Abstract
Many animals have flexible morphological traits that allow them to succeed in differing circumstances with differing diets available to them. For brachyuran crabs, claw height and gut size are diet-specific and largely reflect foraging strategies, while abdomen width reflects relative levels of fecundity. However, the link between claw size and diet has largely been documented only for primarily carnivorous crabs, while the link between diet and fecundity is strong in herbivorous crabs. We sought to determine the nature of the intraspecific relationship between claw size, dietary habits, and fecundity for two primarily herbivorous crab species, Hemigrapsus sanguineus and Aratus pisonii. Specifically, we examined whether claw size and/or abdomen width can be used as reliable measures of individual diet strategy. To test these hypotheses, we collected crabs and measured the dimensions of their claws, abdomens, and guts. By comparing these dimensions for each individual, we found that strongly predictive relationships do not exist between these traits for the primarily herbivorous species in our study. Thus, identifying external morphological features that can be used to assess diets of primarily herbivorous crabs remains elusive.
Introduction
A long-standing goal of ecology is to predict how populations and systems will be affected by future changes to their habitats and resources, changes that continue to become more frequent due to increasing human pressures and environmental change. Making these predictions can be difficult when relying only on past and current population trends, so it has become increasingly common to use individual-based ecology when making predictions. Individual-based ecology focuses on fitness-based decisions of individuals within a population, allowing the development of broader population and ecosystem predictions based on individual traits and processes [1].
Functional traits (i.e., individual level morphological or physiological traits that improve fitness) are useful tools in understanding the complexity of predator-prey relationships [2]. These traits are often highly flexible, so their expression must be considered within the environmental context of the individual. The factors influencing this context include changes in resource quality and consumer pressure [3]. These environmental influences result in differences in functional trait expression within a species due to geographic distribution [4]. Because of the variability of functional traits, they represent a key aspect of understanding the driving influences of predator-prey relationships and their role in ecosystem functioning [2]. By understanding how functional traits of an individual differ in response to resource availability, we can better predict a species’ ability to adapt to changes in the future.
Crabs are an ideal group for considering the role of functional traits in organisms’ responses to changes in resource availability because they have measurable physical traits that can be used to quantify their success as consumers. Individual diet variation is a crucial determinant of reproductive effort in crabs [5–10]. As in other species, diet can be assessed in crabs using standard methods, such as stable isotope analysis or gut content analysis. However, stable isotopes are expensive and time consuming when conducted on large numbers of individuals, and diet identification using gut content analysis is difficult in crabs given their propensity for shredding and masticating their food. In addition, gut content analysis in crabs requires sacrificing animals, and can therefore only be measured a single time per individual.
In carnivorous crabs, well-known proxies exist for assessing diet quickly, cheaply, and nonlethally. For instance, claw size is highly flexible for many crab species, varying in response to resource quality and availability. Among the European green crab Carcinus maenas, captive individuals that were fed snails with harder shells developed larger claws than those fed snails with softer shells [11]. Smith and Palmer [12] similarly demonstrated that crabs raised on shelled prey developed larger and stronger claws than crabs raised on unshelled prey of equivalent nutritional value. These findings show that this trait is variable within an individual’s lifetime, rather than just on an evolutionary timescale. Observational studies have shown the same trend between claw size and shell hardness of the crab’s prey across geographical regions and habitats [4, 13]. Crabs living in regions with harder-shelled prey have greater crushing strength than crabs in regions with softer-shelled prey [4]. Of the various dimensions of crab claws, claw height is the best predictor of strength and crushing ability [4, 14]. The increase in claw height is presumably the result of the crabs’ necessity for greater crushing strength to break through the harder shells of their prey and feed on the soft interior. While this trend has been repeatedly shown in species that are primarily carnivorous, generalist species and species that are primarily herbivorous have been underrepresented in past claw height studies.
Abdomen width is another morphological feature of brachyuran crabs that acts as a functional trait, in this case as a good predictor of fecundity, and therefore of fitness. Studies on multiple crab species have demonstrated strong positive relationships between fecundity and abdomen width [15–17]. Abdomen width can therefore be used as a measure of relative fecundity between individuals of the same species because individuals with greater abdomen widths generally have larger clutch sizes. As noted above, reproductive effort in crabs is highly dependent on diet. To our knowledge, no studies have examined changes in abdomen width with individual diet to determine whether abdomen width could be used as a diet predictor in addition to its role in predicting fecundity.
Yet another functional trait, gut size is a strong predictor of diet in brachyuran crabs (as well as in many other species) and can reliably be used to assess diet difference both between individuals and across sites. In a study of 15 different species of brachyuran crabs, gut size had a strong inverse relationship with the quality of the diet of individual crabs, both within and across species. Specifically, gut size served as a strong predictor (R2 = 0.80 after accounting for phylogenetic relationships among species) of the percent of herbivory in the crab’s diet, with greater herbivory leading to a larger gut [18]. This morphological difference is most likely the result of the low digestibility and nutrient content, especially nitrogen, of many plant materials. Due to the relatively low nutrient content, a greater volume of plant material is needed to meet the same metabolic requirements that can be met with a smaller volume of animal material (i.e., compensatory feeding), which results in a larger gut over time for more herbivorous individuals. Previous studies demonstrate that gut width is a reliable predictor of diet in individual crabs [18, 19], as well as at the population level by comparing across sites with different levels of food availability [20–22].
