Abstract
In 2017, an incident of failed sterilization of dental instruments occurred at a large dental outpatient facility in Singapore. We aim to describe findings of the investigation of the sterilization breach incident, factors related to risk of viral transmission to the potentially affected patients, and the contact tracing process, patient management, and blood test results at a 6-month follow-up.
A full assessment of the incident was immediately carried out. The factors related to risk of viral transmission due to affected instruments were analyzed using 3 keys points: breached step(s) and scale of the incident, prevalence of underlying bloodborne diseases and immunity in the Singapore population, health status of potential source patients, and type of dental procedure performed, and health status of affected patients and type of dental procedure received.
Up to 72 affected instrument sets were used in 714 potentially affected patients who underwent noninvasive dental procedures. The investigation revealed that there was a lapse in the final step of steam sterilization, resulting in the use of incompletely sterilized items. The assessment determined that there was an extremely low risk of bloodborne virus transmission of diseases to the patients. At the 6-month follow-up, there were no infected/colonized cases found related to the incident.
Lapses in the sterilization process for medical and dental instruments can happen, but a risk assessment approach is useful to manage similar incidents. Quick response and proper documentation of the sterilization process can prevent similar incidents.
Keywords: dental, failure of sterilization, patient safety, risk assessment
1. Introduction
Sterilization is a process of complete elimination of all forms of microorganisms to ensure the highest level of decontamination of medical and surgical devices. The sterilization carried out in healthcare facilities includes physical or chemical methods such as steam sterilization, dry heat, ethylene oxide gas, hydrogen peroxide gas plasma, vaporized hydrogen peroxide, and liquid chemicals.[1] Any breach in protocol (either systemic or human error) or equipment failure can lead to incomplete or failure of sterilization, which can potentially result in outbreaks or incidents of transmitting infectious pathogens to patients.
National Dental Centre Singapore is a major referral center that delivers multidisciplinary specialist oral healthcare, with approximately 210,000 patient attendances and 290,000 dental procedures performed each year. We operate a Central Sterile Supplies Department (CSSD), which reprocesses dental instruments using steam sterilization; details of the process can be found under Methods. The breach in our facility occurred at the final step of steam sterilization, due to human error when there was a failure to verify the start and completion of the steam sterilization cycle.
There are a number of sterilization incidents involving medical and dental facilities that resulted from human error, equipment or product failure, or lack of processes.[2] These may lead to transmission of bloodborne pathogens such as hepatitis B virus (HBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), in addition to a variety of bacteria, fungi, and even mycobacteria.
In 2007, Rutala and Weber[2] studied the risk assessment, in a hypothetical setting, of 4 potentially affected patients who had examinations using disinfected but not sterile specula in an Obstetrics–Gynecology Clinic. They found that in noninvasive or category I procedures, the risk of infection of bloodborne diseases following exposure to incompletely sterilized equipment was equal to 8 in 100 trillion for HIV, 1 in 10 billion for HBV, and a value between HIV and HBV for HCV.[2]
In 2013, Cheng et al described an investigation into failed sterilization in a dental clinic due to a rare lapse in monitoring during the autoclaving cycle. A total of 127 sources and 250 exposed patients were involved. The authors noted that a rapid response was crucial in minimizing the impact of such an incident and relieving the anxiety of the exposed patients. They also recommended practicing proper recording and documentation of the autoclaving process.[3]
In 2014, Southworth[4] reviewed 21 articles on outbreaks and incidents associated with inappropriate, inadequate, or unsuccessful decontamination of surgical instruments. The review included 6 incidents associated with reported breaches in sterilization that led to contaminated instruments being exposed to patients (ranging from 1 to 250 cases)[3,5–8] and 15 outbreak incidents with the consequence of infection or colonization, including deaths (ranging from 6 to 302 cases).[9–23] The most common organisms found from inappropriate or inadequate disinfection and sterilization were Proteus mirabilis, Mycobacterium chelonae, and Pseudomonas aeruginosa. Notably, 1 incident was related to arthroscopic equipment in which the debris was inadequately cleaned and steam sterilization was not carried out, which led to a Ps aeruginasa outbreak.[9] Another review reported a Sphingomonas paucimobilis and Burkholderia pickettii outbreak causing keratitis in a patient’s eye as a result of a contaminated steam sterilizer used for cleaning Lasik surgical instruments.[11] Taking these factors into account, the possibility of cross-transmission could be prevalent in an area such as dentistry, where there are large numbers of patients and less stringent controls compared to other types of surgery.
