Abstract
Background and Aims
The erythrocyte sedimentation rate [ESR] as a component of the Truelove and Witts Criteria [TWC] is the traditional inflammatory marker used for the assessment of ulcerative colitis [UC] activity. However, the C-reactive protein [CRP] is preferentially used in contemporary clinical practice. We aimed to determine the equivalent CRP cut-off for an ESR of >30 mm/h in patients presenting with acute severe UC.
Methods
Clinical and pathological data were prospectively collected from 163 presentations of severe UC. A CRP cut-off corresponding to an ESR of >30 mm/h was determined using confusion matrices. A validation cohort of 128 presentations was prospectively collected and analysed.
Results
A CRP cut-off of ≥12 mg/L generated an 85% positive predictive value [PPV] with a sensitivity of 95% and an accuracy of 82% for having a paired ESR of >30 mm/h. There were no statistically significant differences between groups determined by the traditional ESR versus the new CRP-based criterion in the presenting faecal calprotectin, Mayo endoscopic subscore, or the rates of intravenous corticosteroid therapy failure and colectomy-by-discharge. Applying the CRP ≥12 mg/L criterion to a validation cohort of 128 presentations generated a PPV of 83% and a sensitivity of 94%.
Conclusions
The proposed CRP ≥12 mg/L cut-off is an inclusive, sensitive, and very practical alternative to ESR as part of the TWC for defining UC presentation severity. It demonstrated similar performance characteristics to the classical ESR criterion when used for the assessment of acute UC disease activity. These findings were confirmed in a validation cohort.
Keywords: Ulcerative colitis, erythrocyte sedimentation rate, C-reactive protein
1. Introduction
The Truelove and Witts definition of ulcerative colitis [UC] activity remains a relevant instrument providing an objective disease activity assessment that assists clinical decision making.1, 2 Division of disease activity into three categories by objective criteria also has utility in the research and clinical trial settings, by augmenting the ability to perform meaningful inter-study comparison and meta-analyses.
To satisfy the diagnosis of severe UC, the Truelove and Witts criteria [TWC] require a stool frequency of ≥6 bloody stools per 24 h and the presence of at least one of four objective measures of systemic toxicity: one being an erythrocyte sedimentation rate [ESR] of >30 mm/h at the time of presentation.3
Data from Oxford, UK, confirm the intuitive paradigm that the severity of the initial presentation has a large bearing on the eventual outcome of the episode.3,4 This is most evident in the described positive relationship between the number of additional TWC fulfilled on presentation and the incremental risk of colectomy during the course of the admission.4
Not all patients who are admitted to hospital for treatment of acute attacks of UC satisfy the TWC for severe disease. Disease activity in this subgroup usually falls within the category of moderate UC, also called moderately severe UC. The TWC define this group as having between mild and severe disease activity [4-5 bloody stools per day in the presence or absence of any additional criteria].3 With the widespread use of a variety of disease activity indices other than the TWC, there are a number of studies of acute UC that include patients with both TWC severe and TWC moderately active disease.5-8
Over time, the popularity of assessment of systemic inflammation in UC patients with the ESR has waned as ESR has been supplanted by C-reactive protein [CRP].9 The latter is a specific marker of inflammation of hepatic origin which is affected by fewer external influences.10,11 With a half-life of 19 h, the CRP is also more responsive to alterations in the inflammatory burden. It can therefore be used to effectively monitor response to treatment during the course of a hospital admission.1,12
Investigators led by Turner have, in the course of establishing a global activity instrument (paediatric UC activity index; [PUCAI]), evaluated a paediatric cohort to establish the ESR and CRP levels that correlate with varying levels of disease activity as defined by the PUCAI.13 Severe UC was characterised by an ESR of >37 mm/h or a CRP of >9 mg/L. CRP values for severe disease recently proposed in the adult population include the >10 mg/L used in the UK inflammatory bowel disease [IBD] audit14 or >30 mg/L found in the current ECCO e-guidelines.2
As the recognition of a severe flare of UC can be challenging for both the specialist and the non-specialist practitioner, establishing a CRP cut-off value differentiating severe disease will aid in the timely and correct identification of this presentation. This will translate to the institution of an appropriate care pathway befitting the presentation, with a reduction in the mortality, morbidity, and cost generated by these episodes.