Our study considers two brachyuran crab species, the Asian shore crab Hemigrapsus sanguineus and the mangrove tree crab Aratus pisonii. Both species are primarily herbivorous [22, 23]. Hemigrapsus sanguineus has an omnivorous diet that can include macroalgae and detritus, as well as amphipods, polychaetes, and juvenile shelled prey, such as barnacles, bivalves, and gastropods [24]. They primarily consume macroalgae but will consume animal material when it is present [25]. Aratus pisonii is also primarily herbivorous, with most of its diet consisting of the leaves of mangrove trees, especially the red mangrove, Rhizophora mangle [6 and references therein]. In addition, they have been documented feeding on insects, gastropods, dead fish, and other crabs [26, 27]. Like H. sanguineus, A. pisonii has been described as an opportunistic omnivore, feeding on animal material preferentially when it is available, while plant material still makes up the bulk of its diet (Erickson et al., 2008). Finally, gut size is a reliable predictor of diets of individual crabs for both of these species [18–22].
In the present study, we compare intraspecific variation and correlations between claw height and gut size, and between abdomen width and gut size for both H. sanguineus and A. pisonii to determine whether claw height and/or abdomen width can be used as reliable proxies for diet strategy in individuals of these two primarily herbivorous species. Both species readily consume animals with hard outer shells (H. sanguineus: [24, 28]; A. pisonii: [26, 27]). Thus, while both species are primarily herbivorous, consumption of hard-shelled prey by some individuals may still result in variation in claw height (i.e., strength) that reflects individual variation in diet. Fecundity generally increases with the amount of animal tissue included in the diet for crab species in general [5, 7, 8, 10], and for these species in particular [6, 22]. If external morphology is a reliable predictor of diet-driven fitness, we therefore expect claw height and abdomen width to each be inversely correlated with gut width. By describing these relationships within these two primarily herbivorous species, we hope to provide tools to better predict how these species will react to future changes in resource availability.
Materials and methods
Sample sites & crab collection
Collections of A. pisonii were conducted under scientific collection permits from the Florida Department of Environmental Protection (#07101720). Collections of H. sanguineus were collected under permit from the New Hampshire Fish and Game Department (#MFD 1834).
We sampled populations of H. sanguineus and A. pisonii from sites along the East coast of the United States. We only sampled female crabs because we used abdomen width as a metric of fecundity, and this relationship is only present in females. Additionally, measuring female claws can help avoid confounding factors, such as claw size selected for non-feeding purposes, such as for defense and mating, that are more common with males [29].
Hemigrapsus sanguineus samples were gathered in August 2019 from two different sites in Rye, New Hampshire: Fort Stark and Odiorne Point State Parks. Both sites are comprised of boulder fields, and they contain the same group of intertidal algal and animal species. However, Odiorne Point has a much greater density of H. sanguineus than Fort Stark, which has led to a decreased abundance of prey species at Odiorne Point [30]. Because Fort Stark has more animal prey and shelled prey available per capita than Odiorne, we expect differences in diet to exist between the two populations. Hemigrapsus sanguineus samples were collected haphazardly at dawn by overturning boulders in the intertidal region. Only mature crabs (exceeding 12.1 mm carapace width [21, 22]) were collected. Upon collection, the samples were frozen and stored on dry ice at -80° C until dissection. Following dissection, the samples were dried at 65° C (see [22] for a complete description of sampling methods and for a more complete description of these two sites).
Individual A. pisonii were sampled from four sites in Florida along the Atlantic Intracoastal Waterway. Each of the four sites were fringing mangrove habitats that provided abundant access to red mangrove leaves and root-dwelling invertebrates [31], the primary and secondary food sources for A. pisonii; however, they differed in relative human disturbance. We rotated sampling between the four sites across sampling dates to avoid depleting any one population. The samples were originally collected for a study that explored reproduction [23], so they were collected before each spring tide from March-October in order to encompass the time leading up to and during their reproductive season. During sampling, the first 20 female crabs encountered during each sampling date were collected by hand and immediately frozen with dry ice and stored at -80° C until dissection. Following dissection, the samples were dried at 65° C.
Morphological measurements
To compare claw size and fecundity with herbivory levels, we measured dimensions of the claws, abdomen, and cardiac stomach (hereafter ‘gut’) for each crab (see Fig 1 for description of these measurements). After the samples had been dissected and dried, we measured the gut width along the anterior portion of the cardiac stomach to the nearest 0.1 mm using Vernier calipers, using methods described in Griffen and Mosblack [18]. We used the same calipers to measure the abdomen at its widest point. We then removed the claws from the crab body and measured the claw height. For the H. sanguineus samples, only one claw was measured (the left-hand claw, if present) because this species is homochelous. Both claws were measured for A. pisonii because this species is heterochelous.
Fig 1. Dimensions for all size measurements performed as part of this study.
