Abstract
Four isoflavonoids were isolated from stems of Pueraria lobata (Willd.) Ohwi growing in Democratic People’s Republic of Korea and identified as daidzein (1), genistin (2), daidzin (3), and puerarin (4), structures, which were elucidated by means of spectroscopic analysis. Isoflavonoids were isolated using silica gel chromatography and purified with organic solvents. Isoflavonoid contents in P. lobata were determined using reliable high-performance liquid chromatography. The results indicated that the contents of puerarin and genistin in the roots are higher than those in the stems (6.19% and 0.04% vs. 1.15% and 0.02%), whereas the stems have higher contents of daidzin and daidzein than the roots (3.17% and 0.06% vs. 1.72% and 0.05%). Accordingly, the root part of the plant is useful for the isolation of puerarin and the stem part for daidzin. This study suggests that the stem of P. lobata is useful as an alternative source of puerarin, daidzin, genistin, and daidzein. In addition, collection of the stem will not sacrifice the plant and thus is beneficial to the natural ecosystems.
Keywords: daidzin, determination, isoflavonoid, Pueraria lobata, puerarin
1. Introduction
Kudzu [Pueraria lobata (Willd.) Ohwi; Fabaceae] is a medicinal plant used in traditional herbal medicines grown for its roots, similar to Pueraria thomsonii Benth. grown in China, which also has a long history as a medicinal herb [1]. Native Asians such as Koreans, Chinese, and Japanese used the roots both as a health food and as a traditional medicine for relieving fever, treatment of diabetes mellitus, etc.
Kudzu extracts have demonstrated significant estrogen-like activity [2], and antiangiogenic [3,4] and antitumor activities [5]. In particular, kudzu has antioxidant activities similar to other herbal medicines including flavonoids [6–8]. The components isolated from kudzu include isoflavonoids, polysaccharides, saponins, alkaloids, etc. Among them, isoflavonoids include puerarin, daidzein, daidzin, genistein, coumestrol, and formononetin [9,10], which are known to have some important biological activities (Fig. 1). Epidemiologic studies have also shown that the dietary intake of isoflavonoids is associated with some properties beneficial to human health. Isoflavonoids present in the human diet are mainly derived from soybean-based foods [11]. The dietary isoflavonoids, genistein and daidzein, have estrogen-like activity and are classified as phytoestrogens [12]. Because the presence of genistein and daidzein in human urine was related to lower mortality from sex hormone-dependent cancers, genistein and daidzein have excited scientific researchers [13]. Genistein and daidzein may be partly responsible for the ability of soybeans to lower the risk of cardiovascular diseases and prevent bone mineral loss in ovariectomized rats [14]. Puerarin, the C-glycoside of daidzein, has been reported to stimulate the proliferation and differentiation of cultured osteoblasts [15]. It also showed positive action on the prevention of coronary heart disease [16–18]. Puerarin and the other isoflavonoids mentioned are responsible for the antioxidant activity of kudzu, and their contents are highest in the outer bark of the roots in this plant [19]. Puerarin is a unique isoflavonoid C-glycoside isolated and identified from herbal material available in nature. It is present only in the Pueraria species.
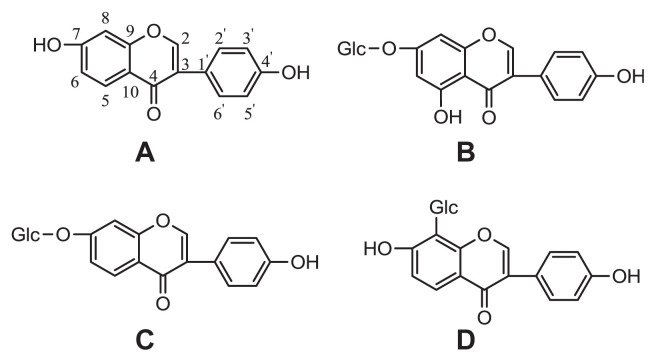
Fig. 1.
Structures of the main isoflavonoids in stems of Pueraria lobata: (A) daidzein; (B) genistin; (C) daidzin; (D) puerarin.
Bulk supply of genistein and daidzein from soybeans is difficult because their contents in this source are low. Much higher mounts of these isoflavonoids exist in Kudzu [20,21]. The biological and pharmacological effects of kudzu can mainly be attributed to puerarin and its derivatives, including 3-methoxypuerarin and puerarin xyloside. The root is rich in puerarin, however, collection of this part of the plant in bulk will damage the wild ecosystems. Therefore, finding new alternative natural sources containing daidzein and puerarin is very important [22]. In order to make use of P. lobata economically and efficiently, evaluation of isoflavonoid contents in other parts of this plant is necessary. Thus, the aim of this current study was to isolate and identify isoflavonoids from stems, and quantitate their contents in stems and roots at different stages of development of P. lobata, growing in Democratic People’s Republic (DPR) of Korea.
