Abstract
Activation of the complement pathway results in the production of bioactive C3a, a product of C3 cleavage, which interacts with membrane-bound receptor C3aR to regulate innate immune cell function and outcome of bacterial infection. Specifically, previous research has identified mechanistically distinct and cell type–specific roles for C3aR in regulating innate immune cell inflammatory state, antimicrobial killing capacity, and metabolism. Historically, the production of C3a has been relegated to the serum; however, recent studies have provided evidence that various cell types can produce intracellular C3a that stimulates intracellular C3aR. In light of these new results, it is imperative that we revisit previous studies regarding the role of C3aR in controlling bacterial infections and analyze these results in the context of both extracellular and intracellular C3a production and C3aR activation. Thus, this review will cover specific roles of C3aR in driving cell type–specific and tissue specific responses during bacterial infections and emphasize the contribution of the C3a–C3aR axis in regulating host resistance to bacterial infection.
Introduction
The complement system plays an essential role in defense against bacterial pathogens, and recent insights into the role of tissue-specific and cell-autonomous complement-mediated immunity have inspired a systemic review of complement literature in the context of these new functions. Specifically, many of the complement pathway effector functions are downstream of the cleavage of complement factor 3 (C3) into biologically active C3a and C3b. C3b acts as an opsonin and activates the lytic pathway. C3a is a soluble factor that activates its cognate receptor C3aR in an autocrine and paracrine fashion on innate immune cells to regulate local and systemic inflammation.
C3aR belongs to the family of inhibitory G protein–coupled 7 transmembrane-containing receptors (Gi) and is expressed both intracellularly and extracellularly on a wide variety of cells [1]. Activation of membrane-bound Gi proteins lead to the release of the α subunit, which suppresses production of intracellular cAMP via inhibition of adenylyl cyclase, and β/γ subunits, which have been shown to regulate ERK1/2 and JNK activation through PI3K/AKT activity in a cell type–specific manner [2]. Further, it has been shown that C3aR undergoes rapid (approximately 15 seconds) inactivation via GPCR kinase (GRK)-dependent phosphorylation in mast cells [3,4] and internalization within granulocytes and epithelial cells [5,6]. Studies that have characterized C3aR as a Gi within innate immune cells are very limited and the effect C3aR activation within innate immune cells on downstream signaling cascades has not been fully defined.
It has been reported in murine dendritic cells (DCs) that intracellular production of cAMP is reduced following stimulation with C3a, with an increase in phosphorylation of the PI3K/AKT and ERK pathways [7]. Similarly, human DCs showed reduced intracellular cAMP production and increased PI3K/AKT, ERK, and NF-κB signaling following stimulation with C3a [8]. Further, murine DCs pretreated with a C3aR agonist saw reduced p38 MAP kinase phosphorylation [9]. In human monocytes and macrophages, C3aR activation leads to enhancement of IL-1β production that is regulated by enhanced ERK1/2 phosphorylation [10]. Further, we have shown blockade of C3aR activation in primary murine and human macrophages using the nonpeptide inhibitor SB290157 leads to reduced TNF and IL-6 secretion and p38 MAP kinase phosphorylation during LPS or IFNβ stimulation [11]. Lastly, in a murine model of spinal cord injury, activation of C3aR by C3a led to increased ERK1/2 phosphorylation in bone marrow–derived neutrophils [12]. These data suggest that C3aR is acting as a canonical Gi within innate immune cells.
Importantly, some Gi proteins can alter Ca2+ mobilization. It was found that treating human neutrophils and monocytes with nanomolar concentrations of exogenous C3a triggered the rapid influx of Ca2+ from the extracellular medium [13,14]. Further, mouse peritoneal macrophages treated with a peptide C3a agonist induced a significant influx of intracellular Ca2+ that was dependent on C3aR [15]. Together, these data partially define the role of C3aR activation in innate immune cell signaling and suggest that C3aR may play a cell type–specific role in regulation of cAMP, PI3K/AKT-dependent signaling cascades, and Ca2+ mobilization. However, all of these studies have assumed that C3aR is located at the plasma membrane and have only analyzed cytoplasmic changes in signaling cascades and secondary messenger molecules, potentially missing important organelle-specific roles of C3aR activation within innate immune cells.
To this point, it was originally thought that production of C3a occurred exclusively within the serum by C3 convertases; however, recent publications have elegantly shown intraphagosomal production of C3a by Cathepsin L and intracellular activation of C3aR within multiple cell types [16–19]. Liszewski and colleagues have shown that in naive T cells, activation of phagosomal C3aR by C3a leads to induction of mTOR activation required for tonic metabolic requirements [18]. This was the first indication that C3aR may be regulating metabolism directly. Further, it was recently shown that in epithelial cells, C3aR is endocytosed upon oxidative stress and is trafficked to the outer mitochondrial membrane [6]. In these studies, activation of C3aR on the mitochondrial membrane increased Ca2+ uptake, which inhibited mitochondrial respiration via blocking state III ADP-driven respiration [6]. These new results have provided a novel paradigm that can now be used as a guide for interpreting past and future findings on C3aR function within the innate immune system.
Importantly, C3aR activation results in pathophysiological changes seen in infection models using both gram-negative and gram-positive bacteria, with both intracellular and/or extracellular life cycles. Surprisingly, little molecular detail underlying C3aR-dependent control of innate immunity is understood. However, many infection models using global C3aR knockout mice (C3aR−/−) have provided exciting insights into the dynamic orchestration of inflammation and infection outcome by C3aR. Here we discuss activation of C3aR during bacterial infection leading to cell type–specific pleiotropic effects, including modulation of signaling pathways and release of cytokines, reprogramming of innate immune cell function, enhanced inflammasome activation, and recruitment of inflammatory cells to infected tissues. Further, we examine how C3aR contributes to the complement system’s role in driving innate immune defenses and host resistance to bacterial infection.
C3aR reprograms neutrophils during systemic and local bacterial infection
Neutrophils are innate effector cells that are rapidly recruited to the sites of infection and are important in the acute phase of bacterial infection. Neutrophils can promote bacterial resistance by clearing bacterial infections, while conversely, they can promote bacterial persistence by inducing tissue damage [20]. C3aR is expressed by neutrophils in the blood, and some reports suggest that serum C3a can enhance neutrophil antimicrobial function and act as a chemoattractant; however, the role of C3aR in neutrophils is currently under debate [21]. Here, we highlight the double-edged sword of enhancing neutrophil recruitment and activation by C3aR during bacterial infection.