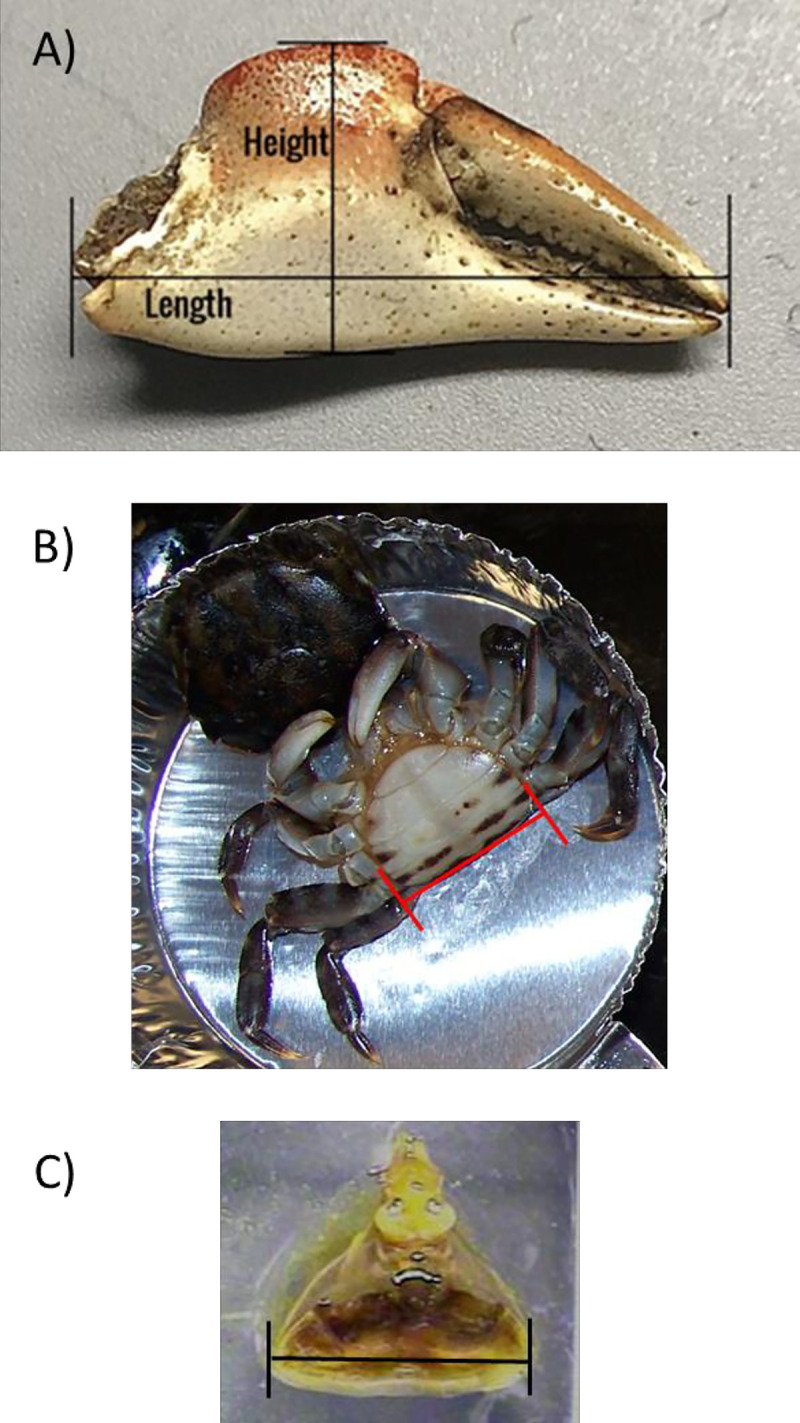
A) Claw dimensions measured. Here we focus only on differences in claw height, a proxy for claw strength in crabs. B) Abdomen width measured at widest point. C) Anterior edge of cardiac stomach.
Analysis
We examined the relationship between claw height (i.e., a proxy for strength) and gut width (i.e., a proxy for diet) between crabs. We also examined the relationship between abdomen width (i.e., a proxy for reproductive potential) and gut width. Each of these measurements is correlated with body size due to allometric relationships. Therefore, in order to avoid spurious correlations between these variables simply because each increases with body size, we controlled for body size in our analysis by regressing each of these variables against carapace width using nonlinear regression, and then using the residuals (i.e., observed data–expected from the nonlinear model) from these regressions in linear models. We analyzed the two species separately, but using the same approach. For both species, we used residual gut width as the response variable, and residual abdomen width and residual claw height as continuous predictor variables, and collection site as a categorical predictor variable. For A. pisonii, residual claw height and residual abdomen width were positively correlated (major claw: R2 = 0.82, minor claw R2 = 0.20). To avoid multicollinearity, we therefore used residual claw height after controlling for both body size and abdomen width. For A. pisonii, significant differences in claw size across collection sites were compared using Tukey’s HSD pairwise post hoc comparisons. We only analyzed the single claw for H. sanguineus (using the left claw, unless it was missing), while for A. pisonii we separately analyzed both the major and minor claws.