2. Methods
2.1. Chemicals and reagents
For chromatographic and chemical analyses, high-performance liquid chromatography (HPLC)-grade methanol was purchased from Merck Co. (Darmstadt, Germany) Ethanol, methanol, chloroform, and benzene, all of analytical grade, were obtained from Pecking Chemical Reagents (Beijing, China). Isoflavonoid standards, daidzin, and puerarin used for assay were purchased from Sigma Chemical Co. (St. Louis, MO, USA) Genistin, puerarin, daidzin, and daidzein (purity >97%) standards were purchased from the National Regulatory Authority, the Ministry of Public Health, DPR of Korea, and used as standards for thin-layer chromatography (TLC) analysis and assay.
2.2. Plant materials
Stems and roots of P. lobata were collected from different locations of DPR of Korea. They were identified by Gwan-Sim Mun, academician candidate, professor, and PhD, working in the Institute of Pharmaceutics, Academy of Medicine Sciences, DPR of Korea. All materials were dried at temperatures <60°C. A reference herbarium was deposited at the National Regulatory Authority, The Ministry of Public Health, DPR of Korea.
2.3. Instruments and general experimental procedures
Melting points were measured using a Yanaco micromelting apparatus (Model I-300). UV data were obtained on a Shimadzu UV3000 spectrophotometer (Beijing, China). A UR-20 spectrophotometer was used for scanning the IR spectra of KBr pellets. Nuclear magnetic resonance (NMR) spectra were recorded on an AM-400 spectrometer (Bruker Avance DRX, Switzerland). Chemical shifts (δ) are expressed in ppm with reference to the solvent (tetramethylsilane, TMS) signals. EI-MS (electrospray ionization-mass spectrum) were measured on a JMS-DX 300 spectrometer. Column chromatography was performed with silica gel (Qingdao Marine Chemical Factory (Qingdao, China)). Fractions were monitored using TLC (Silica gel GF 254; Qingdao Marine Chemical Factory), and spots were visualized using iodine vapor.
2.4. HPLC analysis
2.4.1. Instruments and conditions
HPLC analysis of isoflavonoids was carried out on a Shim-pack VP-ODS (4.6 mm × 250 mm, 5 μm (Shimadzu, Kyoto, Japan)) column coupled to a Shimadzu HPLC system (Model LC 2010HT), under the following conditions: column temperature 30°C, injection volume 10 μL, and detection wavelength 250 nm. Elution was performed at a flow rate of 0.8 mL/min with the following solvent system: methanol–water (gradient elution; 0–10 minutes 20:80, 10–15 minutes 25:75, 15–20 minutes 40:60, 20–22 minutes 45:55, 22–25 minutes 55:45, 25–30 minutes 65:35, 30–35 minutes 72:28, and 35–40 minutes 20:80). All samples were determined under chromatographic conditions described above, and the contents of isoflavonoid compounds were calculated using the method of external standard calibration (Fig. 2).
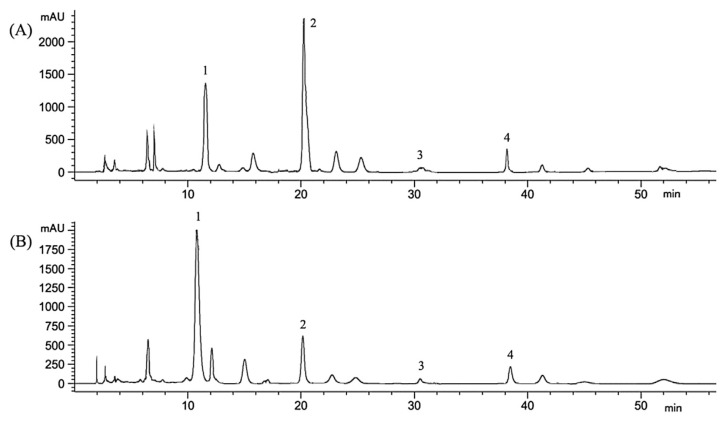
Fig. 2.
HPLC chromatograms of isoflavonoids in (A) stems and (B) roots of Pueraria lobata; 1 = puerarin (11.7 minutes); 2 = daidzin (20.4 minutes); 3 = genistin (30.9 minutes); and 4 = daidzein (38.3 minutes). HPLC = high-performance liquid chromatography.
2.4.2. Preparation of standard for HPLC
Puerarin (10 mg), daidzin (8 mg), daidzein (2 mg), and genistin (1 mg) were accurately weighed and placed in 25-mL flasks, which were filled with methanol to the scale. To the respective 10-mL flasks, 0.1 mL, 0.5 mL, 1.0 mL, 2.0 mL, and 5.0 mL of the solution were added, and they were filled with 30% ethanol to the scale. During HPLC analysis, 10 μL of these final standard solutions were injected and their peak areas measured at 250 nm.
2.4.3. HPLC sample preparation
Each samples dried at temperatures <60°C was accurately weighed (approximately 1 g) and extracted by 50 mL of 80% ethanol and ultrasonic treatment in succession for 30 minutes. The sample solutions were filtered and cooled. The filtered solutions were filtered using a Millipore Mille-HV 13 filter (MA, USA) (0.45 μm) prior to injection.
