Abstract
In folklore systems of medicine, bilberry fruit and leaf extracts have been used for the treatment of diarrhoea, dysentery, diabetes, inflammation, and ulcer. The present study was to determine antioxidant, anti-inflammatory, and antiulcerogenic activities of Vaccinium leschenaultii Wight leaf and fruit. The phenolic, tannin, and flavonoid contents of V. leschenaultii leaf and fruit were quantified and were subjected to assess their antioxidant potential using various in vitro systems such as 1, 1 diphenyl-2-picrylhydrazyl, 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) radical scavenging, phosphomolybdenum, and ferric reducing antioxidant power reduction activities. Based on the antioxidant potential, acetone and methanol extracts of leaf and fruit were used to evaluate the anti-inflammatory activity and protective effect against ethanol-induced gastric damage in a rat model. The quantification of secondary metabolites shows that the phenolic, flavonoid, and tannin contents are higher in methanol extracts of fruit and leaf. The results of antioxidant assays exhibited that the methanol extracts of leaf possesses better 1, 1 diphenyl-2-picrylhydrazyl radical scavenging and ferric reducing power activity. Oral administration of the acetone fruit and leaf extracts of V. leschenaultii were capable of reducing the edema formation in rats against carrageenan and egg albumin induced inflammation. Moreover, leaf and fruit acetone extracts at the dose of 400 mg/kg highly inhibited ulcer formation. The study concluded that the plant substances such as total phenols, flavonoids along with appreciable antioxidant potential could be the supportive evidence to prove both the anti-inflammatory and antiulcer activities of V. leschenaultii. The traditional importance of this plant will help to reveal the potential of plant to provide alternative phytotherapeutics for human health.
Keywords: anti-inflammatory, antioxidant, antiulcer, bilberry, Vaccinium leschenaultii
1. Introduction
Human beings have been using plants for the treatment of diverse ailments for thousands of years. According to the World Health Organization, most populations entrust traditional medicines for their psychological and physical health requirements [1], since they cannot afford the products of Western pharmaceutical industries [2]; these products are also concerned with their side effects and lack of health care facilities [3]. Rural areas of many developing countries still rely on traditional medicine for their primary health care needs and traditional medicines have found a place in day-to-day life. These medicines are relatively safer and cheaper than synthetic or modern medicines in most cases. People living in rural areas from their personal experience know that these traditional remedies are a valuable source of natural products to maintain proper health. Although they are unaware of the science behind these medicines, they knew that some medicinal plants are highly effective only when used at therapeutic doses [4].
The genus Vaccinium is believed to originate from South America and has spread throughout the world [5]. Bilberries grow in temperate woodlands and heaths throughout Europe and produce berries that both animals and humans can eat [6]. Bilberry has been used as a herbal remedy for centuries, all around the globe. Within the genus Vaccinium which belongs to the family Vaccinaceae, there are 450 species of trees, shrubs, sub shrubs, and hemi epiphytes that are mainly distributed in the tropics of the Old World Malaysia, and Southeast Asia [7,8]. More recently, bilberry (Vaccinium myrtillus, Vaccinium arctostaphylos L) fruit extracts have been used for the treatment of diarrhea, dysentery, and mouth and throat inflammations. Bilberry leaf decoctions have been used to lower blood sugar in diabetes. Currently, bilberry research is focused on the treatment of ocular disorders, vascular disorders, and diabetes mellitus [9]. A decoction of the leaves or bark of the root is used as a local application in ulceration of the mouth and throat [10]. Ethnobotanical reviews reveals that Vaccinium leschenaultii (family: Vaccinaceae) has been used for treatment of several disorders such as plough injuries, mouth ulcer, diarrhoea [9], and diabetes [11]. Even though the whole plant possesses many traditionally known medicinal values, extensive research on their phytochemical and pharmacological properties is required.
However, very few experimental studies have been conducted in related to pharmacological and phytochemical aspects of V. leschenaultii. Moreover, there is no report on the antioxidant, anti-inflammatory, and antiulcerogenic activity of V. leschenaultii leaf and fruit. Therefore, the present study has been undertaken to investigate the pharmacological significance of the V. leschenaultii leaf and fruit.
2. Materials and methods
2.1. Chemicals used
Drugs and antioxidant chemicals and standards were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Merch, Himedia (Mumbai, India). All other reagents used were of the highest purity and analytical grade made in India.
2.2. Collection, identification, and extraction of plant material
The fresh plant parts such as leaves and fruit of V. leschenaultii were collected from Kothagiri, Niligris Hills, Tamil Nadu during February–April 2013. The collected plant material was identified and its authenticity was confirmed by comparing to the voucher specimen at the herbarium of Botanical Survey of India, Southern Regional center, Coimbatore, Tamil Nadu (BSI/ SRC/5/23/2012-13/Tech.1889). Freshly collected plant material was cleaned in order to remove adhering dust and then dried in shade for 15–30 days. The air-dried powdered plant materials of V. leschenaultii were extracted separately in Soxhlet extractor with petroleum ether, chloroform, acetone, methanol, and ethanol successively. Each time prior to extracting with the subsequent solvent, the material was dried in a hot air oven below 40°C. Finally, the material was macerated using hot water with occasional stirring for 48 hours and the water extract was filtered. The different solvent extracts were concentrated by rotary vacuum evaporator, air dried, weighed, and stored in desiccators. Further, the extracts were used for various phytochemical and pharmacological studies.
2.3. Determination of total phenolics, flavonoid, and tannin contents
The total phenolic content was determined according to the method described by Siddhuraju and Becker [12]. The results were expressed as gallic acid equivalents. The tannins were estimated after the extracts treated with polyvinyl poly-pyrrolidone (PVPP) [13]. The tannin content of the sample was calculated as follows: Tannins = Total phenolics − Nontannin phenolics. The flavonoid contents of all the extracts were quantified according to the method described by Zhishen et al [14]. All the experiments were done in triplicate and the results expressed in rutin equivalents.
2.4. In vitro antioxidant assays
2.4.1. 1, 1 Diphenyl-2-picrylhydrazyl radical scavenging activity
The antioxidant activity of the extract was determined in terms of hydrogen donating or radical scavenging ability using the stable radical 1, 1 diphenyl-2-picrylhydrazyl (DPPH), according to the method of Braca et al [15]. The results were expressed as IC50, which is the concentration of the sample required to inhibit 50% of DPPH concentration.
2.4.2. 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)•+ scavenging activity
The total antioxidant activity of the samples was measured by 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS)•+ decolorization assay according to the method of Re et al [16]. The unit of total antioxidant activity (TAA) is defined as the concentration of trolox having equivalent antioxidant activity expressed as μM trolox equivalents/g sample extracts.
2.4.3. Phosphomolybdenum assay
The antioxidant activity of samples was evaluated by the green phosphomolybdenum complex formation according to the method of Prieto et al [17]. The results reported are mean values expressed as mg of ascorbic acid equivalents/g extract.