The aim of this study was to determine the concentration of CRP corresponding to an ESR >30 mm/h in adult patients presenting with episodes of severe colitis as defined by the TWC. Following the identification of this cut-off, the clinical outcomes between groups stratified for disease activity on the basis of number of TWC satisfied at presentation, using either the ESR or the newly derived CRP criterion, were assessed. In addition, well-described objective markers of disease activity were compared in order to assess differences between these groups.
2. Materials and Methods
2.1. Inclusion and exclusion criteria
As part of the inflammatory bowel disease [IBD] programme, all patients admitted to the Royal Brisbane and Women’s Hospital [RBWH] with any complications of IBD, including an acute flare of their disease, are referred to the IBD team. Data from these admissions have been prospectively collected on a dedicated software platform, IBD Prime. Patients included in this study were consecutive admissions between 1996 and 2020 with severe UC satisfying the TWC, and with paired ESR and CRP levels taken within 24 h of admission. All pathology tests were performed by the state government pathology service. Re-presentations by the same individual were excluded if they occurred within 12 months of the preceding admission. Presentations not meeting the TWC for severe disease were excluded from this study. The study was approved by the local human research ethics committee [approval HREC/14/QRBW/323].
Patients on concomitant immunomodulator, oral corticosteroid, and/or 5-aminosalicylates at the time of admission were included in the study. For the purpose of maintaining consistency across the cohort, those patients receiving biologic therapy at the time of admission were excluded.
The diagnosis of UC was confirmed by histology together with endoscopic, radiological, and clinical correlation. Patients were treated with a standardised treatment protocol by one of two subspecialist IBD physicians.15 The Day 3 and Day 7 [Oxford] criteria were applied to determine corticosteroid therapy failure.12 When indicated, a choice of either surgery or rescue therapy initially with intravenous cyclosporine A [since 1999] and later with infliximab [since 2001] was offered.15 The choice of rescue agent and/or surgery when appropriate was made in close collaboration with the patient.
The maximum total number of TWC fulfilled was five. This was assessed as a combination of the single mandatory stool frequency criterion with up to four additional criteria.
The Mayo endoscopic subscore [MES] was assigned by the endoscopist at the time of endoscopy and included in the procedure report.
2.2.Training cohort
The training cohort was drawn from prospectively recruited consecutive presentations to the RBWH between January 1997 and September 2017, meeting the above-mentioned criteria.
2.3.Validation cohort
The validation cohort consisted of prospectively recruited and consecutive cases of severe UC, satisfying the TWC, presenting to the RBWH from October 2017 to June 2020, or to one of 11 collaborating hospitals between January 1997 and June 2020. Patient data were collected and stored on the same patient database used for the training cohort [IBD Prime].
2.4.Statistics
R version 3.4.0 was used for all analyses. Each unique CRP level reported in the dataset was tested as a potential cut-off point to approximate an ESR of >30 mm/h. For each tested cut-off, a confusion matrix was generated between ESR >30 mm/h and the CRP cut-off. The positive predictive value [PPV] was then used to establish the lowest CRP cut-off with a PPV >85%. Chi square and Fisher’s exact tests were used to compare corticosteroid therapy failure and colectomy rates between the ESR >30 and CRP ≥12 allocated groups. Mann-Whitney U tests were used to compare presentation faecal calprotectin and MES between inflammatory marker allocation groups.
3. Results
3.1.Training cohort
During the study period to October 2017, 204 presentations of TWC severe UC were recorded. Of these presentations, 163 had paired ESR and CRP levels recorded within 24 h of presentation [training cohort; Table 1]. These presentations were generated by 154 individuals. There were no deaths of hospitalised patients as a consequence of UC, surgery, or any subsequent complications.
Table 1.
