Abstract
Diabetes mellitus is an endocrine disease in which the pancreas does not produce sufficient insulin or the body cannot effectively use the insulin it produces. Insulin therapy has been the best choice for the clinical management of diabetes mellitus. The current insulin therapy is via subcutaneous injection, which often fails to mimic the glucose homeostasis that occurs in normal individuals. This provokes numerous attempts to develop a safe and effective noninvasive route for insulin delivery. Oral delivery is the most convenient administration route. However, insulin cannot be well absorbed orally because of its rapid enzymatic degradation in the gastrointestinal tract. Therefore, nanoparticulate carriers such as polymeric nanoparticles and micelles are employed for the oral delivery of insulin. These nanocarriers protect insulin from degradation and facilitate insulin uptake via a transcellular and/or paracellular pathway. This review article focuses on the application of nanoparticles and micelles in insulin oral delivery. The recent advances in this topic are also reviewed.
Keywords: diabetes mellitus, insulin, micelles, nanoparticles, oral delivery
1. Introduction
Diabetes mellitus is an endocrine disease in which the pancreas does not produce sufficient insulin or the body cannot efficiently use the insulin it produces [1,2]. There are two basic types of diabetes: type I and type II. In type I diabetes, patients produce very little or no insulin. In type II diabetes, patients cannot use insulin efficiently. The number of diabetics will increase to 438 million worldwide by the year 2030, according to the World Health Organization (WHO) [3]. The primary goal for the treatment of type I and type II diabetes is to cure the symptoms related to hyperglycemia [4]. Insulin has an important role in diabetes treatment. Patients with type I diabetes require daily injections of insulin to survive. Patients with type II diabetes may or may not require exogenous insulin for treatment.
Human insulin is composed of 51 amino acid residues. Its molecular formula is C257H387N65O77S6 and the corresponding molecular weight is 5808 daltons. Human insulin consists of two amino acid chains (an A chain and a B chain) that are linked by disulfide bonds, which contain 21 amino acid residues and 30 amino acid residues, respectively. Insulin can be isolated from human, porcine, bovine or sheep sources [5]. The blood glucose level in diabetic patients typically ranges 14–50 mmol/L [6]. By using exogenous insulin, the blood glucose level is reduced and maintained within a narrow range of 3.5–7.0 mmol/L. Insulin promotes the cellular uptake of glucose and increases the synthesis of glycogen, fatty acids, and proteins. Thus, the role of insulin is to convert excess glucose into two storage forms—namely, glycogen and triglycerols—and maintain glucose homeostasis [2]. Insulin cannot be orally administered because it is unstable and degraded by proteolytic enzymes in the gastrointestinal tract [7]. The current insulin therapy is administered by subcutaneous injection, and the major adverse effects of this therapy include hypoglycemia, allergy, insulin resistance, and edema [8]. Multiple and frequent injections of insulin are necessary but burdensome, which leads to poor patient compliance. In addition, there is a risk of infection at the injection site, especially for diabetic patients [6,9]. All of these events have provoked numerous attempts to develop a safe and effective noninvasive route for insulin delivery.
Oral, buccal, rectal, ocular, transdermal, intravaginal, pulmonary, and nasal routes have been explored as noninvasive routes for insulin delivery [6]. Among these delivery routes, the administration of insulin by the oral route has been the goal for many researchers because it minimizes the risk of hypoglycemia while obtaining high patient compliance. However, the delivery of insulin via the oral route is a challenge because of enzymatic instability and the poor absorption of insulin. Among the possible strategies to achieve acceptable bioavailability, polymeric nanoparticles and micelles have immense potential for insulin oral delivery because of their nanosized feature and feasibility for surface modification [9,10]. These nanocarriers possess several advantages over other conventional delivery systems (e.g., tablets, capsules, beads, microparticles, and microemulsions) [11]. The updates on the most promising advances of nanoparticles and micelles in insulin oral delivery are reviewed.
2. Gastrointestinal hurdles in insulin oral delivery
The administration of insulin by the oral route has been the goal of many researchers. However, the oral bioavailability of insulin is very poor. Macromolecular proteins normally cannot cross the intestinal epithelium. They will instead be degraded in the gastrointestinal tract before absorption [12]. Three main obstacles of insulin oral delivery need to be considered: (1) the enzymes present in the gastrointestinal tract, (2) the physiological barrier of the gastrointestinal tract, and (3) the physicochemical property of insulin.
2.1. Enzymatic barrier
The gastrointestinal tract has a variety of enzymatic barriers for insulin oral delivery. The insulin can be degraded by intracellular enzymes (e.g., cathepsins), bacterial flora in the mucus layer and in the epithelial cells of the intestine, and proteolytic enzymes in the stomach and in the intestinal lumen (e.g., pepsin, trypsin, and chymotrypsin) at the brush border membrane (e.g., endopeptidases). These enzymes denature protein drugs. Enzyme inhibitors can slow the insulin degradation rate and increase the insulin that is available for absorption. Sodium cholate and aprotinin reportedly act as enzyme inhibitors and improve insulin absorption in rats [12,13]. However, the use of enzyme inhibitors in long-term diabetic therapy remains questionable because of possible toxicity.