2.4.4. Ferric reducing antioxidant power assay
The antioxidant capacities of different extracts of samples were estimated according to the procedure described by Pulido et al [18]. Equivalent concentration was calculated as the concentration of antioxidant giving an absorbance increase in the ferric reducing antioxidant power (FRAP) assay equivalent to the theoretical absorbance value of a 1mM concentration of Fe (II) solution. Equivalent concentration was calculated as the concentration of antioxidant giving an absorbance increase in the FRAP assay, which is equivalent to the theoretical absorbance value of a 1mM concentration of Fe (II) solution
2.5. In vivo studies
Swiss albino mice of approximately 30 g for acute toxicity studies and Wistar albino rats of 110–160 g for anti-inflammatory and antiulcer studies were used at Nandha College of Pharmacy, Erode, Tamil Nadu, India. The experimental protocol was subjected to the scrutiny of the Institutional Animal Ethics Committee, and was cleared by the same prior to beginning the experiment (No. 688/02/C/CPCSEA).
2.5.1. Acute toxicity
Healthy Swiss albino mice of either sex weighing between 25 g and 30 g were divided into two groups of six animals each. They were housed in different plastic cages and maintained at room temperature, photoperiod of 12 hours and frequent air changes. Mice had free access to tap water and food, except for a short fasting period prior to the treatment with the plant extract. The extracts were administered orally by the doses of 50 mg/kg, 100 mg/kg, 500 mg/kg, 1000 mg/kg, and 2000 mg/kg body weight, which were increased progressively than the preceding one as per Organisation for Economic Co-operation and Development (OECD) guideline [19]. The animals were observed for general behavioral changes, signs of toxicity and mortality continuously for 1 hour after treatment, then intermittently for 4 hours, and thereafter over a period of 24 hours [20].
2.6. Evaluation of anti-inflammatory activity
2.6.1. Carrageenan-induced acute paw edema in rats
The Wistar albino rats were divided into 10 groups, each consisting of six animals of either sex. Paw edema was induced in all the groups of animals by subplantar injection of 0.1 mL of freshly prepared 1% carrageenan suspension into the right hind paw of each rat [21]. They were fasted overnight but had free access to water. A mark was made on the left hind paw just beyond the tibio–tarsal junction. The initial paw thickness of each rat was noted by digital Vernier Caliper. The carrageenan-induced animals were divided in to 10 groups (each comprising 6 rats) namely: Group 1: control (0.6% carboxymethyl cellulose (CMC)); Group 2: oral feeding of indomethacin (10 mg/kg); Group 3: oral feeding of leaf acetone extract: Dose 1 (200 mg/ kg); Group 4: oral feeding of leaf acetone extract dose 2 (400 mg/ kg); Group 5: oral feeding of leaf methanol extract: Dose 1 (200 mg/kg); Group 6: oral feeding of leaf methanol extract: Dose 2 (400 mg/kg); Group 7: oral feeding of fruit acetone extract: Dose 1 (200 mg/kg); Group 8: oral feeding of fruit acetone extract Dose 2 (400 mg/kg); Group 9: oral feeding of fruit methanol extract: Dose 1 (200 mg/kg); and Group 10: oral feeding of fruit methanol extract: Dose 2 (400 mg/kg). The test drug, indomethacin and acetone and methanolic extracts of leaf and fruit were administered orally 1 hour prior to the injection of carrageenan. The linear diameter of the injected paw was measured with the Digital Caliper (Model no. CD- 6′′CSX; Mitutoyo, Kawasaki, Japan) prior to as well as at intervals of 60 minutes, 120 minutes, 180 minutes, and 240 minutes after the injection of carrageenan. The relative potency of the drugs under investigations was calculated based upon the percentage inhibition of the inflammation.
2.6.2. Egg albumin-induced inflammation
To study the anti-inflammatory property against egg albumin-induced hind paw edema in rats, the methodology by Okokon and Nwafor [22] was followed. Wistar albino rats were housed in 10 groups as discussed earlier for the carrageenan-induced method.
2.6.3. Evaluation of antiulcer property by ethanol-induced ulcer experiment
Ulcers were induced by administering ethanol. All the animals were deprived of food for 24 hours prior to the experiment [23], but were allowed free access of drinking water (bottled tap water) up to 2 hours prior to the experiment. The ulcer control group was orally administered with vehicle (CMC, 0.25% w/v), 0.5 mL/animal. The second group was orally administrated with ranitidine (20 mg/kg); the third and fourth groups received acetone leaf extract; the fifth and sixth groups were given methanol leaf extract; the seventh and eighth groups received acetone fruit extract; and the ninth and tenth groups were given methanol extract of V. leschenaultii fruit at the doses of 200 mg/kg and 400 mg/kg. The gastric ulcers were induced in rats by administrating ethanol (80%; 1.0 mL/100g) orally, after 45 minutes of acetone and methanolic extract and ranitidine treatment. Free access to water and food was maintained throughout the experiment. The rats were subjected to euthanasia by cervical dislocation at 60 minutes [24] under an overdose of diethyl ether anesthesia and their stomachs were immediately excised. The length of each gastric lesion was measured and the lesion index was expressed as sum of the length of the entire lesion in mm [25].
where UIC = ulcer index of the control group and UIT = ulcer index of the treatment group. Ulcer index (UI) was calculated using the formula [26]:
where US = mean severity of ulcer score; UN = average number of ulcers per animal; and UP = percentage of animals with ulcer incidence.
2.6.4. Histopathology
Stomach tissues from the rats in each group of the experiment were fixed in 10% formalin for a minimum of 24 hours and then dehydrated by washing in ascending grades of ethanol prior to clearing with xylene and embedding in paraffin wax. The samples were sectioned with a microtome (Spinco, India), stained with hematoxylin and eosin, and mounted on Canada balsam. All sections were examined under light microscope (×10) magnification. Photographs of the lesions were taken with an Olympus photomicroscope (Olympus CH 20i, India) for observation and documentation of histopathologic lesions.
2.7. Statistical analysis
All of the experiments were done in triplicate and the results expressed as mean ± standard deviation. The data were statistically analyzed using one-way analysis of variance (ANOVA) followed by Duncan's test for quantification, in vitro antioxidant studies and Dunnet t test for in vivo anti-inflammatory and antiulcer studies.
3. Results and discussion
Vaccinium leschenaultii is known to possess various therapeutic properties and has been one of the most important plants mentioned in various medicinal systems. In our studies, we went a step ahead and have studied antioxidant, anti-inflammatory, and antiulcer activities of V. leschenaultii leaf and fruit in in vitro and in vivo models.