Severe UC patient characteristics: comparison between training and validation cohorts.
| Training cohort [n = 163] | Validation cohort [n = 128] | p-value | |
|---|---|---|---|
| Gender [male] | 85 [52%] | 71 [55%] | 0.66 |
| Age at admission in years median [IQR] | 33 [25–43] | 29 [22–45] | 0.081 |
| Disease duration in years median [IQR] | 2 [1.2–9.2] | 0.2 [0–3.0] | 6.2 × 10-11 |
| First UC presentation | 23 [14%] | 51 [40%] | 1.1 × 10-6 |
| Relapse | 140 [86%] | 77 [60%] | |
| Disease extent [Montreal classification]a | 0.10 | ||
| Proctitis [E1] | 2 [1%] | 0 [0%] | |
| Left-sided [E2] | 55 [34%] | 32 [25%] | |
| Extensive [E3] | 106 [65%] | 95 [75%] | |
| Immunomodulator on admission | 0.085 | ||
| No | 123 [75%] | 108 [84%] | |
| Yes | 40 [25%] | 20 [16%] | |
| Bowel frequency median [IQR] | 10 [8–15] | 10 [8–15] | 0.84 |
| CRP <mg/L median [IQR] | 58 [28–109] | 75 [44–150] | 0.038 |
| Albumin g/L mean [standard deviation] | 32 [6] | 31 [6] | 0.071 |
CRP, C-reactive protein; IQR, interquartile range.
aE1 and E2 disease were pooled for this analysis. A single case in the validation cohort had an undefined disease distribution.
In the training cohort there was a male predominance of 52% with a median age of 33 years at admission and the median time elapsed between UC diagnosis and admission was 2 years [range 0-45 years]. Two-thirds [65%] of cases had disease proximal to the splenic flexure. One-quarter [25%] of cases were receiving systemic immunosuppression at the time of presentation.
A total of 41 patients considered to have severe UC on clinical grounds did not have a paired ESR recorded within 24 h. Characteristics of this group are detailed in Supplementary Table 1.
3.2.Determining and applying the CRP cut-off
The lowest CRP value that produced a PPV of >85% for having a paired ESR of >30 mm/h was ≥12 mg/L [Table 2]. This threshold captured 95% of cases with an ESR >30 mm/h. The area under the curve was 0.82. Whereas a cut-off of 31 mg/L generated a PPV of 87%, this captured only 81% of cases. The PPV is consistently above 80% for CRP cut-off values of 20-200 mg/L [Figure 1]. The level of accuracy decreased markedly above a cut-off of ≥12 mg/L [Figure 2].
Table 2.
CRP cut-off positive predictive values for a paired ESR of >30 mm/h
| CRP cut-off [≥ mg/L] | PPV [%] | NPV [%] | Sensitivity [%] | Specificity [%] | Accuracy [%] |
|---|---|---|---|---|---|
| 12 | 85 | 63 | 95 | 35 | 82 |
| 18 | 86 | 50 | 88 | 44 | 79 |
| 25 | 86 | 44 | 84 | 47 | 77 |
| 31 | 87 | 43 | 81 | 56 | 75 |
CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; PPV, positive predictive value for CRP cut-off having a paired ESR >30 mm/h; NPV, negative predictive value for CRP cut-off having a paired ESR >30 mm/h.
Figure 1.
Positive predictive value for CRP cut-offs to be paired with an ESR >30 mm/h. CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; PPV, positive predictive value.
Figure 2.
CRP accuracy at varying CRP cut-off values. Vertical line at CRP cut-off of 12 mg/L. CRP, C-reactive protein.
