Abstract

The MolNetEnhancer workflow was applied to molecular networking analysis of the CH2Cl2-soluble fraction of the rhizomes of Curculigo orchioides, which showed a potent inhibitory effect on the lipopolysaccharide (LPS)-induced nitric oxide production. Among the molecular network, clusters of cycloartane-type triterpenoids were classified using the ClassyFire module of MolNetEnhancer, and their structures were predicted by the in silico fragment analysis tool, Network Annotation Propagation (NAP). Using mass spectrometry (MS)-guided isolation methods, six cycloartane-type triterpenoids (1–6) were isolated, and their structures were elucidated based on the interpretation of NMR, HRESIMS, and single-crystal X-ray diffraction. Among the isolates, compounds 1 and 4, which have an α,β-unsaturated carbonyl moiety on the A-ring, exhibited significant inhibitory effects on LPS-induced nitric oxide production in RAW264.7 cells with IC50 values of 12.4 and 11.8 μM, respectively.
Introduction
The rhizomes of Curculigo orchioides Gaertn. (Amaryllidaceae) have been used as a traditional folk medicine in Asia for the treatment of impotence, jaundice, asthma, decline in physical strength, and arthritis.1−3 In the previous phytochemical studies of the genus Curculigo, phenolic compounds including chlorophenolic glycosides,4−6 lignans,7,8 and triterpenoidal saponins9−12 have been reported as the main bioactive constituents. Various pharmacological investigations have revealed the broad biological activities of the extracts and isolates. The ethanol extract of C. orichioides and isolated phenolic compounds such as curculigoside A, curculigoside B, curculigine A, and curculigine D exhibited antiosteoporotic activity.13 In addition, cycloartane glycosides exhibited cytotoxic activity against HL-60 human promyelocytic leukemia cells.12 As part of an ongoing research program aimed at discovering new bioactive constituents in medicinal plants, the MeOH extract of the rhizomes of C. orchioides and the subsequent CH2Cl2-soluble fraction were found to possess inhibitory effect against the lipopolysaccharide (LPS)-induced nitric oxide (NO) production in RAW264.7 cells (IC50 = 27.7 and 20.0 μg/mL, respectively).
Liquid chromatography high-resolution mass spectrometry (LC-HRMS/MS)-based molecular networking analysis by the Global Natural Products Social (GNPS) molecular networking web platform efficiently organized and visualized the cluster of structurally related compounds. Its rapidly growing social library makes it possible to predict the structure of an extract before intensive chromatographic isolation and structure determination.14 In addition, a spectral library could be propagated using in silico analysis tools such as the Network Annotation Propagation (NAP).15 Recently, Nasir et al. applied a combination of molecular networking and LC-MS/MS profiling to annotate phenolic glycosides and norlignans in the rhizomes and leaf extract of C. latifolia.16 However, the molecular network of the CH2Cl2-solube fraction of C. orchioides showed a different pattern, with only a few compounds matching the GNPS library.
In the present study, MolNetEnhancer, which integrates outputs from molecular networking, MS2LDA, in silico tools (NAP or DEREPLICATOR), and automated chemical classification through ClassyFire, was applied to the generated molecular networking analysis.17 The clusters of triterpenoids were classified by MolNetEnhancer, and the detailed structure was predicted to be cycloartane by NAP. Through targeted isolation of these clusters, six cycloartane-type triterpenoids (1–6), including five new compounds, were isolated. Herein, we report the molecular networking-guided isolation and structure determination of compounds 1–6, as well as the structure–activity relationship (SAR) of their inhibitory effects against LPS-induced nitric oxide production in RAW264.7 cells.
