INTRODUCTION
Abnormal filaments of known composition in neurons and glia define many sporadic and hereditary human neurodegenerative diseases. The pathogenic significance of filamentous inclusions became evident when cases of dominantly inherited disease were shown to be associated with mutations in the genes encoding the proteins that make up filaments, be they Tau [1–3], Aβ [4, 5], α-synuclein [6], prion protein [7], TDP-43 [8–10] or FUS [11, 12]. By extrapolation, it follows that a gain of toxic function resulting from the ordered assembly into filaments may also underlie sporadic forms of disease. Assemblies of the microtubule-associated protein Tau into filaments characterize many neurodegenerative diseases. In humans, MAPT, the gene encoding Tau protein, generates six isoforms (352–441 amino acids) by alternative mRNA splicing [13]. They differ by the presence or absence of three inserts encoded by exons 2, 3 and 10. Inclusion of exon 10 results in the production of three isoforms with 4 C-terminal repeats (each repeat is 31 or 32 amino acids long) (4R) and its exclusion in another three isoforms with 3 repeats (3R). Diseases characterised by the intracellular accumulation of Tau filaments can be divided into three groups, based on the isoform composition of filaments (3R, 4R, 3R+4R) [14]. In these diseases, be they sporadic or inherited, Tau is extensively modified post-translationally [15–17].
Tauopathy was coined to describe a dominantly inherited neurodegenerative disease with a +3 mutation in intron 10 of MAPT and abundant filamentous inclusions made of 4R Tau [3, 18]. However, this term is also used in neuropathology and neuroscience to refer to the mere presence of Tau in tissues.
The terms primary and secondary tauopathies are also being used [19–25], even though mutations in MAPT are the only known aetiology for Tauopathies. Primary tauopathy refers to conditions where the presence of Tau filaments is the main or sole known abnormality or where tau pathology is considered the major contributing factor to neurodegeneration interpreted as main ‘driving force of pathogenesis’, as opposed to other proteins such as Aβ [23, 25–28]. Primary tauopathies are also included in the group of frontotemporal lobar degeneration (FTLD). The latter is characterized by the predominant atrophy of frontal and temporal lobes of the cerebral cortex and occurs in association with several different proteinopathies [29]. Secondary tauopathy is used when additional ‘driving pathogenic forces’ are believed to be involved [19]. In our view, cases with mutations in MAPT are the only known example of a primary tauopathy, whereas familial Alzheimer’s disease may be secondary tauopathy. These terms should not be used when referring to diseases of unknown aetiology.
Diseases with pathologic Tau can be classified on the basis Tau isoforms, 3R, 4R or both 3R and 4R being demonstrable with isoform-specific antibodies or Western blot patterns of sarkosyl-insoluble Tau. The anatomical distribution, along with the histological and cytological characterization of neuronal and glial tau immunoreactivities, is also needed for clinicopathological classification [19, 20, 30].
Formation of abnormal Tau filaments is a central event in several neurodegenerative diseases. Like the filaments of other pathologic amyloids, Tau filaments have a cross-β conformation [31]. Recently, the Tau folds of Alzheimer’s disease [32, 33], Pick’s disease [34], chronic traumatic encephalopathy (CTE) [35], corticobasal degeneration (CBD) [36, 37], argyrophilic grain disease (AGD), progressive supranuclear palsy (PSP), and globular glial tauopathy (GGT) [38] were shown to be different. The Tau filament fold of primary age-related tauopathy (PART) was identical to the Alzheimer fold, indicating that it can form in the absence of Aβ deposits [39]. The same filament structures have been found for different individuals with a given disease. It is also noteworthy that Tau folds identical to those of Alzheimer’s disease co-existed with cerebral parenchymal Aβ amyloid, PrP amyloid, Abri and ADan amyloid [38, 40]. Tau filament structures from the brains of intron 10 MAPT mutation carriers were identical to those of AGD. Structural analysis of tau filament folds has also led to the discovery of potentially new disease entities [38].
It is now timely to update the existing terminology and re-examine the grouping of disorders associated with intracellular tau accumulation, since: (1) There is an increased interest in the role of Tau assembly in neurodegeneration. (2) Inconsistent terminology has been used; and (3) High-resolution tau filament structures have been determined. Here we put forward definitions of terms to describe Tau in various conditions, as well as a simple and flexible stratification system that will also allow inclusion of novel pathological entities.
CONCEPTS AND HYPOTHESES
Identification of high-resolution Tau filament structures from brain has provided a compelling argument for reconsidering nomenclature and group organization of conditions in which tau assembly is believed to play a role. Since the term ‘Tauopathy’ has not been rigorously defined, there is a need to establish its precise meaning, so as to avoid inconsistent use.
A new approach may take into consideration the following:
Soluble Tau assembles into insoluble filaments. At some point, as misfolding happens, the gain-of-toxic function that causes neurodegeneration occurs along with a partial loss of physiological function of tau [41].
The Tau isoform composition of filaments can vary between diseases. However, even within a given brain, tau isoforms in filaments may differ, for example when Alzheimer neurofibrillary lesions (3R+4R) co-exist with argyrophilic grains (4R) and ARTAG (4R).
The ordered assembly of Tau occurs through stages, with the so-called ‘pre-aggregates’ evolving into insoluble ubiquitinated inclusions [42]. While silver staining detects insoluble inclusions in histology, some anti-tau antibodies can also identify earlier stages of pathological assembly.
A three-level hierarchical classification of diseases with Tau pathology has been suggested on the basis of the cryo-EM structures of Tau filaments. All known tau folds comprise R3 and R4, as well as 10–13 amino acids after R4. The first level of classification takes into account the structure and composition of tau filament cores (3R, 4R or 3R+4R). At the second level, for diseases with filaments made of 3R+4R Tau isoforms, one can distinguish between Alzheimer and CTE folds, while for diseases with filaments made of 4R Tau, one can distinguish between three-layered (PSP, GGT) and four-layered (CBD, AGD) folds. The third level of classification for 4R tauopathies is based on the differences at the residue level between three- and four-layered folds [38].
Spreading of assembled tau may occur in different and currently undetermined ways in the presence of MAPT mutations, compared to other conditions for which spread may be secondary to local protein aggregation and other factors, be they genetic and/or environmental.
In some cases, Tau assembly may be the only proteinopathy. However, co-pathologies frequently occur; they may be characterised by either additional tau filament folds or the presence of other misfolded proteins.
DEFINITIONS AND NOMENCLATURE
Tau immunoreactivity:
This term refers to the staining for Tau by antibodies and does not provide information directly relevant to the assembly state of the protein. Tau immunoreactivity alone does not necessarily indicate dysfunction of Tau protein or its relevance for a clinicopathological phenotype. Since antibodies to different tau epitopes may give rise to different labelling patterns, knowledge of these epitopes is essential for the interpretation of immunoreactivity.
Tau pathology:
This term implies the presence of assembled Tau, through the interpretation of results obtained using at least one of the following: silver staining, immunohistochemistry, immunofluorescence, electron microscopy, immunoblotting and seeding. Tau pathology describes lesions, not clinicopathological entities.
Tauopathy/Tau proteinopathy:
These terms, which are often used interchangeably, describe conditions that fulfil the following criteria:
Abundant filamentous Tau inclusions made of either 3R, 4R or 3R+4R tau, and
Consistent and typical patterns of cellular Tau pathologies in multiple cases that correlate with clinical signs and neurodegeneration.
This definition acknowledges that each condition is characterized by a spectrum of regional load of filamentous tau deposits (i.e., stages of sequential involvement of brain regions), thus implying that early disease stages may be recognizable in a given disorder. Therefore, early stages of a Tauopathy may be described as Tau pathology. In summary: 1) Cellular immunoreactivities are described as ‘Tau immunoreactivity’ in neurons, astrocytes, oligodendrocytes, etc’; 2) ‘Tau pathology’, but not ‘Tauopathy’, should be used for the definition of lesions revealed by routine diagnostic immunohistochemistry; 3) A condition should not be considered a ‘Tauopathy’ until a consistent staining pattern can be demonstrated in additional cases of disease.