C3aR reprograms neutrophils during systemic Neisseria meningitidis infection
Neisseria meningitidis (meningococcus) is a gram-negative bacterial pathogen that often asymptomatically inhabits the human nasopharynx, yet can cause invasive meningococcal disease (IMD) in susceptible individuals (infants, the elderly, and the immunocompromised) [22]. IMD manifests in meningitis and septicemia that can result in neurological disorders. Upon acquisition of N. meningitidis through inhalation, the organism attaches to and invades the mucosal epithelium in the upper respiratory tract [20]. Mucosal epithelial cells detect N. meningitidis via TLR2, TLR4, and NOD1 to induce production of IL-8, IL-6, TNF, and other inflammatory cytokines [20]. In susceptible individuals, N. meningitidis can evade killing by the mucosal immune response, entering the blood to survive and rapidly replicate [22]. Importantly, infection in the blood stimulates massive recruitment of neutrophils, which is both helpful for clearance and detrimental in causing host tissue damage associated with IMD [20].
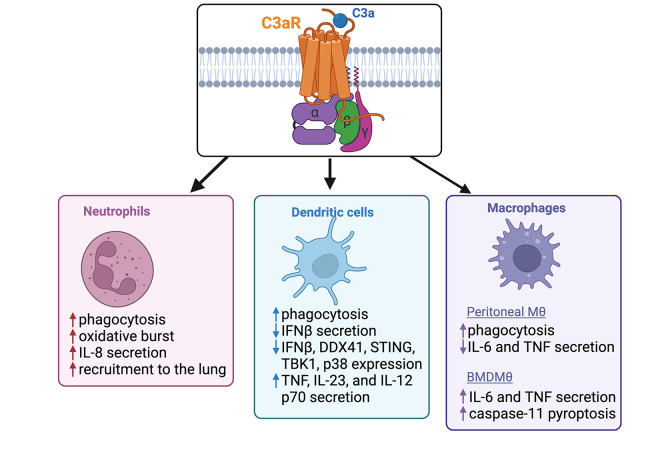
In a murine sepsis model of N. meningitidis, it was found that C3aR−/− mice displayed enhanced disease severity, increased colony-forming unit (CFU) in the blood, and a decrease in survival, compared to wild-type (WT) mice [23]. Considering the important role neutrophils play in driving the response to N. meningitidis, they measured the antimicrobial capacity of neutrophil intrinsic C3aR by pretreating whole human blood with a C3aR antagonist (C3aRi; SB290157) and infecting with N. meningitidis, which resulted in decreased secretion of IL-8 and significant inhibition of oxidative burst and phagocytosis (Fig 1) [23]. These data suggest that the protective effect of C3aR during N. meningitidis infection may be in part explained by its driving enhanced antimicrobial neutrophil activity. Additionally, this group found that early in infection, there was decreased IL-6 in the blood of C3aR−/− mice [23]. Immune cells detect N. meningitidis via TLR2, TLR4, and NOD1 to induce production of inflammatory cytokines [20] and C3aR has been shown to enhance production of inflammatory cytokines downstream of TLR2 and TLR4 activation within macrophages [11]. C3aR may similarly be enhancing inflammation downstream of TLR activation within neutrophils, although this result has not been shown. Future molecular studies understanding the C3aR-dependent signaling cascades required for modulating neutrophil function and enhancing systemic production of IL-6 may provide insight into the protective role of C3aR in N. meningitidis infection.
Fig 1. Schematic of C3aR-dependent innate immune cell function during bacterial infections.
C3aR recruits neutrophils to the lung and promotes Pseudomonas aeruginosa infection
Pseudomonas aeruginosa is a gram-negative pathogen that is often found in the environment and is a major cause of healthcare-associated infections, including pneumonia and infections involving the urinary tract, wounds, burns, and the bloodstream [24]. Colonization of the respiratory tract by P. aeruginosa results in localized inflammation driven by host cell production of cytokines and chemokines, and the subsequent recruitment of neutrophils to the lung. Various P. aeruginosa virulence components (ex: LPS, type III secretion system products, and pili) induce production of inflammatory cytokines by activating pattern recognition receptors (PRRs; typically, TLR4 and TLR5), reviewed elsewhere [25,26].
Numerous studies have worked to demonstrate the role of C3aR and C3a in driving acute inflammation in pulmonary allergy, but not lung infection. Thus, in 2006, Mueller-Ortiz and colleagues modeled P. aeruginosa pulmonary infection via intranasal infection and found C3aR−/− mice displayed decreased recruitment of neutrophils to the lungs and increased bacterial clearance in the blood and lungs of mice, compared to WT mice [27]. These data are the first to suggest that presence of C3aR is deleterious during pulmonary bacterial infection. Furthermore, the C3aR−/− mice showed decreased inflammatory cytokine (IL-6, TNF, and IL-1β) secretion in the bronchoalveolar lavage (BAL) [27]. Interestingly, mice deficient in the TNF receptor [28], IL-1 receptor [29], IFNγ receptor [30], or IL-18 [31] share similar phenotypes as the C3aR−/− mice: less inflammation and decreased neutrophil recruitment to the lungs. It is now appreciated that inflammatory cytokines likely impair bacterial clearance from the pulmonary compartment and enhance tissue damage during P. aeruginosa infection and that C3aR contributes to this damage.
Additionally, these studies correlate with our current knowledge on the function of C3aR in amplifying inflammatory cytokine response during bacterial infection. Napier and colleagues found that C3aR is required for p38 MAP kinase phosphorylation downstream of TLR4 activation by LPS [11]. Recently, Coates and colleagues found that inhibiting p38 MAP kinase activity during P. aeruginosa infection of bronchial epithelial cells depleted IL-6 and CXCL8 production [32]. These findings bolster the hypothesis that C3aR may be enhancing PRR detection of P. aeruginosa and subsequent release of inflammatory cytokines during infection via the p38 MAP kinase pathway in the lung. Further studies will need to be conducted to identify whether neutrophil-intrinsic C3aR activation or bystander C3aR activation is leading to cytokine secretion that regulates neutrophil chemotaxis to the BAL during P. aeruginosa infection of the lung.
C3aR mediates DC inflammatory states to enhance bacterial resistance
DCs play an imperative role in the innate immune response by enhancing inflammation and inducing adaptive immune responses that are critical for clearance of bacteria during infection. The recent development of a C3aR reporter mouse has shown C3aR is expressed in bone marrow–derived dendritic cells (BMDCs), lung-resident CD11b+ conventional DCs (cDCs), and monocyte-derived DCs [1], demonstrating that C3aR is widely expressed on various DC subsets. Here, we review possible mechanisms by which C3aR regulates resistance to 2 bacterial pathogens via dampening expression of type I interferons and enhancing inflammatory cytokines within DCs.