Results and Discussion
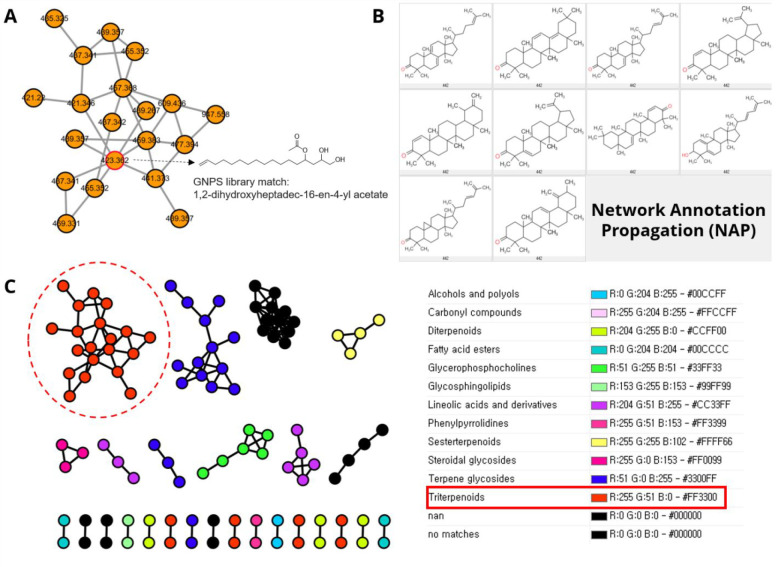
The LC-HRMS/MS data of the CH2Cl2-soluble fraction of C. orchioides were analyzed by classical molecular networking via the GNPS web platform (https://gnps.ucsd.edu). At first, only one node in cluster 1 matched with 1,2-dihydroxyheptadec-16-en-4-yl acetate in the GNPS spectrum library, but the spectrum similarity was not good, with a mass difference of 94.09 and cosine score of 0.75 (Figure 1A). Therefore, an in silico fragment analysis tool (NAP) combined with the structure library of GNPS and SUPER NATURAL 2 (SUPNAT) (Figure 1B) was used to conduct analysis.18 The results of classical molecular networking and NAP were integrated by MolNetEnhancer of the GNPS web platform, which automatically classified the chemical class of each cluster (Figure 1C). Among them, the largest cluster (cluster 1) revealed as that of triterpenoids and the result of NAP also predicted the structure of each node as various triterpenoids containing cycloartane types. Because many cycloartane-type saponins have been previously reported in C. orchioides,9−12 it was reasonable to select cycloartane-type triterpenoids among the predicted candidates. In addition, only a few aglycones were reported,19 and many of which are produced through hydrolysis from isolated saponins. Therefore, cluster 1 was expected to contain new cycloartane-type triterpenoids. Twelve subfractions from silica gel chromatography were tested for their inhibitory effects on NO production in LPS-induced RAW264.7 cells, and the defined subfraction COC9, containing triterpenoids, showed the most potent inhibitory effects (Figure S2). Therefore, this fraction was investigated.
Figure 1.
Molecular networking analysis of the CH2Cl2-soluble fraction of C. orchioides. (A) Spectrum match of the node of molecular networking with GNPS library. (B) Structures of top ranked NAP candidates using GNPS and SUPNAT library. (C) Automatic classification and visualization of each cluster by the MolNetEnhancer. The chemical class of the largest (the cluster filled with red color) clusters were revealed as triterpenoids. The singleton node was excluded in this figure.
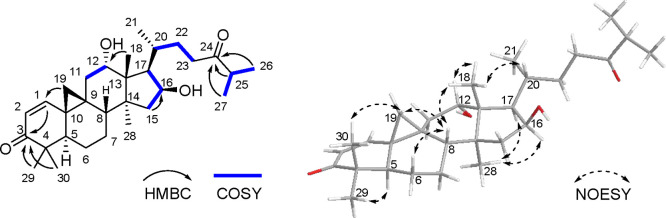
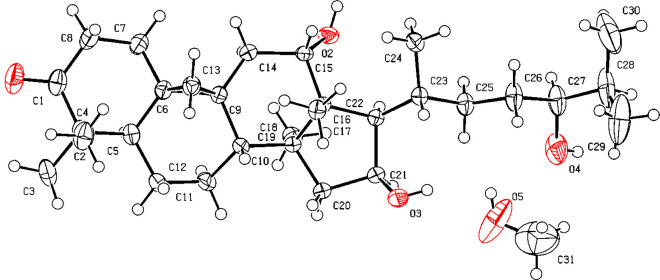
Compound 1 was isolated as a white amorphous powder. Its molecular formula was assigned as C30H48O4 based on HRESIMS data (m/z 473.3624 [M + H]+; calcd 473.3625), indicating seven indices of hydrogen deficiency. The 1H NMR data for 1 displayed two olefinic protons (δH 6.80, 5.96), three oxymethine protons (δH 4.57, 3.97, 3.39), four tertiary methyl protons (δH 1.12, 1.09, 1.03, 0.97), and three secondary methyl protons (δH 1.10, 0.93 × 2) (Table 1). The 13C NMR and HSQC spectra exhibited 30 carbon signals, including one carbonyl, two olefinic carbons, five quaternary carbons, seven methylenes, eight methines, and seven methyl groups (Table 2). The 1H and 13C NMR data of 1 were similar to those of 12α,16β-dihydroxycycloartane-3,24-dione,19 except for the presence of a double bond and an additional oxymethine instead of a carbonyl group. The position of the α,β-unsaturated carbonyl group at the C-1/C-3 was determined by the HMBC correlations from H2-19 (δH 1.25 and 0.95) to olefinic carbon (δC 153.9) and from CH3-29 and CH3-30 (δH 1.12 and 0.97) to a carbonyl carbon (δC 205.1). The proton signals of H2-19 (δH 1.25 and 0.95) of 1 were shifted downfield compared with that of 3 (δH 0.65 and 0.59) because of the anisotropic effect caused by the alkene group between C-1 and C-2. Furthermore, through the HMBC correlations from CH3-18 (δH 1.09) to C-12 (δC 73.1) and from H2-15 (δH 2.06, 1.45) and H-20 (δH 1.89) to C-16 (δC 72.2), and from H-25 (δH 1.67), CH3-26 (δH 0.93), and CH3-27 (δH 0.93) to C-24 (δC 78.7), the planar structure of 1 was determined to be 12,16,24-trihydroxycycloartane-1-en-3-one (Figure 2). The relative configuration of 1 was deduced from the NOESY experiment. The NOE correlations of H2-19/30-CH3, H-6β, H-8, H-8/18-CH3, and 18-CH3/H-20 revealed the β-axial orientations of H-8, 18-CH3, and H-20. In addition, the NOE correlations between H-16 and H-17 and 28-CH3 revealed their α-orientations (Figure 2). Single-crystal X-ray diffraction analysis with Cu Kα radiation [Flack parameter = 0.00(5) and Hooft parameter = 0.02(4)] clearly corroborated the absolute configuration of 1 (Figure 3). Thus, the structure of 1 was determined to be (5R,8S,9R,10S,12S,13R,14S,16S,17R,20R,24S)-12,16,24-trihydroxycycloartane-1-en-3-one and named curculigone A.