CATEGORIES
We propose the following approach for categorizing conditions associated with intracellular Tau accumulation or Tau immunoreactivity of unknown relevance into six groups. It requires knowledge of the following: aetiology, if known, presence of Tau-only pathology versus co-existence with a parenchymal amyloid made of a protein other than Tau, and role of assembled Tau in disease pathogenesis. Each group can then be subdivided according to the Tau isoforms involved, as demonstrated by immunostaining and/or immunoblotting using well-characterized 3R and 4R Tau-specific antibodies. In the absence of such knowledge, we say ‘awaiting isoform classification’. It follows that Tauopathy conditions can be defined on the basis of the spectrum of specific morphological changes, their clinicopathological phenotypes, as well as historically assigned disease names. Finally, based on the cryo-EM structures of Tau folds, Tauopathies can be classified further [38]. Our knowledge of filament folds is still evolving and cryo-EM may lead to the identification of novel Tau folds in previously unrecognised conditions.
Rather than divide disorders into primary and secondary Tauopathies, we propose to classify Tauopathies into five different groups (Table 1). Group 1 includes cases with mutations in MAPT. Some clinicopathological phenotypes and filament structures may be similar to those in sporadic forms of disease [38, 43]. Group 2 includes disorders with filamentous Tau deposits that are pivotal for neuropathological diagnosis and correlate with clinical symptoms and neurodegeneration, but in the absence of mutations in MAPT. Nomenclature of these conditions is based on either clinical presentation (i.e., PSP), anatomical distribution of neurodegeneration (i.e., CBD), morphology of Tau pathologies (i.e., GGT and AGD), or disease eponyms (i.e., Pick’s disease). Groups 1 and 2 are summarized as ‘Main Tauopathies’ (Figure 1). Group 3 includes filamentous Tau deposits in dominantly inherited diseases with mutations in genes encoding the proteins that make up extracellular filamentous deposits (Aβ, prion protein, Bri). Group 4 includes filamentous Tau deposits in idiopathic forms of Alzheimer’s disease. Groups 3 and 4 are summarized as ‘Extracellular filamentous deposit-related Tauopathies’ (Figure 1). Group 5 comprises other Tauopathies that may show overlapping features with those of Group 3. However, they are reported less frequently than those of Group 3. Group 6 includes various conditions with Tau immunoreactivity or Tau pathology. In some cases, Tau immunoreactivity has been described, but filamentous Tau inclusions have not been demonstrated. Tau immunoreactivities or tau pathologies can also be observed in individuals who are clinically normal, where they may represent a preclinical stage and/or an early cytopathological phase of fibril formation [27]. We recommend that ‘Tau pathology’ rather than ‘Tauopathy’ be used for conditions in which filamentous Tau may be detected, but where the following may constitute an obstacle for a more precise characterization: 1) Tau cytopathology is inconsistently detected between cases belonging to the same group of conditions; 2) there are only single case reports; 3) the clinical or pathogenic relevance of Tau cytopathology related to the condition is unclear. Moreover, we also include conditions with neurofibrillary tangles in anatomically circumscribed areas and often unrelated to the leading brain lesions, or constellations of Tau pathologies reported in case reports and not compatible with Tauopathies of groups 1–5. The proposed grouping is summarized in Figure 1. Finally, coexistence of main Tauopathy-like conditions has been described in various disorders with diverse aetiologies (Table 2). However, these conditions can also present without Tauopathy and the direct relation of the primary disorder to a Tauopathy has not been demonstrated.
Table 1.
Stratification of conditions with pathological Tau deposition.
| 1) Tauopathy, autosomal-dominant, associated with MAPT mutation: MAPT-Tauopathies |
| 3R |
| 4R |
| 3R+4R |
| 2) Clinically and pathologically defined idiopathic Tauopathies |
| 3R |
|
| 4R |
|
| 3R+4R |
|
|
3) Tauopathy, obligatory association with extracellular filamentous deposits caused
by genetically determined other proteinopathy |
| 3R+4R |
|
| 4) Tauopathy, obligatory association with extracellular filamentous deposits in idiopathic diseases |
| 3R+4R |
|
| 5) Other Tauopathies |
| 3R |
| 4R |
|
|
| 3R+4R |
|
| Awaiting isoform classification |
|
|
| 6) Tau pathology or Tau immunoreactivity or biochemical alterations of Tau protein |
| 4R |
|
| 3R+4R |
|
| Awaiting isoform classification |
|
NFT: Neurofibrillary Tangle;
conditional, the spectrum to be defined in future studies;
Myotonic Dystrophy: characterized by the preferential aggregation of the smaller 0N3R isoform. However, there are reports where 4R was also detected;
Another study failed to confirm the presence of NFTs in Cockayne syndrome [44].
Figure 1.
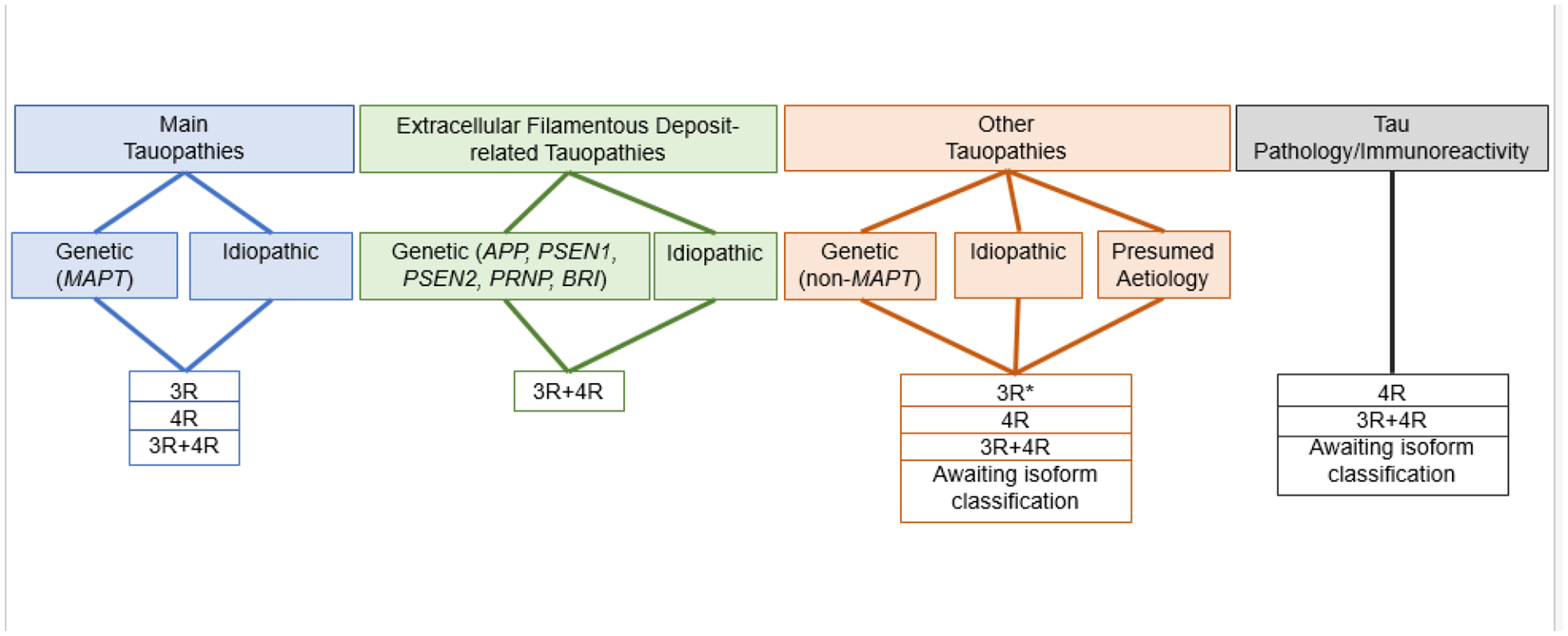
Classification of diseases with accumulation of Tau protein.