C3aR shields against Listeria monocytogenes
Listeria monocytogenes (LM) is a gram-positive facultative intracellular pathogen transmitted via contaminated food and can lead to sepsis and meningitis [33]. After ingestion of contaminated food, LM enters intestinal epithelial cells where it replicates and disseminates via the lymph and blood to target organs (liver and spleen) [33]. Early resistance to LM is dependent on NF-κB and IRF3/7 activation, subsequent production of inflammatory cytokines (TNF, type I-III interferons, etc.), and the recruitment of activated monocytes, macrophages, and neutrophils to the sites of infection [33]. Clearance of infection is typically dependent on CD8+ and CD4+ T-cell responses [33,34]. Additionally, LM induces apoptosis in macrophages, neutrophils, and DCs, which are all important for T cell–mediated immunity and effective clearance of infection [35,36].
To understand the role of C3aR in driving LM infection dynamics, Mueller-Ortiz and colleagues modeled LM infection within C3aR−/− mice and showed a 50% decrease in survival and more severe systemic disease, compared to WT mice [34]. This decrease in survival of C3aR−/− mice correlated with an increase in bacterial burden within the spleen and liver and enhanced production of IFNγ, TNF, and IL-6 in the blood [34]. These results suggest the presence of C3aR acts to reprogram the host inflammatory response to LM and enhance bacterial resistance. However, which cell type that requires C3aR in order to enhance bacterial clearance remains unclear. It is also unknown whether C3aR activation is dampening inflammation directly, or if there are higher concentrations of inflammatory cytokines in the blood of C3aR−/− mice because they have larger bacterial burdens in the liver and spleen.
This same group found that the anaphylatoxin receptor C5aR1, which detects the bioactive C5a molecule, protected against LM infection by dampening type I IFN expression (IFNα/β) [37]. Type I IFNs are induced by cyclic di-AMP from LM stimulating DDX41 and STING, which lead to sensitization of infected cells to apoptosis [9]. There was no report on the effect of C3aR on type I IFNs in their 2013 report; however, a follow-up study from this lab showed that treatment of primary BMDCs with C3a followed by challenge with cyclic-di-AMP or infection with LM resulted in decreased expression and production of the type I IFN, IFNβ [9]. Additionally, they found secretion of inflammatory cytokines TNF and IL-6 were not affected by C3a pretreatment in BMDCs [9]. When LM-derived cyclic di-AMP is detected by DDX41, it complexes with STING and signals to TBK1 and p38 MAPK to induce the type I IFN response [38]. They found pretreatment with an C3aR agonist followed by a cyclic di-AMP challenge reduced expression of key mediators of the type I IFN response (DDX41, STING, TBK1, and p38) [9]. These data suggest that C3aR activation within BMDCs inhibits the expression of key type I IFN response regulators through an unknown mechanism, thus resulting in decreased expression of IFNβ during subsequent challenge with LM (Fig 1). They conclude that this C3aR-dependent decrease in IFNβ may contribute to enhanced survival and lower bacterial burden in WT mice, compared to C3aR−/− mice infected with LM. Future studies using adoptive transfer of C3aR−/− BMDCs into irradiated WT mice will be key in understanding if the DC-intrinsic role of C3aR impacts the host inflammatory state and bacterial resistance to LM.
C3aR makes DCs hungry for Porphyromonas gingivalis
Porphyromonas gingivalis is a gram-negative anaerobic oral bacterium that persists within periodontal pockets and is a key pathogen associated with human periodontitis [39]. Periodontitis is characterized by inflammatory damage of the gums and periodontal support tissues [40]. P. gingivalis–dependent activation of TLR2-phosphoinositide 3 kinase (PI3K) pathway can increase destruction of periodontal tissues and trigger inflammation necessary for further invasiveness [39,40]. Interestingly, TLR polymorphisms are associated with susceptibility of adolescents to periodontitis [41], demonstrating the importance of TLR-mediated innate immune responses in driving P. gingivalis resistance. Typically, P. gingivalis orchestrates inflammatory disease by inducing dysbiosis in the oral microbiome [39]; however, it can also invade and replicate within multiple cell types, including neutrophils, macrophages, and DCs [40].
In a study identifying the requirement for C3aR in the invasion and replication of P. gingivalis within DCs, researchers found WT BMDCs—but not C3aR−/− BMDCs—treated simultaneously with C3a and infected with P. gingivalis showed enhanced bacterial clearance [40]. Further, BMDCs treated with C3a showed enhanced phagocytosis of P. gingivalis and increased secretion of TNF, IL-23, and IL-12 p70 (Fig 1) [39]. These data demonstrate that the C3a–C3aR axis is working to enhance clearance of bacteria and the production of inflammatory cytokines in response to P. gingivalis infection within BMDCs. Interestingly, they found that BMDCs from TLR2−/− mice showed a nearly identical increase in P. gingivalis CFU as the C3aR−/− BMDCs [39]. It has been previously shown that when P. gingivalis enters BMDCs, it uses its Mfa1 fimbriae to interact with DC-SIGN, which directs the bacterium into intracellular vesicles that escape autophagosomal degradation [42]. TLR2 can block the subversion of P. gingivalis autophagosomal degradation in BMDCs, thus enhancing intracellular killing [43]. Currently, there are no studies that identify the potential mechanism of the C3a–C3aR axis enhancing TLR2-dependent autophagosomal degradation of P. gingivalis in BMDCs. Importantly, it had been previously shown that macrophages treated with C3a and infected with P. gingivalis did not have any effect on bacterial burden [44]; however, this study did not investigate the role of phagosomal C3a or the necessity of C3aR activation for P. gingivalis survival within macrophages. Thus, these data show that C3aR-dependent intracellular killing of P. gingivalis is cell type-specific and underline potential differences between inflammatory signaling pathways between cell types [44]. It’s important to note that in vitro studies using C3aR−/− BMDCs may not reflect the phenotypes of cells in vivo and highlights the need to better understand the contributions of different cell types during P. gingivalis infection in vivo. Further, these studies highlight the lack of known mechanisms driving C3aR-dependent intracellular P. gingivalis killing and leave open the possibility that C3aR-enhanced TLR2 signaling may be driving this resistance to bacterial infection.