2.5. Extraction of isoflavonoids from stems
Stems of P. lobate, 800 g collected outside of Pyongyang, DPR of Korea, were extracted by refluxing with 6 L of methanol for 5 hours. Under warm conditions, the extraction solution was filtered and residues were treated twice with 5 L of methanol for 3 hours. After the evaporation of methanol, 85 g of brown extracts (moisture 11%) were obtained. The extracts were extracted with 100 mL of benzene, kept at room temperature for a while, and then filtered. This procedure was repeated five times. Benzene extracts appeared blue black, and benzene-insoluble residues consisted of moist reddish-brown powder. The residues were extracted with 3 L of water in a water bath for 1 hour, and then filtered. The filtrates were extracted eight times with 400 mL of water-saturated butanol to obtain 25 g of reddish brown extracts.
2.6. Isolation of individual isoflavonoids
Butanol extracts were dissolved in methanol, absorbed in 60 g of silica gel for use in column chromatography, and dried. The sample was filled in the column (diameter 5 cm, length 60 cm), which was previously filled with 300 g of the same silica gel. The column was desorbed with chloroform–methanol (9:1, 8:2, and 7:3), receiving 30 mL of fractions one by one. Each fraction was subjected to TLC, and fractions with the same Rf value were collected and evaporated under reduced pressure. Fraction 3, including substances with different Rf values, was further fractionated on a silica gel column with chloroform–methanol–water (9:1:0.1) to give three subfractions (3-1, 3-2, 3-3). Fraction 2 was evaporated, purified with methanol–water (1:1) solution, and then dissolved in warm methanol. It was further purified with charcoal to obtain 0.6 g of compound 1. Subfraction 3-1 was evaporated and purified with water and methanol to give 0.2 g of compound 2. Subfraction 3-2 was evaporated and purified with water and methanol, further purified with charcoal to give 8.4 g of compound 3. Because subfraction 3-3 and fraction 4 had the same Rf values, they were combined, evaporated, and purified with chloroform–methanol (1:4) solution. It was further purified with acetic acid to yield 3.6 g of compound 4 (Fig. 1).
2.7. Isolates
Daidzein (1, purity >95%): white needles; mp 320–321°C; UV(MeOH)λmax 251 nm, 304 nm; IR(KBr)υmax 3200/cm, 1635/cm, 1590/cm, 1510/cm, 1458/cm, 1260/cm, 1100/cm, 1080/cm, 1040/cm; 1H NMR (DMSO-d6, 400 MHz) δ 8.28 (S, H-2), 7.97 (d, J = 8.9 Hz, H-5), 6.81 (d, J = 8.9 Hz, H-6), 6.94 (d, J = 8.2 Hz, H-8), 7.38 (2H, d, J = 8.8 Hz, H-2′, 6′), 6.88 (2H, d, J = 8.8 Hz, H-3′, 5′); 13C NMR (DMSO-d6, 100 MHz) δ 152.812 (C-2), 123.498 (C-3), 174.708 (C-4), 127.302 (C-5), 114.955 (C-6), 162.304 (C-7), 102.038 (C-8), 157.419 (C-9), 116.684 (C-10), 122.584 (C-1′), 130.080 (C-2′), 114.955 (C-3′), 157.024 (C-4′), 114.955 (C-5′), 130.080 (C-6′); EI-MS m/z 254[M+] (calculated for C15H10O4, 254).
Genistin (2, purity >95%): white needles; mp 254–256°C; UV(MeOH)λmax 263 nm, 330 nm; IR(KBr)υmax 3450/cm, 1660/cm, 1620/cm, 1580/cm, 1450/cm, 1260/cm, 1095/cm, 1045/cm; 1H NMR (DMSO-d6, 400 MHz) δ 8.38 (S, H-2), 7.13 (d, J = 2.4 Hz, H-6), 7.07 (d, J = 2.4 Hz, H-8), 7.39 (2H, d, J = 8.5 Hz, H-2′, 6′), 6.81 (2H, d, J = 8.5 Hz, H-3′, 5′), 5.09 (1H, d, J = 7.2 Hz, H-1″), 3.16–3.72 (6H, m, H-2–7″); 13C NMR (DMSO-d6, 100 MHz) δ 153.338 (C-2), 123.709 (C-3), 174.765 (C-4), 126.972 (C-5), 114.991 (C-6), 161.399 (C-7), 103.392 (C-8), 157.257 (C-9), 118.473 (C-10), 122.354 (C-1′), 130.099 (C-2′), 114.991 (C-3′), 157.040 (C-4′), 114.991 (C-5′), 130.099 (C-6′), 99.984 (C-1″), 77.179 (C-2″), 76.328 (C-3″), 73.013 (C-4″), 69.513 (C-5″), 60.535 (C-6″).