Results
Hemigrapsus sanguineus
Our analyses included a total of 188 adult female crabs (13.2–28.3 mm CW), 93 collected from Ft. Stark, and 95 collected from Odiorne Point State Park. After accounting for differences associated with crab body size by using residuals, we found that gut width increased by 0.13 ± 0.03 mm for every 1-mm increase in abdomen width (t = 3.62, P = 0.0004, Fig 2A), but that gut width was not related to claw height (t < -0.18, P = 0.86, Fig 2B), and was larger by 0.11 mm in crabs collected from Odiorne Point than in crabs collected at Fort Stark (t = 1.91, P = 0.057, Fig 2C).
Fig 2.
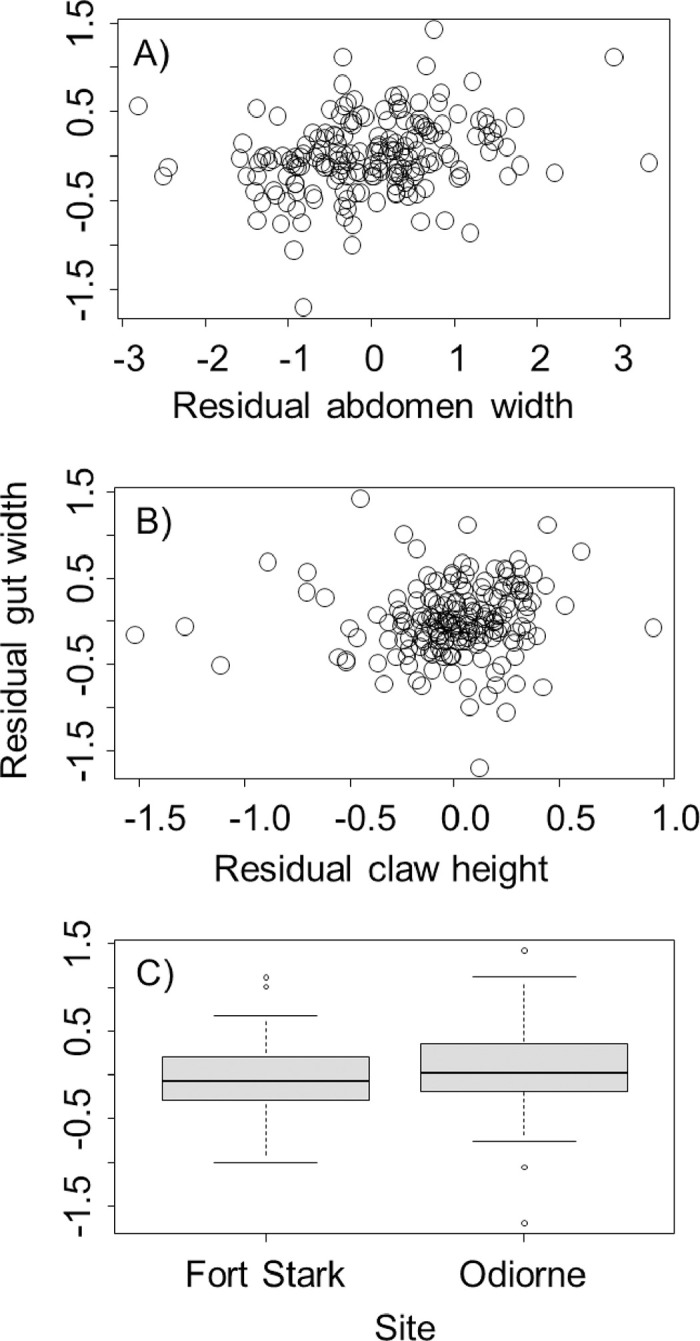
Relationships between residual gut width for Hemigrapsus sanguineus and residual abdomen width (A), residual claw height (B), and collection site (C). All residuals reflect sizes after accounting for differences associated with crab body size. Box plots in part C show the median value (heavy black line), the first to third quartile of the data (box), 95% of the data (whiskers), and outliers that fall outside this range (circles).
Aratus pisonii
We compared the same metrics for 241 A. pisonii samples (11.7–23.6), examining both the major and minor claws. When using the major claw for analysis, after accounting for differences associated with crab body size by using residuals, we found that gut width increased by 0.04 ± 0.01 mm for every 1-mm increase in abdomen width (t = 2.73, P = 0.007, Fig 3A), but was not related to the height of the major claw (t = 0.62, P = 0.53, Fig 3B). We also found differences in gut width between sites. Specifically, crabs collected from Oslo had smaller guts than crabs collected from Round Island (t = 2.66, P = 0.042, Fig 3C) and from Pepper Park (t = 2.65, P = 0.043, Fig 3C). There were no other differences in gut size between sites (P > 0.05).
Fig 3.
