Abstract
To evaluate the efficacy and safety of a gonadotropin-releasing hormone (GnRH) agonist for treating large-sized submucosal leiomyoma before hysteroscopic myomectomy.
The data were retrospectively collected from patients who underwent a hysteroscopic myomectomy for a submucosal leiomyoma >3.5 cm in size from January 2009 to December 2018. The patients were divided into the GnRH group and the control group according to whether they were pretreated before surgery.
A total of 61 patients were included in the study, 31 in the GnRH agonist group and 30 in the control group. At diagnosis, the maximum leiomyoma diameter was similar between the 2 groups (4.67 ± 0.6 cm in the GnRH agonist group vs 3.82 ± 0.6 cm in the control group, P = .061). After pretreatment with the GnRH agonist, the maximum diameter was significantly smaller in the GnRH agonist group compared to the control group (3.82 ± 0.6 vs 4.33 ± 0.8 cm, respectively, P = .004). The leiomyoma volume in the GnRH agonist group decreased by 55.6%, from 41.68 ± 15.7 to 23.19 ± 10.4 cm3, which led to significant differences in leiomyoma volume between the 2 groups (23.19 ± 10.4 cm3 in the GnRH agonist group vs 33.22 ± 24.7 cm3 in the control group, P = .042). The GnRH agonist group showed a shorter operation time (37.7 vs 43.9 minutes, P = .040) and less uterine distention media was used (6800 vs 9373.3 mL, P = .037) compared to the control group. Postoperative complications such as estimated blood loss, remnant leiomyoma, and recurrence were similar between the 2 groups.
Treatment with a GnRH agonist before hysteroscopic myomectomy for large submucosal leiomyoma might decrease the volume of the leiomyoma, reduce operation time, and the amount of uterine-distension media used without surgical complications.
Keywords: gonadotropin-releasing hormone agonist, hysteroscopic myomectomy, large-sized submucosal leiomyoma
1. Introduction
Uterine leiomyomas are the most common benign tumors in the female genital tract. Especially, submucosal leiomyomas are estimated to be the cause of 10% of cases of abnormal uterine bleeding, dysmenorrhea, and infertility.[1,2] Hysteroscopic myomectomy is preferred over the abdominal approach in cases of submucosal leiomyomas because of the shorter duration of hospital stay, and reduced chances of endometrial damage and cesarean section after pregnancy.[3] However, hysteroscopic myomectomy can be difficult when leiomyomas are large and numerous.[4]
Gonadotropin-releasing hormone (GnRH) agonist treatment prior to surgery might reduce operative risks by decreasing leiomyoma size and vascularization of the leiomyoma.[5] GnRH agonists are approved for the pretreatment of leiomyomas in myomectomy. Pretreatment with GnRH agonist improves pre and postoperative hemoglobin (Hb) levels and reduces the uterine and leiomyoma volume.[6]
However, there is a conflict of opinion regarding pretreatment with GnRH agonist for hysteroscopic myomectomy. Some studies revealed that treatment with GnRH agonist before hysteroscopic myomectomy could reduce the size of submucosal leiomyomas, endometrial thickness, and mucous debris which help clear the vision of the surgical field, resulting in decreased surgical morbidity including bleeding, operation time.[7–9] In contrast, some studies showed that treatment with GnRH agonists before hysteroscopic myomectomy caused serious bleeding, prolonged operation time and reduced myometrium thickness, which could lead to uterine perforation.[10–13]
Unfortunately, most studies have included patients with relatively small-sized leiomyomas, and only a few studies using GnRH agonist treatment before the hysteroscopic myomectomy of large-sized leiomyomas have been reported.[14] There are studies that even large-sized submucosal leiomyomas are contraindicated in hysteroscopic myomectomy.[11,15,16] Therefore, the aim of this study was to evaluate the efficacy and safety of using a GnRH agonist before hysteroscopic myomectomy in patients with large-sized submucosal leiomyomas with a diameter >3.5 cm.