2. Objectives
This article aims to report an incident of incomplete sterilization and describes: findings of the investigation of the sterilization breach incident, factors related to the risk of viral and bacterial transmission to the potentially affected patients, and the contact tracing process, patient management, and blood test results at 6-month follow-up.
3. Methods
National Dental Centre Singapore is an ambulatory specialist dental facility comprising 106 dental chairs, 2 general anesthesia operating theaters, and 6 local anesthesia operating theaters. Dental instruments used are sent to the CSSD located in the basement of the Centre. This is a 3-step process comprising washing, thermal disinfection, and steam sterilization before supplies are sent back to clinical floors. The contaminated instruments are first washed in the thermal washer disinfector before being packed and sent to the steam sterilizer. The autoclave must reach and maintain a temperature of 134°C (heat resistant instruments) or 121°C (instruments containing rubber) for at least 30 minutes by using saturated steam under at least 15 psi of pressure. Verified printouts of the parameters during each sterilization cycle, biological and chemical indicators are routinely used to ensure effective sterilization.
Between June 5 and June 6, 2017, we discovered that 1 batch of dental instruments that had not completed the final step of the process (steam sterilization) had been used for patient treatment at outpatient clinics. The incident did not involve the operating theater; hence, surgical instruments were not affected.
An initial instrument recall process was performed by CSSD staff; however, the recall was found to be incomplete. In the late afternoon of day 2, June 6, 2017, the Centre’s Infection Control and Quality Management teams were informed, after which the escalation process was started. The second round of recall led by the Clinical Governance and Quality Management team began on the same day. A complete sweep for unsterile instruments was done on the morning of day 3, June 7, 2017, before the clinics were allowed to resume operation.
A team was assembled for immediate risk assessment and management of the incident. It included the Centre’s Infection Control, and Clinical Governance and Quality Management teams, and the Department of Infection Prevention and Epidemiology and Department of Infectious Diseases of the Singapore General Hospital.
The team commenced the investigation by visiting the CSSD and clinical supply stations at each affected clinical floor. A detailed interview of CSSD and clinical staff was performed to tally the number of instruments that went through the sterilization process before dispatch to the clinical floors. Up to 72 affected instrument sets were used in 714 potentially affected patients who underwent noninvasive dental procedures.
The investigation team made a decision to contact all potentially affected patients to inform them about the incident and risks involved. The attending dentists were tasked to contact their patients and/or caregivers. Patients who remained worried or could not be reassured over the phone were offered face-to-face counseling or advised to call a hotline number for more information. The incident was also communicated to the media, and patients who visited the clinic on June 5 and June 6, 2017, were advised to call the hotline number if they were concerned. Testing for bloodborne viruses, including HBV, HCV, and HIV, was made available for patients who were concerned.
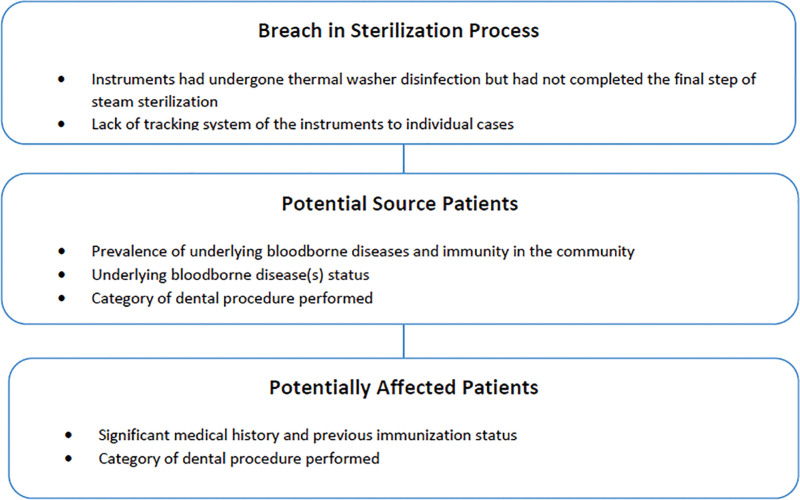
This study was reviewed and approved by SingHealth Centralised Institutional Review Board (Ref: 2020/2990). The investigation involved 3 main components as described in Figure 1.
Figure 1.
Components of Investigations.
3.1. Identification of potentially affected patients
Contact tracing was performed by reviewing the Centre’s records from the time of the failed autoclave until the end of day 2, when nonsterilized instruments had been completely removed from the clinical areas. This involved approximately 1.5 days of clinical sessions on the 4 clinical floors and 72 dental operatories.