Daidzin (3, purity >95%): white needles; mp 235–236°C; UV(MeOH)λmax 254 nm, 305 nm; IR(KBr)υmax 3400/cm, 1630/cm, 1570/cm, 1520/cm, 1450/cm, 1250/cm, 1100/cm, 1050/cm, 1030/cm; 1H NMR (DMSO-d6, 400 MHz) δ 8.38 (S, H-2), 8.03 (d, J = 8.9 Hz, H-5), 7.14 (d, J = 2.4 Hz, H-6), 7.22 (d, J = 2.4 Hz, H-8), 7.39 (2H, d, J = 8.4 Hz, H-2′, 6′), 6.80 (2H, d, J = 8.4 Hz, H-3′, 5′), 5.09 (1H, d, J = 7.2 Hz, H-1″), 3.16–3.68 (6H, m, H-2–7‴); 13C NMR (DMSO-d6, 100 MHz) δ 153.333 (C-2), 123.710 (C-3), 174.765 (C-4), 126.969 (C-5), 115.595 (C-6), 161.400 (C-7), 103.392 (C-8), 157.037 (C-9), 118.476 (C-10), 122.341 (C-1′), 130.095 (C-2′), 114.937 (C-3′), 157.037 (C-4′), 114.937 (C-5′), 130.095 (C-6′), 99.986 (C-1″), 77.179 (C-2″), 76.358 (C-3″), 73.057 (C-4″), 69.562 (C-5″), 60.575 (C-6″).
Puerarin (4, purity >95%): white needles; mp 186–187°C; UV(MeOH)λmax 251 nm, 308 nm; IR(KBr)υmax 3370/cm, 1630/cm, 1580/cm, 1510/cm, 1445/cm, 1260/cm, 1100/cm, 1080/cm, 1040/cm; 1H NMR (DMSO-d6, 400 MHz) δ 8.34 (S, H-2), 7.93 (d, J = 8.8 Hz, H-5), 6.98 (d, J = 8.8 Hz, H-6), 7.38 (2H, d, J = 8.6 Hz, H-2′, 6′), 6.79 (2H, d, J = 8.6 Hz, H-3′, 5′), 5.01 (1H, d, J = 7.2 Hz, H-1″), 3.18–3.71 (6H, m, H-2–7″); 13C NMR (DMSO-d6, 100 MHz) δ 152.886 (C-2), 123.103 (C-3), 174.845 (C-4), 126.281 (C-5), 114.990 (C-6), 161.011 (C-7), 112.642 (C-8), 157.185 (C-9), 116.883 (C-10), 122.556 (C-1′), 130.051 (C-2′), 114.990 (C-3′), 157.185 (C-4′), 114.990 (C-5′), 130.051 (C-6′), 112.642 (C-1″), 81.809 (C-2″), 78.711 (C-3″), 73.439 (C-4″), 70.753 (C-5″), 61.403 (C-6″).
3. Results and discussion
3.1. Methodology validation
Simultaneous determination of isoflavonoids in Pueraria species was tried by many researchers [23–25]. HPLC with an appropriate mobile phase has been efficiently employed for simultaneous determination of flavonoids to evaluate the quality of herbal materials [26,27].
For determination of individual isoflavonoids in herbal material, the simultaneous determination condition of puerarin, daidzin, daidzein, and genistin using HPLC was detected. Sample solution was injected into an HPLC apparatus, and then eluted by eight stages that constituted the gradient ratio. On the basis of the abovementioned condition, peaks of substances could be separated and satisfactory results could be obtained.
For the calibration curve, a series of the mixture of puerarin, daidzin, daidzein, and genistin standards were injected in an HPLC apparatus and the peak areas were measured. Using micrograms of standard (X) as the horizontal axis and the peak area (Y) as the vertical axis, linear correlations were obtained. The regression equations were established using six levels. The results are described in Table 1. The mixture (10 μL) of puerarin, daidzin, daidzein, and genistin standards was injected into an HPLC apparatus, and the peak areas were measured; this procedure was repeated five times for the precision of determination. The results showed that the relative standard deviations of puerarin, daidzin, daidzein, and genistin were 0.87%, 1.13%, 1.26%, and 1.17%, respectively.
Table 1.
Peak areas of standards according to injected amounts.
| Standards | Test range (μg) | Equation | R 2 |
|---|---|---|---|
| Puerarin | 0.4–20 | Y = 148.91X + 15.964 | 0.9999 |
| Daidzin | 0.32–16 | Y = 160.78X + 23.63 | 0.9998 |
| Genistin | 0.04–2 | Y = 341.12X – 4.0496 | 0.9996 |
| Daidzein | 0.08–4 | Y = 177.43X + 1.675 | 0.9999 |
The abovementioned mixture of standards was stored at room temperature for 12 hours, and its contents of puerarin, daidzin, daidzein, and genistin were measured at 2-hour intervals. The results showed that there was no difference between the measured values within 12 hours, that is, the mixture of standard was stable for 12 hours.
