Abstract
Naturally occurring polymers such as alginate (AL) and chitosan (CS) are widely used in biomedical and pharmaceutical fields in various forms such as nanoparticles, capsules, and emulsions. These polymers have attractive applications in drug delivery because of their biodegradability, biocompatibility, and nontoxic nature. The pharmaceutical applications of essential oils such as turmeric oil and lemongrass oil are well-known, and their active components, arturmerone and citral, respectively, are known for their antibacterial, antifungal, antioxidant, antimutagenic, and anticarcinogenic properties. However, these essential oils are unstable, volatile, and insoluble in water, which limits their use for new formulations. Therefore, this study focuses on developing a CS–AL nanocarrier for the encapsulation of essential oils. The effects of process parameters such as the effect of heat and the concentrations of AL and CS were investigated. Various physicochemical characterization techniques such as scanning electron microscopy, Fourier transform infrared spectroscopy, and ultraviolet–visible spectroscopy were performed. Results of characterization studies showed that 0.3 mg/mL AL and 0.6 mg/mL CS produced minimum-sized particles (<300 nm) with good stability. It was also confirmed that the oil-loaded nanocapsules were hemocompatible, suggesting their use for future biomedical and pharmaceutical applications. Furthermore, the antiproliferative activity of turmeric oil- and lemongrass oil-loaded nanocapsules was estimated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay in A549 cell lines and it was found that both the nanoformulations had significant antiproliferative properties than the bare oil.
Keywords: alginate, chitosan, essential oil, nanocapsules
1. Introduction
Nanotechnology, a boon to health care, involves advances in medicine, robotics, cosmetics, communication, and genomics [1–3]. Applications of nanoscience and nanotechnology in health care led to the genesis of a new field called nanomedicine [4,5]. At present, nanomedicine makes use of nanomaterials such as nanoshell, nanobiosensor, nanovaccines, nanorobots, and nanocapsules for various applications such as diagnosis, nervous system tracking, magnetic resonance imaging contrast enhancers, detection of pathogens, and other biomedical applications [6]. Nanotechnology in drug-delivery system provides an opportunity to deliver drugs for a prolonged time with natural affinity [7]. Nanoparticulate systems, dendrimers, micelles, liposomes, and nanoemulsions are some of the nanocarrier systems widely used in drug delivery. These nanocarrier systems help in improving biodistribution of drugs and solubility of hydrophobic compounds, as well as increasing the bioavailability, reducing the number of doses, improving drug targeting, and minimizing toxicity, etc. Among the many nanocarriers, alginate (AL) and chitosan (CS) have gained more attention due to their biocompatibility, biodegradability, mucoadhesiveness, and longer in vivo circulation time [8–10]. AL is a negatively charged, Food and Drug Administration-approved, naturally occurring polysaccharide consisting of d-mannuronic acid and l-guluronic acid arranged as blocks in the polymeric chain. They are known to undergo proton-catalyzed hydrolysis that is dependent on pH and temperature [11]. It is known that at low pH, alginic acid forms an insoluble AL skin, which prevents the release of encapsulated material. However, at higher pH, it becomes a soluble viscous layer, releasing the encapsulated material [12]. By contrast, CS is a positively charged linear copolymer polysaccharide consisting of d-glucosamine and N-acetyl-d-glucosamine with reactive amino and hydroxyl groups [13]. CS is prepared from chitin by deacetylation of chitin using alkaline solutions [14]. It is soluble at low pH but insoluble at high pH [15–17] and has a unique property called the permeation enhancing effect, which makes it a good carrier system. It increases permeation by opening epithelial tight junctions, which is important for the movement of hydrophilic compounds [12]. CS being cationic and AL being anionic make a good polyelectrolyte complex (PEC) [18]. The formation of PEC is known to be influenced by various factors such as pH, molecular weight of the polymers, and the ratio of AL to CS [19,20]. Positive aspects of CS–AL PEC have favored its use as a drug-carrier system [8,21,22].
Essential oils are volatile, aromatic liquids obtained from plants with therapeutic activities. They are widely used in food flavoring agents, perfumes, and pharmaceutical drugs [23,24]. Of the many essential oils, turmeric oil and lemongrass oil are known for their high therapeutic potential. Turmeric belongs to the family Zingiberaceae. Traditionally, it is used for the treatment of sprains, superficial wounds, cough, and fungal infections. Both turmeric and its modified essential oil forms are known to have many therapeutic properties such as anticancer [23,25], antifungal [26], antioxidant [27], anti-inflammatory [28], and antimutagenic [29] effects. Arturmerone, a major ingredient of turmeric oil is well-known for its antimutagenic, antiplatelet, and antifungal properties [30,31]. Lemongrass belongs to the family Poaceae. It cools down body temperature, rheumatisms, muscle cramps, and many gastrointestinal disorders [32]. Some of the therapeutic properties of lemongrass oil include anti-inflammatory [33], antimicrobial [34], anticancer [35], and antiproliferative [36] actions. The major ingredient of lemongrass oil is citral, which is also known for its antifungal, anticancer, antiproliferative, and anticlastogenic properties [37]. However, turmeric oil and lemongrass oil have poor physical properties such as hydrophobicity, susceptible to degradation, and volatility, making them difficult to be used in pharmaceutical applications [30,31,38]. These disadvantages can be overcome to an extent by encapsulating these essential oils in a nanocarrier. There are several potential advantages of conjugating anticancer agents with nanoparticles, including targeted delivery and controlled release at diseased sites by changing their pharmacokinetic profile [39,40].