A total of 714 individuals were identified along with information about the nature of dental treatment(s) performed. The raw data were taken from 2 different sources: the Outpatient Administration System containing patient visit and registration records, and the Electronic Dental Records containing clinical and treatment notes.
3.2. Identification of potential source patients
Due to the lack of radiofrequency identification technology for instrument tracking, we were unable to trace the instruments from end to end. Therefore, potential source patients were manually identified. Potential source patients were preliminarily defined as those who underwent treatment 2 working days before the incident, June 2, 2017 (full day) and June 5, 2017 (morning session). The instruments used by these patients had been sent to CSSD and 1 batch of instruments did not complete the sterilization process.
Instruments were identified according to the clinical floors they were used on during particular time periods. The potentially affected and source patients were classified according to the dental procedures performed and stratified according to the risk of exposure to pathogens, with reference to the Society for Healthcare Epidemiology of America Guidelines[24] (Table 1).
Table 1.
Dental procedures and classification according to the level of risk for bloodborne pathogen transmission (modified from the SHEA Guidelines).
| Exposed patients and classification of risk | ||
|---|---|---|
|
Category I:
Procedures with minimal risk of bloodborne virus transmission |
Category II:
Procedures for which bloodborne virus transmission is theoretically possible but unlikely |
Category III:
Procedures for which there is definite risk of bloodborne virus transmission or that have been classified previously as “exposure-prone” |
| Oral examination Taking dental radiographs Issuance of dental appliances Smoothening of hard dental tissues Plaque removal using hand instruments Impression taking for removal of prostheses Bonding of orthodontic brackets or permanent retainers Removal of surgical stitches Rubber dam placement Placement or removal of bands (including orthodontic band, crown band) Placement of retraction cord Removal of bony sequestration Dental injections Gingival probing Polishing of teeth using polishing cup Pulpectomy Debanding Impression taking for fixed prostheses Removal of temporary anchorage device Dental filling |
Minor surgical procedures Incision and drainage Extractions Scaling and root planning |
General oral surgery, including: Surgical extractions Hard and soft tissue biopsy (if more extensive and/or having difficult access for suturing) Apicoectomy Gingivectomy Periodontal curettage Mucogingival and osseous surgery Alveoplasty or alveoectomy Endosseous implant surgery |
3.3. Risk stratification according to history of bloodborne diseases in community
According to the Ministry of Health (MOH) Singapore, Communicable Diseases Surveillance Report in 2017, the rate of HIV/AIDS in Singapore was 10.9 per 100,000 population or 0.01%, acute hepatitis B was 0.7 per 100,000 population or 0.0007% and acute hepatitis C was 0.4 per 100,000 populations or 0.0004%.[25] The seroprevalence rate of chronic hepatitis B in 2010 was 3.6%,[26,27] which had dropped from 5% to 6% in 1996.[27] Based on MOH internal estimates, the seroprevalence rate of chronic hepatitis C in 2016 was estimated at 0.1%, based on blood donor screening data and cumulative notifications of HCV cases to MOH.[26,27] However, the seroprevalence of chronic hepatitis C could vary among the different groups of Singapore patients, that is, 0.059% in blood donors (recorded in 2011–2014), 2.2% in hemodialysis patients, and 6.9% reported in liver cirrhosis patients at a tertiary liver center.[28]
The medical records of potential source patients were screened for a documented history of bloodborne diseases (e.g., HBV, HCV, and/or HIV infection) to ascertain the risk of cross-infection. Potential risks of transmission of HBV, HCV, and HIV were based on data calculated from parenteral exposure and injection injuries.[29–32]
3.4. Risk stratification according to the prevalence of immunity to HBV in community
Following the introduction of the Singapore National Childhood Immunisation Programme in 1987, Hong et al. conducted a study in 2010 to compare the seroprevalence of hepatitis B surface antigen (HBsAg) and hepatitis B antibodies (anti-HBs ≥10 mIU/mL indicates immunity to hepatitis B). Singapore residents aged 18 to 69 years between 1999 and 2005 were studied to assess the impact of the 4-year catch-up hepatitis B immunization program for adolescents in 2001. The study reported an overall significant decrease in the prevalence of HBsAg and a significant increase in anti-HBs.[33]
Additionally, a national study on hepatitis B seroprevalence in Singaporean citizens aged 18 to 79 years in 2010 confirmed that the prevalence of HBsAg decreased significantly compared between 1998 and 2004, while the prevalence of anti-HBs increased significantly.[34] According to the Singapore Communicable Diseases Surveillance report in 2017, the incidence of indigenous acute hepatitis B for all age groups had significantly declined and was 10 times lower compared with the incidence in 1985 (52 cases or 0.9 per 100,000 population vs 243 cases or 9.5 per 100,000 population). During the same period, the reported number of cases in children aged 15 years or less decreased to 0.[35] Nevertheless, the decision was taken to test patients who were concerned and willing to come back for testing. The following tests were conducted: hepatitis B core total antibody (anti-HBc), anti-HBs, qualitative HBsAg, hepatitis C antibody screening (enzyme immunoassay), HCV RNA polymerase chain reaction (qualitative), and HIV screening. They were advised to have their initial blood tests done within 1 month of the incident to determine their preexisting status for viral infection, and to be followed up at 3- and 6-month intervals to determine if there was a possibility of having been infected through their dental treatment on the day of the incident. The results were interpreted and explained to patients by either an infectious disease specialist or a gastroenterologist from the Singapore General Hospital.