Recovery tests were performed by adding puerarin, daidzin, daidzein, and genistin standards to a known sample whose puerarin, daidzin, daidzein, and genistin contents had already been determined. Recovery of components ranged from 99.25% to 110.38%, and relative standard deviations of puerarin, daidzin, daidzein, and genistin were 2.80%, 1.26%, 1.39%, and 2.27%, respectively.
3.2. Sample analysis
Stems were collected at Mt Hanam, Yangdok County, South Pyongan Province, DPR of Korea, in September, cut into 2–5-mm pieces, dried in the shade until the loss of its weight was <8%, and determined by HPLC (Table 2). According to the age in years and thickness, the contents of puerarin, daidzin, genistin, and daidzein in stems increased. The content of puerarin was higher in small parts of the stems that are 1–4 years old, however, the content of daidzin was higher than that of puerarin in 5-year-old parts. Contents of the other two isoflavonoids were detected as trace amounts in 1-year-old stems, however, their levels increased during developmental stages in ≥ 2-year-old stems.
Table 2.
Contents of isoflavonoids according to the growing years and parts of Pueraria lobata.
| Year | Thickness (mm) | Contents of isoflavonoids (%) | |||
|---|---|---|---|---|---|
|
| |||||
| Daidzein | Genistin | Daidzin | Puerarin | ||
| 1 | 4–5 | Trace | Trace | 0.09 | 0.14 |
| 5–6 | Trace | Trace | 0.36 | 0.66 | |
| 7–8 | 0.01 | Trace | 0.5 | 0.72 | |
| 10–12 | 0.02 | Trace | 0.7 | 1.05 | |
| 15–20 | 0.04 | 0.01 | 0.93 | 1.45 | |
| 2 | 20–25 | 0.07 | 0.02 | 1.22 | 1.86 |
| 3 | 25–30 | 0.1 | 0.03 | 1.70 | 2.15 |
| 4 | 30–35 | 0.17 | 0.04 | 2.00 | 2.34 |
| 5 | 35–50 | 0.14 | 0.03 | 3.17 | 2.40 |
To investigate the dynamics of isoflavonoid contents in stems during the growing period of P. lobata, samples were collected from Mt Taesong, Pyongyang, DPR of Korea, and processed using the same method as mentioned above (Table 3). Contents of individual isoflavonoid compounds increased with the growth of stems, as their biosynthesis by the plant increased; their contents were maximal in the period of appearance of fruit, yellowing of leaves, and maturing of fruits. Contents of individual isoflavonoid compounds were higher in stems collected from August to October. The optimal collection periods of roots as materials for manufacturing isoflavonoids were October, November, and January [28].
Table 3.
Contents of isoflavonoids at different developmental stages of Pueraria lobata stems.
| Year | Month of collection | Appearance | Contents of isoflavonoids (%) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Daidzein | Genistin | Daidzin | Puerarin | |||
| 1 | May | Appearance of leaves and stem | Trace | Trace | 0.03 | 0.12 |
| July | Vigorous growth of leaves and stem | 0.01 | Trace | 0.14 | 0.39 | |
| August | Appearance of flowers | 0.02 | Trace | 0.31 | 0.72 | |
| September | Appearance of fruits | 0.02 | Trace | 0.66 | 1.25 | |
| October | Yellowing of leaves and maturing of fruits | 0.02 | Trace | 0.73 | 1.19 | |
| 2 | January | — | 0.03 | 0.01 | 0.72 | 1.11 |
| March | — | 0.05 | 0.02 | 0.75 | 1.13 | |
| May | Growth of leaves and stem | 0.06 | 0.02 | 0.8 | 1.15 | |
| July | Vigorous growth of leaves and stem | 0.05 | 0.02 | 0.93 | 1.32 | |
| August | Appearance of flowers | 0.08 | 0.03 | 1.13 | 1.72 | |
| September | Appearance of fruits | 0.07 | 0.03 | 1.1 | 1.96 | |
| October | Yellowing of leaves and maturing of fruits | 0.09 | 0.03 | 1.31 | 2.13 | |
| 3 | January | — | 0.07 | 0.02 | 1.27 | 2.05 |
| March | — | 0.08 | 0.03 | 1.28 | 2.07 | |
| May | Growth of leaves and stem | 0.09 | 0.03 | 1.32 | 2.12 | |
| July | Vigorous growth of leaves and stem | 0.1 | 0.04 | 1.37 | 2.19 | |
| August | Appearance of flowers | 0.09 | 0.03 | 1.54 | 2.22 | |
| September | Appearance of fruits | 0.09 | 0.03 | 1.66 | 2.23 | |
| October | Yellowing of leaves and maturing of fruits | 0.12 | 0.04 | 1.69 | 2.23 | |
One- to 2–year-old stems were collected from several regions of DPR of Korea, and the collected samples were investigated using the same method (Table 4). Samples were collected from north, middle, and south regions of DPR of Korea, and contents of isoflavonoids were comparatively detected. There was no significant difference in contents of individual isoflavonoid compounds according to the growing place of stems.