Therefore, in this work, formulation of CS–AL nanocapsules was carried out with the goal of enhancing the physical stability of both the essential oils by encapsulating them into the nanocapsules.
2. Materials and methods
2.1. Chemicals
Sodium AL and CS (degree of acetylation ≥ 75%) were purchased from HiMedia Laboratories (Mumbai, Maharashtra, India). Commercially available turmeric oil (Curcuma longa) and lemongrass oil (Cymbopogon citratus) were purchased from AOS Products Private Limited (New Delhi, India). Thiazolyl blue tetrazolium bromide was purchased from Sigma-Aldrich (Bangalore, Karnataka, India). Human lung adenocarcinoma epithelial cell line (A549) was obtained from the National Centre for Cell Science (Pune, Maharashtra, India). The F-12K 1× nutrient mixture (Kaighn’s modified medium), fetal bovine serum, EDTA–trypsin 0.05%, Dulbecco’s phosphate-buffered saline 1×, and penicillin–streptomycin were procured from Invitrogen (Bangalore, Karnataka, India). Calcium chloride and other solvents used in this study were of analytical grade. The experiments were performed using deionized distilled water and were carried out in triplicates.
2.2. Maintenance of cell culture
Human lung adenocarcinoma epithelial cells (A549) were cultured in F-12K (Kaighn’s) medium containing 10% fetal bovine serum, 100 U/mL penicillin–streptomycin at 37°C in a 5% CO2 humidified atmosphere.
2.3. Preparation of essential oil-loaded CS–AL nanocapsules
Emulsification processes are used generally in the preparation of nanocapsules as well as in other food, cosmetic, and chemical industries [41]. Essential oil-loaded CS–AL nanocapsules were prepared using the method suggested by Lertsutthiwong et al [30], but with some modifications. In brief, the oil in water nanoemulsion was prepared by adding 20 mL aqueous AL solution of various concentrations (0.3 and 0.6 mg/ mL) to 1% (w/v) Tween-80. The pH of aqueous AL solution was maintained between 5 and 5.5 to obtain nano-sized capsules. Subsequently, 0.6 mL of ethanolic turmeric oil solution (20 mg/ mL) was added dropwise into this mixture. Following 15 minutes of sonication, 4 mL of 0.67 mg/mL calcium chloride (CaCl2) solution was added and the emulsion was stirred for 30 minutes. The emulsion was then combined with 4 mL of CS solution of various concentration [0.3 or 0.6 mg/mL in 1% (v/v) acetic acid] and stirred for the next 30 minutes. The pH of CS was maintained in the range of 4.5–5. The resulting suspension was equilibrated overnight and solvent removal was achieved by rotary evaporation at 40°C for 20 minutes. Lemongrass oil-loaded nanocapsules were also synthesized using the same protocol.
2.4. Optimization of process parameters
Various parameters were altered to understand the factors involved in the preparation of nanocapsules. Optimization of essential oil-loaded CS–AL nanocapsules was achieved by varying the concentrations of CS (0.6 or 0.3 mg/mL) and AL (0.6 or 0.3 mg/mL) and by varying the effect of heating the AL solution prior usage for removal of any impurity before use. Furthermore, physicochemical characterization was performed to choose the best parameters for the essential oil-loaded nanocapsules.
2.5. Physicochemical characterization of nanocapsules
2.5.1. Particle size and zeta potential
The hydrodynamic size and zeta potential of nanocapsules were determined by Malvern Zetasizer 3000HS (Malvern Instruments Ltd., UK). Zeta potential provides information about the potential difference between the dispersion medium and the stationary layer of the fluid attached to the dispersed particle, which helps to know about the stability of the nanoformulations. Measurements were made using aqueous diluted samples (2:1 ratio). Using the principle of photon correlation spectrometry, this instrument also gives the measurement of particle-size distributions in the range between 200 and 500 nm.
2.5.2. Scanning electron microscopy
The surface morphology and shape of various nanomaterials and medical and biological samples can be obtained with a field emission scanning electron microscope (JSM-6360, JEOL, Japan) at 15 kV. Approximately 5 mL of the sample was freeze dried and imaged using the scanning electron microscope.
2.5.3. Fourier transform infrared spectroscopy
Oscillations of atoms in molecules were measured by infrared (IR) and Raman spectroscopy. These vibrational spectroscopies provide details about molecular vibrational energy levels. These energy levels are directly proportional to molecular structure, intermolecular interaction, and the chemical bonding [42]. The Fourier transform infrared (FT-IR) spectra of nanoformulations were examined using KBr pellet [1% (w/w) of product in KBr] with a resolution of 4 cm−1 and 100 scans/sample on Perkin Elmer Spectrum RXI FT-IR spectrophotometer. It is a powerful tool for identifying different types of chemical bonds in a molecule by producing an infrared absorption spectrum. The FT-IR spectra of essential oil, bare CS–AL nanocapsules, and essential oil-loaded CS–AL nanocapsules were collected between 4000 and 800 cm−1.