2. Materials and Methods
This retrospective study was conducted at Chungnam National University Hospital and was approved by the Institutional Review Board of the hospital (IRB no. 2020-05-092). The data were collected from patients who underwent a hysteroscopic myomectomy for a submucosal leiomyoma from January 2009 to December 2018. Submucosal leiomyoma was diagnosed based on the patient’s symptoms, physical examination, and ultrasonography. Transvaginal ultrasound examinations were performed to measure the maximal diameter of the leiomyoma and identify the location, number, and type of leiomyoma at the first visit and just before surgery. Submucosal leiomyomas were divided according to the Wamsteker classification and the classification of Lasmar.[17,18] Leiomyoma volume was calculated by multiplying all 3 measures by a constant of 0.52 (length × anteroposterior diameter × transverse diameter × 0.52).[19]
All patients were counseled about the potential benefits and risks of hysteroscopic myomectomy. The inclusion criteria were reproductive age of 18 to 50 years, a submucosal leiomyoma >3.5 cm in the maximum longitudinal diameter on ultrasound, and at least 1 clinical symptom such as hypermenorrhea or dysmenorrhea or infertility. The exclusion criteria were age >50 years or postmenopausal status, age <18 years, the presence of >2 submucosal leiomyomas, concomitant laparoscopic myomectomy, previous hysteroscopic myomectomy history, and the presence of malignancy confirmed by histopathologic examination. Of the 61 patients, 31 underwent GnRH agonist treatment before hysteroscopic myomectomy. The decision to treat with a GnRH agonist before hysteroscopic myomectomy was made preoperatively based on the clinical characteristics of the patient with abundant hypermenorrhea and abnormal uterine bleeding for >7 days.
Demographic information on the patient, obstetric history, characteristics of the leiomyoma (size, location, and weight), preoperative and postoperative symptoms, surgical data (surgical apparatus (bipolar or monopolar), input and output volume of uterine-distending media), short-term outcomes (operation time, estimated blood loss, complications, and duration of hospital stay), and long-term outcomes (symptom scores for 2 years, presence of remnant leiomyoma, recurrence, and pregnancy) were reviewed. For patients in the GnRH agonist group, GnRH agonist (Zoladex®) was administered at 3.75 mg by subcutaneous injection twice before surgery at 4-week intervals. The patients in the control group underwent hysteroscopic myomectomy without any kind of pretreatment. Surgery was performed either under spinal or general anesthesia according to the anesthesiologist’s decision. Four skilled senior gynecologic surgeons conducted the surgeries. Surgery was performed using monopolar or bipolar resectoscopes according to the individual surgeons’ judgment. The uterine-distending media was Urosol® solution (a mixture of 2.7% sorbitol and 0.54% mannitol) in cases with a monopolar resectoscope and normal saline solution for a bipolar resectoscope. The input and output uterine-distending media volume was checked after surgery. The patients were followed up at 1, 6, and 12 months during the first year after hysteroscopic myomectomy, followed by annual visits thereafter. Each time, the patient’s history was obtained and physical examination and transvaginal ultrasonography were performed.
The distribution of the patient characteristics between the GnRH agonist and control groups was compared using the 2-sample t test for continuous variables (or the Wilcoxon rank test when the expected frequency within any cell was <5) and the χ2 test (or Fisher exact test when the expected frequency within any cell was <5) for the categorical variables. Multivariate linear regression analysis was used to determine the factors affecting operative time for hysteroscopic myomectomy.
All statistical analyses were performed using SPSS software version 24.0 (SPSS, Inc., Chicago, IL) and a P value of <.05 was considered statistically significant.