Table 1. 1H NMR (400b, 500c, and 800d MHz) Data for Compounds 1–6a.
| no. | 1b | 2b | 3c | 4d | 5b | 6b |
|---|---|---|---|---|---|---|
| 1 | 6.80 (d, 10.1) | 6.80 (d, 10.1) | 1.77 (m) | 6.78 (d, 10.1) | 2.36 (m) | 1.58 (m) |
| 1.50 (m) | 1.53 (m) | 1.26 (m) | ||||
| 2 | 5.96 (d, 10.0) | 5.95 (d, 10.0) | 2.73 (m) | 5.95 (d, 10.1) | 2.76 (m) | 1.78 (m) |
| 2.35 (m) | 2.32 (m) | 1.58 (m) | ||||
| 3 | 3.31 (m) | |||||
| 4 | ||||||
| 5 | 2.14 (m) | 2.17 (m) | 1.68 (dd, 4.3, 12.5) | 2.07 (dd, 6.9, 10.2) | 1.72 (m) | 1.32 (m) |
| 6 | 1.67 (m) | 1.66 (m) | 1.45 (m) | 1.60 (m) | 1.56 (m) | 1.66 (m) |
| 1.00 (m) | 1.02 (m) | 0.81 (m) | 1.08 (m) | 0.98 (m) | 0.80 (m) | |
| 7 | 1.47 (m) | 1.47 (m) | 1.38 (m) | 1.56 (m) | 1.40 (m) | 1.34 (m) |
| 1.22 (m) | 1.22 (m) | 1.16 (m) | 1.25 (m) | 1.13 (m) | 1.10 (m) | |
| 8 | 1.79 (m) | 1.81 (dd, 5.1, 12.4) | 1.65 (dd, 4.3, 13.0) | 2.13 (dd, 3.9, 12.7) | 1.69 (m) | 1.49 (m) |
| 9 | ||||||
| 10 | ||||||
| 11 | 2.12 (m) | 2.11 (m) | 2.40 (m) | 1.96 (m) | 2.09 (m) | 1.96 (m) |
| 1.97 (m) | 1.99 (m) | 1.93 (m) | 1.54 (m) | 1.19 (m) | 1.76 (m) | |
| 12 | 3.97 (dd, 5.6, 9.1) | 3.94 (dd, 5.6, 9.1) | 4.21 (m) | 1.65 (m) | 1.83 (m) | 3.87 (dd, 6.0, 9.6) |
| 1.60 (m) | ||||||
| 13 | ||||||
| 14 | ||||||
| 15 | 2.06 (m) | 2.03 (m) | 2.27 (dd, 8.1, 12.9) | 2.01 (dd, 8.1, 13.1) | 2.02 (m) | 2.06 (dd, 8.1, 13.2) |
| 1.45 (m) | 1.50 (m) | 1.86 (m) | 1.46 (dd, 4.7, 13.1) | 1.47 (m) | 1.41 (dd, 4.2, 13.2) | |
| 16 | 4.57 (ddd, 2.8, 8.0, 11.6) | 4.64 (ddd, 2.8, 7.7, 12.0) | 4.91 (ddd, 2.8, 7.8, 12.9) | 4.47 (ddd, 2.5, 7.5, 12.5) | 4.48 (ddd, 2.9, 7.8, 12.0) | 4.56 (ddd, 2.8, 7.5, 12.6) |
| 17 | 2.20 (dd, 8.0, 11.6) | 2.13 (m) | 2.91 (dd, 7.8, 11.0) | 1.89 (dd, 7.5, 11.0) | 1.89 (m) | 2.18 (dd, 7.5, 11.2) |
| 18 | 1.09 (s) | 1.08 (s) | 1.48 (s) | 1.19 (s) | 1.23 (s) | 1.08 (s) |
| 19 | 1.25 (m) | 1.25 (d, 4.6) | 0.65 (d, 4.0) | 1.32 (d, 4.6) | 0.83 (d, 4.2) | 0.55 (d, 4.3) |
| 0.95 (m) | 0.94 (d, 4.6) | 0.59 (d, 4.3) | 0.79 (d, 4.6) | 0.60 (d, 4.3) | 0.44 (d, 4.4) | |
| 20 | 1.89 (m) | 1.24 (m) | 2.55 (m) | 2.33 (m) | 2.29 (m) | 1.87 (m) |
| 21 | 1.10 (d, 6.5) | 1.06 (d, 6.8) | 1.52 (d, 6.7) | 0.95 (d, 7.0) | 0.95 (d, 7.1) | 1.08 (d, 7.1) |
| 22 | 1.75 (m) | 2.07 (m) | 2.42 (m) | 4.06 (dt, 2.2, 10.1) | 4.06 (dt, 2.2, 9.8) | 2.36 (m) |
| 1.19 (m) | 1.08 (m) | 1.89 (m) | 1.73 (m) | |||
| 23 | 1.72 (m) | 2.77 (m) | 1.95 (m) | 1.75 (m) | 1.76 (m) | 1.86 (m) |
| 1.30 (m) | 2.56 (m) | 1.81 (m) | 1.68 (m) | 1.67 (m) | 1.72 (m) | |
| 24 | 3.39 (m) | 3.68 (m) | 3.63 (m) | 3.64 (m) | 3.39 (m) | |
| 25 | 1.67 (m) | 2.64 (sept, 6.9) | 1.58 (m) | 1.84 (m) | 1.81 (m) | 1.65 (m) |
| 26 | 0.93 (d, 6.8) | 1.13 (d, 6.8) | 1.12 (d, 6.8) | 1.01 (d, 6.6) | 1.01 (d, 6.6) | 0.93 (d, 6.8) |