2.5.4. Encapsulation efficiency of essential oil-loaded nanocapsules
To determine their encapsulation efficiency (EE%), nanocapsules were first separated by centrifugation (6000 rpm) at 4°C for 10 minutes from the aqueous medium containing nonassociated oil. The supernatant was removed and 3 mL of methanol was added to the pellet, vortexed well, and centrifuged for another 30 minutes. The supernatant was collected and quantified spectrophotometrically. Using a standard curve that was plotted using different concentrations of oil, the concentration of the unknown oil was estimated. The oil EE% of the nanocapsules was calculated using the following equation:
2.5.5. In vitro release studies
An in vitro release study was performed using the dialysis method. In brief, the dialysis bag was soaked in distilled water to remove the preservatives and rinsed with phosphate-buffered saline (PBS) solution. The essential oil-loaded nanocapsules were redispersed in 3 mL of PBS solution and loaded into the dialysis bag, surrounded by 50 mL of PBS containing 20% ethanol at pH 1.5 and 7.4. The use of ethanol helps to minimize aggregation and release oil more uniformly [43]. The time-dependent release study at 0–12 hours was performed. All sets were incubated at 37°C under gentle agitation. At definite time intervals, 3 mL of the medium was removed and was replaced with fresh medium and quantified spectrophotometrically. The release was quantified as follows:
2.5.6. Hemocompatibility assay
The hemocompatibility of essential oil-loaded nanocapsules can be understood by the hemolysis test. Blood was drawn from a healthy human volunteer, with informed consent, to a tube containing anticoagulant solution of EDTA and incubated with 0.9% saline at 37°C. Essential oil-loaded nanocapsules were added to diluted blood and incubated for 60 minutes at 37°C. Blood with distilled water was taken as the positive control and 0.9% saline with blood was taken as the negative control. Samples were centrifuged at 3000 rpm for 5 minutes after incubation.
2.5.7. Cell viability assay
The antiproliferative activity of the cancer cells was observed using yellow tetrazolium dye, which is reduced to purple formazan in live cells. These formazan crystals can then be dissolved using PBS and quantified using a spectrophotometer (1420-040 VICTOR3 multilabel counter, PerkinElmer, USA) at 490 nm. An increase in purple color directly contributes to the increase in the number of cells. Cells were seeded into 96-well plate 24 hours before the treatment. In brief, the cells were exposed to oil-loaded nanocapsules, free oil (dissolved in ethanol), and bare nanocapsules. The relative viability was expressed as a percentage of the control well that was treated with the carrier only. Cell viability (%) was estimated as a ratio of the absorbance of treated cell (Nt) to the absorbance of untreated cells (Nu) multiplied by 100.
3. Results and discussion
3.1. Size and stability of nanocapsules
Essential oil-loaded nanocapsules were prepared using the ionic gelation method. Calcium chloride was used as a crosslinker and ethanol was used to dilute the oil. Particle size and stability was analyzed using the zetasizer. The following three parameters were considered: heating of AL solution and concentrations of AL and CS. Initially, the concentration of CS was kept constant and the concentration of AL was varied. For every concentration, the effect of heat was also observed (Tables 1 and 2). In general, zeta potential values less than −30 mV and +30 mV are considered stable. Considering both particle size and stability, it was observed that the combination of 0.3 mg/mL AL and 0.6 mg/mL CS produced best results with particle sizes of 256 and 226 nm for turmeric oil- and lemongrass oil-loaded nanocapsules, respectively. The zeta potential was greater than +35 mV in both oil-loaded particles, indicating their good stability.
Table 1.
Optimization of process parameters for the encapsulation of turmeric oil and lemongrass oil.
| Concentration of alginate (mg/mL) | Concentration of chitosan (mg/mL) | With heating | Without heating | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Turmeric oil | Lemongrass oil | Turmeric oil | Lemongrass oil | ||||||
|
|
|
|
|
||||||
| Particle size (nm) | Zeta potential (mV) | Particle size (nm) | Zeta potential (mV) | Particle size (nm) | Zeta potential (mV) | Particle size (nm) | Zeta potential (mV) | ||
| 0.3 | 0.6 | 363.5 | 45.5 | 405.3 | 38.8 | 256.6 | 37.3 | 226.4 | 35.7 |
| 0.6 | 0.6 | 399.1 | 40.6 | 702.1 | 38.2 | 895.5 | −21.3 | 786.6 | 40.6 |
Bold values indicate the optimized values.
Table 2.
Optimization of ratios of alginate and chitosan.
| Concentration of alginate (mg/mL) | Concentration of chitosan (mg/mL) | Turmeric oil | Lemongrass oil | ||
|---|---|---|---|---|---|
|
|
|
||||
| Particle size (nm) | Zeta potential (mV) | Particle size (nm) | Zeta potential (mV) | ||
| 0.3 | 0.6 | 256.6 | 37.3 | 226.4 | 35.7 |
| 0.3 | 0.3 | 92.37 | 27.0 | 59.34 | −11.8 |
However, consistent differences in particle size or zeta potential were not revealed due to the heating of AL solutions before the preparation process. Thereafter, heating of AL solutions was not performed. A better size than previous reports [18] was achieved mostly due to maintenance of pH of AL (5–5.3) and CS (4.5–5) solutions [44]. Within this pH, the carboxyl groups were ionized in AL and the amine groups were protonated in CS, which had a major contribution to the formation of complex and optimal interaction for the formation of smaller nanocapsules. Therefore, the concentration of the polymers was optimized and the same concentration was used for further physicochemical characterization studies.