3. Results
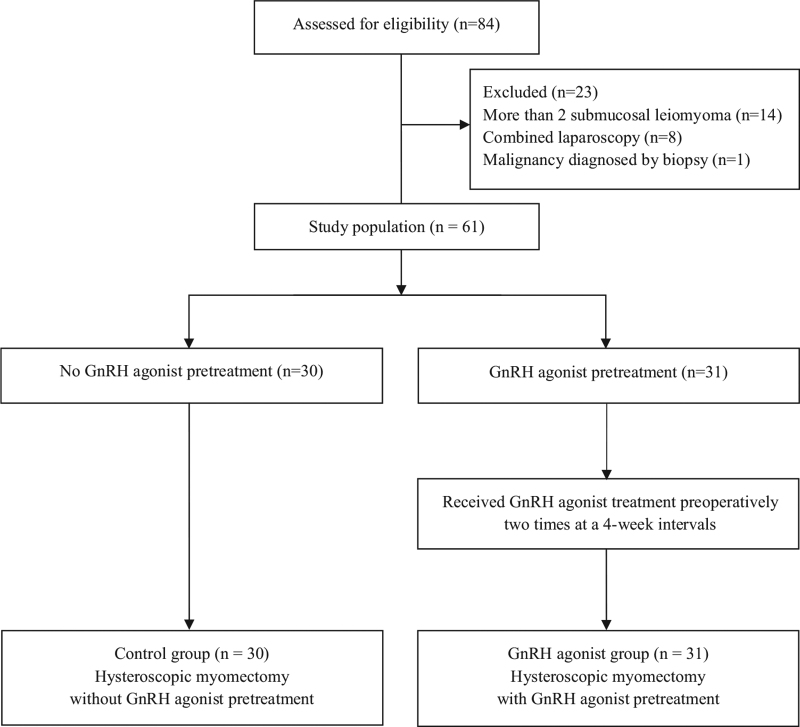
Eighty-four eligible patients underwent hysteroscopic myomectomy. Twenty-three patients were excluded because they had more than 2 submucosal leiomyomas (n = 14), underwent concomitant laparoscopic myomectomy (n = 8), or were diagnosed with malignancy by histopathologic examination (n = 1). Of the 61 patients included in this study, 31 were treated with a GnRH agonist prior to surgery and 30 were not (Fig. 1). The demographic characteristics, preoperative white blood cell count, Hb level, and type, size, and volume of the leiomyomas in the 2 groups are given in Table 1. There was a statistically significant difference between the 2 groups only at the Hb level (P = .020).
Figure 1.
Flow diagram outlining selection of patients. GnRH = gonadotropin-releasing hormone.
Table 1.
Baseline characteristics in 2 groups at the first visit.
| Characteristics | Control group (n = 30) | GnRH agonist group (n = 31) | P value |
|---|---|---|---|
| Age (yr) | 42.07 ± 5.1 | 41.19 ± 5.7 | .530 |
| Diabetes mellitus | 1 (3.3%) | 1 (3.2%) | 1.000 |
| Hypertension | 0 (0%) | 0 (0%) | 1.000 |
| WBC count (/µL) | 6094.67 ± 2172.4 | 5712.90 ± 1424.0 | .419 |
| Hb level (g/dL) | 11.01 ± 1.5 | 10.15 ± 1.2 | .018 |
| Gravidity | 3.13 ± 1.7 | 2.48 ± 1.4 | .106 |
| Parity(%) | .105 | ||
| 0 | 0 (0%) | 4 (12.8%) | |
| 1 | 5 (16.7%) | 3 (9.7%) | |
| ≥2 | 25 (83.3%) | 24 (77.4%) | |
| Wamsteker score | .870 | ||
| G0 | 9 (30.0%) | 10 (32.3%) | |
| G1 | 8 (26.7%) | 8 (25.8%) | |
| G2 | 13 (43.3%) | 13 (41.9%) | |
| Lasmar score, 0–9 | 5.79 ± 1.8 | 6.31 ± 1.6 | .244 |
| Size of leiomyoma at the first visit | |||
| Maximum diameter (cm) | 4.33 ± 0.8 | 4.67 ± 0.6 | .061 |
| Maximum diameter group | <.001 | ||
| 3.5–3.9 cm | 9 (30.0%) | 5 (16.1%) | |
| 4–4.9 cm | 17 (56.7%) | 14 (45.2%) | |
| 5–5.9 cm | 2 (6.7%) | 11 (35.5%) | |
| ≥6 cm | 2 (6.7%) | 1 (3.2%) | |
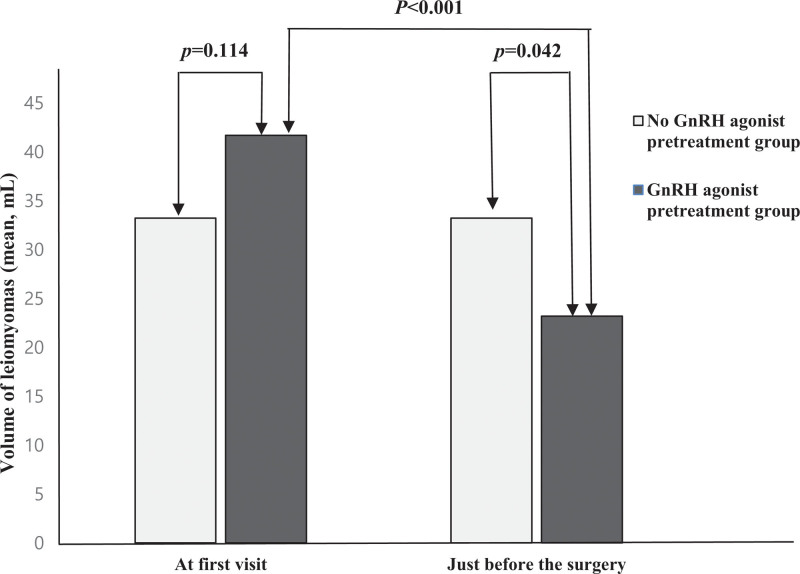
| Volume (cm3) | 33.22 ± 24.7 | 41.68 ± 15.7 | .114 |
As shown in Table 2, the mean maximum diameter of the leiomyomas in the GnRH agonist group decreased from 4.67 ± 0.6 to 3.82 ± 0.6 cm after pretreatment with the GnRH agonist. As a result, the leiomyoma diameters were significantly different between the 2 groups (3.82 ± 0.6 cm in the GnRH agonist group vs 4.33 ± 0.8 cm in the control group, P = .004).