2.2. Physiological barrier
The epithelial cells of the gastrointestinal tract are tightly bound by tight junctions (i.e., the zonulae occludentes) in which the outer surface of the intestinal epithelium is coated by mucus and glycocalyx layers, and thus inhibits the passage of insulin and its subsequent absorption [14]. Absorption may be enhanced by using absorption enhancers such as bile salts, trisodium citrates, EDTA, labrasol, and polymeric materials (e.g., chitosan), which help to open the tight junctions of the intestinal epithelium [13,15,16].
2.3. Physicochemical properties of insulin
The pore radius of the intestinal mucosa ranges 7–15 Å, which is an important barrier for macromolecular insulin translocation. The insulin molecules tend to aggregate at concentrations above 100nM. The transformation of the insulin monomer with a molecular dimension of approximately 12–14 Å into a hexameric conformation impairs its transport across the intestinal epithelium [7]. Temperature, solvents, and additives may disrupt the primary amino acid sequence and tertiary structure of insulin. The alteration in the functional moiety or native charge of insulin has an impact on its intestinal transport. At physiological pH, the carboxylic and amino groups of insulin are entirely ionized, which results in a zwitterionic configuration. It is likely to preclude insulin absorption from transcellular diffusion, unless the charges are neutralized through ion pairs [7]. The large molecular size of insulin nevertheless remains an obstacle to its absorption. Modification of insulin chemical structure against possible enzymatic degradation is an approach to raise its bioavailability. A diacyl derivative of insulin has been shown to maintain insulin biological activity and to increase its intestinal absorption [17]. However, the application of this approach to insulin is very challenged because of the structural complexity of proteins.
3. Delivery systems for insulin oral delivery
The successful oral delivery of insulin requires considering the physiological and biological stability of insulin in formulations, in the gastrointestinal tract, and in the cytosol of enterocytes. The barriers occurring principally in the oral delivery of insulin can be overcome via incorporating functional excipients in the dosage forms. The functional excipients act as a stabilizer, a protease inhibitor, a mucoadhesive agent, and/or a permeation enhancer to maintain insulin stability and enhance its paracellular and/or transcellular transport in terms of increasing its oral bioavailability. Various dosage forms for insulin oral delivery such as nanoparticles, microparticles, hydrogels, tablets, and capsules have been developed worldwide. Among these dosage forms, nanoparticles have stability and the ability to undergo cellular uptake, compared to the other delivery systems, when applied in vivo [4,5]. Nanoparticles are feasible to transport and internalize through the intestinal epithelial membrane, which changes the pharmacokinetic performance of insulin after oral administration [8]. The performance of nanoparticles on insulin oral delivery are affected by several parameters such as the surface charge, particle size, polymer property, polymer–insulin interaction, insulin loading, insulin release performance, residence time at absorption site and the clearance rate from the body [12].
3.1. Nanoparticles and micelles
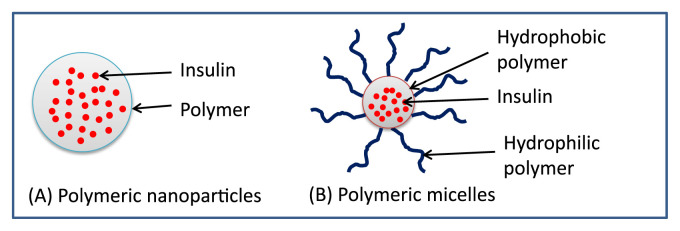
Nanocarriers often refer to particles with a size of 1–100 nm. Various nanocarriers for insulin delivery have been reported such as polymeric nanoparticles, liposomes, solid lipid nanoparticles, and micelles [2]. In this paper, the polymeric nanoparticles and micelles are further discussed. Fig. 1 illustrates their basic conformations. Nanoparticles involve nanospheres and nanocapsules. Nanospheres are a matrix type of particle in which the drug is uniformly dispersed or dissolved in the polymer matrix, whereas nanocapsules are vesicles in which the drug core is surrounded by a polymeric film [18]. Micelles are formed by amphiphilic copolymers which self-assembled to nanosized aggregates above the critical micellar concentration. The hydrophobic moiety forms the core of micelles whereas the hydrophilic moiety forms the corona in the shell of micelles. Micelles possess a dynamic structure where the unimers of the amphiphilic copolymer are interchangeable. There is a wide variety of polymers and preparation methods applied to prepare insulin-loaded nanoparticles and micelles (Table 1). The mechanism of insulin delivered by nanoparticles and micelles is dominated by endocytosis, which is affected by the surface properties of the nanocarriers [18].
Fig. 1.
Diagrammatic illustration of insulin-loaded polymeric nanoparticles and micelles.
Table 1.