Table 4.
Contents of isoflavonoids in stems of Pueraria lobata at different locations.
| Places of collection, (date) | Thickness (mm), (age, y) | Contents of isoflavonoids (%) | |||
|---|---|---|---|---|---|
|
| |||||
| Daidzein | Genistin | Daidzin | Puerarin | ||
| Ryongsong Dist. Pyongyang (September) | 20–25 (2) | 0.05 | 0.02 | 1.32 | 1.72 |
| Yangdok County, South Pyongan Prov. (September) | 20–25 (2) | 0.06 | 0.02 | 1.22 | 1.57 |
| Koksan County, North Huanghae Prov. (September) | 20–25 (2) | 0.03 | 0.01 | 1.49 | 1.90 |
| Unsan County, South Pyongan Prov. (September) | 8–13 (1) | 0.01 | Trace | 0.59 | 1.10 |
| Koksan County, North Huanghae Prov. (September) | 8–13 (1) | 0.01 | Trace | 0.55 | 1.17 |
| Sariwon City, North Huanghae Prov. (September) | 8–13 (1) | 0.02 | Trace | 0.23 | 1.30 |
Dist. = district; Prov. = province.
To determine the drying processes of stems, samples were dried under different conditions, and then the contents of the isoflavonoids were determined (Table 5). Drying conditions were as follows: dried in shade—spread at places at temperatures 20–25°C, relative humidity 55–65%; dried under sunlight—dried under sunlight from morning to early evening and moved inside rooms in the evening; dried in a drier—spread in a drier at temperatures 100–105°C. Moisture content of raw samples was 52%. There was a significant difference in the contents of individual isoflavonoid compounds according to the drying conditions, however, considering economic benefits, it is better to dry under sunlight.
Table 5.
Contents of isoflavonoids in stems of Pueraria lobata under different drying conditions.
| Dry conditions | Time to reach moisture content below 8% | Contents of isoflavonoids (%) | |||
|---|---|---|---|---|---|
|
| |||||
| Daidzein | Genistin | Daidzin | Puerarin | ||
| Raw | — | 0.01 | Trace | 1.19 | 1.83 |
| Drier | 4 h | 0.01 | Trace | 1.13 | 1.81 |
| Sunlight | 3 d | 0.02 | Trace | 1.01 | 1.59 |
| Shade | 6 d | 0.05 | Trace | 0.9 | 1.43 |
Contents of isoflavonoids in different parts of P. lobata were determined (Table 6). The content of puerarin was higher in roots, but lower than that of daidzin in stems. That is, the content of daidzin in stems was two times higher than that in roots. Significant variances were not observed in contents of the other two isoflavonoid compounds. The contents of glycosides in roots and stems were much higher than those of aglycone, the reason for which can be attributed to a novel isoflavone 7-O-glucosyltransferase PlUGT1 isolated from P. lobata. It could convert daidzein to daidzin, genistein to genistin, as well as formononetin to ononin [29].
Table 6.
Isoflavonoid contents in different parts of Pueraria lobata.
| Parts | Contents of isoflavonoids (%) | |||
|---|---|---|---|---|
|
| ||||
| Daidzein | Genistin | Daidzin | Puerarin | |
| Stem | 0.06 | 0.02 | 3.17 | 1.15 |
| Root | 0.05 | 0.04 | 1.72 | 6.19 |
The results suggest that the composition of isoflavonoids in stems was not different from that in roots, and the contents of isoflavonoids in stems were lower than those in roots; however, the amount of stems that can be obtained from a plant is two or three times that of roots, and therefore, stems can be used as a herbal material for manufacturing isoflavonoids.
4. Conclusion
Four isoflavonoids were isolated from the stems of P. lobata growing in DPR of Korea using silica gel column chromatography, and identified by 1H NMR, 13C NMR, UV, infrared spectrum, and mass spectrum spectroscopy. Existence of isoflavonoids in stems could be fundamental in the search for new herbal materials.
For assay of isoflavonoid contents, a much simpler HPLC method was developed and validated. The developed method provided good separation of the homologs, and was linear, precise, stable, and repeatable. The method was verified and found to be suitable for quantifying puerarin, daidzin, genistin, and daidzein in different samples.
Roots are the most important materials for extracting puerarin, which exhibits potential preventive activity against coronary heart disease. Recently, many researchers have paid attention to the miraculous pharmacological activities of puerarin [30]. However, continuous collection of roots resulted in the exhaustion of natural sources and severe breakdown of the environment. Hence, it is very important to discover the dynamics of the contents of puerarin, daidzin, genistin, and daidzein in stems at different stages of development of P. lobata, to elucidate the optimal collection period.
