethyl acetate | Ethanol | Acetone | Ethyl acetate | Ethanol | ||
|
| |||||||
| MIC (mg/mL) | |||||||
| Bacillus subtilis | 1.25 | 1.25 | 5 | 1.25 | 2.5 | 2.5 | 12.5 |
| Klebsiella pneumoniae | 20 | 20 | 10 | 10 | >20 | >20 | 500 |
| Staphylococcus aureus | 20 | 20 | 20 | 10 | >20 | >20 | 1.56 |
| Enterococcus faecalis | 20 | 20 | 20 | 10 | >20 | >20 | 3.12 |
| Pseudomonas aeruginosa | 5 | 10 | 10 | 10 | 20 | >20 | >1000 |
| Proteus mirabilis | 5 | 10 | 5 | 10 | 20 | >20 | >1000 |
| Escherichia coli | 20 | >20 | 20 | >20 | >20 | >20 | 1.56 |
| E. coli a | 10 | 20 | 20 | 10 | 20 | >20 | 6.25 |
| S. aureus b | 1.25 | 1.25 | 2.5 | 1.25 | 2.5 | 2.5 | 6.25 |
| P. aeruginosa c | 10 | 20 | 10 | 10 | 20 | 20 | >1000 |
MIC = minimum inhibitory concentration; S = standard (cephalexin).
E. coli ATCC 25922.
S. aureus ATCC 25923.
P. aeruginosa ATCC 27853.
Table 3.
Antifungal activity of Dorycnium herbaceum and Melilotus albus extracts.
| Species | Dorycnium herbaceum | Melilotus albus | Fluconazole (μg/mL) | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Acetone | Ethyl acetate | Ethanol | Acetone | Ethyl acetate | Ethanol | ||
|
| |||||||
| MIC (mg/mL) | |||||||
| Candida albicans | >20 | >20 | >20 | 20 | >20 | >20 | 62.5 |
| C. albicans a | 20 | 20 | 20 | >20 | >20 | 20 | 31.25 |
| Aspergillus niger | >20 | >20 | >20 | 20 | 20 | 20 | 500 |
| Penicillium italicum | >20 | 20 | 20 | 10 | 10 | 20 | 31.25 |
| Penicillium digitatum | >20 | 20 | 20 | 10 | 10 | 20 | 31.25 |
MIC = minimum inhibitory concentration.
C. albicans ATCC 1023.
Among the D. herbaceum extracts, no statistically significant difference in activity was noticed (p < 0.05). The extracts acted in the interval from 1.25 mg/mL to >20 mg/mL against tested bacteria. The most significant results were obtained for Bacillus subtilis, S. aureus ATCC 25923, P. aeruginosa, and P. mirabilis. For these bacteria, the MIC values were between 1.25 mg/mL and 10 mg/mL. Other bacteria showed sensitivity at approximately the same concentrations (20 mg/mL). The exception was E. coli, which was nonsusceptible to ethyl acetate extract. In addition, the extracts exhibited low antifungal activity on tested yeasts and molds species.
With respect to effectiveness of M. albus extracts, the most active one was acetone extract, followed by ethyl acetate and ethanol extract against tested bacteria. The statistically significant difference in activity among acetone and ethanol extract was observed (p = 0.018). Action interval of extracts was from 1.25 mg/mL to 20 mg/mL. In most of the cases ethanol extract did not act at tested concentrations. If we take into consideration a low content of determined phenolic compounds, a low antibacterial activity of ethanol extract was expected. B. subtilis, S. aureus ATCC 25923 and P. aeruginosa ATCC 27853 showed sensitivity according to tested extracts where B. subtilis and S. aureus ATCC 25923 were the most sensitive (MIC = 1.25 mg/mL and 2.5 mg/mL, respectively). The growth of E. coli was not inhibited by the extracts, whereas Klebsiella pneumoniae, S. aureus and E. faecalis were non-susceptible to the ethanol and ethyl acetate extract. The growth of P. aeruginosa, P. mirabilis, and E. coli ATCC 25922 was not affected by the ethanol extract. M. albus extracts exhibited low or no antifungal activity on tested yeasts and molds species. The MIC values were in the range from 10 mg/mL to >20 mg/mL.