C3aR dampens macrophage function during Uropathogenic Escherichia coli (UPEC) infection
Tissue-resident macrophages are sentinel immune cells critically involved in defending the host from bacterial infection via phagocytosis and destruction of the pathogen and induction of inflammatory cytokines. We have previously shown that macrophage-intrinsic C3aR enhances induction of TLR4-dependent inflammatory cytokines and activation of the caspase-11 inflammasome during Salmonella typhimurium and Shigella flexneri infection, demonstrating a macrophage-intrinsic mechanistic link between C3aR activation and TLR activation [11]. It is currently unclear if macrophage-intrinsic C3aR regulates in vivo bacterial infection. Here, we will review the one study that defines a role of C3aR in controlling UPEC infection by manipulating macrophage function.
C3aR within macrophages destroys tissues during UPEC infection
Uropathogenic E. coli (UPEC) is a gram-negative bacterial pathogen and accounts for roughly 80% of urinary tract infections (UTIs), which can lead to cystitis in the bladder and acute pyelonephritis in the kidneys [45]. Once in the urinary tract, UPEC gains access to superficial bladder epithelial cells (also called facet or umbrella cells) that provides protection from phagocytes [46]. As UPEC replicates intracellularly, it actively suppresses the host inflammatory response, influx of neutrophils, and production of antimicrobial peptides (ex: defensins) [46]. The production of inflammatory cytokines (IL-6, IL-8, and IL-1β) and the neutrophilic response during UPEC infection are critical for host clearance of infection; however, an excessive inflammatory response to UPEC infection can result in deleterious renal pathology [45,46].
To evaluate the role of C3aR in UPEC pathogenesis, Wu and colleagues used a well-established murine model of ascending UTI that leads to pyelonephritis. They found that C3aR−/− mice displayed impaired bacterial clearance in the kidney and more-severe kidney damage, compared to WT mice [45]. They demonstrated that the transfer of WT bone marrow into C3aR−/− mice subsequently infected with UPEC, conferred reduced bacterial burden in the kidneys, suggesting myeloid cell-intrinsic C3aR may be inhibiting UPEC expansion during kidney infection [45]. Additionally, they found within the first 24 hours of infection, expression and secretion of inflammatory cytokines (TNF and IL-6) within the kidney and recruitment of neutrophils to the kidney are enhanced in C3aR−/− mice, compared to WT [45]. Thus, this group demonstrated a protective role of C3aR via dampening the host inflammatory response to UPEC infection of the kidneys.
When defining the mechanism of C3aR-dependent suppression of inflammation and UPEC expansion, they showed in vitro C3a inhibited TNF and IL-6 expression in LPS-treated peritoneal macrophages, which correlated with decreased phosphorylation of ERK1/2 and IκB [45]. It was not tested if UPEC mediated the same C3a-dependent inflammatory signaling response. These data are in opposition to inflammatory data described previously by our lab, that demonstrate C3aR−/− macrophages isolated from the bone marrow of mice and primary human macrophages treated with a C3aR inhibitor exhibit significantly less IL-6 and TNF when challenged with LPS [11]. These conflicting results may be due to the difference in macrophage subclass. Further, they found that C3aR−/− peritoneal phagocytes (neutrophils, monocytes, and macrophages) had an impaired capacity to phagocytose UPEC and C3aR−/− mice infected with UPEC showed decreased bacterial association with phagocytes from the kidney [45]. These results show C3aR is required for efficient UPEC uptake by phagocytes in vitro, which aligns with the results determining C3aR within DCs enhances P. gingivalis phagocytosis and C3aR within neutrophils enhances N. meningitidis phagocytosis (Fig 1) [23,39]. Mechanistic understanding of C3aR activation and how it impacts the physiology of cells will be important to define in order to understand how C3aR increases phagocytosis within multiple innate immune cell types during bacterial infection.
C3aR reorganizes local tissues during early stages of bacterial infection
Complement is considered a serum-specific arm of the innate immune system; however, recent studies have definitively shown C3aR can be found within the phagosomes of T cells and associated with the mitochondria in epithelial cells [6,18]. Additionally, C3a has been shown to be produced by Cathepsin L within the phagosome of T cells [18]. Considering all myeloid cells can express Cathepsin L and C3 [47,48], it is hypothesized that C3a can be produced intracellularly by multiple cell types. These new studies are beginning to define the intracellular role of the C3a–C3aR axis and bolster the nonserum role of complement within specific cell types and tissues. Local tissue reprogramming by the C3a–C3aR axis during bacterial infection was best highlighted by 2 studies that explored the role of C3aR within the lungs during Chlamydia psittaci infection.
C3aR won’t let your system forget Chlamydia
C. psittaci is an obligate intracellular gram-negative avian pathogen that causes psittacosis in birds and can be transmitted to humans [49]. Similar to all other Chlamydia species, C. psittaci possess a unique developmental cycle characterized by extracellular, infectious “elementary bodies” and an intracellular, metabolically active form called “reticulate bodies” within the phagosome [50]. Importantly, chlamydial components can be recognized by TLR2, TLR4, STING, NOD1, and the NLRP3 inflammasome [51,52]. Activation of these receptors triggers cell-autonomous immunity, characterized by the activation of NF-κB and IRF3 and the subsequent release of inflammatory cytokines (IL-6, TNF, and IFNγ) [49]. Further, production of these cytokines initiates a robust adaptive immune response, characterized by both humoral and cellular immunity [49]. To evade these responses, Chlamydia spp. use various strategies to interfere with NF-κB activation including blocking degradation of the NF-κB inhibitor IκBα as well as preventing nuclear translocation of NF-κB [53]. Additionally, Chlamydia spp. employ a protease that degrades the signaling molecule TNF receptor-associated factor 3 (TRAF3) thus blocking the activation of IRF3 and subsequent induction of IFNβ in epithelial cells [54].