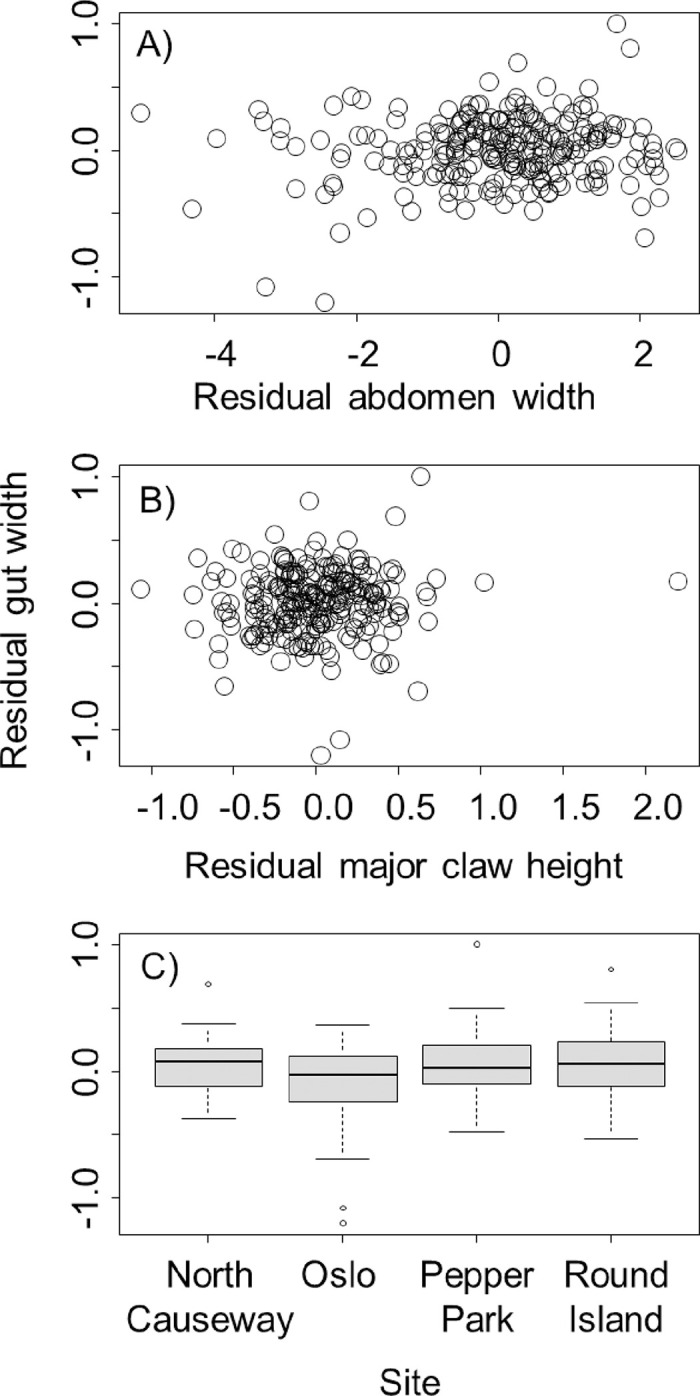
Relationship between residual gut width for Aratus pisonii and residual abdomen width (A), major claw residual height (B), and collection site (C). Residual gut width and abdomen width reflect sizes after accounting for differences associated with crab body size, which residual claw height reflects heights after accounting for correlations with both body size and abdomen width. Boxplots are as described in the caption for Fig 2.
Patterns when the minor claw was used in the analysis were somewhat different. Specifically, we found that the gut width increased by 0.08 ± 0.03 mm for each 1-mm increase in minor claw height (t = 2.56, P = 0.011, Fig 4A). However, there was no relationship between gut width and abdomen width (t = -1.31, P = 0.19, Fig 4B). Finally, for completeness we also included collection site in this analysis, and it returned the same results qualitatively as the analysis with major claw height. Specifically, crabs collected from Oslo had smaller guts than crabs collected from Round Island (t = 2.95, P = 0.019, Fig 4C) and Pepper Park (t = 2.96, P = 0.018, Fig 4C). No other differences in gut size were observed between crabs collected from other sites (P > 0.05).
Fig 4.