Table 2.
Perioperative data and surgical outcomes.
| Control group (n = 30) | GnRH agonists group (n = 31) | P value | |
|---|---|---|---|
| Size of leiomyoma at the time of surgery | |||
| Maximum diameter (cm) | 4.33 ± 0.8 | 3.82 ± 0.6 | .004 |
| Volume (cm3) | 33.22 ± 24.7 | 23.19 ± 10.4 | .042 |
| Weight (g) | 33.20 ± 25.0 | 22.32 ± 11.9 | .033 |
| Type of anesthesia | .202 | ||
| Spinal | 14 (46.7%) | 20 (64.5%) | |
| General | 16 (53.3%) | 11 (35.5%) | |
| Surgical apparatus | 1.000 | ||
| Bipolar | 10 (33.3%) | 11 (35.5%) | |
| Monopolar | 20 (66.7%) | 20 (64.5%%) | |
| Type of media | 1.000 | ||
| Urosol | 20 (66.7%) | 20 (64.5%) | |
| Normal saline | 10 (33.3%) | 11 (35.5%) | |
| Operation time (min) | 43.97 ± 12.9 | 37.71 ± 10.2 | .040 |
| Uterine-distending media (mL) | |||
| Input | 9373.33 ± 5657.8 | 6800.00 ± 3552.4 | .037 |
| Output | 8723.33 ± 5453.9 | 6432.26 ± 3266.1 | .050 |
| Imbalance (input – output) | 650.00 ± 318.1 | 367.74 ± 430.0 | .005 |
| Estimated blood loss (mL) | 27.03 ± 42.1 | 25.84 ± 42.0 | .914 |
| Perioperative WBC count | |||
| Preoperative WBC count (/µL) | 6094.67 ± 2172.4 | 5712.90 ± 1424.0 | .419 |
| Postoperative WBC count (/µL) | 6449.33 ± 1744.9 | 6500.97 ± 1792.56 | .910 |
| Perioperative Hb level | |||
| Preoperative Hb level (g/dL) | 11.01 ± 1.5 | 10.15 ± 1.2 | .018 |
| Postoperative Hb level (g/dL) | 10.83 ± 1.5 | 10.07 ± 1.3 | .039 |
| Hemoglobin decrease (g/dL) | 0.18 ± 1.2 | 0.09 ± 0.8 | .682 |
| Duration of hospital stay (d) | 3.50 ± 1.1 | 3.13 ± 0.3 | .080 |
| Uterine balloon tamponade | 1 (3.3%) | 3 (9.7%) | .612 |
| Remnant leiomyoma | 1 (3.3%) | 3 (9.7%) | .612 |
| Recurrence | 1 (3.3%) | 2 (6.5%) | 1.00 |
| Pregnancy | 2 (6.7%) | 5 (16.1%) | .425 |
Likewise, the leiomyoma volume in the GnRH agonist group decreased by 55.6%, from 41.68 ± 15.7 to 23.19 ± 10.4 cm3 after pretreatment with GnRH agonist, which led to significant differences in leiomyoma volume between the 2 groups (23.19 ± 10.4 cm3 in the GnRH agonist group vs 33.22 ± 24.7 cm3 in the control group, P = .042; Fig. 2).
Figure 2.
Changes in the volume of leiomyomas with or without the GnRH agonist pretreatment. GnRH = gonadotropin-releasing hormone.