3.4. Antibiofilm activity
In order to find a natural compound able to inhibit and prevent bacterial biofilm formation, we tested the effect of the acetone, ethyl acetate, and ethanol extracts of D. herbaceum and M. albus on two P. aeruginosa biofilm positive strains. The examined strains were proven to create moderate biofilm with OD values of 0.72 and 0.83 in extract free conditions, and with the presence of extracts, the possibility of adherence was changed. As presented in Table 4, D. herbaceum and M. albus extracts demonstrated better inhibitory effects on biofilm formation than in disturbing the mature biofilm (p = 0.0002). The effects of the extracts on biofilm formation expressed as BIC showed that inhibitory concentrations of M. albus extracts were 5 mg/mL, whereas for D. herbaceum extracts they were between 5 mg/mL and 20 mg/mL. The extracts exhibited low activity on mature P. aeruginosa biofilms (BIC = 20 mg/mL, >20 mg/mL).
Table 4.
Antibiofilm activity of Dorycnium herbaceum and Melilotus albus extracts.
| Plant extract | Effect on biofilm formation | Effect on established biofilm | ||
|---|---|---|---|---|
|
| ||||
| BIC (mg/mL) | ||||
|
| ||||
| Pseudomonas aeruginosa | P. aeruginosa ATCC 27853 | P. aeruginosa | P. aeruginosa ATCC 27853 | |
| D. herbaceum | ||||
| Acetone | 10 | 5 | >20 | 20 |
| Ethyl acetate | 20 | 5 | 20 | 5 |
| Ethanol | 10 | 10 | >20 | 20 |
| M. albus | ||||
| Acetone | 5 | 5 | >20 | >20 |
| Ethyl acetate | 5 | 5 | 20 | >20 |
| Ethanol | 5 | 5 | 20 | 5 |
BIC = biofilm inhibitory concentration.
4. Discussion
Since the biological activities of D. herbaceum and M. albus extracts have been insufficiently investigated in the current study, antioxidant, antimicrobial, and antibiofilm activity have been analyzed for the first time.
Biological activities of plants including antioxidant and antimicrobial properties could be attributed to different classes of phenolic compounds [21,22] and therefore phytochemical analysis of the tested plant extracts was done. The results indicated that D. herbaceum extracts contained higher level of phenolic compounds than M. albus extracts. In addition, the quantitative estimation showed that the tested extracts, in contrast to tannin content, are rich in total phenols and total flavonoids. Furthermore, the plants are a significant source of flavonoids. Kazantzoglou et al [11] also analyzed the chemical constituents of the aerial parts of D. herbaceum from Greece and the five flavonoids were isolated: myricitrin, quercitrin, kaempferol 3-O-β-glucopyranoside, kaempferol 3-O-(6″-acetyl)-β-glucopyranoside, (+)-dihydromyricetin. Moreover, a new compound, dorycnioside [4-(4′-O-β-d-glucopyranosyl-3′, 5′-dimethoxyphenyl)-2-butanone] was isolated and identified as well as two known phenylbutanone glucosides [(−)-catechin, β-sitosterol], one cyanogenic glucoside (lotaus-tralin), one cyclitol (d-pinitol), and one hydroquinone glucoside (tachioside). According to literature data the phytochemical analysis of M. albus extracts was scarce, and this is the first report of phenolic compound contents.