3.2. Encapsulation efficiency
The EE% was determined using ultraviolet–visible spectroscopy. Using a standard graph (y = 0.044x + 0.030, R2 = 0.991) and (y = 0.0772x + 2.5194, R2 = 0.9846), the concentrations of the unknown turmeric oil and lemongrass oil were determined, respectively. Samples were analyzed in triplicates and it was found that 71.1% of the total turmeric oil and 86.9% of the lemongrass oil were encapsulated into the nanocapsules. This suggests that our carrier is very well suited for encapsulation of hydrophobic drugs.
3.3. FT-IR analysis
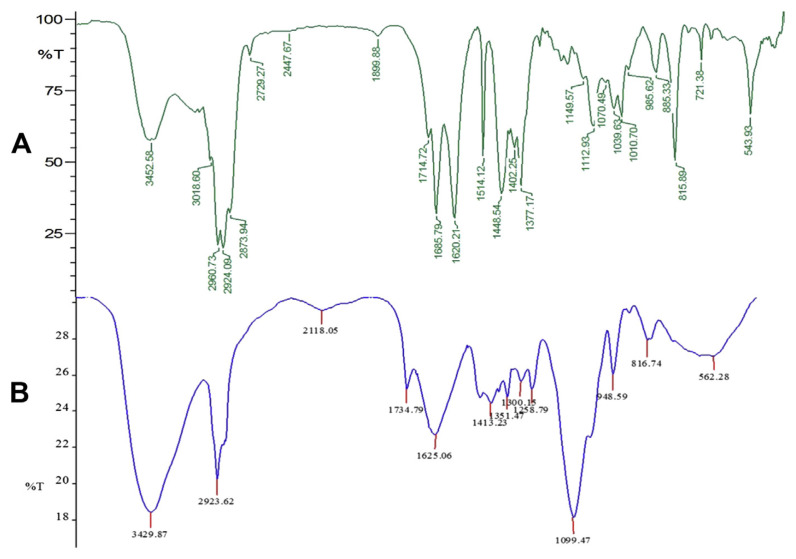
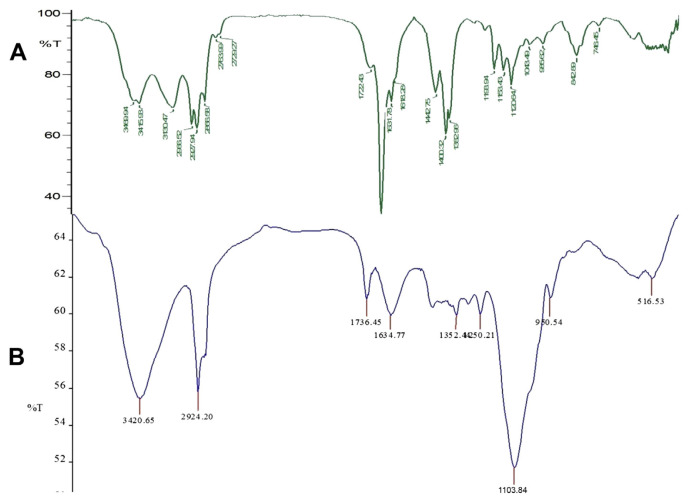
To support the results of zeta potential and EE% of nanocapsules, further studies were carried out by FT-IR analysis to know about the complex formation and interaction of major functional groups involved in the encapsulation of essential oil (Figs. 1A and B and 2A and B). These results helped us in confirming the presence of oil in our nanocapsules. The spectra of bare nanocapsules clearly revealed that the amino groups of CS and the carboxyl groups of AL interact together to form the strong PEC [21]. The peaks at 3428 cm−1 indicate the presence of hydroxyl and amino groups from AL and CS [45]. The peak at 1622.38 cm−1 of blank CS–AL nanocapsules indicates the association of the carboxylate group of AL with CS [46] and the peak at 1080 cm−1 indicates the amino group of CS. It is also evident that the major peaks present in the spectra of bare nanocapsules are also prominent in the loaded nanocapsules. This indicates the strong interaction between CS and AL in oil-loaded nanocapsules.
Fig. 1.
Fourier transform infrared spectra: (A) turmeric oil; (B) turmeric oil-loaded nanocapsules.
Fig. 2.
Fourier transform infrared spectra: (A) lemongrass oil; (B) lemongrass oil-loaded nanocapsules.