Different types of polymers and methods applied to prepare insulin-loaded nanoparticles and micelles.
| Nanocarriers | Preparation method | Material used | Refs |
|---|---|---|---|
| Nanoparticles | Complex coacervation | Chitosan/sodium alginate | [42] |
| Ionic gelation | Chitosan/poly(γ-glutamic acid); Chitosan/alginate | [35,39] | |
| Solvent evaporation | Poly(ɛ-caprolactone)/Eudragit RS 100; Poly(lactide-co-glycolide)/Eudragit RS100 | [14,20] | |
| Ionotropic gelation and polyelectrolyte complexation | Alginate/dextran sulfate | [38,41] | |
| Polymerization of monomers | Chitosan/poly(methyl methacrylate); Chitosan/cyclodextrin/polymethacrylic acid | [40,46] | |
| Micelles | Dialysis method | Poly(ethylene glycol)-b-poly(aspartic acid-co-aspartamidophenylboronic acid) and poly(aspartic acid-co-aspartglucosamine), [PEG-b-P(Asp-co-AspPBA) and P(Asp-co-AGA)] | [26] |
| Film formation | N-octyl-N-arginine chitosan | [27] |
PLGA = poly(lactide-co-glycolide).
3.2. Approaches for insulin oral delivery
Nanocarriers have immense potential for the effective oral delivery of insulin. Designing nanocarriers to improve insulin gastrointestinal absorption may be achieved via modifying the polymer or nanoparticle surface property and applying an enteric coating onto the nanoparticles. These can be combined with enzyme inhibitors or absorption enhancers, as previously mentioned [12]. Table 2 summarizes these strategies to enhance insulin absorption via using nanoparticle delivery systems.
Table 2.
Approaches to enhance insulin oral absorption using various nanocarrier delivery systems.
| Approach | Nanoparticle composition | Pharmacological effect | Refs |
|---|---|---|---|
| Polymeric nanocarrier | Lectin-polystyrene nanoparticles | 2-fold increase in particle absorption | [12,27] |
| Enteric coating | Chitosan/poly(γ-glutamic acid) nanoparticles in an enteric-coated capsule | Increase bioavailability up to 20% | [22] |
| Enzyme inhibitors | Poly(ethylcyanoacrylate) nanoparticles in the presence of protease inhibitors (e.g., glycerrizin, capric acid, deoxycholic acid, hydroxypropyl-β-cyclodextrin and aprotinin) | Reduce and maintain the glucose level below 200 mg/dL | [23,24] |
| Adding complexing agents such as diethylenetriaminepentaacetic acid (DTPA) into the insulin-loaded nanoparticles | Produce substantial protective effect against intestinal proteases | [24] | |
| Permeation enhancers | Fatty acids, surfactants, chelators, zonula occludens toxin Micelle composition | Increase membrane permeability | [12,25] |
| Micelle complex | Complexation of phenylboronic acid-containing block copolymer PEG-b-P (Asp-co-AspPBA) and glycopolymer P(Asp-co-AGA) | Self-regulate insulin delivery in response to physiological glucose level | [26] |
| Conjugation of functional moiety | N-octyl-N-arginine chitosan micelles | Enhance hypoglycemic effect | [27] |
3.2.1. Polymeric nanocarrier approach
The polymeric nanoparticle is an approach to improve insulin absorption from the gastrointestinal tract. Synthetic or natural polymeric materials modulate insulin release and consequent pharmacological activity. Insulin-loaded nanoparticles, which are prepared by using biodegradable polymers such as poly(lactide-co-glycolide), polyanhydride, and polyalkyl cyanoacrylate are absorbed from the intestinal epithelial cells and transport insulin through the intestinal mucosa [12]. Some researchers have developed insulin-loaded nanoparticles using a biodegradable poly(ɛ-caprolactone) combined with a nonbiodegradable acrylic polymer, Eudragit RS100, which is effectively absorbed by the gastrointestinal tract [19,20].
3.2.2. Enteric coating approach
The enteric coating technique has been applied to insulin oral delivery in which the enteric coating polymers possess a pH-dependent property [21]. Polyacrylic polymers (e.g., Eudragit L100-55 and Eudragit S100) and cellulosic polymers (e.g., hydroxypropyl methyl cellulose phthalate) have been widely used for this purpose [20,21]. The increase in insulin bioavailability is achieved by filling the freeze-dried chitosan/ poly(γ-glutamic acid) (CS/γ-PGA) nanoparticles in enteric-coated capsules [22]. The enteric-coated capsules protect the insulin-loaded nanoparticles from acidic gastric fluid and rapidly liberate insulin in the proximal segment of the small intestine. Thus, the absorption of insulin into systemic circulation is improved and the relative bioavailability of insulin is increased.
3.2.3. Enzyme inhibitor approach
Insulin, which is a protein, is easily digested and inactivated by digestive enzymes in the stomach after oral administration. Different protease inhibitors are administered along with the nanoparticles to inhibit the activity of these enzymes [2,4]. Radwan and Aboul-Enein [23] report that the oral administration of insulin-loaded poly(ethylcyanoacrylate) nanoparticles in the presence of protease inhibitors (e.g., glycerrizin, capric acid, deoxycholic acid, hydroxypropyl-β-cyclodextrin, and aprotinin) efficiently reduces and maintains glucose level < 200 mg/dL (i.e., the normal glucose level after a meal). Another approach to inhibit protease activity is by use of cationic metal chelating agents such as diethylenetriaminepentaacetic acid (DTPA) [24]. The addition of the complexing agent DTPA in insulin nanoparticles demonstrates a substantial protective effect against intestinal proteases in which the DTPA binds to cofactors [e.g., calcium (Ca2+) and zinc (Zn2+)] of the enzyme system and cause structural alterations and the loss of enzymatic activity.