Plant phenolic compounds are secondary metabolites with interesting properties for animal or human health. The beneficial effects of those molecules are related to their anti-oxidant activity, particularly their ability to scavenge free radicals, to donate hydrogen atoms or electrons, or to chelate metal cations. Besides, phenolic compounds contribute largely to the color and sensory characteristics of fruits and vegetables. Therefore, it would be valuable to determine the amount of total phenolics present in the plant extracts. Polyphenolic contents of all the sample extracts appear to function as good electron and hydrogen atom donors and therefore should be able to terminate radical chain reaction by converting free radicals to more stable products. The enrichment of phenolic compounds within plant extracts is correlated with their enhanced antioxidant activity. Hence, the presence of higher amount of phenolics in leaf and fruit extracts of V. leschenaultii may indicate higher potentials in antioxidant and other medicinal properties (Table 1). Flavonoids are one of the most important and widespread groups of natural compounds. These compounds possess a broad spectrum of chemical and biological activities including radical scavenging properties [27]. Plant flavonoids have been reported to possess strong antioxidant and good anti-inflammatory activities [28]. Flavonoids are able to scavenge practically all known reactive oxygen species. The flavonoids from bilberry are potent antioxidants and play an important role to protect oxidative damage due to their role in scavenging free radicals [29]. Since V. leschenaultii possess good flavonoid content in leaf and fruit extracts especially in fruits (Table 1), it could be assumed that it has a higher ability of scavenging free radicals, chelating metal ions like iron and copper, and inhibition of enzymes responsible for free radical generation. Tannins are complex moieties produced by the majority of plants as protective substances that have broad pharmacological properties. In addition, tannins can also heal wounded areas when treated with them and at the same time can reduce inflammation. This accentuates the use of herbs containing tannins in compress for cuts and wounds, hemorrhoids, and varicose veins and in medicine for diarrhoea, catarrh, heavy menstrual flows, and inflammatory conditions of the digestive tract [30]. The greater amount of tannins in the acetone and methanol extracts of the leaf and fruit of V. leschenaultii (Table 1) can be due to higher polymerization of existing polyphenolic compounds. Recently, it has been reported that the high molecular weight phenolics such as tannins have more ability to reduce or scavenge free radicals [31]. Therefore the high amount of tannin contents of V. leschenaultii may enhance its free radical scavenging, anti-inflammatory, and antiulcer activities.
Table 1.
Determination of total phenolic, flavonoid and tannin contents in Vaccinium leschenaultia.
| Extracts | Leaf | Fruit | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Total phenolics (mg gallic acid equivalents/ 100 mg extract) | Flavonoids (mg rutin equivalents/ 100 mg extract) | Tannins (mg gallic acid equivalents/ 100 g extract) | Total phenolics (mg gallic acid equivalents/ 100 mg extract) | Flavonoids (mg rutin equivalents/ 100 mg extract) | Tannins (mg gallic acid equivalents/ 100 g extract) | |
| Petroleum ether | 12.40 ± 3.17d | 6.61 ± 1.38 | 9.03 ± 3.16c | 1.27 ± 0.09 | 12.40 ± 0.08 | 1.54 ± 1.90 |
| Chloroform | 11.31 ± 0.32d | 10.68 ± 0.28 | 8.72 ± 0.38c | 3.05 ± 0.29 | 10.18 ± 0.75 | 1.460 ± .08 |
| Acetone | 18.37 ± 0.66c | 43.66 ± 1.14c | 15.36 ± 0.67b | 7.11 ± 0.24 | 10.30 ± 0.10 | 5.91 ± 0.20 |
| Methanol | 27.20 ± 0.97b | 56.25 ± 1.35b | 20.34 ± 0.97a | 30.40 ± 0.47a | 64.50 ± 1.57a | 19.96 ± 0.63a |
| Ethanol | 10.79 ± 0.06d | 36.85 ± 1.41d | 7.08 ± 0.11d | 10.31 ± 1.95d | 21.63 ± 1.82 | 7.15 ± 1.94d |
| Hot water | 11.66 ± 0.34d | 35.26 ± 0.88d | 9.42 ± 0.43c | 2.79 ± 0.40 | 8.33 ± 0.20 | 1.69 ± 0.33 |
Data are presented as mean of triplicate determination ± standard deviation.
Statistically significant at p < 0.05 where a > b > c > d.
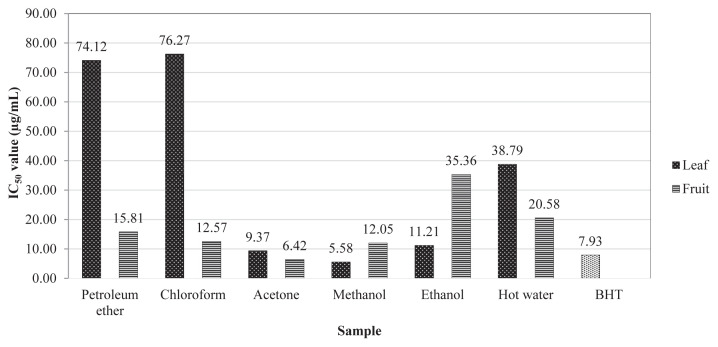
DPPH radical has been widely used to test the ability of compounds to act as free radical scavengers or hydrogen donors and thus to evaluate the antioxidant activity [32]. The results of DPPH assay were expressed in IC50 values. The lower value of IC50 indicates a higher antioxidant activity. Synthetic antioxidant butylhydroxytoluene was used as the reference compound. Among the parts analyzed, the methanol extract of leaf (5.58 μg/mL) and the acetone extract of fruit (6.42 μg/mL) showed better IC50 values compared to other solvent extracts (Fig. 1). Although the phenolic contents were better in methanol extract in both parts, the leaf acetone shows better radical scavenging activity; this might be due to the active phenolics dissolved in acetone. The present study revealed that the DPPH radical scavenging activity was correlated to the higher phenolic contents detected in the fruit methanol extract.
Fig. 1.
1, 1 diphenyl-2-picrylhydrazyl scavenging activity of Vaccinium leschenaultii extracts.
The ABTS assay is an excellent tool to determine the antioxidant activity of hydrogen donating antioxidants (scavenging aqueous phase radicals) and chain breaking antioxidants (scavenging lipid peroxyl radicals). The efficacy of ethanol extract of the leaf showed higher ABTS cation radical scavenging (20,628.58 ± 513.71 mg trolox equivalents/g extract) activity compared to the extracts of fruit (Table 2). Hagerman et al [31] reported that high molecular weight phenolics (tannins) have more ability to quench ABTS•+ and their effectiveness depends on the molecular weight, the number of aromatic rings, and the nature of hydroxyl group's substitution than the specific functional groups. Although the phenolics are lower in ethanol extract than acetone and methanol extracts, high molecular weight phenolic compounds may dissolve in ethanol, which is more active on ABTS radicals. From the result, it is concluded that the extracts of V. leschenaultii have stronghydrogendonating ability and could serve as freeradical quenchers by acting as primary antioxidants. The synthetic antioxidant butylhydroxytoluene was compared for quenching ability where leaf ethanol extract is more significant.
Table 2.