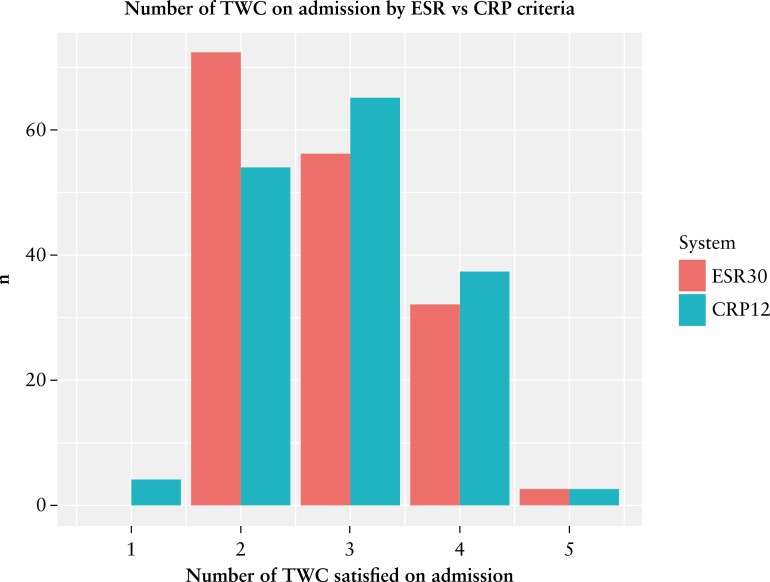
The application of the new CRP ≥12 mg/L cut-off to the training set data altered the total number of TWC satisfied in 18% [29/163] of presentations [Figure 3]. Conversely, 134 or 82% of presentations remained unchanged in their number of TWC satisfied on admission; 22 patients increased their total number of fulfilled TWC by one and seven patients had a decrease of total TWC by one. Overall, there was a net increase in the number of TWC satisfied in 15 [9%] patients when the CRP 12 mg/L criterion was applied. Four patients were downgraded from severe to moderate colitis. Two of these patients required rescue therapy, with one of these patients undergoing colectomy prior to discharge following the failure of rescue therapy.
Figure 3.
Number of Truelove and Witts criteria present at admission by ESR and CRP criteria. The four patients downgraded from TWC severe to moderate colitis when the CRP ≥12 mg/L criterion was applied in place of the ESR >30 mm/h criterion only fulfil the bowel frequency criterion of the TWC and are represented by the bar at the x-axis value of 1. CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; TWC, Truelove and Witts criteria.
3.3. Clinical outcomes
Following the application of the CRP ≥12 mg/L cut-off, there were no statistically significant changes to the rate of corticosteroid therapy failure in any single group separated by the number of TWC met at admission [TWC 2/5, 3/5, or ≥ 4/5]. Those patients who satisfied four or five TWC on presentation were combined to provide sufficient numbers for a statistically meaningful analysis [Figure 4]. When the different inflammatory marker systems were compared, the p-values for the corticosteroid failure rate between TWC categories 2/5, 3/5, and ≥ 4/5 were 1, 0.71, and 0.88, respectively.
Figure 4.
Corticosteroid failure rate by number of TWC and inflammatory marker classification system. TWC, Truelove and Witts criteria.
There were no statistically significant differences by chi square test in the colectomy-by-discharge rate by number of TWC satisfied on admission between the ESR >30 and CRP ≥12 criteria [Figure 5]. The p-values for TWC categories 2/5, 3/5, and ≥4/5 were 1, 1, and 0.96, respectively.
Figure 5.
Colectomy-by-discharge rate by number of TWC fulfilled at admission and inflammatory marker classification system. TWC, Truelove and Witts criteria.
3.4. Objective markers of disease severity present on admission to hospital
Faecal calprotectin results were available for 57 patients with ESR criteria and the 55 patients with CRP criteria qualifying severe colitis [Supplementary Figure 1]. The median calprotectin in the TWC 2/5 group was 1750 μg/g (n = 22, interquartile range [IQR] 870-4325 μg/g] and 2550 μg/g [n = 16, IQR 1135-4400 μg/g] for ESR >30 and CRP ≥12 criteria, respectively. The median calprotectin in the TWC 3/5 group was 1800 μg/g [n = 21, IQR 530-3900 μg/g] and 1600 μg/g [n = 25, IQR 530-4600 μg/g] for ESR >30 and CRP ≥12 criteria, respectively. The median calprotectin in the TWC ≥4/5 group was 2700 μg/g [n = 14, IQR 1475-3950 μg/g] and 2700 μg/g [n = 14, IQR 1475-395 0μg/g] for ESR >30 and CRP ≥12 criteria, respectively.