3.2.4. Permeation enhancers approach
The absorption of insulin from the gastrointestinal tract is improved by the coadministration of permeation enhancers that widen the intercellular junction (e.g., paracellular pathway) and/or perturbate the membrane phospholipids (e.g., transcellular pathway) [4]. Permeation enhancers—which include fatty acids, surfactants, Ca2+-chelating agents, and zonula occludens toxin—are incorporated in the formulations. Fatty acids such as sodium caprate and acyl carnitine improve drug absorption via the transient opening of the tight junctions. Surfactants enhance transcellular transport by disrupting the lipid bilayer. Chelating agents form a complex with calcium ions which rupture the tight junctions and assist the paracellular transport of insulin. Zonula occludens toxin similarly transiently alters the tight junctions for the passage of insulin through the mucosal barrier [25].
3.3. Modification of nanoparticles and micelles for insulin oral delivery
For insulin oral delivery, the copolymers used to form micelles should (1) quickly self-assemble in water, (2) remain stable in the gastrointestinal tract, (3) be nontoxic and biocompatible, and (4) be easy to synthesize on a large scale [18]. Table 2 lists some strategies to prepare stable micelles.
3.3.1. The micelle complex
This strategy involves introducing cross-linkable hydrophilic groups into the hydrophobic polymer to form a stable micelle system [18]. For example, the multifunctional, multiarmed poly(ethylene glycol) is conjugated with biodegradable hydrophobic polymers to form an amphiphilic block copolymer in which poly(ethylene glycol) branches create a cross-linkable structure in the micelle system. The glucose-responsive micelles are formed by the complexation of a phenylboronic acid-containing block copolymer [e.g., poly(-ethylene glycol)-b-poly(aspartic acid-co-aspartamidophenylboronic acid)] and a glycopolymer [e.g., poly(aspartic acid-co-aspartglucosamine)] [26]. The micelle complex has a variety of advantages such as it is stable against aggregation because of the poly(ethylene glycol) shell, it is more sensitive to the glucose level, and there is a fast response to glucose change at the physiological pH.
3.3.2. Conjugation of functional moiety
This strategy involves the development of nanocarriers with a permeation-enhancing property. The specific function moiety is conjugated through the hydrophilic portion of the copolymer. Zhang et al [27] and Yu et al [28] used arginine-rich peptide modified N-octyl-N-arginine chitosan to improve insulin oral absorption. Another method to improve insulin absorption is to attach a specific targeting ligand to the nanoparticle surface. Lectin is widely used for specific targeting to intestinal epithelial cells. The developed lectin-modified polystyrene nanoparticles successfully deliver insulin from the oral administration route and achieve a twofold increase in nanoparticle absorption [12].
3.4. Factors affecting nanoparticle and micelle oral absorption
Physicochemical and biological factors such as particle size, surface charge, nanoparticle stability, the residence time of nanoparticles at the absorption site, and the intestinal contents may all affect nanoparticle or micelle absorption [29].
3.4.1. Particle size
Particle size is a crucial factor to determine the absorption, distribution, and in vivo performance of nanoparticles. In addition, particle size influences the insulin-loading capacity and insulin release performance of nanoparticles. In general, nanoparticles have a higher cellular uptake efficiency than microparticles. Bakhru et al [29] and Panyam and Labhasetwar [30] reported that the cellular uptake of poly(lactide-co-glycolide) (PLGA) nanoparticles with a particle size of 100 nm is 2.5-fold higher than 1-μm microparticles and six-fold higher than 10-μm microparticles in Caco-2 cells. A similar phenomenon has been observed in rats in vivo in which the cellular uptake of PLGA nanoparticles is 15-fold and 250-fold higher than 1-μm and 10-μm microparticles, respectively [30]. A decrease of the particle size to < 1 μm increases particle cellular uptake efficiency. Nanoparticles with a particle size < 100 nm are efficiently taken up in Peyer's patches, and then absorbed into systemic circulation [12].
3.4.2. Surface charge
The surface chemistry of nanoparticles has a profound impact on particles across the absorptive epithelium. Because the intestinal cell membrane is negatively charged, positively charged nanoparticles interact with the intestinal epithelium more strongly, compared to negatively charged and uncharged nanoparticles [29]. By contrast, nanoparticles prepared by using more hydrophobic polymers [e.g., polystyrene, poly(methyl methacrylate), and poly(hydroxybutyrate)] demonstrate better absorption in Peyer's patches, compared to using less hydrophobic polymers [e.g., poly(lactic acid) and poly(lactide-co-glycolide)] [12].
3.4.3. Colloidal stability
Colloidal instability usually leads to nanoparticle and micelle aggregation or flocculation [31]. Chemical stability is very important, especially for nanocarriers bearing specific targeting ligands. The stable colloidal system ensures the binding of nanocarriers to the specific receptor, followed by the efficient internalization of the therapeutic agents into the tumor cells [32].