In mice, C. psittaci infection can be modeled by intranasal infection, and in this model, C3 is required for protection against C. psittaci infection [55]. To determine the contribution of the C3a–C3aR axis in driving C3-protection within this infection model, 2 groups found that C3aR−/− mice infected with C. psittaci displayed enhanced mortality, similar to what is seen in C3−/− mice [55], in comparison with WT [55,56]. Additionally, C3aR−/− showed higher bacterial burden in the lungs and spleen and an increased total number of neutrophils within the BALF [56]. Surprisingly, despite higher CFUs and neutrophils within C3aR−/− lungs and BALF, respectively, C3aR−/− and WT had the same level of inflammatory cytokines (IL-6, TNF, or IFNγ) within the BALF throughout the duration of infection [56]. C3aR has been reported to amplify signaling cascades within various innate immune cells; thus, we suggest that they may have missed the window of time that captures the decreased inflammatory cytokine production in C3aR−/− BALF, since the earliest they checked was 9 days p.i. Further, 9 days p.i. lung-draining lymph nodes (dLNs) within C3aR−/− mice contained significantly fewer B cells, CD4+ T cells, IFNγ+ CD4+ T cells, CD8+ T cells, and Chlamydia-specific IgM and IgG [56]. Considering TLR activation is essential for priming adaptive immune responses, and C3aR enhances TLR2 and TLR4 signaling within in vivo and in vitro models, this may be the reason that the adaptive immune response in the lung-dLNs of C3aR−/− mice is attenuated. Future investigation into cytokine production and innate immune cellular function at earlier time points in C. psittaci infection may provide data imperative for understanding the role of C3aR within this model system.
Building on these findings, Kohn and colleagues elegantly demonstrated that early depletion of serum-C3, via intravenous treatment with cobra venom factor (CVF) 2 days before infection in WT mice, increased disease severity and decreased recovery time, compared to mock-treated mice [55]. Additionally, late depletion of serum-C3 (6d p.i.) did not affect infection outcome, suggesting that C3 and its bioactive forms C3a and C3b, are working to enhance early innate immune function [55]. Interestingly, they confirmed that during C. psittaci lung infection, early serum-C3 depletion correlated with a partial increase in bacterial burden, but did not completely phenocopy the overwhelming increase in bacterial burden in the lungs of infected C3−/− or C3aR−/− mice [55]. Further, depletion of serum-C3 only conferred a minimal, and nonsignificant decrease in survival. These findings are intriguing considering CVF does not deplete intracellular C3, which has been shown to enhance cell-autonomous immunity against the intracellular bacterial pathogen S. typhimurium [57]. We suggest that the inequity of phenotypes between serum depleted C3 and the C3−/− or C3aR−/− mice may be due to the unidentified role of intracellular C3a/C3aR within innate immune cells.
Together, these studies demonstrate the essential role of C3aR in controlling C. psittaci infection within the lung and during dissemination [55,56]. Further, both studies confirm that C3aR is controlling the adaptive immune response to C. psittaci by manipulating innate immune cell function, including enhancing neutrophil migration to the lung and DC migration to the lung-dLNs. However, more mechanistic data describing the requirement of the C3a–C3aR axis in driving innate cell-autonomous functions during C. psittaci infection will be crucial in understanding the requirement of C3aR in resistance to Chlamydia spp.
Conclusions and future directions
Overall, findings in mouse models and human samples demonstrate that C3aR is an important modulator of inflammation, innate immune cell antimicrobial function, and resistance to intracellular and extracellular bacterial pathogens (Table 1). It is clear from the conflicting results obtained thus far with C3aR−/− mice and inflammation during bacterial infection that our understanding of the functional role of C3aR is limited. Additionally, there is a need to better understand how specific innate immune cell subclasses differ in their requirement for C3aR for inflammatory signaling and antimicrobial responses. Currently, there are no data using mice with cell-specific C3aR deficiencies and bacterial infection, only global C3aR knockout mice. Building these mice to investigate how C3aR modulates inflammatory responses during bacterial infection could provide mechanistic insight into how cell-specific C3aR contributes to bacterial resistance.
Table 1. Summary of C3aR-dependent phenotypes in bacterial infection models.
| Pathogen | Gram-stain | Lifecycle | Role of C3aR in vivo; tissues analyzed | Role of C3aR in vitro; cell type analyzed | References |
|---|---|---|---|---|---|
| Listeria monocytogenes | + | Intracellular | Protective; Spleen, liver blood |
BMDCs | [9, 34] |
| Chlamydia psittaci | - | Intracellular | Protective; Lung, liver, spleen | N/A; N/A | [55, 56] |
| Neisseria meningitidis | - | Intracellular/ Extracellular |
Protective; blood blood |
No role; murine whole blood human whole blood |
[23] |
| Porphyromonas gingivalis | - | Intracellular/ Extracellular |
N/A; N/A | Protective; BMDCs BMDCs No role; BMDMs |
[39, 44] |
| Uropathogenic E. coli | - | Intracellular/ Extracellular |
Protective; kidney kidney |
peritoneal macrophages | [45] |
| Pseudomonas aeruginosa | + | Extracellular | Deleterious; lung, blood BAL |
N/A; N/A | [27] |
Red font signifies an inflammatory role for C3aR; blue font signifies an anti-inflammatory role for C3aR.
Further, relatively new studies have identified an intracellular role for C3aR. Intraphagosomal production of C3a via cleavage of phagosomal C3 by hydrolytic enzyme Cathepsin L has been shown to regulate T cell homeostasis and the response to antigens [18]. Additionally, a recent study has shown that C3aR can localize to the mitochondrial membrane within epithelial cells and become activated by intracellular C3a during H2O2-induced oxidative stress [6]. Together, these recent studies conclude intracellular production of C3a and C3aR activation regulates homeostatic and stress responses in various cell types. Thus, it is imperative that we revisit studies looking at C3aR-mediated bacterial resistance with the new understanding that C3a may be coming from within the cell, and not from the serum.
Importantly, there is recent evidence showing that infection of respiratory epithelial cells with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) leads to significant induction of the complement system [58,59]. Specifically, SARS-CoV-2 infection of respiratory epithelial cells generated secreted C3a that acted on bystander immune cells such as macrophages to drive a hyperactivation state that tracked with disease severity in patients with Coronavirus Disease 2019 (COVID-19) [58]. This evidence highlights the need to better distinguish the role of myeloid cell-intrinsic C3aR activation versus bystander C3aR activation, how crosstalk between immune and nonimmune cells contributes to complement hyperactivation, and the signaling events downstream of C3aR involved in promoting pathological complement activation during viral and bacterial infections. Additionally, understanding the role of C3aR in regulating secondary bacterial infections during viral infections, a clinical manifestation common in pandemic respiratory viruses such as SARS-CoV-2 [58–60], may prove beneficial to developing therapeutics for future viral pandemics. Lastly, with the recent evidence of C3aR enhancing antigen uptake and T-cell stimulation in DCs and its effect on phagocytic capacity in phagocytes, future studies on how C3aR primes the immune system for better adaptive responses may be useful in developing better vaccines and combating infectious diseases.