*: Myotonic dystrophy, characterized by the preferential aggregation of 0N3R Tau. There have also been reports of the presence of 4R Tau.
Table 2.
Coexistence of well-characterized or other Tauopathy-like conditions with various disorders.
|
CONCLUSION
As a consequence of developments in our understanding of neurodegenerative diseases and their relation to Tau protein, several terms have been generated, which are widely and inconsistently used. We propose a nomenclature of the various groups of diseases involving Tau that are based on the knowledge of aetiology, relation to non-Tau proteinopathies, presence of Tau isoforms, genetics of MAPT and accumulated experience of clinicopathological correlations, further complemented by high-resolution tau filament structures. We propose a simple system that can easily accommodate future discoveries (i.e., disorders can be moved between groups). This will hopefully allow those working on the understanding of the role of Tau in various conditions to communicate their findings in more concise and unambiguous ways and will inform therapeutic developments.
Key points:
We put forward definitions of terms to describe Tau in various conditions
We propose a flexible stratification system and six groups
Tauopathies are classified into five different groups
A further group includes conditions with Tau immunoreactivity or Tau pathology
Acknowledgements
GGK is supported by the Rossy and the Edmond Safra Foundations. MG is supported by the UK Medical Research Council (MC_U105184291). BG is supported by NIH grants (U01 NS110437 and RF1 AG071177-01A1).
Footnotes
Conflicts of interest
The authors report no conflicts of interest specific to this manuscript.
References
- 1.Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, Andreadis A, Wiederholt WC, Raskind M, Schellenberg GD. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol 1998; 43: 815–25 [DOI] [PubMed] [Google Scholar]
- 2.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998; 393: 702–5 [DOI] [PubMed] [Google Scholar]
- 3.Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci U S A 1998; 95: 7737–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, Mant R, Newton P, Rooke K, Roques P, Talbot C, Pericak-Vance M, Roses A, Williamson R, Rossor M, Owen M, Hardy J. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 1991; 349: 704–6 [DOI] [PubMed] [Google Scholar]
- 5.Murrell J, Farlow M, Ghetti B, Benson MD. A mutation in the amyloid precursor protein associated with hereditary Alzheimer’s disease. Science 1991; 254: 97–9 [DOI] [PubMed] [Google Scholar]
- 6.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 1997; 276: 2045–7 [DOI] [PubMed] [Google Scholar]
- 7.Hsiao K, Baker HF, Crow TJ, Poulter M, Owen F, Terwilliger JD, Westaway D, Ott J, Prusiner SB. Linkage of a prion protein missense variant to Gerstmann-Sträussler syndrome. Nature 1989; 338: 342–5 [DOI] [PubMed] [Google Scholar]
- 8.Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, Bouchard JP, Lacomblez L, Pochigaeva K, Salachas F, Pradat PF, Camu W, Meininger V, Dupre N, Rouleau GA. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet 2008; 40: 572–4 [DOI] [PubMed] [Google Scholar]
- 9.Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, Ackerley S, Durnall JC, Williams KL, Buratti E, Baralle F, de Belleroche J, Mitchell JD, Leigh PN, Al-Chalabi A, Miller CC, Nicholson G, Shaw CE. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 2008; 319: 1668–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Deerlin VM, Leverenz JB, Bekris LM, Bird TD, Yuan W, Elman LB, Clay D, Wood EM, Chen-Plotkin AS, Martinez-Lage M, Steinbart E, McCluskey L, Grossman M, Neumann M, Wu IL, Yang WS, Kalb R, Galasko DR, Montine TJ, Trojanowski JQ, Lee VM, Schellenberg GD, Yu CE. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet Neurol 2008; 7: 409–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwiatkowski TJ Jr., Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, de Jong PJ, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, Siddique T, McKenna-Yasek D, Sapp PC, Horvitz HR, Landers JE, Brown RH Jr. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 2009; 323: 1205–8 [DOI] [PubMed] [Google Scholar]
- 12.Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, Ganesalingam J, Williams KL, Tripathi V, Al-Saraj S, Al-Chalabi A, Leigh PN, Blair IP, Nicholson G, de Belleroche J, Gallo JM, Miller CC, Shaw CE. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 2009; 323: 1208–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron 1989; 3: 519–26 [DOI] [PubMed] [Google Scholar]
- 14.Goedert M, Eisenberg DS, Crowther RA. Propagation of Tau Aggregates and Neurodegeneration. Annu Rev Neurosci 2017; 40: 189–210 [DOI] [PubMed] [Google Scholar]
- 15.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A 1986; 83: 4913–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kametani F, Yoshida M, Matsubara T, Murayama S, Saito Y, Kawakami I, Onaya M, Tanaka H, Kakita A, Robinson AC, Mann DMA, Hasegawa M. Comparison of Common and Disease-Specific Post-translational Modifications of Pathological Tau Associated With a Wide Range of Tauopathies. Front Neurosci 2020; 14: 581936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wesseling H, Mair W, Kumar M, Schlaffner CN, Tang S, Beerepoot P, Fatou B, Guise AJ, Cheng L, Takeda S, Muntel J, Rotunno MS, Dujardin S, Davies P, Kosik KS, Miller BL, Berretta S, Hedreen JC, Grinberg LT, Seeley WW, Hyman BT, Steen H, Steen JA. Tau PTM Profiles Identify Patient Heterogeneity and Stages of Alzheimer’s Disease. Cell 2020; 183: 1699–713 e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spillantini MG, Goedert M, Crowther RA, Murrell JR, Farlow MR, Ghetti B. Familial multiple system tauopathy with presenile dementia: a disease with abundant neuronal and glial tau filaments. Proc Natl Acad Sci U S A 1997; 94: 4113–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung DC, Roemer S, Petrucelli L, Dickson DW. Cellular and pathological heterogeneity of primary tauopathies. Mol Neurodegener 2021; 16: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovacs GG. Invited review: Neuropathology of tauopathies: principles and practice. Neuropathol Appl Neurobiol 2015; 41: 3–23 [DOI] [PubMed] [Google Scholar]
- 21.Rösler TW, Tayaranian Marvian A, Brendel M, Nykänen NP, Höllerhage M, Schwarz SC, Hopfner F, Koeglsperger T, Respondek G, Schweyer K, Levin J, Villemagne VL, Barthel H, Sabri O, Müller U, Meissner WG, Kovacs GG, Höglinger GU. Four-repeat tauopathies. Prog Neurobiol 2019; 180: 101644. [DOI] [PubMed] [Google Scholar]