3.5. Absorption mechanisms of nanoparticle and micelle delivery systems
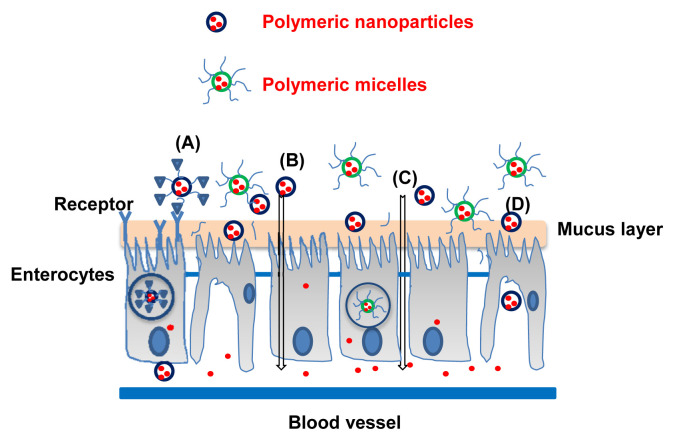
The intestinal epithelium controls the passage of drugs, macromolecules, and particles. The surface area of the small intestine is enlarged because of the villi and microvilli which have a vital role in drug absorption in the gastrointestinal tract. The mechanisms of translocation of nanoparticles and micelles in the intestinal epithelium involve paracellular and/ or transcellular transport [33,34]. Fig. 2 illustrates the intestinal morphology and the translocation routes for nanoparticles and micelles across the intestinal epithelium.
Fig. 2.
Schematic illustration of the different oral delivery pathways of the insulin-loaded polymeric nanoparticles and micelles: (A) receptor-mediated endocytosis, (B) transcellular transport, (C) paracellular transport, and (D) M cell-mediated transport.
3.5.1. Paracellular pathway
The paracellular pathway is the preferred route for the transport of hydrophilic drugs. However, it restricts the passage of macromolecules or particles larger than approximately 1 nm because of a very small intercellular space and because of the tight junctions between the epithelial cells (pore diameter, 3–10 Å) [34]. Therefore, polymeric nanoparticles usually cannot pass through the intestinal barrier via the paracellular route. To improve their paracellular transport, the tight junction must be opened reversibly by using permeation enhancers such as cationic polymers (e.g., chitosan and its derivatives), anionic polymers (e.g., polyacrylic acid and its derivatives), or calcium chelators (e.g., ethylenediaminetetraacetic acid). The width of the tight junction opened by the enhancers is < 20 nm, which still limits the transport of intact nanoparticles into the bloodstream if the nanoparticle is > 20 nm [35]. In this situation, the nanoparticles must be destabilized and disintegrated in the intercellular space when approaching the tight junction between the epithelial cells so that the loaded drug is released and permeated through the opened paracellular pathway. Sonaje et al [35] report that insulin-loaded chitosan/poly(γ-glutamic acid) nanoparticles (CS/γ-PGA NPs) are disintegrated at pH 7.2–7.4 because of the deprotonization of chitosan by which the drug is released and transported through the opened tight junction. Permeation enhancers such as chitosan improve the paracellular transport of drugs and vaccines via interaction of these positively charged polymers with the negatively charged cell membrane [36].
3.5.2. The transcellular pathway
The transcellular transport of nanoparticles occurs when the nanoparticles are taken up by enterocytes or by the M cells of Peyer's patches, and thus overcome presystemic hepatic metabolism and enhance drug bioavailability. The transport of nanoparticles through the cells can be effectively achieved via active transcellular transport [37]. The active transcellular transport involves phagocytosis, macropinocytosis, clathrin-mediated endocytosis, and caveolin-mediated endocytosis [34]. It has been reported that insulin-loaded hyaluronic acid nanoparticles are transported across Caco-2 cell monolayer primarily via a transcellular pathway; the apparent permeability coefficient from the apical to the basolateral side shows a two-fold increase, compared to an insulin solution [9,38].
3.6. Bioavailability of insulin-loaded nanoparticles and micelles
The effectiveness and safety of nanoparticulate delivery systems for insulin have been assessed for their intestinal absorption, bioavailability, physiological response, therapeutic effect, and cytotoxicity. Table 3 [14,20,22,24,35,39–42] summarizes the bioavailability of orally administered insulin-loaded nanoparticles in diabetic rats. The oral delivery of insulin by different kinds of polymeric nanoparticles generally reveals 6–13% bioavailability [14,20,39–41]. For example, oral delivery of insulin-loaded PLGA/Eudragit RS100 nanoparticles in diabetic rats shows 13.2% bioavailability [14]. Several strategies have been applied to improve insulin oral bioavailability. Sonaje et al [35] demonstrated 15% pharmacological availability when orally administering insulin-loaded chitosan/poly(γ-glutamicacid) nanoparticles; its bioavailability was further increased to 20% when the nanoparticles were loaded in the enteric-coated capsule [22]. The oral intake of a capsule containing insulin-loaded DTPA conjugated chitosan/poly(γ-glutamic acid) nanoparticles reveals 19.7 ± 1.3% bioavailability [24]. Thus, DTPA acted as a complexing agent to disrupt intestinal tight junctions and inhibit intestinal proteases. Prusty and Sahu [42] report that the oral bioavailability of insulin-loaded chitosan/sodium alginate nanoparticles is 43.6% (relative to insulin injection) in which chitosan acts as a mucoadhesive agent to transiently and reversibly open the tight junctions between epithelial cells and thereby prominently enhance insulin absorption [42].
Table 3.