There were no statistically significant differences between ESR >30 and CRP ≥12 allocated groups by TWC satisfied at admission. The p-values were 0.68, 0.86, and 0.72 for TWC 2/5, TWC 3/5, and TWC ≥ 4/5, respectively.
3.5. Mayo endoscopic subscore
Mayo endoscopic subscore results were available for 118 TWC severe UC patients by ESR >30 criteria [n = 2, 8, 53, 55 for MES 0-3, respectively] and 114 with TWC severe UC patients by CRP ≥12 criteria [n = 2, 8, 51, 53 for MES 0-3, respectively Figure 6]. Grouped by the number of TWC present at admission, there were n = TWC 2/5: 56, 39; TWC 3/5: 37, 49; TWC ≥4/5: 25, 26; by ESR >30 and CRP ≥12 criteria, respectively.
Figure 6.
Mayo endoscopic subscore at presentation by TWC fulfilled and inflammatory marker classification system. TWC, Truelove and Witts criteria; MES, Mayo endoscopic subscore.
There were no statistically significant differences between ESR >30 and CRP ≥12 allocated groups by TWC satisfied at admission. The p-values were 0.57, 0.70, and 0.66 for TWC 2/5, TWC 3/5, and TWC ≥ 4/5, respectively.
3.6. Validation cohort
The validation cohort comprised 128 presentations. This group consisted of 101 [79%] cases who presented to collaborating hospitals and 27 [21%] cases who presented to the RBWH from October 2017. The presenting ESR was >30 mm/h in 101/128 [79%] cases.
Whereas the training and validation cohorts were very similar in terms of demographics and baseline disease characteristics, differences included an increased rate of index UC presentations with a resultant reduction in disease duration in the validation cohort [Table 1]. Significantly higher CRP levels were observed, with a median concentration of 75 mg/L versus 58 mg/L in the training cohort.
When applied to the validation cohort, the CRP ≥12 mg/L cut-off produced a sensitivity and PPV of 94% and 83%, respectively.
Six cases with ESR >30 mm/h had a paired CRP of <12 mg/L. Two of these presentations were downgraded to TWC moderate from the TWC severe category. One of these downgraded presentations did go on to require rescue medical therapy and colectomy during the admission. The other case had a presenting ESR of 31 mm/h and a paired CRP of 8.8 mg/L. This patient responded to intravenous corticosteroid therapy.
Twenty cases were found to have ESR rates <31 mm/h and paired CRP levels of ≥12 mg/L. The median CRP in this group was 66 mg/L [IQR 38-93 mg/L]. Fourteen [70%] of this group received rescue medical therapy during the acute admission, with eight [40%] of these cases proceeding to colectomy-by-discharge.
Overall, there were 14 [11%] more patients who met the CRP ≥12 mg/L inflammatory marker criteria who previously would not have met the ESR >30 mm/h inflammatory marker criteria. All of these patients fulfilled at least one additional TWC criterion, hence their inclusion in the study which was limited to severe cases as assessed by the classical TWC.
4.Discussion
The primary finding of this observational study is an evidence-based CRP equivalent to the previously used ESR >30 mm/h cut-off proposed by Truelove and Witts in 1955 to define severe UC. This derivation was performed in a real-world dataset of 163 TWC-qualifying presentations of severe UC. This CRP cut-off was then tested in a validation cohort of severe UC patients, predominantly sourced from collaborating hospitals. The broad spectrum of predominantly smaller regional hospitals that contributed patients to the validation cohort confers a high degree of external validity on the findings of this study.
The proposed CRP cut-off of ≥12 mg/L is an inclusive and conservative additional Truelove and Witts criterion capturing 95% of patients with a paired ESR of >30 mm/h. When compared with the classical ESR-based system, it resulted in a net increase of 9% in the number of presentations satisfying the Truelove and Witts inflammatory marker criterion.
When the important clinical outcomes of corticosteroid therapy failure and inpatient colectomy were considered, the disparities between the ESR >30 and CRP ≥12 allocated groups did not reach statistical significance. From this result it can be inferred that the 18% of patients who underwent a change in their total TWC allocation underwent a balanced redistribution along the TWC severity continuum.