The probability of collecting a higher amount of isoflavonoid compounds, including puerarin, daidzin, genistin, and daidzein, from the stems of one whole plant of P. lobata indicates that it could be an alternative source of preparation of isoflavonoid compounds to roots, in which the content of daidzin is much lower. The results of this study offer new ideas for comprehensive utilization of P. lobata resources with great social and economic benefits. Moreover, we have also demonstrated the dynamics of the contents of puerarin, daidzin, genistin, and daidzein in stems according to the growing period of P. lobata and elucidated the optimal collection period for it to be useful as a natural resource for manufacturing isoflavonoid compounds, including puerarin, daidzin, genistin, and daidzein.
Acknowledgments
The corresponding author appreciates Professor and Dr Gwan-Sim Mun’s effort for identification of herbal materials.
Footnotes
Conflicts of interest
All authors declare that no conflicts of interest exist regarding this study.
REFERENCES
- 1. Chen YG, Song YL, Wang Y, Yuan YF, Huang XJ, Ye WC, Wang YT, Zhang QW. Metabolic differentiations of Pueraria lobata and Pueraria thomsonii using 1H NMR spectroscopy and multivariate statistical analysis. J Pharm Biomed Anal. 2014;93:51–8. doi: 10.1016/j.jpba.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 2. Kayano SI, Yoko M, Yoko K, Kobayashi M, Nagayama A, Kawabata N, Kikuzaki H, Kitada Y. Isoflavonoid C-glycosides isolated from the root of kudzu (Pueraria lobata) and their estrogenic activities. Food Chem. 2012;134:282–7. [Google Scholar]
- 3. Gao Q, Yang B, Ye ZG, Wang J, Bruce IC, Xia Q. Opening the calcium-activated potassium channel participates in the cardioprotective effect of puerarin. Eur J Pharmacol. 2007;574:179–84. doi: 10.1016/j.ejphar.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 4. Mu YL, Xie YY, Wang FW, Zhong Y, Li J, Hu ZL, Wang YS, Zhang XM. Protective effect of methylamine irisolidone, a novel compound, on acute myocardial ischemia in anesthetized dogs. JFDA. 2009;17:11–6. [Google Scholar]
- 5. Lin YJ, Hou YC, Lin CH, Hsu YA, Sheu JJ, Lai CH, Chen BH, Lee Chao PD, Wan L, Tsai FJ. Puerariae Radix isoflavonoids and their metabolites inhibit growth and induce apoptosis in breast cancer cells. Biochem Biophys Res Commun. 2009;378:683–8. doi: 10.1016/j.bbrc.2008.10.178. [DOI] [PubMed] [Google Scholar]
- 6. Yu WL, Zhao YP, Shu B. The radical scavenging activities of Radix Puerariae isoflavonoids: a chemiluminescence study. Food Chem. 2004;86:525–9. [Google Scholar]
- 7. Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju Y-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. JFDA. 2014;22:296–302. doi: 10.1016/j.jfda.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lou SN, Hsu YS, Ho CT. Flavonoid compositions and antioxidant activity of calamondin extracts prepared using different solvents. JFDA. 2014;22:290–5. doi: 10.1016/j.jfda.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boue SM, Wiese TE, Nehls S, Burow ME, Elliott S, Carter-Wientjes CH, Shih BY, McLachlan JA, Cleveland TE. Evaluation of the estrogenic effects of legume extracts containing phytoestrogens. J Agric Food Chem. 2003;51:2193–9. doi: 10.1021/jf021114s. [DOI] [PubMed] [Google Scholar]
- 10. Zhang Y, Chen J, Zhang C, Wu W, Liang X. Analysis of the estrogenic component in kudzu root by bioassay and high performance liquid chromatography. J Steroid Biochem Mol Biol. 2005;94:375–81. doi: 10.1016/j.jsbmb.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 11. Prasain JK, Jones K, Kirk M, Wilson L, Smith-Johnson M, Weaver C, Barnes S. Profiling and quantification of isoflavonoids in kudzu dietary supplements by high-performance liquid chromatography and electrospray ionization tandem mass spectrometry. J Agric Food Chem. 2003;51:4213–8. doi: 10.1021/jf030174a. [DOI] [PubMed] [Google Scholar]
- 12. Tanatorn S, Tanapat P, Suchinda M. Anti-osteoclastrogenic, estrogenic, and antioxidant activities of cell suspension cultures and tuber root extracts from Pueraria mirifica. Food Sci Biotech. 2014;23:1253–9. [Google Scholar]
- 13. Hou YC, Lee Chao PD, Yeh YR, Yang SY, Tsai SY. Comparison of urinary kinetics between traditional decoction and concentrated powders of Puerariae radix in healthy men. JFDA. 2011;19:517–22. [Google Scholar]