The oral bioavailability of various insulin-loaded nanoparticle formulations in diabetic rats.
| Nanoparticle formulation | Dose (IU/kg) | Bioavailability (%) | Refs |
|---|---|---|---|
| Alginate/chitosan nanoparticles | 50 | 5.1 ± 2.1 | [39] |
| PLGA/HPMCP-55 nanoparticles | 50 | 6.27 | [20] |
| PLGA/Eudragit RS100 nanoparticles in an enteric-coated capsule | 50 | 9.2 | [20] |
| Carboxylated chitosan-grafted poly(methylmethacrylate) nanoparticles | 25 | 9.7 | [40] |
| Alginate/dextran sulfate nanoparticles | 50 | 10.7 | [41] |
| Poly(ɛ-caprolactone)/Eudragit RS 100 nanoparticles | 50 | 13.2 | [14] |
| Chitosan/poly(γ-glutamic acid) nanoparticles | 30 | 15 | [35] |
| Chitosan/poly(γ-glutamic acid) nanoparticles in an enteric-coated capsule | 30 | 20 | [22] |
| Chitosan/poly(γ-glutamic acid)-DTPA nanoparticles | 30 | 19.7 ± 1.3 | [24] |
| Chitosan/sodium alginate nanoparticles | 10 | 43.6 | [42] |
DTPA = diethylenetriaminepentaacetic acid; PLGA = poly(lactide-co-glycolide).
3.7. Possible toxicity of nanoparticles in use and in future study
Several researchers have proven that nanocarriers administered daily to patients are safe, nontoxic, biocompatible, and biodegradable, and they evoke reproducible biological effects. By contrast, there are some literature reports on the toxicological impacts of nanocarriers on human health. Recent literature suggests that the cytotoxicity of nanoparticles is associated with oxidative stress and with proinflammatory gene activation; however, the exact underlying mechanism remains unknown [43]. Therefore, there is a need to consider the usefulness of nanocarriers and the unpredictable and adverse consequences of nanocarriers to human health. Nanocarriers have different properties from their bulk conformation, which allows them to pass through cell membranes and biological barriers at the cellular level in tissues and organs. It is widely known that the nanocarriers toxicity depends on several physiochemical parameters such as particle size, shape, surface charge, composition, and subsequent stability of the nanocarriers [44,45]. In addition, the administration dose, administration route, and tissue distribution seem to be important factors relevant to nanocarrier cytotoxicity. For the safe development and safe use of nanoparticles, investigations regarding the cellular toxicity of nanocarriers has become more important and necessary.
4. Conclusion and future perspectives
The protection of insulin from the harsh gastrointestinal environment makes nanoparticles and micelles an alternative promising delivery system for insulin. Recent overall findings reveal that advances in insulin oral delivery mostly focus on the development of the following: (1) biocompatible polymers with mucoadhesive and absorption-enhancing properties [46], (2) nanocarriers with enhanced transport ability [35], and (3) nanoparticles bearing specific ligands for effective oral delivery of insulin [12]. All of these advances highlight the importance of designing nanomedicines for insulin. Many studies indeed demonstrate that polymeric nanoparticles and micelles enhance insulin oral bioavailability in the preclinical animal stage. Whether these insulin-loaded nanocarriers can be launched in the market in the future still has many challenges. Therefore, continuous investigation and optimization of nanoparticle-based delivery systems for insulin oral delivery in diabetics are anticipated.
Footnotes
Conflicts of interest
The authors have no conflicts of interest to declare.
References
- 1. Sonia TA, Sharma CP. An overview of natural polymers for oral insulin delivery. Drug Discov Today. 2012;17:784–92. doi: 10.1016/j.drudis.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 2. Ramesan RM, Sharma CP. Challenges and advances in nanoparticle-based oral insulin delivery. Expert Rev Med Devic. 2009;6:665–76. doi: 10.1586/erd.09.43. [DOI] [PubMed] [Google Scholar]
- 3. Card JW, Magnuson BA. A review of the efficacy and safety of nanoparticle-based oral insulin delivery systems. Am J Physiol Gastrointest Liver Physiol. 2011;301:G956–67. doi: 10.1152/ajpgi.00107.2011. [DOI] [PubMed] [Google Scholar]
- 4. Iyer H, Khedkar A, Verma M. Oral insulin—a review of current status. Diabetes Obes Metab. 2010;12:179–85. doi: 10.1111/j.1463-1326.2009.01150.x. [DOI] [PubMed] [Google Scholar]
- 5. Sarmento B, Martins S, Ferreira D, Souto EB. Oral insulin delivery by means of solid lipid nanoparticles. Int J Nanomed. 2007;2:743–9. [PMC free article] [PubMed] [Google Scholar]