| 27 | 0.93 (d, 6.8) | 1.12 (d, 6.8) | 1.10 (d, 6.8) | 0.90 (d, 6.8) | 0.91 (d, 6.8) | 0.91 (d, 6.8) |
| 28 | 1.03 (s) | 1.04 (s) | 1.34 (s) | 0.92 (s) | 0.92 (s) | 0.99 (s) |
| 29 | 1.12 (s) | 1.12 (s) | 1.17 (s) | 1.11 (s) | 1.11 (s) | 0.98 (s) |
| 30 | 0.97 (s) | 0.97 (s) | 1.07 (s) | 0.97 (s) | 1.05 (s) | 0.81 (s) |
Assignments were based on COSY and HSQC experiments. Compound 3 was dissolved with pyridine-d5, and others used CDCl3.
Table 2. 13C NMR (100b, 125c, and 200d MHz) Data for Compounds 1–6a.
| no. | 1b | 2b | 3c | 4d | 5b | 6b |
|---|---|---|---|---|---|---|
| 1 | 153.9, CH | 154.0, CH | 33.6, CH2 | 153.9, CH | 33.4, CH2 | 32.2, CH2 |
| 2 | 126.9, CH | 126.9, CH | 37.6, CH2 | 126.7, CH | 37.5, CH2 | 30.4, CH2 |
| 3 | 205.1, C | 205.3, C | 215.0, C | 205.3, C | 216.6, C | 78.7, CH |
| 4 | 46.3, C | 46.3, C | 50.3, C | 46.0, C | 50.3, C | 40.5, C |
| 5 | 45.7, CH | 45.7, CH | 48.7, CH | 44.3, CH | 48.5, CH | 47.3, CH |
| 6 | 20.5, CH2 | 20.5, CH2 | 21.8, CH2 | 19.7, CH2 | 21.5, CH2 | 21.2, CH2 |
| 7 | 25.0, CH2 | 25.0, CH2 | 26.6, CH2 | 23.8, CH2 | 26.1, CH2 | 26.3, CH2 |
| 8 | 47.3, CH | 47.3, CH | 49.1, CH | 44.7, CH | 48.0, CH | 48.6, CH |
| 9 | 23.1, C | 23.2, C | 21.2, C | 24.4, C | 21.0, C | 19.6, C |
| 10 | 29.2, C | 29.0, C | 26.0, C | 29.9, C | 26.0, C | 26.0, C |
| 11 | 39.2, CH2 | 39.6, CH2 | 40.3, CH2 | 27.6, CH2 | 26.5, CH2 | 38.3, CH2 |
| 12 | 73.1, CH | 73.2, CH | 72.4, CH | 32.6, CH2 | 33.0, CH2 | 73.6, CH |
| 13 | 49.6, C | 49.6, C | 50.1, C | 45.8, C | 45.8, C | 49.5, C |
| 14 | 46.8, C | 46.7, C | 47.1, C | 47.3, C | 46.9, C | 46.8, C |
| 15 | 48.4, CH2 | 45.7, CH2 | 50.8, CH2 | 46.0, CH2 | 47.1, CH2 | 49.0, CH2 |
| 16 | 72.2, CH | 71.7, CH | 71.7, CH | 71.7, CH | 72.1, CH | 72.6, CH |
| 17 | 49.2, CH | 49.6, CH | 49.3, CH | 52.0, CH | 52.3, CH | 49.1, CH |
| 18 | 17.9, CH3 | 17.7, CH3 | 18.6, CH3 | 17.8, CH3 | 18.9, CH3 | 17.7, CH3 |
| 19 | 32.2, CH2 | 32.2, CH2 | 29.6, CH2 | 30.1, CH2 | 29.8, CH2 | 30.0, CH2 |
| 20 | 31.2, CH | 29.4, CH | 31.6, CH | 34.9, CH | 35.0, CH | 31.3, CH |
| 21 | 17.7, CH3 | 17.0, CH3 | 17.8, CH3 | 16.1, CH3 | 15.9, CH3 | 17.8, CH3 |
| 22 | 33.5, CH2 | 29.3, CH2 | 34.1, CH2 | 74.2, CH | 74.2, CH | 33.5, CH2 |
| 23 | 30.9, CH2 | 36.7, CH2 | 32.3, CH2 | 34.3, CH | 34.5, CH2 | 30.9, CH2 |
| 24 | 78.7, CH | 217.1, C | 77.4, CH | 76.2, CH | 76.1, CH | 78.7, CH |
| 25 | 34.0, CH | 41.0, CH | 34.2, CH | 32.5, CH | 32.6, CH | 33.9, CH |
| 26 | 18.7, CH3 | 18.4, CH3 | 19.8, CH3 | 18.8, CH3 | 18.7, CH3 | 18.8, CH3 |
| 27 | 17.4, CH3 | 18.3, CH3 | 17.7, CH3 | 18.7, CH3 | 19.0, CH3 | 17.4, CH3 |
| 28 | 21.1, CH3 | 21.3, CH3 | 22.1, CH3 | 19.6, CH3 | 20.3, CH3 | 21.4, CH3 |
| 29 | 21.6, CH3 | 21.6, CH3 | 22.7, CH3 | 21.4, CH3 | 22.2, CH3 | 25.4, CH3 |