- 14. Udomsuk L, Chatuphonprasert W, Monthakantirat O, Churikhit Y, Jarukamjorn K. Impact of Pueraria candollei var. mirifica and its potent phytoestrogen miroestrol on expression of bone-specific genes in ovariectomized mice. Fitoterapia. 2012;83:1687–92. doi: 10.1016/j.fitote.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 15. Wong R, Rabie B. Effect of puerarin on bone formation. Osteoarthr Cartil. 2007;15:894–9. doi: 10.1016/j.joca.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 16. Tang L, Liu D, Yi XQ, Xu TT, Liu Y, Luo YC, Dong Yin D, He M. The protective effects of puerarin in cardiomyocytes from anoxia/reoxygenation injury are mediated by PKCɛ. Cell Biochem Funct. 2014;32:378–86. doi: 10.1002/cbf.3026. [DOI] [PubMed] [Google Scholar]
- 17. Xu X, Zhang Z. Effects of puerarin on synaptic structural modification in hippocampus of ovariectomized mice. Planta Med. 2007;73:1047–53. doi: 10.1055/s-2007-981564. [DOI] [PubMed] [Google Scholar]
- 18. Jaroenporn S, Urasopon N, Watanabe G, Malaivijitnond S. Improvements of vaginal atrophy without systemic side effects after topical application of Pueraria mirifica, a phytoestrogen-rich herb, in postmenopausal cynomolgus macaques. J Reprod Develop. 2014;60:238–45. doi: 10.1262/jrd.2013-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen TG, Shih SC, Ping HP, Wei QK. Antioxidant activity and isoflavonoid components in different sections of Pueraria lobata root. JFDA. 2012;20:681–5. [Google Scholar]
- 20. Zhang CL, Ding XP, Hu ZF, Wang XT, Chen LL, Qi J, Yu BY. Comparative study of Pueraria lobata and Pueraria thomsonii by HPLC–diode array detection–flow injection–chemiluminescence coupled with HPLC–electrospray ionization–MS. Chem Pharm Bull. 2011;59:541–5. doi: 10.1248/cpb.59.541. [DOI] [PubMed] [Google Scholar]
- 21. Zeng AG, Xing JF, Wang CH, Song J, Li C, Yang X, Yang G. Simultaneous analysis and retention behavior of major isoflavonoids in Radix Puerariae lobatae and Radix Puerariae thomsonii by high performance liquid chromatography with cyclodextrins as a mobile phase modifier. Anal Chim Acta. 2012;712:145–51. doi: 10.1016/j.aca.2011.10.061. [DOI] [PubMed] [Google Scholar]
- 22. Jutarmas J, Dhiani AB, Wichai C. Pueraria mirifica leaves, an alternative potential isoflavonoid source. Biosci Biotech Biochem. 2014;78:917–26. doi: 10.1080/09168451.2014.910091. [DOI] [PubMed] [Google Scholar]
- 23. Chen SB, Liu HP, Tian RT, Yang DJ, Chen SL, Xu HX, Chan AS, Xie PS. High-performance thin-layer chromatographic fingerprints of isoflavonoids for distinguishing between Radix Puerariae lobatae and Radix Puerariae Thomsonii. J Chromatogr A. 2006;1121:114–9. doi: 10.1016/j.chroma.2006.04.082. [DOI] [PubMed] [Google Scholar]
- 24. Du G, Zhao HY, Zhang QW, Li GH, Yang FQ, Wang Y, Li YC, Wang YT. A rapid method for simultaneous determination of 14 phenolic compounds in Radix Puerariae using microwave-assisted extraction and ultra high performance liquid chromatography coupled with diode array detection and time-of-flight mass spectrometry. J Chromatogr A. 2011;1217:705–14. doi: 10.1016/j.chroma.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 25. Wang JX, Mu JN, Ma J, Yang Y, Wang M, Zhu L, Du X. Determination of rutin and puerarin in teas and pharmaceutical preparations using poly (Evans blue) film-modified electrodes. JFDA. 2012;20:611–6. [Google Scholar]
- 26. Xie DW, Li YH, Zhao L, Ding G, Yuan SW, Xu J, Xiao W, Wang ZZ. Simultaneous quantification of five compound from Nauclea officinalis leaves by high performance liquid chromatography. JFDA. 2012;20:489–94. [Google Scholar]
- 27. Hsu BY, Inbaraj BS, Chen BH. Analysis of soy isoflavones in foods and biological fluids: an overview. JFDA. 2010;18:141–54. [Google Scholar]
- 28. Cherdshewasart W, Subtang S, Dahlan W. Major isoflavonoid content of the phytoestrogen rich-herb Pueraria mirifica in comparison with Pueraria lobata. J Pharmaceut Biomed Anal. 2007;43:428–34. doi: 10.1016/j.jpba.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 29. Li J, Li ZB, Li CF, Gou J, Zhang Y. Molecular cloning and characterization of an isoflavonoid 7-O-glucosyltransferase from Pueraria lobata. Plant Cell Rep. 2014;33:1173–85. doi: 10.1007/s00299-014-1606-7. [DOI] [PubMed] [Google Scholar]
- 30. Malaivijitnond S, Tungmunnithum D, Gittarasanee S, Kawin K, Limjunyawong N. Puerarin exhibits weak estrogenic activity in female rats. Fitoterapia. 2010;81:569–76. doi: 10.1016/j.fitote.2010.01.019. [DOI] [PubMed] [Google Scholar]