- 22.Lebouvier T, Pasquier F, Buee L. Update on tauopathies. Curr Opin Neurol 2017; 30: 589–98 [DOI] [PubMed] [Google Scholar]
- 23.Revesz T, Holton JL. Anatamopathological spectrum of tauopathies. Mov Disord 2003; 18 Suppl 6: S13–20 [DOI] [PubMed] [Google Scholar]
- 24.Gotz J, Halliday G, Nisbet RM. Molecular Pathogenesis of the Tauopathies. Annu Rev Pathol 2019; 14: 239–61 [DOI] [PubMed] [Google Scholar]
- 25.Arendt T, Stieler JT, Holzer M. Tau and tauopathies. Brain Res Bull 2016; 126: 238–92 [DOI] [PubMed] [Google Scholar]
- 26.Dickson DW, Kouri N, Murray ME, Josephs KA. Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci 2011; 45: 384–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovacs GG. Molecular pathology of neurodegenerative diseases: principles and practice. J Clin Pathol 2019; 72: 725–35 [DOI] [PubMed] [Google Scholar]
- 28.Mann DM, Hardy J. Amyloid or tau: the chicken or the egg? Acta Neuropathol 2013; 126: 609–13 [DOI] [PubMed] [Google Scholar]
- 29.Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL 3rd, Schneider JA, Grinberg LT, Halliday G, Duyckaerts C, Lowe JS, Holm IE, Tolnay M, Okamoto K, Yokoo H, Murayama S, Woulfe J, Munoz DG, Dickson DW, Ince PG, Trojanowski JQ, Mann DM, Consortium for Frontotemporal Lobar D. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 2007; 114: 5–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forrest SL, Kril JJ, Halliday GM. Cellular and regional vulnerability in frontotemporal tauopathies. Acta Neuropathol 2019; 138: 705–27 [DOI] [PubMed] [Google Scholar]
- 31.Berriman J, Serpell LC, Oberg KA, Fink AL, Goedert M, Crowther RA. Tau filaments from human brain and from in vitro assembly of recombinant protein show cross-beta structure. Proc Natl Acad Sci U S A 2003; 100: 9034–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitzpatrick AWP, Falcon B, He S, Murzin AG, Murshudov G, Garringer HJ, Crowther RA, Ghetti B, Goedert M, Scheres SHW. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 2017; 547: 185–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falcon B, Zhang W, Schweighauser M, Murzin AG, Vidal R, Garringer HJ, Ghetti B, Scheres SHW, Goedert M. Tau filaments from multiple cases of sporadic and inherited Alzheimer’s disease adopt a common fold. Acta Neuropathol 2018; 136: 699–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falcon B, Zhang W, Murzin AG, Murshudov G, Garringer HJ, Vidal R, Crowther RA, Ghetti B, Scheres SHW, Goedert M. Structures of filaments from Pick’s disease reveal a novel tau protein fold. Nature 2018; 561: 137–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falcon B, Zivanov J, Zhang W, Murzin AG, Garringer HJ, Vidal R, Crowther RA, Newell KL, Ghetti B, Goedert M, Scheres SHW. Novel tau filament fold in chronic traumatic encephalopathy encloses hydrophobic molecules. Nature 2019; 568: 420–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arakhamia T, Lee CE, Carlomagno Y, Duong DM, Kundinger SR, Wang K, Williams D, DeTure M, Dickson DW, Cook CN, Seyfried NT, Petrucelli L, Fitzpatrick AWP. Posttranslational Modifications Mediate the Structural Diversity of Tauopathy Strains. Cell 2020; 180: 633–44 e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W, Tarutani A, Newell KL, Murzin AG, Matsubara T, Falcon B, Vidal R, Garringer HJ, Shi Y, Ikeuchi T, Murayama S, Ghetti B, Hasegawa M, Goedert M, Scheres SHW. Novel tau filament fold in corticobasal degeneration. Nature 2020; 580: 283–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi Y, Zhang W, Yang Y, Murzin AG, Falcon B, Kotecha A, van Beers M, Tarutani A, Kametani F, Garringer HJ, Vidal R, Hallinan GI, Lashley T, Saito Y, Murayama S, Yoshida M, Tanaka H, Kakita A, Ikeuchi T, Robinson AC, Mann DMA, Kovacs GG, Revesz T, Ghetti B, Hasegawa M, Goedert M, Scheres SHW. Structure-based classification of tauopathies. Nature 2021; 598: 359–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y, Murzin AG, Falcon B, Epstein A, Machin J, Tempest P, Newell KL, Vidal R, Garringer HJ, Sahara N, Higuchi M, Ghetti B, Jang MK, Scheres SHW, Goedert M. Cryo-EM structures of tau filaments from Alzheimer’s disease with PET ligand APN-1607. Acta Neuropathol 2021; 141: 697–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hallinan GI, Hoq MR, Ghosh M, Vago FS, Fernandez A, Garringer HJ, Vidal R, Jiang W, Ghetti B. Structure of Tau filaments in Prion protein amyloidoses. Acta Neuropathol 2021; 142: 227–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goedert M The ordered assembly of tau is the gain-of-toxic function that causes human tauopathies. Alzheimers Dement 2016; 12: 1040–50 [DOI] [PubMed] [Google Scholar]
- 42.Bancher C, Brunner C, Lassmann H, Budka H, Jellinger K, Wiche G, Seitelberger F, Grundke-Iqbal I, Iqbal K, Wisniewski HM. Accumulation of abnormally phosphorylated tau precedes the formation of neurofibrillary tangles in Alzheimer’s disease. Brain Res 1989; 477: 90–9 [DOI] [PubMed] [Google Scholar]
- 43.Forrest SL, Kril JJ, Stevens CH, Kwok JB, Hallupp M, Kim WS, Huang Y, McGinley CV, Werka H, Kiernan MC, Gotz J, Spillantini MG, Hodges JR, Ittner LM, Halliday GM. Retiring the term FTDP-17 as MAPT mutations are genetic forms of sporadic frontotemporal tauopathies. Brain 2018; 141: 521–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Itoh M, Hayashi M, Shioda K, Minagawa M, Isa F, Tamagawa K, Morimatsu Y, Oda M. Neurodegeneration in hereditary nucleotide repair disorders. Brain Dev 1999; 21: 326–33 [DOI] [PubMed] [Google Scholar]
- 45.Caillet-Boudin ML, Fernandez-Gomez FJ, Tran H, Dhaenens CM, Buée L, Sergeant N. Brain pathology in myotonic dystrophy: when tauopathy meets spliceopathy and RNAopathy. Front Mol Neurosci 2014; 6: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maurage CA, Udd B, Ruchoux MM, Vermersch P, Kalimo H, Krahe R, Delacourte A, Sergeant N. Similar brain tau pathology in DM2/PROMM and DM1/Steinert disease. Neurology 2005; 65: 1636–8 [DOI] [PubMed] [Google Scholar]
- 47.Klotz S, Fischer P, Hinterberger M, Ricken G, Honigschnabl S, Gelpi E, Kovacs GG. Multiple system aging-related tau astrogliopathy with complex proteinopathy in an oligosymptomatic octogenarian. Neuropathology 2020; 41: 72–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kovacs GG, Molnar K, Laszlo L, Strobel T, Botond G, Honigschnabl S, Reiner-Concin A, Palkovits M, Fischer P, Budka H. A peculiar constellation of tau pathology defines a subset of dementia in the elderly. Acta Neuropathol 2011; 122: 205–22 [DOI] [PubMed] [Google Scholar]