ABTS•+, phosphomolybdenum reduction and ferric reducing antioxidant power activity.
| Extracts | Leaf | Fruit | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| (1)ABTS•+ (mg trolox equivalents/g) | Phosphomolybdenum (mg ascorbic acid equivalents/g) | (2)FRAP(mg Fe(II)equivalents/100 mg) | (1)ABTS•+ (mg trolox equivalents/g) | Phosphomolybdenum (mg ascorbic acid equivalents/g) | (2)FRAP(mg Fe(II) equivalents/100 mg) | |
| Petroleum ether | 540.68 ± 49.31 | 385.96 ± 2.60 | 5.68 ± 0.60 | 552.67 ± 3.05 | 425.26 ± 16.13d | 23.28 ± 1.29 |
| Chloroform | 826.34 ± 61.71 | 389.65 ± 0.80 | 51.23 ± 6.28 | 3589.03 ± 455.67 | 476.32 ± 17.99c | 52.55 ± 4.91 |
| Acetone | 16,100.68 ± 1087.55b | 624.21 ± 16.57a | 118.32 ± 1.93a | 3495.81 ± 777.78 | 539.47 ± 10.95b | 26.13 ± 3.16 |
| Methanol | 11,745.90 ± 513.71d | 435.79 ± 21.19d | 122.04 ± 1.85a | 10,400.85 ± 100.54 | 467.37 ± 12.26c | 122.54 ± 2.22a |
| Ethanol | 20,628.58 ± 513.71a | 436.32 ± 2.93d | 102.65 ± 1.49c | 5966.17 ± 926.32 | 398.42 ± 4.59 | 104.79 ± 4.42b |
| Hot water | 17,285.92 ± 1201.44b | 388.95 ± 0.53 | 101.78 ± 0.50c | 3748.83 ± 827.58 | 387.72 ± 2.90 | 110.89 ± 5.09b |
| BHT | 28213.02 | — | 423 ± 1.43 | 28,213.02 | — | 423 ± 1.43 |
Data are presented as mean of triplicate determination ± standard deviation.
Statistically significant at p < 0.05 where a > b > c > d.
ABTS•+ = 2, 2′-azino-bis-(3-ethyl-benzothiozoline)-6-sulfonic acid diammonium salt cation radical; BHT = butylhydroxytoluene; FRAP = ferric reducing antioxidant power.
The phosphomolybdenum assay is successfully used to determine the ability of extracts to reduce Mo (VI) to Mo (V) and subsequent formation of green phosphate/Mo (V) complex at an acid pH. It was also used to quantify vitamin E in seeds, being simple and independent of other antioxidant measurements commonly employed, it was decided to extend to plant extracts to analyze the potential of phosphomolybdenum reducing ability [17]. Moreover, antioxidant activity is expressed as equivalents of ascorbic acid. Thus, the total antioxidant capacity observed for the extracts of V. leschenaultii can be correlated with natural antioxidant ascorbic acid. The experimental data reveal that acetone extracts of the leaf and fruit showed higher antioxidant activity by the reduction of phosphomolybdenum complex and were 624.21 ± 16.57 mg and 539.47 ± 10.95 mg ascorbic acid equivalents/g extract respectively. The reduction of Mo (VI) to Mo (V) by the leaf and fruit extracts of V. leschenaultii may be due to the electron transfer or hydrogen ion transfer by the bioactive compounds, specifically phenolics and flavonoids present in the respective parts. Even though the quantity of both phenolics and flavonoids are lower than methanol extract, again the acetone dissolved compounds are more responsible in this case.
The FRAP activity has been widely used to evaluate the antioxidant activity of plant extracts and foods. The reducing capacity of a compound might serve as a significant indicator of its potential antioxidant capacity. The FRAP assay measures the ability of antioxidants to reduce the 2,4,6- tripyridyls-triazine (TPTZ)–Fe(III) complex to the intensely blue colored TPTZ–Fe(II) complex in acidic medium. The data on the ferric reducing potential of V. leschenaultii leaf and fruit extracts presented in Table 2 indicate that methanol extracts of the fruit (122.54 mg Fe II equivalents/100 mg extract) and leaf (122.04 mg Fe II equivalents/100 mg extract) showed higher ferric reducing power than other extracts. These results suggest that extracts have the ability to donate electrons and thus could scavenge free radicals. Antioxidant compounds that act as reducing agents exert their effect by donating a hydrogen atom to the ferric complex and thus breaking the radical chain reaction [33].
The free radical scavenging activity of the crude extracts tested might be also considered as one of the possible mechanisms of its anti-inflammatory and gastroprotective effect observed. Based on the quantification of secondary metabolites and various in vitro antioxidant assay results, the acetone and methanol extracts of V. leschenaultii leaf and fruit were selected for further in vivo studies. Different solvents dissolve according to their binding and reactivity nature, those were used to separate the active parts from a pool of compounds. In the acute toxicity study, the extracts did not alter the general behavior of animals and did not produce any mortality even at the highest dose of 2000 mg/kg. Since the extract was found to be safe, the selected plant parts might be considered as nontoxic. Based on this acute toxicity study, two different doses were selected to assess anti-inflammatory and anti-ulcerogenic activity in rat models.
Carrageenan-induced paw edema as an in vivo model for inflammation has been frequently used to assess the anti-inflammatory effect of natural products. In this biphasic event, the early phase is accompanied by the release of histamine and serotonin, while in the late phase prostaglandins, proteases and lysozymes are released [34]. Carrageenan-induced paw edema remained even 4 hours after its injection into the subplantar region of rat paw. Researchers have demonstrated that the inflammatory effect induced by carrageenan is associated with free radicals. The carrageenan-induced inflammatory response has been linked to neutrophil infiltration and the production of neutrophil-derived free radicals, such as hydrogen peroxide, superoxide, and hydroxyl radicals, as well as the release of other neutrophil-derived mediators [35]. Oral pretreatment of animals with acetone and methanol extracts of the fruit and leaf has resulted in a significant inhibition of carrageenan evoked hind paw edema because these extracts have also shown renounced activity in in-vitro antioxidant assays.
The most widely used primary test to screen new anti-inflammatory agent measures the ability of a compound to reduce local edema induced in the rat paw by the injection of an irritant agent. The test was carried out using a phlogistic agent (fresh undiluted egg albumin), which induced rat hind paw edema as a model of acute inflammation. This is mediated by many chemical mediators such as histamine, serotonin, kinins, and prostanoids. This model is widely accepted for the evaluation of antiedemal or anti-inflammatory effect of drugs [21,36].
The inflammatory response is a physiological characteristic of vascularized tissues [37]. Exudation, which is a consequence of increased vascular permeability, is considered as a major feature of acute inflammation [38]. The paw edema that was induced by an injection of egg albumin was peaked after 1 hour and then progressively declined with time. It is similar to carrageenan used for the induction of edema in an experimental animal model for acute inflammation and is believed to be biphasic [39,40]. The plant, Chinese water berry exhibited a significant effect on the acute inflammatory processes induced by egg albumin [41]. In the present study, acetone fruit and leaf extracts (400 mg/kg) were capable of reducing the edema formation in rats against carrageenan-induced inflammation after 4 hours by 97.6 and 94.8% respectively (Table 3). Oral administration of V. leschenaultii fruit (92.6%) and leaf acetone (87.2%) extracts on egg albumin-induced edema in rats caused a significant and dose dependent anti-inflammatory activity (Table 4). This can be achieved in both the anti-inflammation models concurrently and concluded the fruit acetone extract have edema reducing potency. It could be said that the V. leschenaultii anti-inflammatory effects may be possibly mediated through their antioxidant properties.
Table 3.