Acknowledgments
We would like to thank Rael Dornfest (Portland State University) for critical editing of this manuscript.
Funding Statement
This research was funded by grants to B.A.N. from the Collins Medical Trust (Portland, Oregon) and the Medical Research Foundation (Oregon Health & Sciences University, Portland, Oregon). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Quell KM, Karsten CM, Kordowski A, Almeida LN, Briukhovetska D, Wiese AV, et al. Monitoring C3aR Expression Using a Floxed tdTomato-C3aR Reporter Knock-in Mouse. J Immunol. 2017;199(2):688–706. Epub 2017/06/16. doi: 10.4049/jimmunol.1700318 . [DOI] [PubMed] [Google Scholar]
- 2.Goldsmith ZG, Dhanasekaran DN. G protein regulation of MAPK networks. Oncogene. 2007;26(22):3122–3142. doi: 10.1038/sj.onc.1210407 . [DOI] [PubMed] [Google Scholar]
- 3.Langkabel P, Zwirner J, Oppermann M. Ligand-induced phosphorylation of anaphylatoxin receptors C3aR and C5aR is mediated by "G protein-coupled receptor kinases. Eur J Immunol. 1999;29(9):3035–3046. doi: . [DOI] [PubMed] [Google Scholar]
- 4.Gupta K, Subramanian H, Klos A, Ali H. Phosphorylation of C3a receptor at multiple sites mediates desensitization, β-arrestin-2 recruitment and inhibition of NF-κB activity in mast cells. PLoS ONE. 2012;7(10):e46369. Epub 20121015. doi: 10.1371/journal.pone.0046369 ; PubMed Central PMCID: PMC3471852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Settmacher B, Bock D, Saad H, Gärtner S, Rheinheimer C, Köhl J, et al. Modulation of C3a activity: internalization of the human C3a receptor and its inhibition by C5a. J Immunol. 1999;162(12):7409–7416. . [PubMed] [Google Scholar]
- 6.Ishii M, Beeson G, Beeson C, Rohrer B. Mitochondrial C3a Receptor Activation in Oxidatively Stressed Epithelial Cells Reduces Mitochondrial Respiration and Metabolism. Front Immunol. 2021;12:628062. Epub 20210305. doi: 10.3389/fimmu.2021.628062 ; PubMed Central PMCID: PMC7973370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li K, Anderson KJ, Peng Q, Noble A, Lu B, Kelly AP, et al. Cyclic AMP plays a critical role in C3a-receptor-mediated regulation of dendritic cells in antigen uptake and T-cell stimulation. Blood. 2008;112(13):5084–94. Epub 2008/09/23. doi: 10.1182/blood-2008-05-156646 . [DOI] [PubMed] [Google Scholar]
- 8.Li K, Fazekasova H, Wang N, Peng Q, Sacks SH, Lombardi G, et al. Functional modulation of human monocytes derived DCs by anaphylatoxins C3a and C5a. Immunobiology. 2012;217(1):65–73. Epub 20110805. doi: 10.1016/j.imbio.2011.07.033 ; PubMed Central PMCID: PMC3234345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mueller-Ortiz SL, Calame DG, Shenoi N, Li YD, Wetsel RA. The Complement Anaphylatoxins C5a and C3a Suppress IFN-β Production in Response to. J Immunol. 2017;198(8):3237–44. Epub 20170308. doi: 10.4049/jimmunol.1601420 ; PubMed Central PMCID: PMC5398560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asgari E, Le Friec G, Yamamoto H, Perucha E, Sacks SS, Köhl J, et al. C3a modulates IL-1β secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood. 2013;122(20):3473–81. Epub 20130722. doi: 10.1182/blood-2013-05-502229 . [DOI] [PubMed] [Google Scholar]
- 11.Napier BA, Brubaker SW, Sweeney TE, Monette P, Rothmeier GH, Gertsvolf NA, et al. Complement pathway amplifies caspase-11-dependent cell death and endotoxin-induced sepsis severity. J Exp Med. 2016. doi: 10.1084/jem.20160027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brennan FH, Jogia T, Gillespie ER, Blomster LV, Li XX, Nowlan B, et al. Complement receptor C3aR1 controls neutrophil mobilization following spinal cord injury through physiological antagonism of CXCR2. JCI Insight. 2019;4(9). Epub 20190502. doi: 10.1172/jci.insight.98254 ; PubMed Central PMCID: PMC6538362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norgauer J, Dobos G, Kownatzki E, Dahinden C, Burger R, Kupper R, et al. Complement fragment C3a stimulates Ca2+ influx in neutrophils via a pertussis-toxin-sensitive G protein. Eur J Biochem. 1993;217(1):289–294. doi: 10.1111/j.1432-1033.1993.tb18245.x . [DOI] [PubMed] [Google Scholar]
- 14.Martin U, Bock D, Arseniev L, Tornetta MA, Ames RS, Bautsch W, et al. The human C3a receptor is expressed on neutrophils and monocytes, but not on B or T lymphocytes. J Exp Med. 1997;186(2):199–207. doi: 10.1084/jem.186.2.199 ; PubMed Central PMCID: PMC2198980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu MC, Brennan FH, Lynch JP, Mantovani S, Phipps S, Wetsel RA, et al. The receptor for complement component C3a mediates protection from intestinal ischemia-reperfusion injuries by inhibiting neutrophil mobilization. Proc Natl Acad Sci U S A. 2013;110(23):9439–44. Epub 20130521. doi: 10.1073/pnas.1218815110 ; PubMed Central PMCID: PMC3677481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Ye J, Ren Y, Zuo J, Dai W, He Y, et al. Intracellular activation of complement C3 in Paneth cells improves repair of intestinal epithelia during acute injury. Immunotherapy. 2018;10(15):1325–36. Epub 20181101. doi: 10.2217/imt-2018-0122 . [DOI] [PubMed] [Google Scholar]
- 17.Satyam A, Kannan L, Matsumoto N, Geha M, Lapchak PH, Bosse R, et al. Intracellular Activation of Complement 3 Is Responsible for Intestinal Tissue Damage during Mesenteric Ischemia. J Immunol. 2017;198(2):788–97. Epub 20161202. doi: 10.4049/jimmunol.1502287 . [DOI] [PubMed] [Google Scholar]