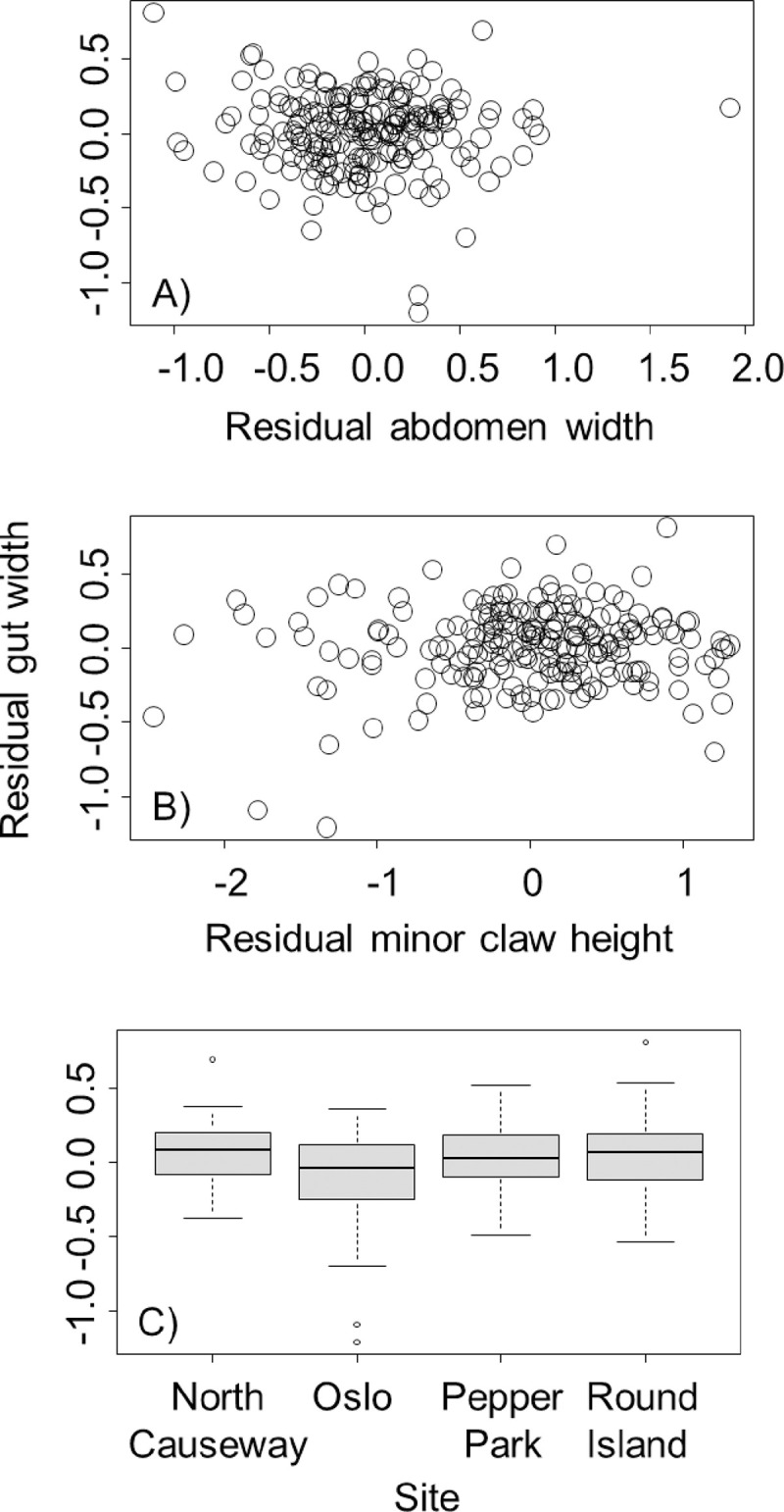
Relationship between residual gut width for Aratus pisonii and residual abdomen width (A), minor claw residual height (B), and collection site (C). Residual gut width and abdomen width reflect sizes after accounting for differences associated with crab body size, which residual claw height reflects heights after accounting for correlations with both body size and abdomen width. Boxplots are as described in the caption for Fig 2.
Discussion
In this study, we examined morphometric data of two primarily herbivorous species of brachyuran crabs to determine whether claw height and/or abdomen width can be used as reliable proxies for diet strategy in individuals of these species. We used gut size as a measure of the relative percent herbivory in the individual’s diet. Our results indicate that weak relationships exist between abdomen width and gut size for both species, but these relationships are likely not strong enough to use abdomen width as a reliable proxy for individual diet strategy. Further, the weak positive relationship between gut width and abdomen width is opposite to the negative correlation that we expected. No relationship was found between claw height and gut size for either species, other than a small correlation with the minor claw of A. pisonii. Thus, neither of these external morphological features provide a good proxy for individual diet strategy in these two species.
The results for H. sanguineus and A. pisonii indicate that claw height was not related to percent herbivory of the crab’s diet for either species. Among H. sanguineus, those collected from the site with less shelled and animal prey available (Odiorne) had greater claw heights than those collected from the site with more of these prey available (Fort Stark), counter to expectations based on studies of carnivorous species [4, 12]. For primarily carnivorous species, diet and claw size can differ across sites due to differences in prey availability (e.g., [32, 33]). The differences between sites that existed among the A. pisonii samples were minimal, and they were likely unrelated to percent herbivory because all the sites presumably had similar levels of animal resource availability. These results show that the relationship between claw height and herbivory for these species does not follow the trends found in other crab species that are primarily carnivorous. This may be expected, as herbivorous species do not use their claws to break into hard-shelled prey. Thus, despite the ready consumption of animal prey when available, breaking into hard-shelled prey is not a measurable driver of claw size in H. sanguineus or A. pisonii. These findings are consistent with an experimental study performed by [29], which found that claw dimensions were strong predictors of closing force for two species that specialized in shell breaking (Lophopenopeus bellus and Cancer productus), but not for a generalist species (Hemigrapsus nudus) that does not specialize on hard-shelled prey. Our results suggest that H. sanguineus and A. pisonii may meet metabolic nitrogen requirements by scavenging dead animals [34], and/or consuming relatively soft animals (worms, gammarids, etc.).