4.1. Antioxidant activity
It has been reported that phenolic compounds possess the ideal chemistry for antioxidant activity because they have high reactivity as hydrogen or electron donors and also they are capable of chelating metal ions. In addition, the synergism between the individual phenolic compounds in the mixture makes the antioxidant activity not only dependant on the concentration, but also on the structure and the interaction between the compounds [23]. The antioxidant activity could be evaluated using different methods, among which total antioxidant activity, reducing power, DPPH assay, and metal chelating assay are most commonly used for testing of anti-oxidant activities of plant extracts [24]. In this study, the free radicals scavenging ability and the reducing capacity of different D. herbaceum and M. albus extracts were determined. The antioxidant activity increased in a concentration-dependent manner. The ethanol extract of D. herbaceum was the most active, and the activity was several times higher than the other tested extracts. It is suggested that the phenolic compounds may contribute directly to the antioxidant activity due to their hydroxyl groups [25]. In this study, a positive relationship between the contents of phenolic compounds and antioxidant activity of the tested extracts was found. The results obtained are in good agreement with the literature data where the authors confirmed correlation between anti-oxidant activity and content of phenolic compounds [25–27].
4.2. Antibacterial and antifungal activity
Ten bacterial species and five fungal species were used to screen the possible antimicrobial activity of D. herbaceum and M. albus extracts. The intensity of antimicrobial activity varied depending on: (1) plant species, (2) the type of plant extract, and (3) the species of microorganisms. It was observed that tested plants exhibited better antibacterial than antifungal activity and antibacterial activity of D. herbaceum extracts was higher. Among the tested extracts, ethanol extracts exhibited lower or equal antimicrobial activity in relation to other tested extracts. The noticeable results were that the extracts inhibited the growth of gram-negative bacteria and fungi at the highest tested concentrations. This lack of activity against tested microorganisms, in case of M. albus extracts, also, has been observed by the other research groups. Aćimović-Djoković et al [28] tested antibacterial activity of petrol ether and ethyl acetate extract of Melilotus officinale, Melilotus albus, and Melitis melissophyllum, using disk-diffusion method, in relation to E. coli, P. mirabilis, Salmonella enteritidis, P. aeruginosa, Streptococcushaemoliticus A, S. aureus, and Candida albicans. M. albus extracts were less efficient than other tested plants. Karakaş et al [29] also noticed low antibacterial activity of M. albus water, ethanol, and methanol extract originate from Turkey. By contrast, the extracts exhibited good or moderate activity against gram-positive bacteria. The effect of D. herbaceum extracts on medicinal important human pathogenic bacteria was presented for the first time in this study. Previously, a group of scientists tested anti-Helicobacter pilory effect of medicinal plants of Greek traditional medicine, among which was D. herbaceum, but it was not active [10].
4.3. Antibiofilm activity
The bacteria have the ability to form biofilms. Biofilms are defined as a surface attached community of bacteria embedded in an organic polymer matrix of bacterial origin. They are involved in major problems associated with the food industry, medicine, and everyday life. The risk becomes even more serious because bacteria within biofilms have been shown to have a decreased susceptibility to antimicrobial agents compared with those in the planktonic form [30]. In this study, for the first time, the antibiofilm activity of D. herbaceum and M. albus extracts on two P. aeruginosa biofilm positive strains was investigated. Tested extracts showed better efficacy in preventing of biofilm formation than in disturbing the mature biofilm. Moreover, P. aeruginosa within mature biofilms showed lower susceptible than that in planktonic growth approving the findings that bacteria in biofilm are more resistant to antimicrobial agents than free-living cells. The success of plant extracts in inhibiting bacterial biofilm formation has been documented [31–33]. This could be a promising tool for reducing microbial colonization of surfaces and epithelial mucosa which subsequently leads to infections; therefore, it is interesting to carry out investigations on both sessile and planktonic cells to ensure that plant extracts possess a broader inhibitory activity.
5. Conclusion
In vitro antimicrobial, antibiofilm, and antioxidant activity of acetone, ethyl acetate, and ethanol extracts of M. albus and D. herbaceum with quantification of the total phenols, flavonoids, and tannins were determined. The plants possessed marked antibacterial activity against B. subtilis, S. aureus ATCC 25923, P. Aeruginosa, and P. mirabilis. Moreover, the extracts were able to inhibit and prevent P. aeruginosa biofilm formation. Furthermore, the extracts were rich in phenolic compounds and all the extracts were found to possess DPPH radicals scavenging activity and reducing power. Great potential as antioxidant agent was shown by D. herbaceum ethanol extract. Antioxidant activity correlated well with the content of phenolic compounds, which suggests an important role of these compounds in overall antioxidant activity of investigated plants. Biological and pharmacological potential of M. albus and D. herbaceum has not yet been fully explored so the results obtained contribute to a better understanding of their biotic properties and potential application as antimicrobial and antioxidant agents.