Anti-inflammatory effect of Vaccinium leschenaultii on carrageenan-induced rats.
| Groups | Dose (mg/kg) | Edema induced by carrageenan (mm) | % inhibition | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0 h | 1 h | 2 h | 3 h | 4 h | |||
| Control | — | 4.52 ± 0.78 | 5.03 ± 0.25 | 6.0 ± 0.02 | 6.54 ± 0.31 | 6.67 ± 0.32 | — |
| Indomethacin | 10 | 3.43 ± 0.33 | 4.74 ± 0.3 | 5.28 ± 0.2 | 4.43 ± 0.28 | 3.46 ± 0.31*,** | 98.6 |
| Leaf acetone | 200 | 4.21 ± 0.51 | 5.64 ± 0.51 | 5.9 ± 0.42 | 5.96 ± 0.42 | 5.85 ± 0.79 | 23.7 |
| 400 | 4.31 ± 0.39 | 5.89 ± 0.96 | 5.92 ± 0.89 | 5.47 ± 0.85 | 4.42 ± 0.82*,** | 94.8 | |
| Leaf methanol | 200 | 3.91 ± 0.2 | 5.32 ± 0.89 | 5.49 ± 0.91 | 5.09 ± 0.72 | 4.87 ± 0.77 | 55.3 |
| 400 | 4.44 ± 0.82 | 5.94 ± 0.73 | 6.01 ± 0.76 | 5.21 ± 0.76 | 4.77 ± 0.87** | 84.6 | |
| Fruit acetone | 200 | 4.12 ± 0.19 | 5.02 ± 0.43 | 5.5 ± 0.42 | 5.21 ± 0.49 | 4.71 ± 0.6** | 72.5 |
| 400 | 4.27 ± 0.28 | 5.9 ± 0.8 | 5.81 ± 0.3 | 5.32 ± 0.34 | 4.32 ± 0.4*,** | 97.6 | |
| Fruit methanol | 200 | 3.54 ± 0.21 | 5.74 ± 0.4 | 5.98 ± 0.34 | 5.53 ± 0.45 | 5.1 ± 0.51 | 27.4 |
| 400 | 4.44 ± 0.32 | 5.45 ± 0.22 | 5.74 ± 0.33 | 5.49 ± 0.43 | 4.83 ± 0.49 | 81.8 | |
Data are presented as mean ± standard error of the mean.
Statistically significant at
p < 0.001 and
p < 0.005.
Table 4.
Effect of Vaccinium leschenaultii on egg albumin-induced edema in rats.
| Groups | Dose (mg/kg) | Egg albumin-induced edema (mm) | % inhibition | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 0 h | 1 h | 2 h | 3 h | 4 h | |||
| Control | — | 4.44 ± 0.09 | 5.06 ± 0.07 | 5.2 ± 0.1 | 5.6 ± 0.17 | 5.93 ± 0.2 | — |
| Indomethacin | 10 | 4.45 ± 0.08 | 5.53 ± 0.11 | 5.59 ± 0.13 | 4.92 ± 0.17 | 4.53 ± 0.21*,** | 94.63 |
| Leaf acetone | 200 | 4.23 ± 0.67 | 5.35 ± 0.67 | 5.46 ± 0.63 | 5.23 ± 0.5 | 5.2 ± 0.42** | 34.89 |
| 400 | 4.69 ± 0.05 | 5.71 ± 0.12 | 5.82 ± 0.07 | 5.32 ± 0.52 | 4.88 ± 0.2*,** | 87.24 | |
| Leaf methanol | 200 | 4.94 ± 0.1 | 5.71 ± 0.15 | 5.78 ± 0.09 | 5.57 ± 0.16 | 5.28 ± 0.1 | 77.18 |
| 400 | 4.23 ± 0.2 | 5.15 ± 0.07 | 5.6 ± 0.04 | 5.27 ± 0.03 | 4.95 ± 0.05** | 51.67 | |
| Fruit acetone | 200 | 4.69 ± 0.01 | 5.73 ± 0.09 | 5.82 ± 0.07 | 5.89 ± 0.04 | 5.42 ± 0.11 | 51.00 |
| 400 | 4.47 ± 0.15 | 5.41 ± 0.1 | 5.72 ± 0.19 | 5.21 ± 0.13 | 4.58 ± 0.12*,** | 92.61 | |
| Fruit methanol | 200 | 4.04 ± 0.28 | 5.21 ± 0.25 | 5.43 ± 0.13 | 5.54 ± 0.2 | 5.22 ± 0.2 | 20.80 |
| 400 | 4.48 ± 0.11 | 5.53 ± 0.11 | 5.66 ± 0.06 | 5.57 ± 0.05 | 4.84 ± 0.07** | 75.83 | |
Data are presented as mean ± standard error of the mean.
Statistically significant at
p < 0.001 and
p < 0.005.
Gastric hyperacidity and ulcer are very common, causing tremendous human sufferings nowadays. Although prolonged anxiety, emotional stress, hemorrhagic surgical shock, burns, and trauma are known to cause severe gastric irritation, the mechanism however, is still very poorly understood [42]. The maintenance of gastric mucosal integrity under an adverse environment of luminal contents depends on a delicate balance of factors regulating the cellular proliferation as well as the process of cell death [43]. Indeed, the results of recent investigations indicate that the increase in gastric epithelial cell apoptosis is a prominent feature associated with mucosal injury by nonsteroidal anti-inflammatory agents such as indomethacin, and an acute gastritis evoked by Helicobacter pylori lipopolysaccharide [44]. As gastric mucosal injury is a common feature of acute ethanol ingestion observed in alcoholics and since available antiulcer agents displaying protective effects against ethanol damage [43] there is a need to develop highly effective and safer antiulcer agents.
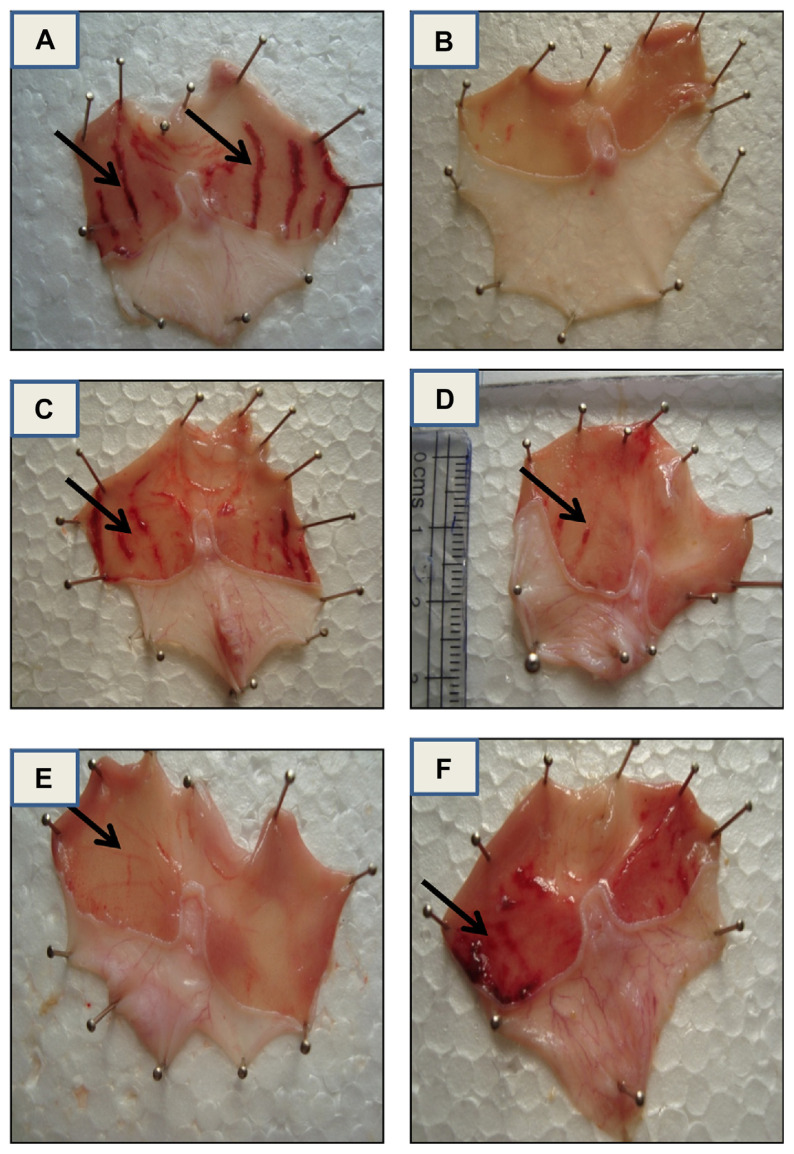
The results of the present study demonstrated that an animal pretreated with V. leschenaultii leaf and fruit (acetone and methanol extracts; Fig. 2D–F), presented a dose dependent protection of mucosal layer damage and color similar to the control rat (Fig. 2A). Ethanol consumption is known to be one of many factors responsible for gastric ulcer formation due to the generation of oxygen-derived free radicals such as superoxide anions, hydroxyl radicals, and lipid peroxides [45,46]. The obtained results suggest that the V. leschenaultii leaf and fruit (acetone and methanol extracts) exhibiting strong antioxidant and radical scavenging potential, has remarkably restricted ethanol-induced depletion of gastric mucosa in rat.