These differences, which were diagnosed by ultrasonography, were confirmed by comparing the weight of the removed leiomyoma after surgery between the 2 groups (22.32 ± 11.9 g in the GnRH agonist group vs 33.20 ± 25.0 g in the control group, P = .033).
Surgical outcomes were compared between the 2 groups (Table 2). The operation time was shorter in the GnRH agonist group compared to the control group (37.71 ± 10.2 vs 43.97 ± 12.9 minutes, respectively, P = .040). The input volume was smaller, and the imbalance (input minus output) in uterine-distending media was less in the GnRH agonist group compared to the control group (P = .037 and P = .005, respectively). Neither the estimated blood loss nor the Hb decrease was significantly different between the 2 groups.
In the 2 groups, there was no significant increase before and after surgery in white blood cell count, and there was no significant difference between the 2 groups in the duration of hospital stay.
Uterine balloon tamponade was maintained in 3 patients in the GnRH agonist group and 1 patient in the control group due to intraoperative bleeding. There were no complications such as uterine perforation or intravasation of the fluid during surgery. Remnant leiomyomas were only present in cases where uterine balloon tamponade was performed. Recurrence occurred in 2 patients in the GnRH agonist group and 1 patient in the control group. Five patients in the GnRH agonist group and 2 patients in the control group achieved spontaneous pregnancy during the 2-year follow-up period.
Using Pearson correlation analysis, we determined that longer operation time was significantly correlated with other adverse surgical outcomes such as greater fluid imbalance, more blood loss, and longer hospital stay. Therefore, a predictive model for operation time was developed using multiple linear regression analysis (Table 3). Operation time was determined to be significantly related to the posttreatment volume of the leiomyoma, and a regression-based equation to predict operation time was obtained as follows: predicted operation time = 0.250 × posttreatment myoma volume + 33.753
Table 3.
Univariate and multivariate analyses of operation time in hysteroscopic surgery.