Acknowledgments
This investigation was supported by the Ministry of Education and Science of the Republic of Serbia, Grant Numbers 41010 and 173032.
Funding Statement
This investigation was supported by the Ministry of Education and Science of the Republic of Serbia, Grant Numbers 41010 and 173032.
Footnotes
Conflicts of interest
The authors declare that they have no competing interests.
REFERENCES
- 1. Gyawali R, Salam AI. Natural products as antimicrobial agents. Food Control. 2014;46:412–29. [Google Scholar]
- 2. Krishnaiah D, Sarbatly R, Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod Process. 2011;89:217–33. [Google Scholar]
- 3. Luo Y, Cai Q, Sun M, Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004;74:2157–84. doi: 10.1016/j.lfs.2003.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumar S, Bajwa BS, Singh K, Kalia AN. Anti – inflammatory activity of herbal plants: a review. Int J Adv Pharm Biol Chem. 2013;2:272–81. [Google Scholar]
- 5. Atta-Ur-Rahman, Zaman K. Medicinal plants with hypoglycemic activity. J Etnopharmacol. 1989;26:1–55. doi: 10.1016/0378-8741(89)90112-8. [DOI] [PubMed] [Google Scholar]
- 6.Josifovic M. Flora of SR Serbia. Belgrade: Serbian Academy of Sciences and Arts; 1972. [Google Scholar]
- 7. Stoker JR. The biosynthesis of coumarin in Melilotus alba. Biochem Biophys Res Commun. 1963;14:17–20. doi: 10.1016/0006-291x(63)90203-1. [DOI] [PubMed] [Google Scholar]
- 8.Saric M, editor. Medicinal plants of SR Serbia. Belgrade: Serbian Academy of Sciences and Arts; 1989. [Google Scholar]
- 9. Khodakov GV, Akimov YA, Shashkov AS, Kintia PK, Grishkovets VI. Triterpene and steroid saponins isolated from two Melilotus species. Adv Exp Med Biol. 1996;405:211–22. doi: 10.1007/978-1-4613-0413-5_18. [DOI] [PubMed] [Google Scholar]
- 10. Stamatis G, Kyriazopoulos P, Golegou S, Basayiannis A, Skaltsa S, Skaltsa H. In vitro anti-Helicobacter pylori activity of Greek herbal medicines. J Ethnopharmacol. 2003;88:175–9. doi: 10.1016/s0378-8741(03)00217-4. [DOI] [PubMed] [Google Scholar]
- 11. Kazantzoglou G, Magiatis P, Panoutsopoulos G, Skaltsounis AL. Dorycnioside, a new phenylbutanone glucoside from Dorycnium pentaphyllum subsp. herbaceum. Z Natuforschung B. 2004;59c:23–6. doi: 10.1515/znc-2004-1-205. [DOI] [PubMed] [Google Scholar]
- 12. Wootton-Beard PC, Moran A, Ryan L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by FRAP, DPPH, ABTS and Folin-Ciocalteu methods. Food Res Int. 2011;44:217–24. [Google Scholar]
- 13. Quettier-Deleu C, Gressier B, Vasseur J, Dine T, Brunet C, Luyckx M, Cazin M, Cazin JC, Bailleul F, Trotin F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J Ethnopharmacol. 2000;72:35–42. doi: 10.1016/s0378-8741(00)00196-3. [DOI] [PubMed] [Google Scholar]
- 14. Makkar HPS, Blummel M, Borowy NK, Becker K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J Sci Food Agr. 1993;61:161–5. [Google Scholar]
- 15. Porter LJ, Hrstich LN, Chan BG. The conversion of proanthocyanidins and delphinidins to cyaniding and delphinidin. Phytochem. 1986;25:223–30. [Google Scholar]
- 16. Takao T, Kitatani F, Watanabe N, Yagi A, Sakata K. A simple screening method for antioxidants and isolation of several antioxidants produced by marine bacteria from fish and shellfish. Biosci Biotechnol Biochem. 1994;58:1780–3. [Google Scholar]