| 30 | 19.1, CH3 | 19.1, CH3 | 20.8, CH3 | 19.1, CH3 | 20.8, CH3 | 14.0, CH3 |
Assignments were based on COSY and HSQC experiments. Compound 3 was dissolved with pyridine-d5, and others used CDCl3.
Figure 2.
Key HMBC, COSY, and NOESY correlations of compound 1.
Figure 3.

X-ray ORTEP plot for the molecular structure of compound 1.
Compound 2 was obtained as a white amorphous powder. The molecular formula of 2 was deduced from the HRESIMS data as C30H46O4 (m/z 471.3467 [M + H]+; calcd 471.3469), indicating eight indices of hydrogen deficiency. A comparison of the 1H and 13C NMR data of 1 and 2 indicated that 2 was similar to 1 except the hydroxyl group was replaced by a carbonyl group (δC 217.1) in 2 (Tables 1 and 2). The positions of the two carbonyl groups at C-3 and C-24 were determined by the HMBC correlations of H-1 (δH 6.80), CH3-29 (δH 1.12), and CH3-30 (δH 0.97)/C-3 (δC 205.3) and H-25 (δH 2.64), CH3-26 (δH 1.13), and CH3-27 (δH 1.12)/C-24 (δC 217.1) (Figure 4). The relative configuration of 2 was determined to be the same as that of 1 based on their similar NOE correlations (Figure 4). Furthermore, single-crystal X-ray diffraction analysis using Cu Kα radiation was performed on 2, and the Flack parameter −0.14(8) and Hooft parameter −0.16(7) permitted the absolute configuration (Figure 5). Thus, the structure of 2 was determined to be (5R,8S,9R,10S,12S,13R,14S,16S,17R,20R)-12,16-dihydroxycycloartane-1-en-3,24-one and named curculigone B.
Figure 4.
Key HMBC, COSY, and NOESY correlations of compound 2.
Figure 5.

X-ray ORTEP plot for the molecular structure of compound 2.
Compound 3 was obtained as a white amorphous powder. Its molecular formula was determined by the HRESIMS data as C30H50O4 (m/z 497.3598 [M + Na]+; calcd 497.3601), indicating six indices of hydrogen deficiency, one less than that of 1. In the 1H NMR and 13C NMR data, the signals corresponding to the double bond disappeared, indicating the reduction of the double bond between C-1 and C-2 in 1 (Tables 1 and 2). The positions of the carbonyl group and three hydroxy groups were determined by the HMBC correlations of CH3-29, CH3-30/C-3, H2-11, CH3-18/C-12, H2-15, H-20/C-16, and H-25, CH3-26, CH3-27/C-24 (Figure 6). As with compounds 1 and 2, the relative and absolute configurations of 3 were determined by NOESY experiment and single-crystal X-ray diffraction analysis [Flack parameter = 0.08(5) and Hooft parameter = 0.07(4)] (Figure 7). Thus, the structure of 3 was determined as (5R,8S,9R,10R,12S,13R,14S,16S,17R,20R,24S)-12,16,24-trihydroxycycloartane-3-one and named curculigone C.
Figure 6.
Key HMBC, COSY, and NOESY correlations of compound 3.
Figure 7.
X-ray ORTEP plot for the molecular structure of compound 3.