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Regression coefficient | SE | P value | Regression coefficient | SE | P value | |
| Intercept | 33.753 | 2.501 | .000* | |||
| Age | –0.567 | 0.280 | .047* | –0.294 | 0.288 | .311 |
| Gravidity | 1.012 | 0.982 | .307 | |||
| Parity | 1.283 | 2.203 | .562 | |||
| Lasmar score | 1.654 | 0.946 | .086 | 0.889 | 1.048 | .400 |
| Posttreatment leiomyoma volume | 0.250 | 0.073 | .001* | 0.250 | 0.073 | .001* |
| Leiomyoma weight | 0.208 | 0.072 | .006* | 0.043 | 0.147 | .769 |
4. Discussion
In the present study, treatment with a GnRH agonist before hysteroscopic myomectomy for a large submucosal leiomyoma with a diameter >3.5 cm decreased the size of the leiomyoma, and reduced operation time and the amount of uterine-distension media used. Indman[11] reported a large-sized leiomyoma as a contraindication for GnRH agonist pretreatment in hysteroscopic surgery because of the possibility of severe hemorrhaging. Other authors suggested pretreatment with GnRH agonists particularly for fibroids with a diameter of >3 cm as well as for patients with secondary anemia.[20–22] In the present study, the administration of a GnRH agonist before hysteroscopic myomectomy significantly reduced the size of the leiomyoma, operation time, and input volume of uterine-distending media without increasing surgical morbidity. These results should be further verified in studies based on a large prospective cohort.
The size of the leiomyoma was reported to be one of the crucial factors in 1-step hysteroscopic myomectomy.[23] According to a previous study, 1-step hysteroscopic myomectomy was curative for only 61.9% of the patients with leiomyomas with a diameter >3 cm.[24] In the present study, nearly all leiomyomas with a diameter of >3.5 cm were treated with 1-step hysteroscopy except for 3 cases. All 3 hysteroscopic myomectomy patients had leiomyomas with a diameter of >5 cm (3 in the GnRH agonist group and 1 in the control group). The high success rate of 1-step hysteroscopic myomectomy in the patients with large-sized leiomyomas in our study might indicate the role of GnRH agonists in such cases by decreasing the size of the leiomyomas.
The intravasation of fluid is an important complication when performing hysteroscopic myomectomy.[25] Excessive fluid absorption occurs through the open veins of the leiomyoma and through transperitoneal absorption from possible retrograde flow through the fallopian tubes. The influencing factors include the size of the leiomyoma,[26,27] duration of the operation, and total inflow volume.[28] In the present study, pretreatment with a GnRH agonist reduced the size and volume of the leiomyoma, led to shorter operation times, and subsequently, reduced the amount of total inflow volume. As a result, none of the patients experienced excessive intravasation of the uterine-distending media. Pretreatment with GnRH agonists might be an effective strategy to reduce the rate of intravasation during hysteroscopic myomectomy in patients who have risk factors for intravasation such as large uterine leiomyomas.
Our study had several limitations. First, there was a possibility of selection bias because of the retrospective nature of the study. Second, the volume of the leiomyomas was estimated using a formula rather than by direct measurement of the volume using magnetic resonance imaging or 3-dimensional ultrasound. Third, there were no data comparing the administration of 1 or 2 injections of the GnRH agonist. Despite these limitations, this was the first study to evaluate the efficacy and safety of GnRH agonist pretreatment in patients with large submucosal leiomyomas with a mean diameter >3.5 cm, who are expected to derive the maximum benefit from preoperative GnRH agonist.
In conclusion, in cases of large submucosal leiomyomas with a diameter of >3.5 cm, administering a GnRH agonist before hysteroscopic myomectomy might reduce the volume of the leiomyoma, which leads to shorter operation time and the use of less uterine-distension solution without surgical complications.
Abbreviations:
- GnRH =
- gonadotropin-releasing hormone
- Hb =
- hemoglobin
MP, MSS, and BHK contributed equally to this work.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The study was approved by the Institutional Review Board of Chungnam National University Hospital (IRB no. 2020-05-092). The need for informed consent was waived due to the retrospective nature of this study. All information obtained from the patients’ medical records was anonymized and de-identified prior to analysis.
How to cite this article: Park M, Song MS, Kang BH, Song SY, Lee GW, Jung YW, Shin WK, Ko YB, Lee KH, Yoo HJ. The efficacy of gonadotropin-releasing hormone agonist treatment before hysteroscopic myomectomy for large-sized submucosal leiomyoma. Medicine 2022;101:31(e29726).