- 18.Liszewski MK, Kolev M, Le Friec G, Leung M, Bertram PG, Fara AF, et al. Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity. 2013;39(6):1143–57. Epub 20131205. doi: 10.1016/j.immuni.2013.10.018 ; PubMed Central PMCID: PMC3865363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulkarni HS, Elvington ML, Perng YC, Liszewski MK, Byers DE, Farkouh C, et al. Intracellular C3 Protects Human Airway Epithelial Cells from Stress-associated Cell Death. Am J Respir Cell Mol Biol. 2019;60(2):144–157. doi: 10.1165/rcmb.2017-0405OC ; PubMed Central PMCID: PMC6376412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Criss AK, Seifert HS. A bacterial siren song: intimate interactions between Neisseria and neutrophils. Nat Rev Microbiol. 2012;10(3):178–90. Epub 20120131. doi: 10.1038/nrmicro2713 ; PubMed Central PMCID: PMC3569855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metzemaekers M, Gouwy M, Proost P. Neutrophil chemoattractant receptors in health and disease: double-edged swords. Cell Mol Immunol. 2020;17(5):433–50. Epub 20200401. doi: 10.1038/s41423-020-0412-0 ; PubMed Central PMCID: PMC7192912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Virji M. Pathogenic neisseriae: surface modulation, pathogenesis and infection control. Nat Rev Microbiol. 2009;7(4):274–286. doi: 10.1038/nrmicro2097 . [DOI] [PubMed] [Google Scholar]
- 23.Muenstermann M, Strobel L, Klos A, Wetsel RA, Woodruff TM, Köhl J, et al. Distinct roles of the anaphylatoxin receptors C3aR, C5aR1 and C5aR2 in experimental meningococcal infections. Virulence. 2019;10(1):677–694. doi: 10.1080/21505594.2019.1640035 ; PubMed Central PMCID: PMC6650196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berube BJ, Rangel SM, Hauser AR. Pseudomonas aeruginosa: breaking down barriers. Curr Genet. 2016;62(1):109–13. Epub 20150925. doi: 10.1007/s00294-015-0522-x ; PubMed Central PMCID: PMC4724561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol. 2009;7(9):654–665. doi: 10.1038/nrmicro2199 ; PubMed Central PMCID: PMC2766515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lavoie EG, Wangdi T, Kazmierczak BI. Innate immune responses to Pseudomonas aeruginosa infection. Microbes Infect. 2011;13(14–15):1133–45. Epub 20110802. doi: 10.1016/j.micinf.2011.07.011 ; PubMed Central PMCID: PMC3221798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mueller-Ortiz SL, Hollmann TJ, Haviland DL, Wetsel RA. Ablation of the complement C3a anaphylatoxin receptor causes enhanced killing of Pseudomonas aeruginosa in a mouse model of pneumonia. Am J Physiol Lung Cell Mol Physiol. 2006;291(2):L157–L165. doi: 10.1152/ajplung.00358.2005 . [DOI] [PubMed] [Google Scholar]
- 28.Skerrett SJ, Martin TR, Chi EY, Peschon JJ, Mohler KM, Wilson CB. Role of the type 1 TNF receptor in lung inflammation after inhalation of endotoxin or Pseudomonas aeruginosa. Am J Physiol. 1999;276(5):L715–L727. doi: 10.1152/ajplung.1999.276.5.L715 . [DOI] [PubMed] [Google Scholar]
- 29.Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest. 2007;117(12):3786–3799. doi: 10.1172/JCI32285 ; PubMed Central PMCID: PMC2066195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultz MJ, Rijneveld AW, Speelman P, van Deventer SJ, van der Poll T. Endogenous interferon-gamma impairs bacterial clearance from lungs during Pseudomonas aeruginosa pneumonia. Eur Cytokine Netw. 2001;12(1):39–44. . [PubMed] [Google Scholar]
- 31.Schultz MJ, Knapp S, Florquin S, Pater J, Takeda K, Akira S, et al. Interleukin-18 impairs the pulmonary host response to Pseudomonas aeruginosa. Infect Immun. 2003;71(4):1630–1634. doi: 10.1128/IAI.71.4.1630-1634.2003 ; PubMed Central PMCID: PMC152024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coates MS, Alton EWFW, Rapeport GW, Davies JC, Ito K. Pseudomonas aeruginosa induces p38MAP kinase-dependent IL-6 and CXCL8 release from bronchial epithelial cells via a Syk kinase pathway. PLoS ONE. 2021;16(2):e0246050. Epub 20210201. doi: 10.1371/journal.pone.0246050 ; PubMed Central PMCID: PMC7850485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radoshevich L, Cossart P. Listeria monocytogenes: towards a complete picture of its physiology and pathogenesis. Nat Rev Microbiol. 2018;16(1):32–46. Epub 20171127. doi: 10.1038/nrmicro.2017.126 . [DOI] [PubMed] [Google Scholar]
- 34.Mueller-Ortiz SL, Morales JE, Wetsel RA. The receptor for the complement C3a anaphylatoxin (C3aR) provides host protection against Listeria monocytogenes-induced apoptosis. J Immunol. 2014;193(3):1278–89. Epub 2014/06/30. doi: 10.4049/jimmunol.1302787 ; PubMed Central PMCID: PMC4122265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119(2):299–309. doi: 10.1016/j.cell.2004.09.032 . [DOI] [PubMed] [Google Scholar]
- 36.Guzmán CA, Domann E, Rohde M, Bruder D, Darji A, Weiss S, et al. Apoptosis of mouse dendritic cells is triggered by listeriolysin, the major virulence determinant of Listeria monocytogenes. Mol Microbiol. 1996;20(1):119–126. doi: 10.1111/j.1365-2958.1996.tb02494.x [DOI] [PubMed] [Google Scholar]
- 37.Calame DG, Mueller-Ortiz SL, Morales JE, Wetsel RA. The C5a anaphylatoxin receptor (C5aR1) protects against Listeria monocytogenes infection by inhibiting type 1 IFN expression. J Immunol. 2014;193(10):5099–107. Epub 20141008. doi: 10.4049/jimmunol.1401750 ; PubMed Central PMCID: PMC4295506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, Yuan B, Bao M, Lu N, Kim T, Liu YJ. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol. 2011;12(10):959–65. Epub 20110904. doi: 10.1038/ni.2091 ; PubMed Central PMCID: PMC3671854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hajishengallis G, Krauss JL, Jotwani R, Lambris JD. Differential capacity for complement receptor-mediated immune evasion by Porphyromonas gingivalis depending on the type of innate leukocyte. Mol Oral Microbiol. 2017;32(2):154–65. Epub 20160518. doi: 10.1111/omi.12161 ; PubMed Central PMCID: PMC5065733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jia L, Han N, Du J, Guo L, Luo Z, Liu Y. Pathogenesis of Important Virulence Factors of. Front Cell Infect Microbiol. 2019;9:262. Epub 20190718. doi: 10.3389/fcimb.2019.00262 ; PubMed Central PMCID: PMC6657652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leite FRM, Enevold C, Bendtzen K, Baelum V, López R. Pattern recognition receptor polymorphisms in early periodontitis. J Periodontol. 2019;90(6):647–54. Epub 20181219. doi: 10.1002/JPER.18-0547 . [DOI] [PubMed] [Google Scholar]