Compound 4 was obtained as a white amorphous powder and exhibited an m/z value of 473.3623, corresponding to the molecular formula of C30H48O4 ([M + H]+, calcd 473.3625), with seven indices of hydrogen deficiency. Its molecular weight was the same as that of 1, but the chemical shifts of the oxymethines showed a slight difference in 1H and 13C NMR data (Tables 1 and 2). The positions of the three hydroxy groups were determined to be C-16, C-22, and C-24 by the HMBC correlations of H2-15 (δH 2.01 and 1.46), H-20 (δH 2.33)/C-16 (δC 71.7), H-20 (δH 2.33), CH3-21 (δH 0.95)/C-22 (δC 74.2), and H-25 (δH 1.84), CH3-26 (δH 1.01), CH3-27 (δH 0.90)/C-24 (δC 76.2). In addition, the 1H–1H COSY correlations of H-22/H2-23/H-24 verified the positions of two hydroxy groups at C-22 and C-24. The relative configuration of 4 was elucidated by the ROESY experiment with the aid of computational 3D modeling after MM2 energy minimization (Figure 8). As with compounds 1–3, the relative configurations of H-8β, CH3-18β, CH3-30β, H-5α, and CH3-28α were deduced from their ROESY correlations. In addition, the large coupling constant between H-17 and H-20 (J = 11.0 Hz), together with the ROESY correlation between CH3-18β and H-20, clearly determined the β-configuration of H-20. The coupling constant between H-20 and H-22 exhibited a large value (J = 10.1 Hz), and two possible configurations were simulated using computational 3D modeling for the preferred match. In the case of the 3D model of 20S*,22R*, the dihedral angle between H-20 and H-22 was −163.3°, whereas that of the model of 20S*,22S* was 69.4°. Thus, the configurations of H-20 and H-22 were determined to be 20S*,22R* according to the Karplus equation (Figure 8A).20 Furthermore, in the computational model for the 22R*,24R* configuration, the distance between H-22 and H-24 was 3.83 Å, which is too far to show the NOE effect. For 22R*,24S*, the distance of H-22 and H-24 (2.66 Å) corresponded with the observed ROESY correlation between H-22 (δH 4.06) and H-24 (δH 3.63) (Figure 8B).21 The optical rotation of compound 4 was measured as +3.5 (c 0.5, methanol), which has the same polarity as compounds 1–3. Therefore, the structure of 4 was determined as (5R,8S,9S,10S,13R,14S,16S,17R,20S,22R,24S)-16,22,24-trihydroxycycloartane-1-en-3-one and named curculigone D.
Figure 8.

3D model simulation after MM2 minimization (minimum RMS gradient = 0.01) for the comparison of relative configuration of compound 4. (A) Comparison of 3D computational models of (20S*,22R*)-4 and (20S*,22S*)-4 and 3J values upon dihedral angle. (B) Comparison of (20S*,22R*,24S*)-4 and (20S*,22R*,24R*)-4 and calculated interproton distances.
Compound 5 was obtained as a white amorphous powder and assigned the molecular formula C30H50O4 based on its HRESIMS data (m/z 475.3777 [M + H]+, calcd 475.3782) and 13C NMR data. With one less hydrogen deficiency than 4, along with the disappearance of the olefinic moiety in 1H and 13C NMR data revealed the reduction of the double bond between C-1 and C-2 of 4 (Tables 1 and 2). The planar structure of 5 was determined to be 16,22,24-trihydroxycycloartane-3-one by HMBC experiment. The large coupling constant between H-20 and H-22 (J = 9.8 Hz) matched well with the 3D modeling for 20S*,22R*, which showed a dihedral angle of −163.9°. Moreover, the observed NOESY correlation of H-22 and H-24 permitted the configuration of 22R*,24S* (2.66 Å) rather than 22R*,24R* (3.83 Å) (Figure 9). The positive optical rotation value of +5.9 (c 0.5, methanol) confirmed the absolute configuration of 5. Therefore, the structure of 5 was determined as (5R,8S,9S,10R,13R,14S,16S,17R,20S,22R,24S)-16,22,24-trihydroxycycloartane-3-one and named curculigone D.
Figure 9.

3D computational model for the compound 5 and key NOESY correlation.
Compound 6 was obtained as a white amorphous powder. Its molecular formula was determined by the HRESIMS data as C30H52O4 ([m/z 499.3757 [M + Na]+; calcd 499.3758). The 1H and 13C NMR data of 6 resembled those of 3, except for the absence of the carbonyl group and the presence of an additional hydroxyl group (Tables 1 and 2). These data were consistent with those of (24S)-9,19-cyclolanostane-3β,12α,16β,24-tetrol, which was produced by the enzymatic hydrolysis of (24S)-12α,16β,24-trihydroxy-9,19-cyclolanostan-3β-hydroxyl-β-d-glucopyranoside.12
After structure determination, the clusters classified as triterpenoids by MolNetEnhancer were annotated according to their structures (Figure 10). The structures predicted by the NAP module with GNPS and the SUPNAT library were close with the actual structure, except for the position of double bond, and/or the number of hydroxyl group. The isolated compounds were mostly present as adducts of [M + H – H2O]+ or [M + H – 2H2O]+, whereas most of the predicted candidates were present as protonated or a sodium adduct in the spectrum library. This gap in the adducts may result in a difference between predicted and actual structures.
Figure 10.
Annotation of compounds 1–6 on the triterpenoid clusters.