However, there are many minor peaks and some major peaks, which are formed due to the presence of essential oil as shown in Figs. 1B and 2B. In the case of turmeric oil-loaded nanocapsules, the peak at 1734 cm−1 in Fig. 2B corresponds to the 1714 cm−1 peak present in Fig. 1A and indicates the presence of C=O groups probably from the active component, turmerones [47]. Similarly, the peak at 1736 cm−1 in Fig. 2B also corresponds to the peak at 1722 cm−1 in Fig. 2A, indicating the presence of citral, a highly therapeutic compound in lemongrass oil. The shift in the peaks indicates the possible interaction and successful encapsulation of oil into the carrier. Major peaks at 3429, 2923, 1625, and 1099 cm−1 in turmeric oil-loaded nanocapsules and at 3428, 2924, 1622, and 1080 cm−1 in lemongrass oil-loaded nanocapsules indicate the presence of N–H, O–H, C=O, and CH–OH functional groups, respectively.
3.4. Scanning electron microscopy
To obtain information about the morphology of the turmeric oil- and lemongrass oil-loaded nanocapsules, scanning electron microscopy (SEM) analysis was performed. SEM analysis was carried out to visualize the size and shape of the nanocapsules. It can be inferred from Fig. 3A and B that the essential oil-loaded nanocapsules have spherical surface morphology and their mean size was found to be below 300 nm.
Fig. 3.
Scanning electron microscopic images: (A) turmeric oil-loaded nanocapsules; (B) lemongrass oil-loaded nanocapsules.
3.5. In vitro release study
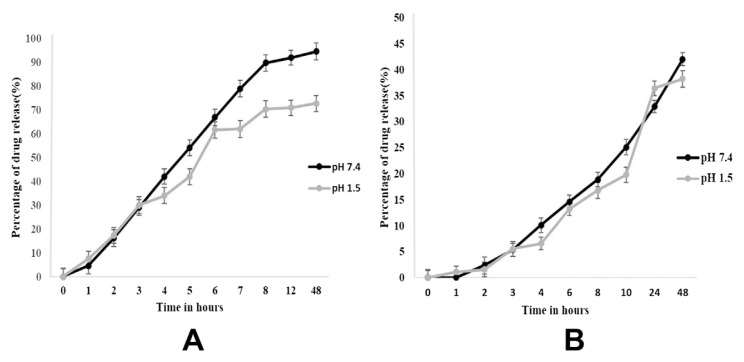
Fig. 4 indicates the release of both the essential oil-loaded CS–AL nanocapsules at pH 7.4 and 1.5. It was observed that at pH 7.4, there was a better sustained release than at pH 1.5. At pH 7.4, approximately 90% of the encapsulated turmeric oil was released, whereas only 42% of the encapsulated lemongrass oil was released for a period of 48 hours. At pH 1.5, the release was less than 70% and 38% for both the encapsulated turmeric oil and lemongrass oil, respectively (Fig. 4A and B). This might be attributed to the formation of insoluble alginic skin at acidic pH. This suggests that the drug release of CS–AL nanoparticles is pH sensitive and more of the oil will be released in the blood system (at pH 7.4) than in the acidic stomach (at pH 1.5). Therefore, more of the oil will be absorbed into the blood system and in turn transported in the blood circulatory system to the site of infection more efficiently than when the oil is not encapsulated in the nanoparticles.
Fig. 4.
Essential oil release kinetics. Drug-release profile of (A) turmeric oil-loaded nanocapsules at pH 7.4 and 1.5. (B) Lemongrass oil-loaded nanocapsules at pH 7.4 and 1.5.
3.6. Hemocompatibility
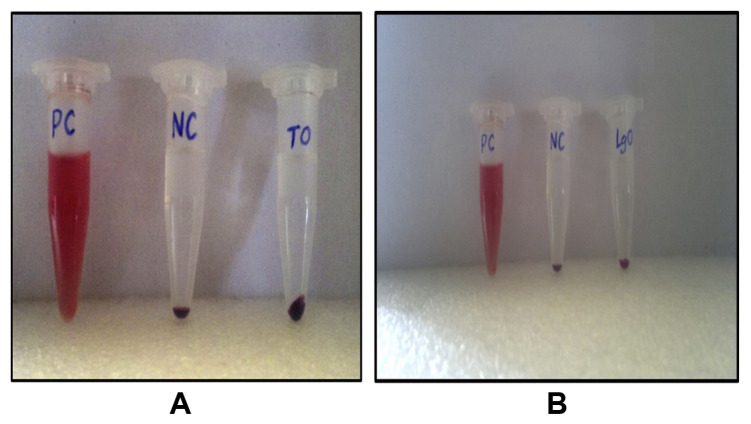
Both the essential oil-loaded nanocapsules were incubated with blood samples and the results were observed. The obtained image clearly demonstrates that there was no lysis in the supernatant and the red blood cells settled down as pellets. Absence of lysis in the incubated sample demonstrated that this formulation was perfectly suitable for circulation in blood (Fig. 5A and B). Distilled water was used as the positive control to produce lysis of red blood cells, which contributes to the red supernatant. Saline, which is used as the negative control, did not show any hemolysis or toxicity to red blood cells, and therefore, the percentage of lysis is 0, whereas it is 100 for distilled water. The blood samples incubated with oil-loaded nanocapsules showed results similar to those of saline, and therefore, it can be concluded that they have 0% hemolysis. Thus, it is proved that both turmeric oil- and lemongrass oil-loaded nanocapsules were hemocompatible and could be suitable for further drug-delivery applications.
Fig. 5.