3.5. Risk stratification according to bacterial spore/bacterial infection
More than 700 bacterial species have been detected in the oral cavity.[36] However, in this incident, the instruments had completed the earlier steps of thermal disinfection in the sterilization process, which would have removed 99.99% of organisms of concern; hence, the risk of bacterial infection to patients was assessed to be extremely low. There was a very small risk related to bacterial spores causing diarrhea, including a very small risk of tetanus.
In Singapore, the National Childhood Immunization Programme (recorded between years 2008 and 2017) provided tetanus toxoid vaccine (tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine and pediatric diphtheria and tetanus toxoid and acellular pertussis) for each child born in Singapore. The coverage rate was reported as 96.0% to 97.5% for the primary vaccination course, 89.8.3% to 92.8% for the first booster dose, and 91.4% to 96.0% for the second booster dose.[37] The possibility of bacterial spores causing diarrhea and risk of tetanus infection were graded as low or unlikely. The human tetanus immunoglobulin was available upon request but was not necessary under the treatment and prevention protocol.
4. Results
4.1. The causes of sterilization breach and a delay in escalation process
The investigations identified human error as the cause of the incident whereby the steam sterilizer had not been activated by the staff on duty. Up to 72 packs of dental instruments that did not complete the final step of steam sterilization had been sent to the outpatient clinics by the same staff.
The instruments were used on patients in the afternoon session of clinics on the same day and the whole of the following day. The recall process was initially started by CSSD staff on the first day of the incident. However, the recall was incomplete due to lack of understanding of the distribution and usage of the instruments in the outpatient clinics. Procedural weaknesses and a lack of vigilance of staff involved led to delays in escalation of the incident to senior management, which only happened toward the end of day 2 of the incident.
4.2. Identification of the risk of potentially affected patients
Up to 72 packs of instruments were possibly used for dental treatment in 714 patients, resulting in potentially 1 out of 10 patients being exposed to disinfected but nonsterilized instruments. However, this is likely to be an overestimation as >1 pack of instruments may be used on a single patient.
Out of the 714 potentially affected patients, 552 underwent category I procedures, while 162 underwent category II procedures. The incident did not involve any category III or the “definite risk” dental treatment (Table 2).
Table 2.
Number of potentially affected and potential source patients according to category of treatment received.
| Group of patients | Classification of risk of dental treatment | ||
|---|---|---|---|
| Category I | Category II | Category III | |
| Potentially affected patients (total 714 patients, 714 dental treatment visits) | 552 | 162 | 0 |
| Potential source patients (total 723 patients,743 dental treatment visits) | 490 | 221 | 32 |
4.3. Identification of potential source patients
There were 723 potential source patients who made up a total of 743 dental visits and 2771 dental instruments used on these patients prior to the failed sterilization cycle were identified. The dental treatment procedures given to the potential source patients were categorized (Table 2).
Of these potential source patients, 13 patients (1.79%) had chronic HBV infection with positive HBsAg, 1 patient (0.14%) had chronic HCV infection, and 1 patient (0.14%) was positive for HCV by enzyme immunoassay (previous exposure or false positive). None of the potential source patients was known to be HIV-positive. Some patients may not have declared their condition or might not have been aware of their status; thus, this is likely to be an underestimation.
4.4. HBV and HBV immunity profile of affected patients
From the risk estimation, it was concluded that following the implementation of the childhood immunization and catch-up immunization programs, hepatitis B immunity is high among the adult population aged ≤30 years—the level of immunity increased from 27.9% in 1998 to 43.3% in 2010.[34]
It was found that 384 cases (53.78%) of all potentially affected cases were in the age range of ≤30 years. This patient group would have benefitted from the Singapore Hepatitis B National Childhood Immunisation Programme.