- 49.Llibre-Guerra JJ, Lee SE, Suemoto CK, Ehrenberg AJ, Kovacs GG, Karydas A, Staffaroni A, De Paula Franca Resende E, Kim EJ, Hwang JH, Ramos EM, Wojta KJ, Pasquini L, Pang SY, Spina S, Allen IE, Kramer J, Miller BL, Seeley WW, Grinberg LT. A novel temporal-predominant neuro-astroglial tauopathy associated with TMEM106B gene polymorphism in FTLD/ALS-TDP. Brain Pathol 2021; 31: 267–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borrego-Ecija S, Grau-Rivera O, Colom-Cadena M, Molinuevo JL, Tolosa E, Sanchez-Valle R, Gelpi E. Tauopathy with Hippocampal 4-Repeat Tau Immunoreactive Spherical Inclusions in a Patient with PSP. Brain Pathol 2018; 28: 284–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kovacs GG, Kwong LK, Grossman M, Irwin DJ, Lee EB, Robinson JL, Suh E, Van Deerlin VM, Lee VM, Trojanowski JQ. Tauopathy with hippocampal 4-repeat tau immunoreactive spherical inclusions: a report of three cases. Brain Pathol 2018; 28: 274–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miki Y, Mori F, Hori E, Kaimori M, Wakabayashi K. Hippocampal sclerosis with four-repeat tau-positive round inclusions in the dentate gyrus: a new type of four-repeat tauopathy. Acta Neuropathol 2009; 117: 713–8 [DOI] [PubMed] [Google Scholar]
- 53.Ukai K, Kosaka K. Diffuse neurofibrillary tangles with calcification (Kosaka-Shibayama disease) in Japan. Psychiatry Clin Neurosci 2016; 70: 131–40 [DOI] [PubMed] [Google Scholar]
- 54.Mimuro M, Yoshida M, Kuzuhara S, Kokubo Y. Amyotrophic lateral sclerosis and parkinsonism-dementia complex of the Hohara focus of the Kii Peninsula: A multiple proteinopathy? Neuropathology 2018; 38: 98–107 [DOI] [PubMed] [Google Scholar]
- 55.Shinotoh H, Shimada H, Kokubo Y, Tagai K, Niwa F, Kitamura S, Endo H, Ono M, Kimura Y, Hirano S, Mimuro M, Ichise M, Sahara N, Zhang MR, Suhara T, Higuchi M. Tau imaging detects distinctive distribution of tau pathology in ALS/PDC on the Kii Peninsula. Neurology 2019; 92: e136–e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buée-Scherrer V, Buée L, Hof PR, Leveugle B, Gilles C, Loerzel AJ, Perl DP, Delacourte A. Neurofibrillary degeneration in amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam. Immunochemical characterization of tau proteins. Am J Pathol 1995; 146: 924–32 [PMC free article] [PubMed] [Google Scholar]
- 57.Joachim CL, Morris JH, Kosik KS, Selkoe DJ. Tau antisera recognize neurofibrillary tangles in a range of neurodegenerative disorders. Ann Neurol 1987; 22: 514–20 [DOI] [PubMed] [Google Scholar]
- 58.Pollanen MS, Onzivua S, Robertson J, McKeever PM, Olawa F, Kitara DL, Fong A. Nodding syndrome in Uganda is a tauopathy. Acta Neuropathol 2018; 136: 691–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gelpi E, Höftberger R, Graus F, Ling H, Holton JL, Dawson T, Popovic M, Pretnar-Oblak J, Hogl B, Schmutzhard E, Poewe W, Ricken G, Santamaria J, Dalmau J, Budka H, Revesz T, Kovacs GG. Neuropathological criteria of anti-IgLON5-related tauopathy. Acta Neuropathol 2016; 132: 531–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jellinger KA. Absence of alpha-synuclein pathology in postencephalitic parkinsonism. Acta Neuropathol 2009; 118: 371–9 [DOI] [PubMed] [Google Scholar]
- 61.Ferrer I, Legati A, Garcia-Monco JC, Gomez-Beldarrain M, Carmona M, Blanco R, Seeley WW, Coppola G. Familial behavioral variant frontotemporal dementia associated with astrocyte-predominant tauopathy. J Neuropathol Exp Neurol 2015; 74: 370–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gelpi E, Aldecoa I, Lopez-Villegas D, Abellan-Vidal MT, Mercadel-Fananas P, Fortea J, Ribosa R, Morenas E, Gomez-Anson B, Molina-Porcel L, Ximelis T, Borrego S, Antonell A, Rovelet-Lecrux A, Klotz S, Andres-Benito P, Sanchez-Valle R, Ferrer I. Atypical astroglial pTDP-43 pathology in astroglial predominant tauopathy. Neuropathol Appl Neurobiol 2021: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Darwich NF, Phan JM, Kim B, Suh E, Papatriantafyllou JD, Changolkar L, Nguyen AT, O’Rourke CM, He Z, Porta S, Gibbons GS, Luk KC, Papageorgiou SG, Grossman M, Massimo L, Irwin DJ, McMillan CT, Nasrallah IM, Toro C, Aguirre GK, Van Deerlin VM, Lee EB. Autosomal dominant VCP hypomorph mutation impairs disaggregation of PHF-tau. Science 2020; 370: eaay8826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Auer IA, Schmidt ML, Lee VM, Curry B, Suzuki K, Shin RW, Pentchev PG, Carstea ED, Trojanowski JQ. Paired helical filament tau (PHFtau) in Niemann-Pick type C disease is similar to PHFtau in Alzheimer’s disease. Acta Neuropathol 1995; 90: 547–51 [DOI] [PubMed] [Google Scholar]
- 65.Sherriff FE, Bridges LR, De Souza DS. Non-Alzheimer neurofibrillary tangles show beta-amyloid-like immunoreactivity. Neuroreport 1994; 5: 1897–900 [DOI] [PubMed] [Google Scholar]
- 66.Suzuki K, Parker CC, Pentchev PG, Katz D, Ghetti B, D’Agostino AN, Carstea ED. Neurofibrillary tangles in Niemann-Pick disease type C. Acta Neuropathol 1995; 89: 227–38 [DOI] [PubMed] [Google Scholar]
- 67.Love S, Bridges LR, Case CP. Neurofibrillary tangles in Niemann-Pick disease type C. Brain 1995; 118 ( Pt 1): 119–29 [DOI] [PubMed] [Google Scholar]
- 68.Gao AF, Faust-Socher A, Al-Murshed M, Del Bigio MR, Lang AE, Munoz DG. Progressive ataxia and palatal tremor: Two autopsy cases of a novel tauopathy. Mov Disord 2017; 32: 1465–73 [DOI] [PubMed] [Google Scholar]
- 69.Mari Z, Halls AJM, Vortmeyer A, Zhukareva V, Uryu K, Lee VM, Hallett M. Clinico-Pathological Correlation in Progressive Ataxia and Palatal Tremor: A Novel Tauopathy. Mov Disord Clin Pract 2014; 1: 50–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ikeda K, Akiyama H, Kondo H, Arai T, Arai N, Yagishita S. Numerous glial fibrillary tangles in oligodendroglia in cases of subacute sclerosing panencephalitis with neurofibrillary tangles. Neurosci Lett 1995; 194: 133–5 [DOI] [PubMed] [Google Scholar]
- 71.Malamud N, Haymaker W, Pinkerton H. Inclusion encephalitis; with a clinicopathologic report of three cases. Am J Pathol 1950; 26: 133–53, incl 5 pl [PMC free article] [PubMed] [Google Scholar]
- 72.Mandybur TI, Nagpaul AS, Pappas Z, Niklowitz WJ. Alzheimer neurofibrillary change in subacute sclerosing panencephalitis. Ann Neurol 1977; 1: 103–7 [DOI] [PubMed] [Google Scholar]
- 73.Spillantini MG, Tolnay M, Love S, Goedert M. Microtubule-associated protein tau, heparan sulphate and alpha-synuclein in several neurodegenerative diseases with dementia. Acta Neuropathol 1999; 97: 585–94 [DOI] [PubMed] [Google Scholar]
- 74.Paula-Barbosa MM, Brito R, Silva CA, Faria R, Cruz C. Neurofibrillary changes in the cerebral cortex of a patient with subacute sclerosing panencephalitis (SSPE). Acta Neuropathol 1979; 48: 157–60 [DOI] [PubMed] [Google Scholar]