GnRH = gonadotropin-releasing hormone, Hb = hemoglobin, WBC = white blood cell.
GnRH = gonadotropin-releasing hormone, Hb = hemoglobin, WBC = white blood cell.
Adjusted R2 = 0.150, P = .001. Dubin–Watson = 1.717.
SE = standard error.
P value <.05.
Contributor Information
Mia Park, Email: mia86@cnuh.co.kr.
Min Soon Song, Email: sysong@cnuh.co.kr.
Byung Hun Kang, Email: missinglime@cnuh.co.kr.
Soo Youn Song, Email: sysong@cnuh.co.kr.
Geon Woo Lee, Email: oldfox@cnuh.co.kr.
Won Kyo Shin, Email: bluered120@cnuh.co.kr.
Young Bok Ko, Email: koyoung@cnuh.co.kr.
Ki Hwan Lee, Email: oldfox@cnuh.co.kr.
References
- [1].Boosz AS, Reimer P, Matzko M, et al. The conservative and interventional treatment of fibroids. Dtsch Arztebl Int. 2014;111:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wallach EE, Vlahos NF. Uterine myomas: an overview of development, clinical features, and management. Obstet Gynecol. 2004;104:393–406. [DOI] [PubMed] [Google Scholar]
- [3].Capmas P, Levaillant JM, Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr Opin Obstet Gynecol. 2013;25:332–8. [DOI] [PubMed] [Google Scholar]
- [4].Namkung J, Kang SY, Chung YJ, et al. Multidisciplinary approach in large-sized submucosal myoma: hysteroscopic myomectomy after uterine artery embolization. J Minim Invasive Gynecol. 2019;26:643–7. [DOI] [PubMed] [Google Scholar]
- [5].de Milliano I, Huirne JAF, Thurkow AL, et al. Ulipristal acetate vs gonadotropin-releasing hormone agonists prior to laparoscopic myomectomy (MYOMEX trial): short-term results of a double-blind randomized controlled trial. Acta Obstet Gynecol Scand. 2020;99:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lethaby A, Vollenhoven B, Sowter M. Pre-operative GnRH analogue therapy before hysterectomy or myomectomy for uterine fibroids. Cochrane Database Syst Rev. 0547;2001:CD00. [DOI] [PubMed] [Google Scholar]
- [7].Di Spiezio Sardo A, Mazzon I, Bramante S, et al. Hysteroscopic myomectomy: a comprehensive review of surgical techniques. Hum Reprod Update. 2008;14:101–19. [DOI] [PubMed] [Google Scholar]
- [8].Mavrelos D, Ben-Nagi J, Davies A, et al. The value of pre-operative treatment with GnRH analogues in women with submucous fibroids: a double-blind, placebo-controlled randomized trial. Hum Reprod. 2010;25:2264–9. [DOI] [PubMed] [Google Scholar]
- [9].Muzii L, Boni T, Bellati F, et al. GnRH analogue treatment before hysteroscopic resection of submucous myomas: a prospective, randomized, multicenter study. Fertil Steril. 2010;94:1496–9. [DOI] [PubMed] [Google Scholar]
- [10].Bradley LD. Complications in hysteroscopy: prevention, treatment and legal risk. Curr Opin Obstet Gynecol. 2002;14:409–15. [DOI] [PubMed] [Google Scholar]
- [11].Indman PD. Hysteroscopic treatment of menorrhagia associated with uterine leiomyomas. Obstet Gynecol. 1993;81(5 Pt 1):716–20. [PubMed] [Google Scholar]
- [12].Campo S, Campo V, Gambadauro P. Short-term and long-term results of resectoscopic myomectomy with and without pretreatment with GnRH analogs in premenopausal women. Acta Obstet Gynecol Scand. 2005;84:756–60. [DOI] [PubMed] [Google Scholar]