All compounds were tested for their inhibitory effects on LPS-induced NO production in RAW264.7 macrophages using aminoguanidine as a positive control (Table 3). The cytotoxicity of the compounds was examined using the MTT reagent, and none of the test compounds showed significant cytotoxicity at their effective concentrations for the inhibition of NO production (Figure S41, Supporting Information). A comparison of the IC50 values of compounds 1–6 suggested the α,β-unsaturated carbonyl moiety on the A-ring was critical for NO inhibition. The lack of an inhibitory effect on NO production in compound 2, despite having an α,β-unsaturated carbonyl moiety in the A-ring, could be due to the introduction of an additional carbonyl group at C-24.
Table 3. Inhibitory Effects of Compounds 1–6 against LPS-Induced NO Production in RAW264.7 Cells and Their Cytotoxicitya.
| compound | IC50 (μM)b | CC50 (μM)c |
|---|---|---|
| 1 | 12.4 ± 0.9 | 76.7 ± 1.0 |
| 2 | >100 | >100 |
| 3 | >100 | >100 |
| 4 | 11.8 ± 1.2 | 66.9 ± 3.1 |
| 5 | 29.4 ± 0.2 | 89.1 ± 2.6 |
| 6 | 41.7 ± 0.7 | 54.8 ± 4.6 |
Aminoguanidine was used as the positive control (IC50 = 15.9 ± 1.7 μM). Results are expressed as the mean IC50 values in μM from triplicate experiments.
IC50: 50% inhibitory concentration.
CC50: 50% cytotoxic concentration.
In conclusion, the MolNetEnhacer module, coupled with the in silico fragment analysis tool NAP, was applied to the molecular networking analysis of the rhizomes of C. orchioides. The workflow of MolNetEnhancer efficiently classified the chemical class of each cluster, and the largest cluster in the molecular network was revealed to be triterpenoid. Targeted isolation of this cluster afforded six cycloartane-type triterpenoids (1–6). Among them, the cylcloartane-1-en-3-one-type compound (1 and 4) exhibited potent inhibitory effects against LPS-induced NO production in RAW264.7 cells. Hence, cycloartane-type triterpenoids from the rhizomes of C. orchioides may have potential for the treatment of inflammation-associated diseases and are worthy of further study for their mechanism of action.
Experimental Section
General Experimental Procedures
The optical rotations were measured using a JASCO DIP-1000 polarimeter. UV and IR spectra were obtained using a JASCO UV-550 spectrophotometer and JASCO FT-IR 4100 spectrometer, respectively. NMR spectra were recorded on Bruker AVANCE 400, 500, and 800 MHz spectrometers using CDCl3 and pyridine-d5 as solvents. HR-ESI-MS and UPLC-MS/MS analyses were performed using an Orbitrap Exploris 120 mass spectrometer coupled with a Vanquish UHPLC system (ThermoFisher Scientific). Chromatographic elution was conducted on a YMC Triart C18 (100 × 2.1 mm, 1.9 μm, 0.3 mL/min) column at a temperature of 30 °C, with a mobile phase of water +0.1% formic acid (A) and CH3CN + 0.1% formic acid (B), and the gradient consisted of a linear gradient of 10–100% B (0–10 min). Mass detection was performed in the m/z range of 200–2000, and the resolution of the Orbitrap mass analyzer was fixed at 60,000 for the full MS scan and 15,000 for the data-dependent MSn scan. The following parameters were used during MS measurements: a spray voltage of 3.5/2.5 kV for the positive/negative modes, ion transfer tube temperature of 320 °C, HESI probe vaporizer temperature of 275 °C, RF lens 70 (%). Ultrapure nitrogen (>99.999%) was used as both sheath and auxiliary gas of the HESI probe and set to 50 and 15 arb, respectively. A normalized higher-energy collision dissociation energy of 30% was used for the collision of ions in the Orbitrap detector. MS/MS fragmentation was obtained by the data-dependent MSn mode to obtain an MS2 spectrum of the four most intense ions, with a dynamic exclusion filter to exclude the repeated fragmentation of ions within 2.5 s after acquiring the MS2 spectrum. Column chromatography was performed using a silica gel column (Merck, 70-230 mesh). MPLC was performed using a Biotage Isolera Prime chromatography system with Lichroprep RP-18 (Merck, 40-63 μm). Preparative HPLC was performed using a Waters HPLC system equipped with two Waters 515 pumps, a 2996 photodiode array detector, and a YMC J’sphere ODS H-80 column (4 μm, 150 × 20 mm, i.d., flow rate 6.0 mL/min). Thin layer chromatography was performed using precoated silica gel 60 F254 (0.25 mm, Merck) plates, and spots were detected using a 10% vanillin-H2SO4 aqueous spray reagent.
Plant Material
Dried rhizomes of C. orchioides were purchased from the Kyungdong herbal market (Seoul, South Korea) in May 2021 and were identified by one of the authors (B.Y.H.). A voucher specimen (CBNU-2021-05-CO) was deposited at the Herbarium of the College of Pharmacy, Chungbuk National University, South Korea.