The fact that one patient from each of the training and validation cohorts was reclassified from severe to moderate UC and underwent colectomy prior to discharge is of interest but is not an unexpected outcome; nor is it an outcome reaching statistical significance. Operative management and the initiation of biologic therapy are not uncommon clinical outcomes in this clinically important but frequently overlooked group with a moderate but still clinically significant inflammatory burden.
The new CRP-based criterion did not produce any statistically significant difference in the number of patients experiencing either corticosteroid therapy failure or colectomy-by-discharge.
Consistency between the ESR >30 and CRP ≥12 allocated groups is further evidenced by the lack of statistically significant differences observed in objective markers of disease activity.16 These included the presenting faecal calprotectin and the Mayo endoscopic subscore as assessed in 57 and 118 patients, respectively. We acknowledge that the inability to reject the null is not statistically the same as accepting the null, but p-values reported for alterations in TWC categories by switching ESR >30 for CRP ≥12 were in all cases >0.5. This suggests that it is extremely unlikely that a subsequent study with the same sample size would report a significant difference.
The establishment of a CRP cut-off is of clinical importance given the relative accessibility of this test in contemporary clinical practice, the ongoing applicability of the TWC in clinical decision making, and the importance of the inflammatory marker criterion within this instrument. In the training cohort of 163 presentations, 79% had an ESR >30 mm/h, and for 46 [28% overall] of these cases an ESR of >30 mm/h was the only additional Truelove and Witts criterion, apart from stool frequency, admitting them into the severe disease category.
The important comparison of our data with the Turner et al. study yields low validity. This paediatric series assessed disease activity after 3 days of intravenous corticosteroid therapy to obtain a more heterogeneous pool of results.13 Additionally, the gold standard of disease activity was determined by a very different instrument [PUCAI] that contains a paucity of objective measures of disease activity. Finally, baseline CRP values are likely to be higher with increasing age and body mass index encountered in adult series.17, 18
The described CRP cut-off of ≥12 mg/L lies between the previously described cut-offs of >10 mg/L and >30 mg/L reported in the UK IBD Audit and the ECCO e-guidelines, respectively.2,14 At the time of writing, neither of these cut-offs has been supported by an evidence base.
Paired ESR and CRP results were reported in the study of 167 TWC severe UC patients which generated the Edinburgh risk score.19 The mean ESR was 44.8 mm/h [standard deviation 26.5 mm/h] and the median CRP was 4.4 mg/L [IQR: 2.1–13.3 mg/L].
There is a handful of smaller studies in moderate-severe UC which reported both ESR and CRP results, including a 1982 study of 50 patients at Hammersmith Hospital, London.20 Nine patients were considered to have severe disease as per the TWC. Only four of the nine were admitted to hospital in this study. The median CRP in this small inpatient subgroup was 12 mg/L [range 2-33 mg/L] whereas the median ESR was 28 mm/h [range 10-59 mg/L]. Investigators in India reported a CRP of ≥12 mg/L in 14 of 17 [82%] of patients admitted with severe UC.21
A CRP of ≥12 mg/L may seem to be a low threshold to define severe colitis, but the UC inflammatory cytokine milieu, unlike that seen with Crohn’s disease and rheumatoid arthritis, is not typically a prolific inducer of CRP production.22 An analogous situation can be found in systemic lupus erythematosus [SLE], another inflammatory disease that usually produces low CRP concentrations. A tipping point is reached when a critical inflammatory burden is accumulated resulting in a significant increase in CRP production. In SLE this situation may arise when the disease is complicated by serositis, whereas in UC, this point may be reached when there is extensive involvement of the submucosa and deeper layers of the colonic wall as seen in severe disease.
Some investigators have proposed using either or both of ESR and CRP in the assessment of acute IBD.23 As the purpose of this study was to supersede the ESR assay in favour of the CRP, this approach was not taken.