The abdomen width also did not provide a clear proxy for assessing diet strategy. As explained in this Introduction, we anticipated a negative relationship between abdomen width and gut width. Instead, we observed weak positive relationships between abdomen width and gut width for both species. This surprising result may potentially be explained by anatomical constraints that disproportionately affect crab species that are primarily herbivorous. We have previously demonstrated that residual gut size increases with percent herbivory in these species [18, 20, 21], but this relationship appears to break down at very high levels of herbivory (>60%) [35]. This may result from the limited space inside the carapace and the tradeoff in space use amongst the gut and other organs (gills, hepatopancreas, gonads, etc.). Because both of the species in our study are highly herbivorous, they are both likely affected by this constraint, thus preventing the expected negative relationship between gut size and abdomen width.
In conclusion, multiple studies demonstrate a clear relationships between individual diet strategy and claw size among primarily carnivorous crab species [11, 12], but our study demonstrates that neither claw height nor abdomen width can reliably be used to estimate individual diet strategy for these two primarily herbivorous crab species. Reliable morphological proxies of long-term diet strategies in herbivorous crustaceans therefore remain elusive.
Data Availability
All data from this study deposited in Dryad: https://doi.org/10.5061/dryad.8cz8w9gtw.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Stillman RA, Railsback SF, Giske J, Berger UT, Grimm V. Making predictions in a changing world: the benefits of individual-based ecology. BioScience. 2015. Feb 1;65(2):140–50. doi: 10.1093/biosci/biu192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmitz O. Predator and prey functional traits: understanding the adaptive machinery driving predator–prey interactions. F1000Research. 2017;6. doi: 10.12688/f1000research.11813.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitz OJ, Buchkowski RW, Burghardt KT, Donihue CM. Functional traits and trait-mediated interactions: connecting community-level interactions with ecosystem functioning. InAdvances in ecological research 2015. Jan 1 (Vol. 52, pp. 319–343). Academic Press. [Google Scholar]
- 4.Smith LD. Biogeographic differences in claw size and performance in an introduced crab predator Carcinus maenas. Marine Ecology Progress Series. 2004. Aug 2;276:209–22. [Google Scholar]
- 5.Griffen BD. Linking individual diet variation and fecundity in an omnivorous marine consumer. Oecologia. 2014. Jan;174(1):121–30. doi: 10.1007/s00442-013-2751-3 [DOI] [PubMed] [Google Scholar]
- 6.Riley ME, Vogel M, Griffen BD. Fitness-associated consequences of an omnivorous diet for the mangrove tree crab Aratus pisonii. Aquatic Biology. 2014. Jan 13;20(1):35–43. [Google Scholar]
- 7.Griffen BD, Norelli AP. Spatially variable habitat quality contributes to within‐population variation in reproductive success. Ecology and Evolution. 2015. Apr;5(7):1474–83. doi: 10.1002/ece3.1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffen BD, Riley ME. Potential impacts of invasive crabs on one life history strategy of native rock crabs in the Gulf of Maine. Biological Invasions. 2015. Sep;17(9):2533–44. [Google Scholar]
- 9.Belgrad BA, Griffen BD. The influence of diet composition on fitness of the blue crab, Callinectes sapidus. PloS one. 2016. Jan 19;11(1):e0145481. doi: 10.1371/journal.pone.0145481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gül MR, Griffen BD. Diet, energy storage, and reproductive condition in a bioindicator species across beaches with different levels of human disturbance. Ecological Indicators. 2020. Oct 1;117:106636. [Google Scholar]
- 11.Edgell TC, Rochette R. Prey-induced changes to a predator’s behaviour and morphology: Implications for shell–claw covariance in the northwest Atlantic. Journal of Experimental Marine Biology and Ecology. 2009. Dec 31;382(1):1–7. [Google Scholar]
- 12.Smith LD, Palmer AR. Effects of manipulated diet on size and performance of brachyuran crab claws. Science. 1994. Apr 29;264(5159):710–2. doi: 10.1126/science.264.5159.710 [DOI] [PubMed] [Google Scholar]
- 13.Silva AC, Silva IC, Hawkins SJ, Boaventura DM, Thompson RC. Cheliped morphological variation of the intertidal crab Eriphia verrucosa across shores of differing exposure to wave action. Journal of Experimental Marine Biology and Ecology. 2010. Aug 15;391(1–2):84–91. [Google Scholar]
- 14.Wilcox M, Rochette R. Does claw morphology of the green crab Carcinus maenas vary in relation to its diet on rocky versus fine-sediment shores of southwest New Brunswick, Bay of Fundy, Canada?. Journal of Experimental Marine Biology and Ecology. 2015. Apr 1;465:121–9. [Google Scholar]