4.5. Risk of viral transmission due to affected instruments in this incident
Table 3 provides the calculated estimated individual risk of potentially affected patients according to the category of treatment.
Table 3.
Individual risk calculated in relation to the incident in our center.
| Dental treatment category (SHEA Guidelines) |
Prevalence of bloodborne diseases
in Singapore[25–28,38] |
Risk of transmission via oral mucosa membrane contact[1]
and injection injuries[29–32] |
Likelihood of nonsterilized instrument used | Efficacy of washer/disinfector | Effect of drying | Individual risk | |
|---|---|---|---|---|---|---|---|
| Category I | HIV | ~0.109:1000 | ~1:1000 | 1:10 | 1:100,000 | 1:100 | 1.09 × 10–15 |
| HBV | ~36:1000 to 60:1000 |
~1:100 | 1:10 | 1:100,000 | 1:1 | 3.6 × 10–10 to 6.0 × 10–10 | |
| HCV | ~1:1000 | ~1:100 | 1:10 | 1:100,000 | 1:1 | 1 × 10–11 | |
| Category II | HIV | ~0.109:1000 | ~2.38:100 | 1:10 | 1:100,000 | 1:100 | 2.59 × 10–14 |
| HBV | ~36:1000 to 60:1000 |
~37:100 | 1:10 | 1:100,000 | 1:1 | 1.33 × 10–8 to 2.22 × 10–8 | |
| HCV | ~1:1000 | ~5:100 | 1:10 | 1:100,000 | 1:1 | 5.0 × 10–11 | |
4.6. Patient management
4.6.1. HBV, HCV, and HIV.
Taking into account the nature of the treatment at the outpatient clinics (of which none was invasive) and the completion of the earlier steps of thermal disinfection in the sterilization process, which would have removed 99.99% of organisms of concern, the risk of infection to patients was assessed to be extremely low. Postexposure prophylaxis with hepatitis B vaccination and hepatitis B immunoglobulin were not indicated due to the very low risk of disease transmission. Similarly, as no potential source patient was HIV-positive, the decision was taken not to offer postexposure prophylaxis for HIV to anyone.
Extensive contact tracing of 714 patients was done. Communication to patients was performed by the attending or senior clinicians to explain or provide guidance to the individual patient and/or caregivers regarding concerns and risks associated with the incident. Majority of them (576 cases or 80.7%) were satisfied with the information provided, understood the situation, and did not express any concern or need for further support. However, 138 patients (19.32%) were concerned and walked in for face-to-face counseling at the Centre.
Upon counseling and reassurance, blood tests were performed on 114 potentially affected patients as a baseline. Only 100 cases continued with the follow-up for the second and third blood tests at 3 and 6 months.
In Table 4, baseline results showed that 95 patients (83.3%) were negative to anti-HBc. Nineteen patients had positive anti-HBc, which indicated past infection with hepatitis B. Two patients had positive HBsAg, which indicated that they were hepatitis B carriers. Hepatitis B carriers were further counseled regarding their status and medical management. Postexposure prophylaxis with hepatitis B vaccination and hepatitis B immunoglobulin were not indicated due to the very low risk of disease transmission.
Table 4.
Results of serology tests of potentially affected patients who underwent testing at 3 timelines: first blood test at baseline or those with a preexisting condition, second blood test at 12 wk after the incident, and third or last blood test at 24 wk after the incident.
| Total number of potentially affected patients identified and contacted | 714 | |||||||
|---|---|---|---|---|---|---|---|---|
| Total number of potentially affected patients concerned and counseled | 138 | |||||||
| Blood test | Number of patients who went for blood test | HBV |
HCV (anti-HCV) |
HIV (HIV antibodies screening) |
||||
|
Negative
anti-HBc Total antibodies |
Positive
anti-HBc Total antibodies |
Positive
HBsAg |
Negative | Positive | Negative | Positive | ||
| First | 114 | 95 | 19* | (2)† | 114 | 0 | 114 | 0 |
| Second | 100 | 82 | 0‡ | 0‡ | 100 | 0 | 100 | 0 |
| Third | 100 | 82 | 0‡ | 0‡ | 100 | 0 | 100 | 0 |
At the 3 and 6 months postincident blood test, none of the 100 patients who continued with the follow-up had a positive serology test for any of the 3 viruses.