Hemolysis assay. Hemocompatibility of (A) turmeric oil-loaded nanocapsules; (B) lemongrass oil-loaded nanocapsules. LGO = lemongrass oil; NC = negative control; PC = positive control; TO = turmeric oil.
3.7. Cell viability assay
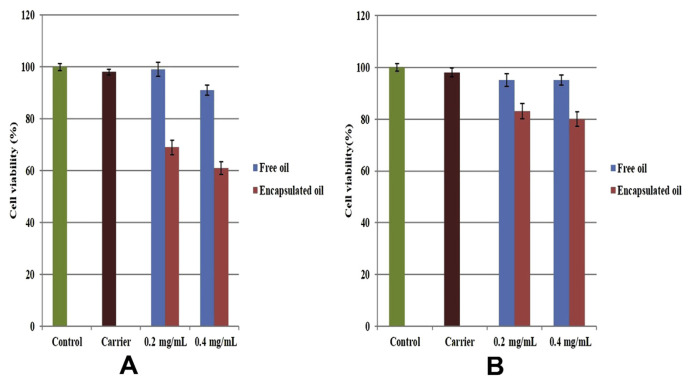
The antiproliferative effect of both turmeric oil- and lemongrass oil-loaded nanocapsules was evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. In brief, cultured A549 cells were exposed to carrier alone, free oil, and encapsulated oil at various concentrations (0.4 and 0.2 mg/mL) for 24 hours. To find the viability of cells, MTT reagent (thiazolyl blue tetrazolium bromide) was added to all the wells and incubated for 24 hours. As a result, formazan crystals were dissolved using PBS solution and the absorbance was noted using a spectrophotometer.
Our results revealed that there was a significant decline in cancer cell viability. When treated with 0.4 mg/mL turmeric oil-loaded CS–AL nanocapsules, there was about 40% decline in viability. At a lesser concentration (0.2 mg/mL turmeric oil-loaded nanocapsules), the treated cells showed 31% decline in cancer cell viability. By contrast, at the same concentration, lemongrass oil-loaded nanocapsules showed toxic effects with activity of 20% and 17% for 0.4 and 0.2 mg/mL concentrations, respectively (Fig. 6A and B). Concentration-dependent cell viability was observed in both free and encapsulated oil. Compared with lemongrass oil, turmeric oil showed a high level of inhibition in cell viability. This might be attributed to the presence of various powerful anticancer components (turmerone), which were responsible for reduction in cancer cell viability. This has led the path for turmeric oil and its components to be tested in various clinical trials.
Fig. 6.
Cytotoxicity of essential oil-loaded nanocapsules in A549 cells using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay: (A) turmeric oil; (B) lemongrass oil.
Free oil of both the concentrations showed less than 10% toxic activity. This may be due to the fact that encapsulated oil could reach to individual cells and deliver the oil to them. However, it was not possible for the free oil to reach the cells individually owing to its hydrophobicity. The nontoxic nature of carrier was confirmed with the viability of treated cells, which was 98%. Higher loading of turmeric oil was expected to increase toxic activity and our carrier was very well suited for these kinds of drug-delivery applications, which were also supported by our hemocompatibility results.
4. Conclusion
CS–AL nanocapsules were synthesized using an ionic gelation method and the size of the particles was below 300 nm with good stability. SEM results suggest that the morphology of both the essential oil-loaded nanocapsules was spherical. EE% was found to be 71% and 86.9% for turmeric oil- and lemongrass oil-loaded nanocapsules, respectively. FT-IR results showed the functional groups, which played a role in the interaction. The drug-release profile showed a slow and sustained release at neutral pH for 48 hours. The antiproliferative activity of essential oil-loaded nanocapsules was estimated using MTT assay and it was found that the activity of turmeric oil is retained in the carrier. The carrier showed high cell viability percentage, which indicates that it is nontoxic. It was also confirmed from the clear supernatant in hemolysis assay that our oil-loaded nanocapsules were hemocompatible, which suggests that they are a suitable carrier for further biomedical applications.
Acknowledgments
We would like to acknowledge the Department of Science and Technology for providing the external funding to carry out the project entitled “Biocompatibility of Surface Modified and Unmodified Graphene Oxide Nanoparticles” (No. SR/FT/LS-18). We would also like to thank the Centre for NanoTechnology and Advanced Biomaterials (CeNTAB) and the Centre for Advanced Research in Indian System of Medicine (CARISM), SASTRA University for providing the facilities to carry out this work.
Funding Statement
We would like to acknowledge the Department of Science and Technology for providing the external funding to carry out the project entitled “Biocompatibility of Surface Modified and Unmodified Graphene Oxide Nanoparticles” (No. SR/FT/LS-18).