- 75.Kovacs GG, Ferrer I, Grinberg LT, Alafuzoff I, Attems J, Budka H, Cairns NJ, Crary JF, Duyckaerts C, Ghetti B, Halliday GM, Ironside JW, Love S, Mackenzie IR, Munoz DG, Murray ME, Nelson PT, Takahashi H, Trojanowski JQ, Ansorge O, Arzberger T, Baborie A, Beach TG, Bieniek KF, Bigio EH, Bodi I, Dugger BN, Feany M, Gelpi E, Gentleman SM, Giaccone G, Hatanpaa KJ, Heale R, Hof PR, Hofer M, Hortobagyi T, Jellinger K, Jicha GA, Ince P, Kofler J, Kovari E, Kril JJ, Mann DM, Matej R, McKee AC, McLean C, Milenkovic I, Montine TJ, Murayama S, Lee EB, Rahimi J, Rodriguez RD, Rozemuller A, Schneider JA, Schultz C, Seeley W, Seilhean D, Smith C, Tagliavini F, Takao M, Thal DR, Toledo JB, Tolnay M, Troncoso JC, Vinters HV, Weis S, Wharton SB, White CL 3rd, Wisniewski T, Woulfe JM, Yamada M, Dickson DW. Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol 2016; 131: 87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakano M, Riku Y, Nishioka K, Hasegawa M, Washimi Y, Arahata Y, Takeda A, Horibe K, Yamaoka A, Suzuki K, Tsujimoto M, Li Y, Yoshino H, Hattori N, Akagi A, Miyahara H, Iwasaki Y, Yoshida M. Unclassified four-repeat tauopathy associated with familial parkinsonism and progressive respiratory failure. Acta Neuropathol Commun 2020; 8: 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garbern JY, Neumann M, Trojanowski JQ, Lee VM, Feldman G, Norris JW, Friez MJ, Schwartz CE, Stevenson R, Sima AA. A mutation affecting the sodium/proton exchanger, SLC9A6, causes mental retardation with tau deposition. Brain 2010; 133: 1391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thal DR, Zuchner S, Gierer S, Schulte C, Schöls L, Schule R, Synofzik M. Abnormal Paraplegin Expression in Swollen Neurites, tau- and alpha-Synuclein Pathology in a Case of Hereditary Spastic Paraplegia SPG7 with an Ala510Val Mutation. Int J Mol Sci 2015; 16: 25050–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Korvatska O, Strand NS, Berndt JD, Strovas T, Chen DH, Leverenz JB, Kiianitsa K, Mata IF, Karakoc E, Greenup JL, Bonkowski E, Chuang J, Moon RT, Eichler EE, Nickerson DA, Zabetian CP, Kraemer BC, Bird TD, Raskind WH. Altered splicing of ATP6AP2 causes X-linked parkinsonism with spasticity (XPDS). Hum Mol Genet 2013; 22: 3259–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Poorkaj P, Raskind WH, Leverenz JB, Matsushita M, Zabetian CP, Samii A, Kim S, Gazi N, Nutt JG, Wolff J, Yearout D, Greenup JL, Steinbart EJ, Bird TD. A novel X-linked four-repeat tauopathy with Parkinsonism and spasticity. Mov Disord 2010; 25: 1409–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dyment DA, Smith AC, Humphreys P, Schwartzentruber J, Beaulieu CL, Consortium FC, Bulman DE, Majewski J, Woulfe J, Michaud J, Boycott KM. Homozygous nonsense mutation in SYNJ1 associated with intractable epilepsy and tau pathology. Neurobiol Aging 2015; 36: 1222 e1–5 [DOI] [PubMed] [Google Scholar]
- 82.Gelpi E, van der Zee J, Turon Estrada A, Van Broeckhoven C, Sanchez-Valle R. TARDBP mutation p.Ile383Val associated with semantic dementia and complex proteinopathy. Neuropathol Appl Neurobiol 2014; 40: 225–30 [DOI] [PubMed] [Google Scholar]
- 83.Moreno F, Rabinovici GD, Karydas A, Miller Z, Hsu SC, Legati A, Fong J, Schonhaut D, Esselmann H, Watson C, Stephens ML, Kramer J, Wiltfang J, Seeley WW, Miller BL, Coppola G, Grinberg LT. A novel mutation P112H in the TARDBP gene associated with frontotemporal lobar degeneration without motor neuron disease and abundant neuritic amyloid plaques. Acta Neuropathol Commun 2015; 3: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jansen C, Parchi P, Jelles B, Gouw AA, Beunders G, van Spaendonk RM, van de Kamp JM, Lemstra AW, Capellari S, Rozemuller AJ. The first case of fatal familial insomnia (FFI) in the Netherlands: a patient from Egyptian descent with concurrent four repeat tau deposits. Neuropathol Appl Neurobiol 2011; 37: 549–53 [DOI] [PubMed] [Google Scholar]
- 85.Kovacs GG, Rahimi J, Strobel T, Lutz MI, Regelsberger G, Streichenberger N, Perret-Liaudet A, Höftberger R, Liberski PP, Budka H, Sikorska B. Tau pathology in Creutzfeldt-Jakob disease revisited. Brain Pathol 2017; 27: 332–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fernandez-Nogales M, Cabrera JR, Santos-Galindo M, Hoozemans JJ, Ferrer I, Rozemuller AJ, Hernandez F, Avila J, Lucas JJ. Huntington’s disease is a four-repeat tauopathy with tau nuclear rods. Nat Med 2014; 20: 881–5 [DOI] [PubMed] [Google Scholar]
- 87.Ichihara K, Uchihara T, Nakamura A, Suzuki Y, Mizutani T. Selective deposition of 4-repeat tau in cerebral infarcts. J Neuropathol Exp Neurol 2009; 68: 1029–36 [DOI] [PubMed] [Google Scholar]
- 88.Chen DH, Latimer CS, Spencer M, Karna P, Gonzalez-Cuyar LF, Davis MY, Keene CD, Bird TD, Raskind WH. Hyperphosphorylated Tau, Increased Adenylate Cyclase 5 (ADCY5) Immunoreactivity, but No Neuronal Loss in ADCY5-Dyskinesia. Mov Disord Clin Pract 2020; 7: 70–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tai XY, Koepp M, Duncan JS, Fox N, Thompson P, Baxendale S, Liu JY, Reeves C, Michalak Z, Thom M. Hyperphosphorylated tau in patients with refractory epilepsy correlates with cognitive decline: a study of temporal lobe resections. Brain 2016; 139: 2441–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thom M, Liu JY, Thompson P, Phadke R, Narkiewicz M, Martinian L, Marsdon D, Koepp M, Caboclo L, Catarino CB, Sisodiya SM. Neurofibrillary tangle pathology and Braak staging in chronic epilepsy in relation to traumatic brain injury and hippocampal sclerosis: a post-mortem study. Brain 2011; 134: 2969–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li A, Paudel R, Johnson R, Courtney R, Lees AJ, Holton JL, Hardy J, Revesz T, Houlden H. Pantothenate kinase-associated neurodegeneration is not a synucleinopathy. Neuropathol Appl Neurobiol 2013; 39: 121–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paudel R, Li A, Wiethoff S, Bandopadhyay R, Bhatia K, de Silva R, Houlden H, Holton JL. Neuropathology of Beta-propeller protein associated neurodegeneration (BPAN): a new tauopathy. Acta Neuropathol Commun 2015; 3: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kovacs GG, Seguin J, Quadrio I, Höftberger R, Kapas I, Streichenberger N, Biacabe AG, Meyronet D, Sciot R, Vandenberghe R, Majtenyi K, Laszlo L, Strobel T, Budka H, Perret-Liaudet A. Genetic Creutzfeldt-Jakob disease associated with the E200K mutation: characterization of a complex proteinopathy. Acta Neuropathol 2011; 121: 39–57 [DOI] [PubMed] [Google Scholar]
- 94.Anthony IC, Norrby KE, Dingwall T, Carnie FW, Millar T, Arango JC, Robertson R, Bell JE. Predisposition to accelerated Alzheimer-related changes in the brains of human immunodeficiency virus negative opiate abusers. Brain 2010; 133: 3685–98 [DOI] [PubMed] [Google Scholar]