Fig. 2.
The different extract-treated rats' stomachs were dissected and treated. (A) Ethanol-treated control rat stomach is displayed, (B) standard ranitidine-treated rat stomach (20 mg/kg), (C) leaf acetone extract-treated (400 mg/kg), (D) leaf methanol extract-treated (400 mg/kg), (E) fruit acetone extract-treated (400 mg/kg), and (F) fruit methanol extract-treated (400 mg/kg).
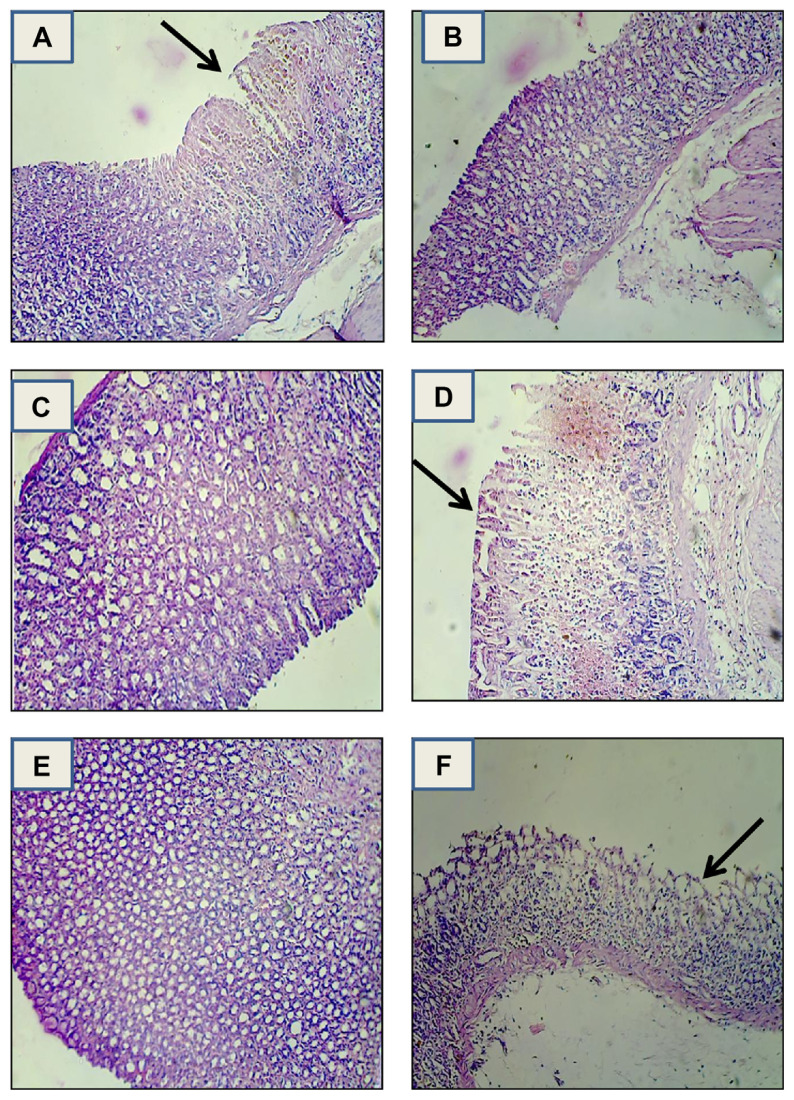
In order to confirm the results of the antiulcer experiment, the stomachs of experimental animals were also evaluated by histopathological examination. In histological observation, the stomach of normal animals showed no damage (Fig. 3A). However, rats treated with absolute ethanol showed disrupted epithelial cells from the upper portion of fundic glands, vacuolization, necrosis, and deep dilated interglandular spaces (Fig. 3B). Following V. leschenaultii leaf and fruit (acetone and methanol extracts) pretreatment, the pathological injuries were markedly attenuated, with only slight damage observed in the superficial epithelium (Fig. 3C–F). In reducing the epithelial cells damage, V. leschenaultii leaf and fruit efficacy was slightly higher than the standard drug.
Fig. 3.
Histopathological evaluation of ulcer in rats. (A) Ethanol-treated control rat stomach, (B) standard ranitidine-treated rat stomach (20 mg/kg), (C) leaf acetone extract-treated (400 mg/kg), (D) leaf methanol extract-treated (400 mg/kg), (E) fruit acetone extract-treated (400 mg/kg), and (F) fruit methanol extract-treated (400 mg/kg).
The screening of the plant potential against ethanol damage was observed in this study. The animals treated with V. leschenaultii leaf and fruit extracts prior to the administration of ethanol exhibited a significant reduction in the numbers of ulcer spots and ulcer index. The ulceration index for the control group was found to be 32.87. Moreover, the acetone extracts of leaf and fruit at the dose of 400 mg/kg inhibited ulcer formation (70% and 71%, respectively) whereas in ranitidine treated animals 98% ulcer inhibition was observed (Table 5). The extract reduced ethanol-induced ulcer significantly. This may be due to the cytoprotective effect of the extract via an antioxidant effect. The extract showed protection against characteristic lesions produced by ethanol administration. The significant increase in the antiulcer activity of V. leschenaultii could be attributed to the presence of flavonoids and tannins. Flavonoids are among the cytoprotective material for which antiulcerogenic efficacy has been extensively confirmed. It is suggested that these active compounds would be able to stimulate mucous bicarbonate and to inhibit prostaglandin secretion and counteract with the liberating effect of reactive oxygen in the gastrointestinal lumen [47]. The result of the present study suggests that the methanol and acetone extracts of V. leschenaultii leaf and fruit may be beneficial in the treatment of inflammatory and gastric lesions. Development of a novel nontoxic potent antioxidant, anti-inflammatory, and antiulcer compound will prevent oxidative stress and it will be a suitable way to control the human sufferings.