- 17. Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr. 1986;44:307–15. [Google Scholar]
- 18. Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42:321–4. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stepanović S, Vuković D, Dakić I, Savić B, Švabić-Vlahović M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods. 2000;40:175–9. doi: 10.1016/s0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 20. Nostro A, Roccaro AS, Bisignano G, Marino A, Cannatelli MA, Pizzimenti FC, Cioni PL, Procopio F, Blanco AR. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Med Microbiol. 2007;56:519–23. doi: 10.1099/jmm.0.46804-0. [DOI] [PubMed] [Google Scholar]
- 21. Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12:564–82. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stevenson DE, Hurst RD. Polyphenolic phytochemicals – just antioxidants or much more? Cell Mol Life Sci. 2007;64:2900–16. doi: 10.1007/s00018-007-7237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rice-Evans CA, Miller NJ, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–9. [Google Scholar]
- 24. Gülçin İ, Küfrevioǧlu Öİ, Oktay M, Büyükokuroǧlu ME. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.) J Ethnopharmacol. 2004;90:205–15. doi: 10.1016/j.jep.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 25. Hatano T, Edamatsu R, Mori A, Fujita Y, Yasuhara E. Effect of interaction of tannins with co-existing substances. VI. Effects of tannins and related polyphenols on superoxide anion radical and on DPPH radical. Chem Pharm Bull. 1989;37:2016–21. [Google Scholar]
- 26. Trigui M, Hsouna AB, Tounsi S, Jaoua S. Chemical composition and evaluation of antioxidant and antimicrobial activities of Tunisian Thymelaea hirsuta with special reference to its mode of action. Ind Crop Prod. 2013;4:150–7. [Google Scholar]
- 27. Boudjou S, Oomah BD, Zaidi F, Hosseinian F. Phenolics content and antioxidant and anti-inflammatory activities of legume fractions. Food Chem. 2013;138:1543–50. doi: 10.1016/j.foodchem.2012.11.108. [DOI] [PubMed] [Google Scholar]
- 28. Aćamović-Djoković G, Djukić D, Mandić L, Kalinić S, Bošković T. Antimicrobial activity of the petrol-ether and ethyl-acetate extracts of Melilotus officinalis (L.) Pall, Melilotus albus Medic. and Melitis melissophyllum L. Lekovite sirovine. 2002;22:59–63. [In Serbian, English abstract] [Google Scholar]
- 29. Karakaş FP, Yildirim A, Türker A. Biological screening of various medicinal plant extracts for antibacterial and antitumor activities. Turk J Biol. 2012;36:641–52. [Google Scholar]
- 30. Gilbert P, Mcbain AJ, Rickard AH. Formation of microbial biofilm in hygienic situations: a problem of control. Int Biodeter Biodegr. 2003;51:245–8. [Google Scholar]
- 31. Chaieb K, Kouidhi B, Jrah H, Mahdouani K, Bakhrouf A. Antibacterial activity of thymoquinone, an active principle of Nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complement Altern Med. 2011;11:29–35. doi: 10.1186/1472-6882-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sandasi M, Leonard CM, Viljoen AM. The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes. Lett Appl Microbiol. 2009;50:30–5. doi: 10.1111/j.1472-765X.2009.02747.x. [DOI] [PubMed] [Google Scholar]
- 33. Sandasi M, Leonard CM, Van Vuuren SF, Viljoen AM. Peppermint (Mentha piperita) inhibits microbial biofilms in vitro. S Afr J Bot. 2011;77:80–5. [Google Scholar]