- 6. Wong TW. Design of oral insulin delivery systems. J Drug Target. 2010;18:79–92. doi: 10.3109/10611860903302815. [DOI] [PubMed] [Google Scholar]
- 7. Damge C, Reis CP, Maincent P. Nanoparticle strategies for the oral delivery of insulin. Expert Opin Drug Deliv. 2008;5:45–68. doi: 10.1517/17425247.5.1.45. [DOI] [PubMed] [Google Scholar]
- 8. Mukhopadhyay P, Mishra R, Rana D, Kundu PP. Strategies for effective oral insulin delivery with modified chitosan nanoparticles: a review. Prog Polym Sci. 2012;37:1457–75. [Google Scholar]
- 9. Han LN, Zhao YF, Yin LF, Li RM, Liang Y, Huang H, Pan SR, Wu CB, Feng M. Insulin-loaded pH-sensitive hyaluronic acid nanoparticles enhance transcellular delivery. AAPS PharmSciTech. 2012;13:836–45. doi: 10.1208/s12249-012-9807-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaudhury A, Das S. Recent advancement of chitosan-based nanoparticles for oral controlled delivery of insulin and other therapeutic agents. AAPS PharmSciTech. 2011;12:10–20. doi: 10.1208/s12249-010-9561-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiu GN, Wong MY, Ling LU, Shaikh IM, Tan KB, Chaudhury A, Tan BJ. Lipid-based nanoparticulate systems for the delivery of anti-cancer drug cocktails: implications on pharmacokinetics and drug toxicities. Curr Drug Metab. 2009;10:861–74. doi: 10.2174/138920009790274531. [DOI] [PubMed] [Google Scholar]
- 12. Woitiski CB, Carvalho RA, Ribeiro AJ, Neufeld RJ, Veiga F. Strategies toward the improved oral delivery of insulin nanoparticles via gastrointestinal uptake and translocation. BioDrugs. 2008;22:223–37. doi: 10.2165/00063030-200822040-00002. [DOI] [PubMed] [Google Scholar]
- 13. Herrero EP, Alonso MJ, Saba N. Polymer-based oral peptide nanomedicines. Ther Deliv. 2012;3:657–68. doi: 10.4155/tde.12.40. [DOI] [PubMed] [Google Scholar]
- 14. Damge C, Maincent P, Ubrich N. Oral delivery of insulin associated to polymeric nanoparticles in diabetic rats. J Control Release. 2007;117:163–70. doi: 10.1016/j.jconrel.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 15. Li CL, Deng YJ. Oil-based formulations for oral delivery of insulin. J Pharm Pharmacol. 2004;56:1101–7. doi: 10.1211/0022357044175. [DOI] [PubMed] [Google Scholar]
- 16. Eaimtrakarn S, Rama Prasad YV, Ohno T, Konishi T, Yoshikawa Y, Shibata N, Takada K. Absorption enhancing effect of Labrasol on the intestinal absorption of insulin in rats. J Drug Target. 2002;10:255–60. doi: 10.1080/10611860290022688. [DOI] [PubMed] [Google Scholar]
- 17. Kinesh VP, Neelam DP, Punit BP, Bhavesh SB, Pragna KS. Novel approaches for oral delivery of insulin and current status of oral insulin products. Int J Pharm Sci Nanotech. 2010;3:1058–64. [Google Scholar]
- 18. Plapied L, Duhem N, des Rieux A, Preat V. Fate of polymeric nanocarriers for oral drug delivery. Curr Opin Colloid Interface Sci. 2011;16:228–37. [Google Scholar]
- 19. Socha M, Sapin A, Damge C, Maincent P. Influence of polymers ratio on insulin-loaded nanoparticles based on poly-epsilon-caprolactone and Eudragit (R) RS for oral administration. Drug Deliv. 2009;16:430–6. doi: 10.3109/10717540903223442. [DOI] [PubMed] [Google Scholar]
- 20. Wu ZM, Zhou LY, Guo XD, Jiang W, Ling L, Qian Y, Luo KQ, Zhang LJ. HP55-coated capsule containing PLGA/RS nanoparticles for oral delivery of insulin. Int J Pharm. 2012;425:1–8. doi: 10.1016/j.ijpharm.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 21. Chen MC, Mi FL, Liao ZX, Hsiao CW, Sonaje K, Chung MF, Hsu LW, Sung HW. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv Drug Deliver Rev. 2013;6:865–79. doi: 10.1016/j.addr.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 22. Sonaje K, Chen YJ, Chen HL, Wey SP, Juang JH, Nguyen HN, Hsu CW, Lin KJ, Sung HW. Enteric-coated capsules filled with freeze-dried chitosan/poly(gamma-glutamic acid) nanoparticles for oral insulin delivery. Biomaterials. 2010;31:3384–94. doi: 10.1016/j.biomaterials.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 23. Radwan MA, Aboul-Enein HY. The effect of absorption enhancers on the initial degradation kinetics of insulin by alpha-chymotrypsin. Int J Pharm. 2001;217:111–20. doi: 10.1016/s0378-5173(01)00595-6. [DOI] [PubMed] [Google Scholar]
- 24. Su FY, Lin KJ, Sonaje K, Wey SP, Yen TC, Ho YC, Panda N, Chuang EY, Maiti B, Sung HW. Protease inhibition and absorption enhancement by functional nanoparticles for effective oral insulin delivery. Biomaterials. 2012;33:2801–11. doi: 10.1016/j.biomaterials.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 25. Park K, Kwon IC, Park K. Oral protein delivery: current status and future prospect. React Funct Polym. 2011;71:280–7. [Google Scholar]