4.6.2. Bacterial spore/bacterial infection.
There was no report of patients experiencing symptoms related to spore-forming microbes, for example, diarrhea, wound infection, or tetanus related to exposure to Clostridium tetani, Bacillus species. One patient requested and received human antitetanus immunoglobulin.
5. Discussion
Possible failures in disinfection or sterilization processes may occur as a result of human error, equipment malfunction, or system failure. Healthcare institutions must have a comprehensive work plan for dealing with such events, including a response timeline, access to laboratory for large numbers of blood tests, and patient call-back mechanism.
Immediate response and management of affected patients are crucial. We adapted the Society for Healthcare Epidemiology of America guidelines[24] with 3 main investigation components in the risk assessment: identification of breached step in the sterilization process, potentially affected patients, and potential source patients. The assessment determined that there was an extremely low risk in transmission of disease, which guided the strategy to manage the potentially affected patients. As it turned out, no one among those who chose to be tested seroconverted, suggesting that using tie risk stratification of Rutala and Weber,[2] and combining it with our knowledge of the prevalence of HBV, HCV, and HIV in Singapore, was a useful strategy. Six hundred patients did not come forward for testing or chose not to be tested after counseling. Their status remains unknown. However, all affected patients had been contacted and informed of the incident. Given the increasing awareness of medical litigation in Singapore, it is unlikely for the Centre not to have been informed of a seroconversion.
For the 20% who were concerned, blood tests and medical counseling were provided without cost. The results of the blood tests confirmed that there was no transmission of disease to affected patients.
A thorough review of the processes at the CSSD was carried out using the Asia Pacific Society of Infection Control checklist.[39] Immediate measures were instituted to address the gaps followed by sustained quality improvement activities and audits.
Acknowledgments
The author wishes to thank all supports from Infectious Diseases Department, Pathology Department, Centre for Digestive and Liver Diseases, Singapore General Hospital, and SingHealth Office for Insights & Analytics group.
Author contributions
All authors contributed to the writing of the manuscript. Main authors are N Chanchareonsook, ML Ling, BH Tan and CY Poon.
Abbreviations:
- AIDS =
- Acquired Immunodeficiency Syndrome
- anti-HBc =
- Hepatitis B core total antibody
- anti-HBs =
- Hepatitis B antibodies
- CSSD
- = Central Sterile Supplies Department
- HBC =
- Hepatitis C Virus
- HBsAg =
- Hepatitis B Surface Antigen
- HBV =
- Hepatitis B Virus
- HIV =
- Human Immunodeficiency Virus
- MOH =
- Ministry of Health
- RNA =
- Ribonucleic acid
How to cite this article: Chanchareonsook N, Ling ML, Sim QX, Teoh KH, Tan K, Tan BH, Fong KY, Poon CY. Failure of sterilization in a dental outpatient facility: Investigation, risk assessment and management. Medicine 2022;101:31(e29815).
Supplemental Digital Content is available for this article.
All authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
SHEA = Society for Healthcare Epidemiology of America.
The classification is based on a modification of the SHEA Guidelines.[24]
Classification of risk of dental treatment: category I: procedures with minimal risk of bloodborne virus transmission; category II: procedures for which bloodborne virus transmission is theoretically possible but unlikely; and category III: procedures for which there is definite risk of bloodborne virus transmission or that have been classified previously as “exposure-prone.”
SHEA = Society for Healthcare Epidemiology of America.
HBV = hepatitis B virus, HCV = hepatitis C virus, HIV = human immunodeficiency virus, Individual risk = estimated individual risk of infection, SHEA = Society for Healthcare Epidemiology of America, Dental treatment.
Anti-HBc = hepatitis B core antibody, anti-HCV = hepatitis C antibody, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, HCV = hepatitis C virus, HIV = human immunodeficiency virus.
Ninteen patients consulted a gastroenterologist; past infection/natural immunity.
Two patients are HBV carriers.
Eighteen patients who were anti-HBc and HBsAg positive at first blood test were not tested for HBV.
Contributor Information
N Chanchareonsook, Email: cnattharee@yahoo.com.
ML Ling, Email: ling.moi.lin@singhealth.com.sg.
QX Sim, Email: chelsia.sim.q.x@singhealth.com.sg.
KH Teoh, Email: teoh.khim.hean@singhealth.com.sg.
K Tan, Email: tan.ban.hock@singhealth.com.sg.
BH Tan, Email: tan.ban.hock@singhealth.com.sg.