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
References
- 1. Sahoo SK, Parveen S, Panda JJ. The present and future of nanotechnology in human health care. Nanomedicine. 2007;3:20–31. doi: 10.1016/j.nano.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 2. Fu PP, Xia Q, Hwang HM, Ray PC, Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal. 2014;22:64–75. doi: 10.1016/j.jfda.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marcos ADN, Isao K, Mitsutoshi N. Nanotechnology for bioactives delivery systems. J Food Drug Anal. 2012;20:184–8. [Google Scholar]
- 4. Freitas RA., Jr What is nanomedicine? Nanomedicine. 2005;1:2–9. doi: 10.1016/j.nano.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 5. He W, Liu Y, Wamer WG, Yin JJ. Electron spin resonance spectroscopy for the study of nanomaterial-mediated generation of reactive oxygen species. J Food Drug Anal. 2014;22:49–63. doi: 10.1016/j.jfda.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yadav A, Ghune M, Jain DK. Nano-medicine based drug delivery system. J Adv Pharm Techol Res. 2011;1:201–13. [Google Scholar]
- 7. Mastrobattista E. Advanced drug delivery in motion. Int J Pharm. 2013;454:517–20. doi: 10.1016/j.ijpharm.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 8. Sarmento B, Ribeiro A, Veiga F, Sampaio P, Neufeld R, Ferreira D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm Res. 2007;24:2198–206. doi: 10.1007/s11095-007-9367-4. [DOI] [PubMed] [Google Scholar]
- 9. Goycoolea FM, Lollo G, Remuñán-López C, Quaglia F, Alonso MJ. Chitosan-alginate blended nanoparticles as carriers for the transmucosal delivery of macromolecules. Biomacromolecules. 2009;10:1736–43. doi: 10.1021/bm9001377. [DOI] [PubMed] [Google Scholar]
- 10. Gazori T, Khoshayand MR, Azizi E, Yazdizade P, Nomani A, Haririan I. Evaluation of alginate/chitosan nanoparticles as antisense delivery vector: formulation, optimization and in vitro characterization. Carbohydr Polym. 2009;77:599–606. [Google Scholar]
- 11. Tønnesen HH, Karlsen J. Alginate in drug delivery systems. Drug Dev Ind Pharm. 2002;28:621–30. doi: 10.1081/ddc-120003853. [DOI] [PubMed] [Google Scholar]
- 12. George M, Abraham TE. Polyionic hydrocolloids for the intestinal delivery of protein drugs: alginate and chitosan—a review. J Control Release. 2006;114:1–14. doi: 10.1016/j.jconrel.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 13. Dutta PK, Dutta J, Tripathi VS. Chitin and chitosan: chemistry, properties and applications. J Sci Ind Res (India) 2004;63:20–31. [Google Scholar]
- 14. Trung TS, Phuong PTD. Bioactive compounds from byproducts of shrimp processing industry in Vietnam. J Food Drug Anal. 2012;20:194–7. [Google Scholar]
- 15. Chen MC, Mi FL, Liao ZX, Hsiao CW, Sonaje K, Chung MF, Hsu LW, Sung HW. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv Drug Deliv Rev. 2013;65:865–79. doi: 10.1016/j.addr.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 16. Feng C, Wang Z, Jiang C, Kong M, Zhou X, Li Y, Cheng X, Chen X. Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: in vitro and in vivo evaluation. Int J Pharm. 2013;457:158–67. doi: 10.1016/j.ijpharm.2013.07.079. [DOI] [PubMed] [Google Scholar]
- 17. Ahn S, Lee IH, Lee E, Kim H, Kim YC, Jon S. Oral delivery of an anti-diabetic peptide drug via conjugation and complexation with low molecular weight chitosan. J Control Release. 2013;170:226–32. doi: 10.1016/j.jconrel.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 18. Luo Y, Wang Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int J Biol Macromol. 2014;64:353–67. doi: 10.1016/j.ijbiomac.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 19. Simsek-Ege FA, Bond GM, Stringer J. Polyelectrolye complex formation between alginate and chitosan as a function of pH. J Appl Polym Sci. 2003;88:346–51. [Google Scholar]
- 20. Douglas KL, Tabrizian M. Effect of experimental parameters on the formation of alginate–chitosan nanoparticles and evaluation of their potential application as DNA carrier. J Biomater Sci Polym Ed. 2005;16:43–56. doi: 10.1163/1568562052843339. [DOI] [PubMed] [Google Scholar]
- 21. Li P, Dai YN, Zhang JP, Wang AQ, Wei Q. Chitosan-alginate nanoparticles as a novel drug delivery system for nifedipine. Int J Biomed Sci. 2008;4:221–8. [PMC free article] [PubMed] [Google Scholar]
- 22. Lacerda L, Parize AL, Fávere V, Laranjeira MC, Stulzer HK. Development and evaluation of pH-sensitive sodium alginate/chitosan microparticles containing the antituberculosis drug rifampicin. Mater Sci Eng C Mater Biol Appl. 2014;39:161–7. doi: 10.1016/j.msec.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 23. Burt S. Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol. 2004;94:223–53. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 24. Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem. 2003;10:813–29. doi: 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]