Limitations of this study include the long study duration. The treatment of acute severe UC has evolved extensively over the past two decades. Ameliorating this bias are: the focus on objective admission indices; inherent consistencies including the limited pool of two treating IBD clinicians; the application of a heavily protocolised inpatient management algorithm; and the exclusion of patients with recent biologic therapy. The exclusion of patients actively on biologic therapy may limit the validity of the findings of this study to that patient group.
This work may influence clinical practice by relegating ESR as an admission blood test in acute presentations of UC. Utilising the CRP-based criteria will improve accessibility to the clinical information required for a complete assessment of the TWC. This will enable a shorter turn-around time for critical decision making regarding patient disposition and management.
A more sensitive CRP cut-off is likely to lead to an increase in recommendations for admission for intravenous corticosteroids. Indeed, an increase in the total number of TWC satisfied was observed in 9% of training cohort patients.
Analysis of unpublished data from 37 TWC moderate cases admitted to our centre has found that after applying the CRP ≥12 mg/L criteria, 13/37 cases were upgraded to severe. Six of these cases were corticosteroid responsive, another six required rescue therapy, and one proceeded to inpatient colectomy following the failure of corticosteroid therapy.
Finally, the inclusive nature of the CRP criteria will enhance access to infliximab therapy in health care settings where satisfying the TWC for severe disease is mandatory for reimbursement.
In summary, a CRP threshold of ≥12 mg/L was found to be an inclusive and sensitive cut-off that, when incorporated into the Truelove and Witts criteria and replacing the traditional ESR >30 mm/h criterion, had similar performance characteristics when applied to the assessment of UC disease activity. These findings were validated in a similarly sized cohort that was predominantly drawn from acute UC presentations to smaller, collaborating hospitals.
Supplementary Material
Acknowledgements
We acknowledge previous IBD Fellows who have contributed to data collection, including Dr Desmond Patrick and Dr James Irwin; also medical and nursing staff from collaborating hospitals in Metro North HHS and contributing regional hospital and health services.
Contributor Information
Anthony Croft, Department of Gastroenterology & Hepatology, Royal Brisbane and Women’s Hospital, Brisbane, Australia; QIMR-Berghofer Medical Research Institute, Brisbane, Australia; Faculty of Medicine, University of Queensland, Brisbane, Australia.
Anton Lord, QIMR-Berghofer Medical Research Institute, Brisbane, Australia; Centre for Health Services Research, University of Queensland, Brisbane, Australia.
Graham Radford-Smith, Department of Gastroenterology & Hepatology, Royal Brisbane and Women’s Hospital, Brisbane, Australia; QIMR-Berghofer Medical Research Institute, Brisbane, Australia; Faculty of Medicine, University of Queensland, Brisbane, Australia.
Funding
This work was supported by the Royal Brisbane and Women’s Hospital Foundation with the provision of a Postgraduate Scholarship used to support AC in 2017.
Conflict of Interest
AC and AL have no competing interests to declare. GR-S is on advisory boards for Janssen, Novartis, Takeda, Ferring, Abbvie, and Pfizer, has received research funding from Janssen, and has received speaker’s fees from Takeda and Novartis.
Author Contributions
AC is the guarantor of this article. Author contributions: AC: concept, data curation, formal analysis, funding acquisition, investigation, methodology, validation, visualisation, writing—original draft, review, and editing. AL: formal analysis, methodology, supervision, software, visualisation, writing—review and editing. GR-S: concept, data curation, funding application, methodology, project administration, resources, supervision, writing—review and editing. All authors have approved the final version of the submitted manuscript. Presented [in part] at Digestive Disease Week, Washington DC, 2018.