KY Fong, Email: fong.kok.yong@singhealth.com.sg.
References
- [1].Rutala WA, Weber DJ. Disinfection, sterilization, and control of hospital waste. Bennett JE, Dolin R, Blaser MJ, eds. In: Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. United States of America: Elsevier; 2015:3294–3309.e4. [Google Scholar]
- [2].Rutala WA, Weber DJ. How to assess risk of disease transmission to patients when there is a failure to follow recommended disinfection and sterilization guidelines. Infect Control Hosp Epidemiol. 2007;28:146–55. [DOI] [PubMed] [Google Scholar]
- [3].Cheng VC, Wong SC, Sridhar S, et al. Management of an incident of failed sterilization of surgical instruments in a dental clinic in Hong Kong. J Formos Med Assoc. 2013;112:666–75. [DOI] [PubMed] [Google Scholar]
- [4].Southworth PM. Infections and exposures: reported incidents associated with unsuccessful decontamination of reusable surgical instruments. J Hosp Infect. 2014;88:127–31. [DOI] [PubMed] [Google Scholar]
- [5].Young M. Unprocessed tray incident prompts investigation, leads to process improvements. OR Manager. 2013;29:16–7. [PubMed] [Google Scholar]
- [6].Cooper E, Breckon K. Sterilization breach. J Hosp Infect. 2005;60:379–81. [DOI] [PubMed] [Google Scholar]
- [7].Zinn C. Nine hospital patients may have been exposed to CJD. BMJ. 2000;320:1296. [PMC free article] [PubMed] [Google Scholar]
- [8].Kumar S. Delhi hospital lambasted for use of contaminated surgical equipment. Lancet. 2000;356:576. [DOI] [PubMed] [Google Scholar]
- [9].Tosh PK, Disbot M, Duffy JM, et al. Outbreak of Pseudomonas aeruginosa surgical site infections after arthroscopic procedures: Texas, 2009. Infect Control Hosp Epidemiol. 2011;32:1179–86. [DOI] [PubMed] [Google Scholar]
- [10].Unal M, Yucel I, Akar Y, et al. Outbreak of toxic anterior segment syndrome associated with glutaraldehyde after cataract surgery. J Cataract Refract Surg. 2006;32:1696–701. [DOI] [PubMed] [Google Scholar]
- [11].Villarrubia A, Palacin E, Gomez del Rio M, et al. Description, etiology, and prevention of an outbreak of diffuse lamellar keratitis after LASIK. J Refract Surg. 2007;23:482–6. [DOI] [PubMed] [Google Scholar]
- [12].Hellinger WC, Hasan SA, Bacalis LP, et al. Outbreak of toxic anterior segment syndrome following cataract surgery associated with impurities in autoclave steam moisture. Infect Control Hosp Epidemiol. 2006;27:294–8. [DOI] [PubMed] [Google Scholar]
- [13].Duffy RE, Brown SE, Caldwell KL, et al. An epidemic of corneal destruction caused by plasma gas sterilization. The Toxic Cell Destruction Syndrome Investigative Team. Arch Ophthalmol. 2000;118:1167–76. [DOI] [PubMed] [Google Scholar]
- [14].Rutala WA, Weber DJ, Thomann CA. Outbreak of wound infections following outpatient podiatric surgery due to contaminated bone drills. Foot Ankle. 1987;7:350–4. [DOI] [PubMed] [Google Scholar]
- [15].Vijayaraghavan R, Chandrashekhar R, Sujatha Y, et al. Hospital outbreak of atypical mycobacterial infection of port sites after laparoscopic surgery. J Hosp Infect. 2006;64:344–7. [DOI] [PubMed] [Google Scholar]
- [16].Meyers H, Brown-Elliott BA, Moore D, et al. An outbreak of Mycobacterium chelonae infection following liposuction. Clin Infect Dis. 2002;34:1500–7. [DOI] [PubMed] [Google Scholar]
- [17].Courtright P, Lewallen S, Holland SP, et al. Corneal decompensation after cataract surgery: an outbreak investigation in Asia. Ophthalmology. 1995;102:1461–5. [DOI] [PubMed] [Google Scholar]
- [18].Breebaart AC, Nuyts RA, Pels E, et al. Toxic endothelial cell destruction of the cornea after routine extracapsular cataract surgery. Archs Ophthalmol. 1990;108:1121–5. [DOI] [PubMed] [Google Scholar]
- [19].Centers for Disease Control and Prevention (CDC). Rapidly growing mycobacterial infection following liposuction and liposculpture Caracas, Venezuela, 1996e1998. Morb Mortal Wkly Rep. 1998;47:1065–7. [PubMed] [Google Scholar]
- [20].Pednekar SN, Dohe VB, Deshpande SM, et al. An outbreak of Pseudomonas aeruginosa in a burn unit. Burns. 2010;36:e130–1. [DOI] [PubMed] [Google Scholar]