- 25. Manosroi J, Dhumtanom P, Manosroi A. Anti-proliferative activity of essential oil extracted from Thai medicinal plants on KB and P388 cell lines. Cancer Lett. 2006;235:114–20. doi: 10.1016/j.canlet.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 26. Apisariyakul A, Vanittanakom N, Buddhasukh D. Antifungal activity of turmeric oil extracted from Curcuma longa (Zingiberaceae) J Ethnopharmacol. 1995;49:163–9. doi: 10.1016/0378-8741(95)01320-2. [DOI] [PubMed] [Google Scholar]
- 27. Ling J, Wei B, Lv G, Ji H, Li S. Anti-hyperlipidaemic and antioxidant effects of turmeric oil in hyperlipidaemic rats. Food Chem. 2012;130:229–35. [Google Scholar]
- 28. Liju VB, Jeena K, Kuttan R. An evaluation of antioxidant, anti-inflammatory, and antinociceptive activities of essential oil from Curcuma longa. L. Indian J Pharmacol. 2011;43:526–31. doi: 10.4103/0253-7613.84961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liju VB, Jeena K, Kuttan R. Acute and subchronic toxicity as well as mutagenic evaluation of essential oil from turmeric (Curcuma longa L) Food Chem Toxicol. 2013;53:52–61. doi: 10.1016/j.fct.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 30. Lertsutthiwong P, Rojsitthisak P, Nimmannit U. Preparation of turmeric oil-loaded chitosan-alginate biopolymeric nanocapsules. Mater Sci Eng C Mater Biol Appl. 2009;29:856–60. [Google Scholar]
- 31. Lertsutthiwong P, Noomun K, Jongaroonngamsang N, Rojsitthisak P, Nimmannit U. Preparation of alginate nanocapsules containing turmeric oil. Carbohydr Polym. 2008;74:209–14. [Google Scholar]
- 32. Shah G, Shri R, Panchal V, Sharma N, Singh B, Mann AS. Scientific basis for the therapeutic use of Cymbopogon citratus, Stapf (lemon grass) J Adv Pharm Technol Res. 2011;2:3–8. doi: 10.4103/2231-4040.79796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alitonou GA, Avlessi F, Sohounhloue DK, Agnaniet H, Bessiere JM, Menut C. Investigations on the essential oil of Cymbopogon giganteus from Benin for its potential use as an anti-inflammatory agent. Int J Aroma Ther. 2006;16:37–41. [Google Scholar]
- 34. Leimann FV, Gonçalves OH, Machado RAF, Bolzan A. Antimicrobial activity of microencapsulated lemongrass essential oil and the effect of experimental parameters on microcapsules size and morphology. Mater Sci Eng C Mater Biol Appl. 2009;29:430–6. [Google Scholar]
- 35. Sharma PR, Mondhe DM, Muthiah S, Pal HC, Shahi AK, Saxena AK, Qazi GN. Anticancer activity of an essential oil from Cymbopogon flexuosus. Chem Biol Interact. 2009;179:160–8. doi: 10.1016/j.cbi.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 36. Santin MR, dos Santos AO, Nakamura CV, Dias Filho BP, Ferreira IC, Ueda-Nakamura T. In vitro activity of the essential oil of Cymbopogon citratus and its major component (citral) on Leishmania amazonensis. Parasitol Res. 2009;105:1489–96. doi: 10.1007/s00436-009-1578-7. [DOI] [PubMed] [Google Scholar]
- 37. Ganjewala D. Cymbopogon essential oils: chemical compositions and bioactivities. Int J Essent Oil Ther. 2009;3:56–65. [Google Scholar]
- 38. Weisheimer V, Miron D, Silva CB, Guterres SS, Schapoval EE. Microparticles containing lemongrass volatile oil: preparation, characterization and thermal stability. Pharmazie. 2010;65:885–90. [PubMed] [Google Scholar]
- 39. Fan Z, Fu PP, Yu H, Ray PC. Theranostic nanomedicine for cancer detection and treatment. J Food Drug Anal. 2014;22:3–17. doi: 10.1016/j.jfda.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu CL, Wang XW, Lin ZZ, Xie ZH, Wang XR. Cell microenvironment stimuli-responsive controlled-release delivery systems based on mesoporous silica nanoparticles. J Food Drug Anal. 2014;22:18–28. doi: 10.1016/j.jfda.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhong F, Xu W, Fu T, Li Y. Preparation and characterization of functional compounds encapsulated microemulsion with nonionic surfactants. J Food Drug Anal. 2012;20:203–7. [Google Scholar]
- 42. Li YS, Church JS. Raman spectroscopy in the analysis of food and pharmaceutical nanomaterials. J Food Drug Anal. 2014;22:29–48. doi: 10.1016/j.jfda.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Parris N, Cooke PH, Hicks KB. Encapsulation of essential oils in zein nanospherical particles. J Agric Food Chem. 2005;15:4788–92. doi: 10.1021/jf040492p. [DOI] [PubMed] [Google Scholar]
- 44. Motwani SK, Chopra S, Talegaonkar S, Kohli K, Ahmad FJ, Khar RK. Chitosan–sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: formulation, optimization and in vitro characterization. Eur J Pharm Biopharm. 2008;68:513–25. doi: 10.1016/j.ejpb.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 45. Das RK, Kasoju N, Bora U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine. 2010;6:153–60. doi: 10.1016/j.nano.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 46. Lawrie G, Keen I, Drew B, Chandler-Temple A, Rintoul L, Fredericks P, Grøndahl L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules. 2007;8:2533–41. doi: 10.1021/bm070014y. [DOI] [PubMed] [Google Scholar]
- 47. Hong CH, Kim Y, Lee SK. Sesquiterpenoids from the rhizome of Curcuma zedoaria. Arch Pharm Res. 2001;24:424–6. doi: 10.1007/BF02975188. [DOI] [PubMed] [Google Scholar]