Table 5.
Effect of Vaccinium leschenaultii on ethanol-induced ulcer in rats.
| Groups | Dose (mg/kg) | No. of ulcer spots | Ulcer index | % Ulcer inhibition |
|---|---|---|---|---|
| Control | — | 11 | 32.9 ± 1.7 | — |
| Ranitidine | 20 | 2 | 2.14 ± 0.8* | 93.49 |
| Leaf acetone | 200 | 7 | 16.72 ± 1.4 | 49.17 |
| 400 | 5 | 9.78 ± 1.9* | 70.27 | |
| Leaf methanol | 200 | 7 | 21.53 ± 1.3 | 34.55 |
| 400 | 6 | 14.26 ± 0.5 | 56.65 | |
| Fruit acetone | 200 | 6 | 18.87 ± 0.7 | 42.64 |
| 400 | 4 | 9.26 ± 1.2* | 71.85 | |
| Fruit methanol | 200 | 8 | 13.24 ± 1.2 | 59.75 |
| 400 | 5 | 11.25 ± 0.6 | 65.80 |
Data are presented as mean ± standard error of the mean.
Statistically significant at
p < 0.001.
4. Conclusion
The results of the present study clearly indicated that acetone, methanol extracts of V. leschenaultii leaf and fruit exhibited higher anti-inflammatory and antiulcerogenic activity. The quantification of secondary metabolites shows that the total phenolics, flavonoid, and tannin content are higher in methanol followed by acetone extracts of the fruit and leaf. The acetone and methanol extracts of the leaf and fruit substantiates a good antioxidant potential and hence, these two extracts were screened for in vivo studies. The acetone fruit and leaf extracts (400 mg/kg) were capable of reducing the edema formation in rats against carrageenan and egg albumin induced inflammation after 4 hours. Moreover, the acetone extracts of the leaf and fruit inhibit the ulcer formation at the dose of 400 mg/kg (by 70% and 71%, respectively). The study concluded that the plant substances such as total phenols, flavonoids, along with appreciable antioxidant potential could be the supportive evidence to prove both the anti-inflammatory and antiulcer activities of V. leschenaultii. The importance of this plant will help to reveal its potential to provide phytotherapeuticals for human health. Further, detailed investigation, chemical studies, and screening for medicinal properties will provide cost effective and reliable source of medicine for the welfare of humanity.
Acknowledgments
The authors are grateful to Dr. T. Sivakumar, Principal, Dr. S. Sengottuvelu, Head, Department of Pharmacology, Mr. S. Haja Sherif, Lecturer, Mr. G. Thamodharan, Lecturer, and Mrs. Lalitha, Lecturer, Nandha College of Pharmacy, Erode for giving permission and for their enormous advice and help during the in vivo studies. The authors would like to express their special acknowledgments to all colleagues for their general help.
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1. Rabe T, Van Staden J. Isolation of an antibacterial sesquiterpenoid from Warburgia salutaris. J Ethnopharmacol. 2000;73:171–4. doi: 10.1016/s0378-8741(00)00293-2. [DOI] [PubMed] [Google Scholar]
- 2. Salie F, Eagles PFK, Leng HMJ. Preliminary antimicrobial screening of four South African Asteraceae species. J Ethnopharmacol. 1996;76:347–54. doi: 10.1016/0378-8741(96)01381-5. [DOI] [PubMed] [Google Scholar]
- 3. Griggs JK, Manander HP, Towers GHN, Talor RSI. The effects of storage on the biological activity of medicinal plants from Nepal. J Ethnopharmacol. 2001;77:247–52. doi: 10.1016/s0378-8741(01)00291-4. [DOI] [PubMed] [Google Scholar]
- 4.Van Wyk BE, Gericke N. People's Plants. A guide to useful plants of Southern Africa. Pretoria: Briza Publications; 2000. [Google Scholar]
- 5. Camp WH. The North American blueberries with notes on other groups of Vacciniaceae. Brittonia. 1945;5:203–75. [Google Scholar]
- 6. Albert T, Raspe O, Jacquemart AL. Clonal diversity and genetic structure in Vaccinium myrtillus populations from different habitats. Belg J Bot. 2004;137:155–62. [Google Scholar]
- 7. Luteyn J. Diversity, adaptation, and endemism in neotropical Ericaceae: Biogeographical patterns in the Vaccinieae. Bot Rev. 2002;68:55–87. [Google Scholar]
- 8. Vander Kloet SP, Dickinson TA. A subgeneric classification of the genus Vaccinium and the metamorphosis of Vaccinium section Bracteata Nakai: more terrestrial and less epiphytic in habit, more continental and less insular in distribution. J Plant Res. 2009;122:253–68. doi: 10.1007/s10265-008-0211-7. [DOI] [PubMed] [Google Scholar]
- 9. Anonymous. Vaccinium myrtillus (Bilberry) – Monograph. Alternative Medicine Review. 2001;6:500–4. http://www.altmedrev.com/publications/6/5/500.pdf . [PubMed] [Google Scholar]
- 10. Taherpour A, Noorabadi P, Abedi F, Taherpour AA. Effect of aqueous cranberry (Vaccinium arctostaphylos L.) extract accompanied with antibiotics on urinary tract infections caused by Escherichia coli in vitro. J Pure Appl Microbiol. 2008;2:135–8. [Google Scholar]
- 11. Marles RJ, Farnsworth NR. Antidiabetic plants and their active constituents. Phytomed. 1995;2:137–89. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- 12. Siddhuraju P, Becker K. Studies on antioxidant activities of Mucuna seed (Mucuna pruriens var. utilis) extracts and certain non-protein amino/imino acids through in vitro models J Agric Food Chem 2003. 51 2144 55 12670148 [Google Scholar]
- 13. Siddhuraju P, Manian S. The antioxidant and free radical scavenging capacity of dietary phenolic extracts from horse gram (Macrotyloma uniflorum (Lam.) Verdc.) seeds. Food Chem. 2007;105:950–8. [Google Scholar]
- 14. Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–9. [Google Scholar]
- 15. Braca A, Tommasi ND, Bari LD, Pizza C, Politi M, Morelli I. Antioxidant principles from Bauhinia terapotensis. J Nat Prod. 2001;64:892–5. doi: 10.1021/np0100845. [DOI] [PubMed] [Google Scholar]
- 16. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolourization assay. Free Radical Bio Med. 1999;26:1231–7. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 17. Prieto P, Pineda M, Aguilar M. Spectrophotometric quantity of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–41. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 18. Pulido R, Bravo L, Sauro-Calixto F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem. 2000;48:3396–402. doi: 10.1021/jf9913458. [DOI] [PubMed] [Google Scholar]