- [21].Kayabas U, Bayraktar M, Otlu B, et al. An outbreak of Pseudomonas aeruginosa because of inadequate disinfection procedures in a urology unit: a pulsed-field gel electrophoresis-based epidemiologic study. Am J Infect Control. 2008;36:33–8. [DOI] [PubMed] [Google Scholar]
- [22].Duarte RS, Lourenço MC, Fonseca Lde S, et al. Epidemic of postsurgical infections caused by Mycobacterium massiliense. J Clin Microbiol. 2009;47:2149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Soto LE, Bobadilla M, Villalobos Y, et al. Post-surgical nasal cellulitis outbreak due to Mycobacterium chelonae. J Hosp Infect. 1991;19:99–106. [DOI] [PubMed] [Google Scholar]
- [24].Henderson DK, Dembry L, Fishman NO, et al. SHEA guideline for management of healthcare workers who are infected with hepatitis B virus, hepatitis C virus, and/or human immunodeficiency virus. Infect Control Hosp Epidemiol. 2010;31:203–32. [DOI] [PubMed] [Google Scholar]
- [25].Ministry of Health (MOH) Singapore. Communicable diseases surveillance in Singapore, chapter V blood borne & sexually transmitted disease. Published 2017. Available at: https://www.moh.gov.sg/docs/librariesprovider5/diseases-updates/blood-borne-and-sexually-transmitted-2017a3322a92d7c34c4280013fc8bc83fff7.pdf [access date September 28, 2020].
- [26].Muthiah M, Chong C H, Lim S G. Liver disease in Singapore. Euroasian J Hepatogastroenterol. 2018;8:66–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lim SG. Time for action on viral hepatitis. Ann Acad Med Singapore. 2016;45:27–30. [PubMed] [Google Scholar]
- [28].Soh BYM, Kumar R, Ekstrom VSM, et al. Prevalence of hepatitis C virus infection and the IL28B genotype polymorphism among blood donors and high-risk populations. Singapore Med J. 2019;60:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Baggaley RF, Boily MC, White RG, et al. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis. AIDS. 2006;20:805–12. [DOI] [PubMed] [Google Scholar]
- [30].Werner B, Grady GF. Accidental hepatitis-B-surface-antigen-positive inoculations: use of e antigen to estimate infectivity. Ann Intern Med. 1982;97:367–9. [DOI] [PubMed] [Google Scholar]
- [31].U.S. Public Health Service. Updated U.S. Public Health Service Guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2001;50:1–52. [PubMed] [Google Scholar]
- [32].Pépin J, Abou Chakra CN, Pépin E, et al. Evolution of the global burden of viral infections from unsafe medical injections, 2000-2010. PLoS One. 2014;9:e99677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hong WW, Ang LW, Cutter J, et al. Changing sero-prevalence of hepatitis B virus markers of adults in Singapore. Ann Acad Med Singapore. 2010;39:591–8. [PubMed] [Google Scholar]
- [34].Ang LW, Cutter J, James L, et al. Seroepidemiology of hepatitis B virus infection among adults in Singapore: a 12-year review. Vaccine. 2013;32:103–10. [DOI] [PubMed] [Google Scholar]
- [35].Ministry of Health (MOH) Singapore. Communicable diseases surveillance in Singapore, chapter VII childhood immunization. Published 2017. Available at: https://www.moh.gov.sg/docs/librariesprovider5/diseases-updates/childhood-immunisation-2017d0b895c2b9464bfd9296deb0cf73e709.pdf [access date September 28, 2019].
- [36].Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ministry of Health (MOH) Singapore. Communicable Diseases Surveillance in Singapore Childhood Immunization. 2017. Available at: https://www.moh.gov.sg/docs/librariesprovider5/diseases-updates/childhood-immunisation-2017d0b895c2b9464bfd9296deb0cf73e709.pdf [access date September 28, 2020].
- [38].Guan R. Hepatitis B virus infection in Singapore. Gut. 1996;38(Suppl 2):S13–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ling ML, Ching P, Widitaputra A, et al. APSIC guidelines for disinfection and sterilization of instruments in health care facilities. Antimicrob Resist Infect Control. 2018;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]