- 95.Kovacs GG, Horvath MC, Majtenyi K, Lutz MI, Hurd YL, Keller E. Heroin abuse exaggerates age-related deposition of hyperphosphorylated tau and p62-positive inclusions. Neurobiol Aging 2015; 36: 3100–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saito Y, Motoyoshi Y, Kashima T, Izumiyama-Shimomura N, Toda T, Nakano I, Hasegawa M, Murayama S. Unique tauopathy in Fukuyama-type congenital muscular dystrophy. J Neuropathol Exp Neurol 2005; 64: 1118–26 [DOI] [PubMed] [Google Scholar]
- 97.Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol 2006; 111: 529–38 [DOI] [PubMed] [Google Scholar]
- 98.Kurzawa-Akanbi M, Keogh M, Tsefou E, Ramsay L, Johnson M, Keers S, Wsa Ochieng L, McNair A, Singh P, Khan A, Pyle A, Hudson G, Ince PG, Attems J, Burn J, Chinnery PF, Morris CM. Neuropathological and biochemical investigation of Hereditary Ferritinopathy cases with ferritin light chain mutation: Prominent protein aggregation in the absence of major mitochondrial or oxidative stress. Neuropathol Appl Neurobiol 2021; 47: 26–42 [DOI] [PubMed] [Google Scholar]
- 99.Chou SM, Thompson HG. Electron microscopy of storage cytosomes in Kufs’ disease. Arch Neurol 1970; 23: 489–501 [DOI] [PubMed] [Google Scholar]
- 100.Autio-Harmainen H, Oldfors A, Sourander P, Renlund M, Dammert K, Simila S. Neuropathology of Salla disease. Acta Neuropathol 1988; 75: 481–90 [DOI] [PubMed] [Google Scholar]
- 101.Soffer D, Grotsky HW, Rapin I, Suzuki K. Cockayne syndrome: unusual neuropathological findings and review of the literature. Ann Neurol 1979; 6: 340–8 [DOI] [PubMed] [Google Scholar]
- 102.Paisan-Ruiz C, Li A, Schneider SA, Holton JL, Johnson R, Kidd D, Chataway J, Bhatia KP, Lees AJ, Hardy J, Revesz T, Houlden H. Widespread Lewy body and tau accumulation in childhood and adult onset dystonia-parkinsonism cases with PLA2G6 mutations. Neurobiol Aging 2012; 33: 814–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Riku Y, Ikeuchi T, Yoshino H, Mimuro M, Mano K, Goto Y, Hattori N, Sobue G, Yoshida M. Extensive aggregation of alpha-synuclein and tau in juvenile-onset neuroaxonal dystrophy: an autopsied individual with a novel mutation in the PLA2G6 gene-splicing site. Acta Neuropathol Commun 2013; 1: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Balicza P, Bencsik R, Lengyel A, Gal A, Grosz Z, Csaban D, Rudas G, Danics K, Kovacs GG, Molnar MJ. Novel dominant MPAN family with a complex genetic architecture as a basis for phenotypic variability. Neurol Genet 2020; 6: e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gregory A, Lotia M, Jeong SY, Fox R, Zhen D, Sanford L, Hamada J, Jahic A, Beetz C, Freed A, Kurian MA, Cullup T, van der Weijden MCM, Nguyen V, Setthavongsack N, Garcia D, Krajbich V, Pham T, Woltjer R, George BP, Minks KQ, Paciorkowski AR, Hogarth P, Jankovic J, Hayflick SJ. Autosomal dominant mitochondrial membrane protein-associated neurodegeneration (MPAN). Mol Genet Genomic Med 2019; 7: e00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wharton SB, McDermott CJ, Grierson AJ, Wood JD, Gelsthorpe C, Ince PG, Shaw PJ. The cellular and molecular pathology of the motor system in hereditary spastic paraparesis due to mutation of the spastin gene. J Neuropathol Exp Neurol 2003; 62: 1166–77 [DOI] [PubMed] [Google Scholar]
- 107.White KD, Ince PG, Lusher M, Lindsey J, Cookson M, Bashir R, Shaw PJ, Bushby KM. Clinical and pathologic findings in hereditary spastic paraparesis with spastin mutation. Neurology 2000; 55: 89–94 [DOI] [PubMed] [Google Scholar]
- 108.Schafernak KT, Bigio EH. West Nile virus encephalomyelitis with polio-like paralysis & nigral degeneration. Can J Neurol Sci 2006; 33: 407–10 [DOI] [PubMed] [Google Scholar]
- 109.Houlden H, Johnson J, Gardner-Thorpe C, Lashley T, Hernandez D, Worth P, Singleton AB, Hilton DA, Holton J, Revesz T, Davis MB, Giunti P, Wood NW. Mutations in TTBK2, encoding a kinase implicated in tau phosphorylation, segregate with spinocerebellar ataxia type 11. Nat Genet 2007; 39: 1434–6 [DOI] [PubMed] [Google Scholar]
- 110.Danics K, Forrest SL, Kapas I, Erber I, Schmid S, Toro K, Majtenyi K, Kovacs GG. Neurodegenerative proteinopathies associated with neuroinfections. J Neural Transm (Vienna) 2021; 128: 1551–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Soffer D, Umansky F, Goldman JE. Ganglioglioma with neurofibrillary tangles (NFTs): neoplastic NFTs share antigenic determinants with NFTs of Alzheimer’s disease. Acta Neuropathol 1995; 89: 451–3 [DOI] [PubMed] [Google Scholar]
- 112.Halper J, Scheithauer BW, Okazaki H, Laws ER, Jr. Meningio-angiomatosis: a report of six cases with special reference to the occurrence of neurofibrillary tangles. J Neuropathol Exp Neurol 1986; 45: 426–46 [PubMed] [Google Scholar]
- 113.Batra A, Prayson RA. Meningioangiomatosis associated with focal cortical dysplasia and neurofibrillary tangles. Clin Neuropathol 2013; 32: 37–41 [DOI] [PubMed] [Google Scholar]
- 114.Goates JJ, Dickson DW, Horoupian DS. Meningioangiomatosis: an immunocytochemical study. Acta Neuropathol 1991; 82: 527–32 [DOI] [PubMed] [Google Scholar]
- 115.Sarnat H, Flores-Sarnat L, Crino P, Hader W, Bello-Espinosa L. Hemimegalencephaly: foetal tauopathy with mTOR hyperactivation and neuronal lipidosis. Folia Neuropathol 2012; 50: 330–45 [DOI] [PubMed] [Google Scholar]
- 116.Sarnat HB, Flores-Sarnat L. Infantile tauopathies: Hemimegalencephaly; tuberous sclerosis complex; focal cortical dysplasia 2; ganglioglioma. Brain Dev 2015; 37: 553–62 [DOI] [PubMed] [Google Scholar]
- 117.Anderson JM, Patani R, Reynolds R, Nicholas R, Compston A, Spillantini MG, Chandran S. Evidence for abnormal tau phosphorylation in early aggressive multiple sclerosis. Acta Neuropathol 2009; 117: 583–9 [DOI] [PubMed] [Google Scholar]
- 118.Anderson JM, Hampton DW, Patani R, Pryce G, Crowther RA, Reynolds R, Franklin RJ, Giovannoni G, Compston DA, Baker D, Spillantini MG, Chandran S. Abnormally phosphorylated tau is associated with neuronal and axonal loss in experimental autoimmune encephalomyelitis and multiple sclerosis. Brain 2008; 131: 1736–48 [DOI] [PubMed] [Google Scholar]
- 119.St-Amour I, Turgeon A, Goupil C, Planel E, Hebert SS. Co-occurrence of mixed proteinopathies in late-stage Huntington’s disease. Acta Neuropathol 2018; 135: 249–65 [DOI] [PubMed] [Google Scholar]