- 15.Rodrigues MA, Heberle MF, D’Incao F. Fecundity variation and abundance of female blue crab Callinectes sapidus Rathbun, 1896 (Decapoda, Brachyura, Portunidae) in the Patos Lagoon estuary, RS, Brazil. Atlântica. 2011. 33:141–148. [Google Scholar]
- 16.Ali MH, Al-Maliky TH. Fecundity of the crab, Potamon mesopotamicum Brandis, Storch & Turkay, 1998 from the Mesopotamian Marshlands, Iraq. Journal of Fisheries and Environment. 2017. Oct 25;41(3):6–11. [Google Scholar]
- 17.Litulo C. Fecundity of the pantropical fiddler crab Uca annulipes (H. Milne Edwards, 1837)(Brachyura: Ocypodidae) at costa do sol mangrove, Maputo Bay, Southern Mozambique. Western Indian Ocean Journal of Marine Science. 2004. 3:87–91. [Google Scholar]
- 18.Griffen BD, Mosblack H. Predicting diet and consumption rate differences between and within species using gut ecomorphology. Journal of Animal Ecology. 2011. Jul;80(4):854–63. doi: 10.1111/j.1365-2656.2011.01832.x [DOI] [PubMed] [Google Scholar]
- 19.Griffen BD, Altman I, Bess BM, Hurley J, Penfield A. The role of foraging in the success of invasive Asian shore crabs in New England. Biological Invasions. 2012. Dec;14(12):2545–58. [Google Scholar]
- 20.Cannizzo ZJ, Dixon SR, Griffen BD. An anthropogenic habitat within a suboptimal colonized ecosystem provides improved conditions for a range‐shifting species. Ecology and Evolution. 2018. Feb;8(3):1521–33. doi: 10.1002/ece3.3739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cannizzo ZJ, Lang SQ, Benitez-Nelson B, Griffen BD. An artificial habitat increases the reproductive fitness of a range-shifting species within a newly colonized ecosystem. Scientific reports. 2020. Jan 17;10(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffen BD, Bailey J, Carver J, Vernier A, DiNuzzo ER, Anderson L, et al. Mechanisms of possible self-limitation in the invasive Asian shore crab Hemigrapsus sanguineus. Scientific Reports. 2020. Oct 9;10(1):1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffen BD, Cannizzo ZJ, Carver J, Meidell M. Reproductive and energetic costs of injury in the mangrove tree crab. Marine Ecology Progress Series. 2020. Apr 23;640:127–37. [Google Scholar]
- 24.Brousseau DJ, Goldberg R. Effect of predation by the invasive crab Hemigrapsus sanguineus on recruiting barnacles Semibalanus balanoides in western Long Island Sound, USA. Marine Ecology Progress Series. 2007. Jun 6;339:221–8. [Google Scholar]
- 25.Brousseau DJ, Baglivo JA. Laboratory investigations of food selection by the Asian shore crab, Hemigrapsus sanguineus: algal versus animal preference. Journal of Crustacean Biology. 2005. Jan 1;25(1):130–4. [Google Scholar]
- 26.Beever JW, Simberloff D, King LL. Herbivory and predation by the mangrove tree crab Aratus pisonii. Oecologia. 1979. Dec;43(3):317–28. doi: 10.1007/BF00344958 [DOI] [PubMed] [Google Scholar]
- 27.Erickson AA, Feller IC, Paul VJ, Kwiatkowski LM, Lee W. Selection of an omnivorous diet by the mangrove tree crab Aratus pisonii in laboratory experiments. Journal of Sea Research. 2008. Feb 1;59(1–2):59–69. [Google Scholar]
- 28.Lohrer AM, Whitlatch RB. Relative impacts of two exotic brachyuran species on blue mussel populations in Long Island Sound. Marine Ecology Progress Series. 2002. Feb 13;227:135–44. [Google Scholar]
- 29.Yamada SB, Boulding EG. Claw morphology, prey size selection and foraging efficiency in generalist and specialist shell-breaking crabs. Journal of Experimental Marine Biology and Ecology. 1998. Feb 1;220(2):191–211. [Google Scholar]
- 30.Griffen BD, van den Akker D, DiNuzzo ER, Anderson L, Vernier A. Comparing methods for predicting the impacts of invasive species. Biological Invasions. 2021. Feb;23(2):491–505. [Google Scholar]
- 31.Riley ME, Griffen BD. Habitat-specific differences alter traditional biogeographic patterns of life history in a climate-change induced range expansion. PLoS One. 2017. May 4;12(5):e0176263. doi: 10.1371/journal.pone.0176263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yong AY, Lim SS. Plasticity of foraging strategies adopted by the painted ghost crab, Ocypode gaudichaudii, in response to in situ food resource manipulation experiments. Zoological Studies. 2021;60. doi: 10.6620/ZS.2021.60-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gül MR, Griffen BD. Changes in claw morphology of a bioindicator species across habitats that differ in human disturbance. Hydrobiologia. 2020. Aug;847(14):3025–37. [Google Scholar]
- 34.Wolcott TG. Ecological role of ghost crabs, Ocypode quadrata (Fabricius) on an ocean beach: scavengers or predators?. Journal of Experimental Marine Biology and Ecology. 1978. Jan 1;31(1):67–82. [Google Scholar]
- 35.Quezada-Villa K, Fletcher LS, Smith N, Stancil C, McMullin A, Orocu B, et al. In review Predicting diet using external morphology. Forthcoming. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data from this study deposited in Dryad: https://doi.org/10.5061/dryad.8cz8w9gtw.