References
- 1. Travis S, Satsangi J, Lemann M. Predicting the need for colectomy in severe ulcerative colitis: a critical appraisal of clinical parameters and currently available biomarkers. Gut 2011;60:3–9. [DOI] [PubMed] [Google Scholar]
- 2. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017;11:649–70. [DOI] [PubMed] [Google Scholar]
- 3. Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 1955;2:1041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dinesen LC, Walsh AJ, Protic MN, et al. The pattern and outcome of acute severe colitis. J Crohns Colitis 2010;4:431–7. [DOI] [PubMed] [Google Scholar]
- 5. Järnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005;128:1805–11. [DOI] [PubMed] [Google Scholar]
- 6. Aceituno M, Garcia-Planella E, Heredia C, et al. Steroid-refractory ulcerative colitis: predictive factors of response to cyclosporine and validation in an independent cohort. Inflamm Bowel Dis 2008;14:347–52. [DOI] [PubMed] [Google Scholar]
- 7. Sjoberg D, Holmstrom T, Larsson M, et al. Incidence and natural history of ulcerative colitis in the Uppsala Region of Sweden 2005-2009: results from the IBD cohort of the Uppsala Region [ICURE]. J Crohns Colitis 2013;7:e351–7. [DOI] [PubMed] [Google Scholar]
- 8. Saito K, Katsuno T, Nakagawa T, et al. Predictive factors of response to intravenous ciclosporin in severe ulcerative colitis: the development of a novel prediction formula. Aliment Pharmacol Ther 2012;36:744–54. [DOI] [PubMed] [Google Scholar]
- 9. Harrison M. Abnormal laboratory results: Erythrocyte sedimentation rate and C-reactive protein. Aust Prescr 2015;38:93–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut 2006;55:426–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cioffi M, Rosa AD, Serao R, et al. Laboratory markers in ulcerative colitis: Current insights and future advances. World J Gastrointest Pathophysiol 2015;6:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Travis SPL, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut 1996;38:905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turner D, Mack DR, Hyams J, et al. C-reactive protein [CRP], erythrocyte sedimentation rate [ESR] or both? A systematic evaluation in paediatric ulcerative colitis. J Crohns Colitis 2011;5:423–9. [DOI] [PubMed] [Google Scholar]
- 14. Lynch RW, Lowe D, Protheroe A, et al. Outcomes of rescue therapy in acute severe ulcerative colitis: data from the United Kingdom inflammatory bowel disease audit. Aliment Pharmacol Ther 2013;38:935–45. [DOI] [PubMed] [Google Scholar]
- 15. Croft A, Walsh A, Doecke J, et al. Outcomes of salvage therapy for steroid-refractory acute severe ulcerative colitis: ciclosporin vs. infliximab. Aliment Pharmacol Ther 2013;38:294–302. [DOI] [PubMed] [Google Scholar]
- 16. Ho GT, Lee HM, Brydon G, et al. Fecal calprotectin predicts the clinical course of acute severe ulcerative colitis. Am J Gastroenterol 2009;104:673–8. [DOI] [PubMed] [Google Scholar]
- 17. Siemons L, ten Klooster P M, Vonkeman HE, et al. How age and sex affect the erythrocyte sedimentation rate and C-reactive protein in early rheumatoid arthritis. BMC Musculoskelet Disord 2014;15:368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Visser M, Bouter L, McQuillan G. Elevated C-reactive protein levels in overweight and obese adults. JAMA 1999;282:2131–5. [DOI] [PubMed] [Google Scholar]
- 19. Ho GT, Mowat C, Goddard CJ, et al. Predicting the outcome of severe ulcerative colitis: development of a novel risk score to aid early selection of patients for second-line medical therapy or surgery. Aliment Pharmacol Ther 2004;19:1079–87. [DOI] [PubMed] [Google Scholar]
- 20. Fagan EA, Dyck RF, Maton PN, et al. Serum levels of C-reactive protein in Crohn’s disease and ulcerative colitis. Eur J Clin Invest 1982;12:351–9. [DOI] [PubMed] [Google Scholar]
- 21. Chouhan S, Gahlot S, Pokharna RK, et al. Severity and extent of ulcerative colitis: role of C-reactive protein. Indian J Gastroenterol 2006;25:46–7. [PubMed] [Google Scholar]
- 22. Adelstein S, Baker A. Making sense of inflammatory markers. J Gastroenterol Hepatol 2014;33[Suppl 3]: 7. –8. [Google Scholar]
- 23. Consigny Y, Modigliani R, Colombel J-F, et al. A simple biological score for predicting low risk of short term relapse in Crohns disease. Inflamm Bowel Dis 2006;12:551–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.