- 120.Vuono R, Winder-Rhodes S, de Silva R, Cisbani G, Drouin-Ouellet J, Network RIotEHsD, Spillantini MG, Cicchetti F, Barker RA. The role of tau in the pathological process and clinical expression of Huntington’s disease. Brain 2015; 138: 1907–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kovacs GG. Are comorbidities compatible with a molecular pathological classification of neurodegenerative diseases? Curr Opin Neurol 2019; 32: 279–91 [DOI] [PubMed] [Google Scholar]
- 122.Head MW, Lowrie S, Chohan G, Knight R, Scoones DJ, Ironside JW. Variably protease-sensitive prionopathy in a PRNP codon 129 heterozygous UK patient with co-existing tau, alpha synuclein and Abeta pathology. Acta Neuropathol 2010; 120: 821–3 [DOI] [PubMed] [Google Scholar]
- 123.Kovacs GG, Robinson JL, Xie SX, Lee EB, Grossman M, Wolk DA, Irwin DJ, Weintraub D, Kim CF, Schuck T, Yousef A, Wagner ST, Suh E, Van Deerlin VM, Lee VM, Trojanowski JQ. Evaluating the Patterns of Aging-Related Tau Astrogliopathy Unravels Novel Insights Into Brain Aging and Neurodegenerative Diseases. J Neuropathol Exp Neurol 2017; 76: 270–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kapas I, Katko M, Harangi M, Paragh G, Balogh I, Koczi Z, Regelsberger G, Molnar MJ, Kovacs GG. Cerebrotendinous xanthomatosis with the c.379C>T (p.R127W) mutation in the CYP27A1 gene associated with premature age-associated limbic tauopathy. Neuropathol Appl Neurobiol 2014; 40: 345–50 [DOI] [PubMed] [Google Scholar]
- 125.Zadori D, Szalardy L, Reisz Z, Kovacs GG, Maszlag-Torok R, Ajeawung NF, Vecsei L, Campeau PM, Klivenyi P. Clinicopathological Relationships in an Aged Case of DOORS Syndrome With a p.Arg506X Mutation in the ATP6V1B2 Gene. Front Neurol 2020; 11: 767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Koga S, Eric Ahlskog J, DeTure MA, Baker M, Roemer SF, Konno T, Rademakers R, Ross OA, Dickson DW. Coexistence of Progressive Supranuclear Palsy With Pontocerebellar Atrophy and Myotonic Dystrophy Type 1. J Neuropathol Exp Neurol 2019; 78: 756–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mizuno Y, Maeda N, Hamasaki H, Arahata H, Sasagasako N, Honda H, Fujii N, Iwaki T. Four-repeat tau dominant pathology in a congenital myotonic dystrophy type 1 patient with mental retardation. Brain Pathol 2018; 28: 431–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rajput A, Dickson DW, Robinson CA, Ross OA, Dachsel JC, Lincoln SJ, Cobb SA, Rajput ML, Farrer MJ. Parkinsonism, Lrrk2 G2019S, and tau neuropathology. Neurology 2006; 67: 1506–8 [DOI] [PubMed] [Google Scholar]
- 129.Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 2004; 44: 601–7 [DOI] [PubMed] [Google Scholar]
- 130.Sanchez MP, Gonzalo I, Avila J, De Yebenes JG. Progressive supranuclear palsy and tau hyperphosphorylation in a patient with a C212Y parkin mutation. J Alzheimers Dis 2002; 4: 399–404 [DOI] [PubMed] [Google Scholar]
- 131.Bieniek KF, Murray ME, Rutherford NJ, Castanedes-Casey M, DeJesus-Hernandez M, Liesinger AM, Baker MC, Boylan KB, Rademakers R, Dickson DW. Tau pathology in frontotemporal lobar degeneration with C9ORF72 hexanucleotide repeat expansion. Acta Neuropathol 2013; 125: 289–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leverenz JB, Yu CE, Montine TJ, Steinbart E, Bekris LM, Zabetian C, Kwong LK, Lee VM, Schellenberg GD, Bird TD. A novel progranulin mutation associated with variable clinical presentation and tau, TDP43 and alpha-synuclein pathology. Brain 2007; 130: 1360–74 [DOI] [PubMed] [Google Scholar]
- 133.Kiely AP, Asi YT, Kara E, Limousin P, Ling H, Lewis P, Proukakis C, Quinn N, Lees AJ, Hardy J, Revesz T, Houlden H, Holton JL. alpha-Synucleinopathy associated with G51D SNCA mutation: a link between Parkinson’s disease and multiple system atrophy? Acta Neuropathol 2013; 125: 753–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Duda JE, Giasson BI, Mabon ME, Miller DC, Golbe LI, Lee VM, Trojanowski JQ. Concurrence of alpha-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol 2002; 104: 7–11 [DOI] [PubMed] [Google Scholar]
- 135.Ikeuchi T, Kakita A, Shiga A, Kasuga K, Kaneko H, Tan CF, Idezuka J, Wakabayashi K, Onodera O, Iwatsubo T, Nishizawa M, Takahashi H, Ishikawa A. Patients homozygous and heterozygous for SNCA duplication in a family with parkinsonism and dementia. Arch Neurol 2008; 65: 514–9 [DOI] [PubMed] [Google Scholar]
- 136.Markopoulou K, Dickson DW, McComb RD, Wszolek ZK, Katechalidou L, Avery L, Stansbury MS, Chase BA. Clinical, neuropathological and genotypic variability in SNCA A53T familial Parkinson’s disease. Variability in familial Parkinson’s disease. Acta Neuropathol 2008; 116: 25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Seidel K, Schöls L, Nuber S, Petrasch-Parwez E, Gierga K, Wszolek Z, Dickson D, Gai WP, Bornemann A, Riess O, Rami A, Den Dunnen WF, Deller T, Rub U, Kruger R. First appraisal of brain pathology owing to A30P mutant alpha-synuclein. Ann Neurol 2010; 67: 684–9 [DOI] [PubMed] [Google Scholar]
- 138.Baskota SU, Lopez OL, Greenamyre JT, Kofler J. Spectrum of tau pathologies in Huntington’s disease. Lab Invest 2019; 99: 1068–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Davis MY, Keene CD, Jayadev S, Bird T. The co-occurrence of Alzheimer’s disease and Huntington’s disease: a neuropathological study of 15 elderly Huntington’s disease subjects. J Huntingtons Dis 2014; 3: 209–17 [DOI] [PubMed] [Google Scholar]
- 140.Jellinger KA. Alzheimer-type lesions in Huntington’s disease. J Neural Transm (Vienna) 1998; 105: 787–99 [DOI] [PubMed] [Google Scholar]
- 141.Yoshida Y, Nunomura J, Shimohata T, Nanjo H, Miyata H. Benign hereditary chorea 2: pathological findings in an autopsy case. Neuropathology 2012; 32: 557–65 [DOI] [PubMed] [Google Scholar]
- 142.Dermaut B, Kumar-Singh S, Engelborghs S, Theuns J, Rademakers R, Saerens J, Pickut BA, Peeters K, van den Broeck M, Vennekens K, Claes S, Cruts M, Cras P, Martin JJ, Van Broeckhoven C, De Deyn PP. A novel presenilin 1 mutation associated with Pick’s disease but not beta-amyloid plaques. Ann Neurol 2004; 55: 617–26 [DOI] [PubMed] [Google Scholar]
- 143.Halliday GM, Song YJ, Lepar G, Brooks WS, Kwok JB, Kersaitis C, Gregory G, Shepherd CE, Rahimi F, Schofield PR, Kril JJ. Pick bodies in a family with presenilin-1 Alzheimer’s disease. Ann Neurol 2005; 57: 139–43 [DOI] [PubMed] [Google Scholar]
- 144.King A, Al-Sarraj S, Troakes C, Smith BN, Maekawa S, Iovino M, Spillantini MG, Shaw CE. Mixed tau, TDP-43 and p62 pathology in FTLD associated with a C9ORF72 repeat expansion and p.Ala239Thr MAPT (tau) variant. Acta Neuropathol 2013; 125: 303–10 [DOI] [PubMed] [Google Scholar]
- 145.Adachi T, Kitayama M, Nakano T, Adachi Y, Kato S, Nakashima K. Autopsy case of spinocerebellar ataxia type 31 with severe dementia at the terminal stage. Neuropathology 2015; 35: 273–9 [DOI] [PubMed] [Google Scholar]