- [13].Favilli A, Mazzon I, Grasso M, et al. Intraoperative effect of preoperative gonadotropin-releasing hormone analogue administration in women undergoing cold loop hysteroscopic myomectomy: a randomized controlled trial. J Minim Invasive Gynecol. 2018;25:706–14. [DOI] [PubMed] [Google Scholar]
- [14].Camanni M, Bonino L, Delpiano EM, et al. Hysteroscopic management of large symptomatic submucous uterine myomas. J Minim Invasive Gynecol. 2010;17:59–65. [DOI] [PubMed] [Google Scholar]
- [15].Donnez J, Polet R, Smets M, et al. Hysteroscopic myomectomy. Curr Opin Obstet Gynecol. 1995;7:311–6. [PubMed] [Google Scholar]
- [16].Wang C-J, Yu H-T, Wu P-J, et al. Laparoscopic myomectomy instead of hysteroscopic myomectomy for large submucous fibroids. 2013;2:93–5. [Google Scholar]
- [17].Wamsteker K, Emanuel MH. de Kruif JH. Transcervical hysteroscopic resection of submucous fibroids for abnormal uterine bleeding: results regarding the degree of intramural extension. Obstet Gynecol. 1993;82:736–40. [PubMed] [Google Scholar]
- [18].Lasmar RB, Barrozo PR, Dias R, et al. Submucous myomas: a new presurgical classification to evaluate the viability of hysteroscopic surgical treatment—preliminary report. J Minim Invasive Gynecol. 2005;12:308–11. [DOI] [PubMed] [Google Scholar]
- [19].Zivkovic N, Zivkovic K, Despot A, et al. Measuring the volume of uterine fibroids using 2- and 3-dimensional ultrasound and comparison with histopathology. Acta Clin Croat. 2012;51:579–89. [PubMed] [Google Scholar]
- [20].Romer T. Benefit of GnRH analogue pretreatment for hysteroscopic surgery in patients with bleeding disorders. Gynecol Obstet Invest. 1998;45(Suppl 1):12–20. [DOI] [PubMed] [Google Scholar]
- [21].Romer T, Schmidt T, Foth D. Pre- and postoperative hormonal treatment in patients with hysteroscopic surgery. Contrib Gynecol Obstet. 2000;20:1–12. [DOI] [PubMed] [Google Scholar]
- [22].Valle RF. Development of hysteroscopy: from a dream to a reality, and its linkage to the present and future. J Minim Invasive Gynecol. 2007;14:407–18. [DOI] [PubMed] [Google Scholar]
- [23].Saccardi C, Conte L, Fabris A, et al. Hysteroscopic enucleation in toto of submucous type 2 myomas: long-term follow-up in women affected by menorrhagia. J Minim Invasive Gynecol. 2014;21:426–30. [DOI] [PubMed] [Google Scholar]
- [24].Keskin M, Çakmak D, Yarci Gürsoy A, et al. Single-step hysteroscopic myomectomy for submucous leiomyoma. Turk J Obstet Gynecol. 2020;17:139–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Murakami T, Tachibana M, Hoshiai T, et al. Successful strategy for the hysteroscopic myomectomy of a submucous myoma arising from the uterine fundus. Fertil Steril. 2006;86:1513e19–22. [DOI] [PubMed] [Google Scholar]
- [26].Maher PJ, Hill DJ. Transcervical endometrial resection for abnormal uterine bleeding--report of 100 cases and review of the literature. Aust N Z J Obstet Gynaecol. 1990;30:357–60. [DOI] [PubMed] [Google Scholar]
- [27].Loffer FD. Preliminary experience with the versapoint bipolar resectoscope using a vaporizing electrode in a saline distending medium. J Am Assoc Gynecol Laparosc. 2000;7:498–502. [DOI] [PubMed] [Google Scholar]
- [28].Corson SL, Brooks PG, Serden SP, et al. Effects of vasopressin administration during hysteroscopic surgery. J Reprod Med. 1994;39:419–23. [PubMed] [Google Scholar]