- 26. Yang H, Sun X, Liu G, Ma R, Li Z, An Y, Shi L. Glucose-responsive complex micelles for self-regulated release of insulin under physiological conditions. Soft Matter. 2013;9:8589–99. [Google Scholar]
- 27. Zhang ZH, Abbad S, Pan RR, Waddad AY, Hou LL, Lv HX, Zhou JP. N-octyl-N-arginine chitosan micelles as an oral delivery system of insulin. J Biomed Nanotechnol. 2013;9:601–9. doi: 10.1166/jbn.2013.1572. [DOI] [PubMed] [Google Scholar]
- 28. Yu F, He C, Waddad AY, Munyendo WL, Lv H, Zhou J, Zhang Q. N-octyl-N-arginine-chitosan (OACS) micelles for gambogic acid oral delivery: preparation, characterization and its study on in situ intestinal perfusion. Drug Dev Ind Pharm. 2014;40:774–82. doi: 10.3109/03639045.2013.786723. [DOI] [PubMed] [Google Scholar]
- 29. Bakhru SH, Furtado S, Morello AP, Mathiowitz E. Oral delivery of proteins by biodegradable nanoparticles. Adv Drug Deliv Rev. 2013;65:811–21. doi: 10.1016/j.addr.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 30. Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 31. Florence AT. “Targeting” nanoparticles: the constraints of physical laws and physical barriers”. J Control Release. 2012;164:115–24. doi: 10.1016/j.jconrel.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 32. Florence AT. Nanoparticle uptake by the oral route: fulfilling its potential? Drug Discov Today Technol. 2005;2:75–81. doi: 10.1016/j.ddtec.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 33. Florence AT. Issues in oral nanoparticle drug carrier uptake and targeting. J Drug Target. 2004;12:65–70. doi: 10.1080/10611860410001693706. [DOI] [PubMed] [Google Scholar]
- 34. Chen MC, Sonaje K, Chen KJ, Sung HW. A review of the prospects for polymeric nanoparticle platforms in oral insulin delivery. Biomaterials. 2011;32:9826–38. doi: 10.1016/j.biomaterials.2011.08.087. [DOI] [PubMed] [Google Scholar]
- 35. Sonaje K, Lin YH, Juang JH, Wey SP, Chen CT, Sung HW. In vivo evaluation of safety and efficacy of self-assembled nanoparticles for oral insulin delivery. Biomaterials. 2009;30:2329–39. doi: 10.1016/j.biomaterials.2008.12.066. [DOI] [PubMed] [Google Scholar]
- 36. Shakweh M, Ponchel G, Fattal E. Particle uptake by Peyer's patches: a pathway for drug and vaccine delivery. Expert Opin Drug Deliv. 2004;1:141–63. doi: 10.1517/17425247.1.1.141. [DOI] [PubMed] [Google Scholar]
- 37. Shahbazi MA, Santos HA. Improving oral absorption via drug-loaded nanocarriers: absorption mechanisms, intestinal models and rational fabrication. Curr Drug Metab. 2013;14:28–56. [PubMed] [Google Scholar]
- 38. Woitiski CB, Sarmento B, Carvalho RA, Neufeld RJ, Veiga F. Facilitated nanoscale delivery of insulin across intestinal membrane models. Int J Pharm. 2011;412:123–31. doi: 10.1016/j.ijpharm.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 39. Sarmento B, Ribeiro A, Veiga F, Sampaio P, Neufeld R, Ferreira D. Alginate/chitosan nanoparticles are effective for oral insulin delivery. Pharm Res. 2007;24:2198–206. doi: 10.1007/s11095-007-9367-4. [DOI] [PubMed] [Google Scholar]
- 40. Cui FY, Qian F, Zhao ZM, Yin LC, Tang C, Yin CH. Preparation, characterization, and oral delivery of insulin loaded carboxylated chitosan grafted poly(methyl methacrylate) nanoparticles. Biomacromolecules. 2009;10:1253–8. doi: 10.1021/bm900035u. [DOI] [PubMed] [Google Scholar]
- 41. Woitiski CB, Neufeld RJ, Veiga F, Carvalho RA, Figueiredo IV. Pharmacological effect of orally delivered insulin facilitated by multilayered stable nanoparticles. Eur J Pharm Sci. 2010;41:556–63. doi: 10.1016/j.ejps.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 42. Prusty AK, Sahu SK. Development and evaluation of insulin incorporated nanoparticles for oral administration. ISRN Nanotechnology. 2013;2013:1–6. [Google Scholar]
- 43. Karmakar A, Zhang Q, Zhang Y. Neurotoxicity of nanoscale materials. J Food Drug Anal. 2014;22:147–60. doi: 10.1016/j.jfda.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. He X, Aker WG, Leszczynski J, Hwang HM. Using a holistic approach to assess the impact of engineered nanomaterials inducing toxicity in aquatic systems. J Food Drug Anal. 2014;22:128–46. doi: 10.1016/j.jfda.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fu PP, Xia X, Hwang HM, Ray PC, Yu H. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal. 2014;22:64–75. doi: 10.1016/j.jfda.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sajeesh S, Sharma CP. Cyclodextrin-insulin complex encapsulated polymethacrylic acid based nanoparticles for oral insulin delivery. Int J Pharm. 2006;325:147–54. doi: 10.1016/j.ijpharm.2006.06.019. [DOI] [PubMed] [Google Scholar]