- 19. Kennedy GL, Ferenz RL, Burgess BA. Estimation of acute oral toxicity in rats by the determination of the approximate lethal dose rather than the LD50. J Appl Toxicol. 1986;6:145–8. doi: 10.1002/jat.2550060302. [DOI] [PubMed] [Google Scholar]
- 20. Twaij HAA, Kery A, Al Khazraji NK. Some pharmacological, toxicological and phytochemical investigation on Centaurea phyllocephala. J Ethnopharmacol. 1983;9:299–314. doi: 10.1016/0378-8741(83)90037-5. [DOI] [PubMed] [Google Scholar]
- 21. Winter CA, Risley EA, Nuss W. Carrageenan induced edema in hind paw of rats as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 22. Okokon JE, Nwafor PA. Antiinflammatory, analgesic and antipyretic activities of ethanolic root extract of Croton zambesicus. Pak J Pharm Sci. 2010;23:385–92. [PubMed] [Google Scholar]
- 23. Abdulla MA, Ahmed KAA, Al Bayaty FH, Masood Y. Gastroprotective effect of Phyllanthus niruri leaf extract against ethanol-induced gastric mucosal injury in rats. Afr J Pharma Pharmacol. 2010;4:226–30. [Google Scholar]
- 24. Paiva LAF, Rao VSN, Gramosa NV, Silveira ER. Gastroprotective effect of Copaifera langsdorffii oleo-resin on experimental gastric ulcer models in rats. J Ethnopharmacol. 1998;62:73–8. doi: 10.1016/s0378-8741(98)00058-0. [DOI] [PubMed] [Google Scholar]
- 25.Valle DL. Peptic ulcer diseases and related disorders. In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, editors. Harrison's principles of internal medicine. 15th ed. New York: McGraw-Hill; 2002. pp. 1649–65. [Google Scholar]
- 26.Gerhard Vogel H. Drug discovery and evaluation. 2nd ed. Berlin: Springer –Verlag; 2002. p. 868. [Google Scholar]
- 27. Amarowicz R, Pegg R, Rahimi MP, Barl B, Weil JA. Free-radical scavenging capacity antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004;84:551–62. [Google Scholar]
- 28. Raj KJ, Shalini K. Flavonoids: a review of biological activities. Indian Drugs. 1999;36:668–76. [Google Scholar]
- 29. Dunn WB, Bailey NJC, Johnson HE. Measuring the metabolome: current analytical technologies. Analyst. 2005;130:606–25. doi: 10.1039/b418288j. [DOI] [PubMed] [Google Scholar]
- 30.Trease GE, Evans WC. Textbook of pharmacognosy. 13th ed. London: Balliere Tindall; 1989. [Google Scholar]
- 31. Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, Riechel TL. High molecular weight plant polyphenolics (Tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–92. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- 32. Jao CH, Ko WC. 1, 1 Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging by protein hydrolysates from tuna cooking juice. J Fishery Sci. 2002;68:430–5. [Google Scholar]
- 33. Shakirin FH, Nagendra Prasad K, Ismail A, Lau CY, Azrina A. Antioxidant capacity of underutilized Malaysian Canarium odontophyllum (dabai) Miq. Fruit. J Food Comp Anal. 2010;23:777–81. [Google Scholar]
- 34. Zhang L, Hu JJ, Lin JW, Fanf WS, Du GH. Anti-inflammatory and analgesic effects of ethanol and aqueous extracts of Pterocephalus hookeri (C.B., Clarke) Höeck. J Ethnopharmacol. 2009;123:510–4. doi: 10.1016/j.jep.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 35. Dawson J, Sedgwick AD, Edwards JCW, Lees P. A comparative study of the cellular, exudative, and histological responses to carrageenan, dextran, and zymosan in the mouse. Int J Tissue Reac. 1991;13:171–85. [PubMed] [Google Scholar]
- 36. Marsha-Lyn M, Mckoy G, Everton T, Oswald S. Preliminary investigation of the anti-inflammatory properties of an aqueous extract from Morinda citrifolia (Noni) Proceedings of West Pharmacology Society. 2002;45:76–8. [PubMed] [Google Scholar]
- 37.Rang HP, Dale MM, Ritter JM. Textbook of pharmacology. 6th ed. London: Churchill Livingstone; 2007. Local hormones, inflammation and immune reactions; pp. 202–26. [Google Scholar]
- 38.Hiley P, Barber PC. Homepage of the Pathology. Department Medical School, University of Birmingham; 2000. Acute inflammation. [Google Scholar]
- 39. Brito ARMS, Antonio MA. Oral antiinflammatory and antiulcerogenic activities of a hydroalcoholic extract and partitioned fractions of Turnera ulmifolia (Turneraceae) J Ethnopharmacol. 1998;61:215–28. doi: 10.1016/s0378-8741(98)00049-x. [DOI] [PubMed] [Google Scholar]
- 40. Gupta M, Mazumder UK, Gomathi P, Thamil Selvan V. Anti-inflammatory evaluation of leaves of Plumeria acuminate. BMC Complement Altern Med. 2006;6:36. doi: 10.1186/1472-6882-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ezeonwumelu AN, Omar AM, Ajayi AG, Okoruwa AG, Tanayen JK, Kiplagat DM, Okpanachi OA, Abba S, Ezekiel I, Onchweri AN, Okonkwo CO, Byarugaba F. Phytochemical screening, acute toxicity, anti-inflammatory and antipyretic studies of aqueous extract of the root of Flueggea virosa (Roxb. ex Willd.) in rats. Int J Biomed Pharma Sci. 2012;3:128–35. [Google Scholar]
- 42. Rao CV, Verma AR, Vijayakumar M, Rastogi S. Gastroprotective effect of standardized extract of Ficus glomerata fruit on experimental gastric ulcers in rats. J Ethnopharmacol. 2008;115:323–6. doi: 10.1016/j.jep.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 43. Slomiany BL, Piotrowski J, Murty VLN, Slomiany A. Mechanism of ebrotidine protection against gastric mucosal injury induced by ethanol. Gen Pharmacol. 1992;23:719–27. doi: 10.1016/0306-3623(92)90155-d. [DOI] [PubMed] [Google Scholar]
- 44. Piotrowski J, Skrodzka D, Slomiany A, Slomiany BL. Helicobacter pylori lipopolysaccharide induces gastric epithelial cell apoptosis. Biochem Mol Biol Int. 1996;40:597–602. doi: 10.1080/15216549600201183. [DOI] [PubMed] [Google Scholar]
- 45. Li CY, Xu HD, Zhao BT, Chang HI, Rhee HI. Gastroprotective effect of cyanidin 3-glucoside on ethanol-induced gastric lesions in rats. Alcohol. 2008;42:683–7. doi: 10.1016/j.alcohol.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 46. Pan JS, He SZ, Xu HZ, Zhan XJ, Yang XN, Xiao HM, Shi HX, Ren JL. Oxidative stress disturbs energy metabolism of mitochondria in ethanol-induced gastric mucosa injury. World J Gastroenterol. 2008;14:5857–67. doi: 10.3748/wjg.14.5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sakat SS, Juvekar RA. Antiulcer activity of methanol extract of Erythrina indica Lam. leaves in experimental animals. Pharmacognosy Res. 2009;1:396–401. [Google Scholar]