- 42.Carrion J, Scisci E, Miles B, Sabino GJ, Zeituni AE, Gu Y, et al. Microbial carriage state of peripheral blood dendritic cells (DCs) in chronic periodontitis influences DC differentiation, atherogenic potential. J Immunol. 2012;189(6):3178–87. Epub 20120813. doi: 10.4049/jimmunol.1201053 ; PubMed Central PMCID: PMC3459682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Awady AR, Miles B, Scisci E, Kurago ZB, Palani CD, Arce RM, et al. Porphyromonas gingivalis evasion of autophagy and intracellular killing by human myeloid dendritic cells involves DC-SIGN-TLR2 crosstalk. PLoS Pathog. 2015;10(2):e1004647. Epub 20150213. doi: 10.1371/journal.ppat.1004647 ; PubMed Central PMCID: PMC4352937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang M, Krauss JL, Domon H, Hosur KB, Liang S, Magotti P, et al. Microbial hijacking of complement-toll-like receptor crosstalk. Sci Signal. 2010;3(109):ra11. Epub 20100216. doi: 10.1126/scisignal.2000697 ; PubMed Central PMCID: PMC2824906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu KY, Zhang T, Zhao GX, Ma N, Zhao SJ, Wang N, et al. The C3a/C3aR axis mediates anti-inflammatory activity and protects against uropathogenic E coli-induced kidney injury in mice. Kidney Int. 2019;96(3):612–27. Epub 20190321. doi: 10.1016/j.kint.2019.03.005 . [DOI] [PubMed] [Google Scholar]
- 46.Olson PD, Hunstad DA. Subversion of Host Innate Immunity by Uropathogenic Escherichia coli. Pathogens. 2016;5(1). Epub 20160104. doi: 10.3390/pathogens5010002 ; PubMed Central PMCID: PMC4810123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307(5715):1630–1634. doi: 10.1126/science.1108003 . [DOI] [PubMed] [Google Scholar]
- 48.Lubbers R, van Essen MF, van Kooten C, Trouw LA. Production of complement components by cells of the immune system. Clin Exp Immunol. 2017;188(2):183–94. Epub 20170324. doi: 10.1111/cei.12952 ; PubMed Central PMCID: PMC5383442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elwell C, Mirrashidi K, Engel J. Chlamydia cell biology and pathogenesis. Nat Rev Microbiol. 2016;14(6):385–400. Epub 20160425. doi: 10.1038/nrmicro.2016.30 ; PubMed Central PMCID: PMC4886739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdelrahman YM, Belland RJ. The chlamydial developmental cycle. FEMS Microbiol Rev. 2005;29(5):949–959. doi: 10.1016/j.femsre.2005.03.002 . [DOI] [PubMed] [Google Scholar]
- 51.Rusconi B, Greub G. Chlamydiales and the innate immune response: friend or foe? FEMS Immunol Med Microbiol. 2011;61(3):231–44. Epub 20110118. doi: 10.1111/j.1574-695X.2010.00772.x . [DOI] [PubMed] [Google Scholar]
- 52.Stephens RS. The cellular paradigm of chlamydial pathogenesis. Trends Microbiol. 2003;11(1):44–51. doi: 10.1016/s0966-842x(02)00011-2 . [DOI] [PubMed] [Google Scholar]
- 53.Le Negrate G, Krieg A, Faustin B, Loeffler M, Godzik A, Krajewski S, et al. ChlaDub1 of Chlamydia trachomatis suppresses NF-kappaB activation and inhibits IkappaBalpha ubiquitination and degradation. Cell Microbiol. 2008;10(9):1879–92. Epub 20080628. doi: 10.1111/j.1462-5822.2008.01178.x . [DOI] [PubMed] [Google Scholar]
- 54.Wolf K, Fields KA. Chlamydia pneumoniae impairs the innate immune response in infected epithelial cells by targeting TRAF3. J Immunol. 2013;190(4):1695–701. Epub 20130109. doi: 10.4049/jimmunol.1202443 ; PubMed Central PMCID: PMC3563728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kohn M, Lanfermann C, Laudeley R, Glage S, Rheinheimer C, Klos A. Complement and. Front Immunol. 2021;12:580594. Epub 20210309. doi: 10.3389/fimmu.2021.580594 ; PubMed Central PMCID: PMC7986412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dutow P, Fehlhaber B, Bode J, Laudeley R, Rheinheimer C, Glage S, et al. The complement C3a receptor is critical in defense against Chlamydia psittaci in mouse lung infection and required for antibody and optimal T cell response. J Infect Dis. 2014;209(8):1269–78. Epub 20131123. doi: 10.1093/infdis/jit640 ; PubMed Central PMCID: PMC3969542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tam JC, Bidgood SR, McEwan WA, James LC. Intracellular sensing of complement C3 activates cell autonomous immunity. Science. 2014;345(6201):1256070. Epub 20140904. doi: 10.1126/science.1256070 ; PubMed Central PMCID: PMC4172439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan B, Freiwald T, Chauss D, Wang L, West E, Mirabelli C, et al. SARS-CoV-2 drives JAK1/2-dependent local complement hyperactivation. Sci Immunol. 2021;6(58). doi: 10.1126/sciimmunol.abg0833 ; PubMed Central PMCID: PMC8139422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Afzali B, Noris M, Lambrecht BN, Kemper C. The state of complement in COVID-19. Nat Rev Immunol. 2022;22(2):77–84. Epub 20211215. doi: 10.1038/s41577-021-00665-1 ; PubMed Central PMCID: PMC8672651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manohar P, Loh B, Nachimuthu R, Hua X, Welburn SC, Leptihn S. Secondary Bacterial Infections in Patients With Viral Pneumonia. Front Med (Lausanne). 2020;7:420. Epub 20200805. doi: 10.3389/fmed.2020.00420 ; PubMed Central PMCID: PMC7419580. [DOI] [PMC free article] [PubMed] [Google Scholar]