Molecular Networking-Based Analysis
LC-MS/MS data were uploaded to the GNPS (http://gnps.ucsd.edu) for classical molecular networking. The product ion and fragment ion mass tolerances were set to 0.02 Da. The edge between the nodes was created when they displayed cosine scores above 0.7 and more than six matched peaks. Furthermore, these edges were kept in the network if and only if each of the nodes appeared in each of the top 10 most similar nodes. The network was downloaded and visualized using Cytoscape 3.8.2,22 and the NAP tool in the GNPS web platform was used for in silico analysis of the molecular network. The following parameters were used for this purpose: 10 first candidates, mass tolerance of 5 ppm, database of GNPS and SUPNAT2 (Supernatural II). After analysis, the resulting file was exported to Cytoscape, and the structures were visualized using the ChemViz2 plug-in. The workflow and results of classical molecular networking and NAP could be browsed on the following GNPS Web site links:
https://gnps.ucsd.edu/ProteoSAFe/status.jsp?task=e68dc185eac44e4889272286f5e971b8
https://proteomics2.ucsd.edu/ProteoSAFe/status.jsp?task=d5b5890d96df4b4b85df42566c7e5916
The MolNetEnhancer workflow was conducted via “Advanced Views-Experimental Views” tab of the result of classical networking with the job ID of NAP above. The result (https://proteomics2.ucsd.edu/ProteoSAFe/status.jsp?task=d5b5890d96df4b4b85df42566c7e5916) was downloaded and imported to Cytoscape, and the color of the clusters was automatically filled upon the chemical subclass.
Extraction and Isolation
The dried rhizomes of C. orchioides (3.0 kg) were extracted with MeOH (3 × 18 L) by maceration for 3 days at room temperature (25 °C) and then filtered and evaporated under reduced pressure to obtain a MeOH extract. A suspension of the extract (150 g) in distilled water was partitioned sequentially with n-hexane (2 × 2 L), CH2Cl2 (2 × 2 L), EtOAc (2 × 2 L), and n-BuOH (2 × 2L). The CH2Cl2-soluble fraction (8.3 g) was subjected to silica gel column chromatography and eluted using a CH2Cl2–MeOH gradient system (30:1 to 0:1) to yield 12 fractions (COC1–COC12). COC9 (2.5 g) was further separated by RP-MPLC and eluted with a MeOH–H2O gradient system (20:80 to 100:0) to obtain 18 subfractions (COC9-1–COC9-18). COC9-12 (134.2 mg) was further purified by preparative HPLC (MeCN–H2O, 57:43, isocratic) to yield (24S)-9,19-cyclolanostane-3β,12α,16β,24-tetrol (6) (tR = 22.5 min, 2.1 mg), compound 1 (tR = 27.1 min, 4.6 mg), and compound 2 (tR = 49.0 min, 12.2 mg). COC9-13 (116.8 mg) was isolated by preparative HPLC (MeCN–H2O, 55:45, isocratic) to obtain compounds 4 (tR = 41.5 min, 1.6 mg) and 3 (tR = 43.0 min, 7.2 mg). COC9-14 (80.4 mg) was purified using preparative HPLC (MeCN–H2O, 65:35, isocratic) to yield compound 5 (tR = 32.8 min, 2.5 mg).
Curculigone A (1):
White amorphous powder; [α]D25 +5.5 (c 1.0, MeOH); UV (MeOH) λmax 270 nm; IR νmax (film) 3390, 2956, 2925, 2854, 1705, 1384, and 1102 cm–1; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3), see Tables 1 and 2; HRESIMS m/z 473.3624 [M + H]+ (calcd for C30H49O4, 473.3625).
Curculigone B (2):
White amorphous powder; [α]D25 +3.2 (c 0.5, MeOH); UV (MeOH) λmax 269.2 nm; IR νmax (film) 3398, 2950, 2915, 2856, 1711, 1705, 1384, and 1102 cm–1; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3), see Tables 1 and 2; HRESIMS m/z 471.3467 [M + H]+ (calcd for C30H47O4, 471.3469).
Curculigone C (3):
White amorphous powder; [α]D25 +14.3 (c 1.0, MeOH); UV (MeOH) λmax 210 nm; IR νmax (film) 3405, 2951, 2925, 2848, 1710, and 1036 cm–1; 1H NMR (500 MHz, pyridine-d5) and 13C NMR (125 MHz, pyridine-d5), see Tables 1 and 2; HRESIMS m/z 497.3598 [M + Na]+ (calcd for C30H50NaO4, 497.3601).
Curculigone D (4):
White amorphous powder; [α]D25 +3.5 (c 0.5, MeOH); UV (MeOH) λmax 270 nm; IR νmax (film) 3393, 2947, 2922, 2858, 1704, and 1106 cm–1; 1H NMR (800 MHz, CDCl3) and 13C NMR (200 MHz, CDCl3), see Tables 1 and 2; HRESIMS m/z 473.3623 [M + H]+ (calcd for C30H49O4, 473.3625).
Curculigone E (5):
White amorphous powder; [α]D25 +5.9 (c 0.5, MeOH); UV (MeOH) λmax 210 nm; IR νmax (film) 3383, 2957, 2930, 2850, 1709, and 1098 cm–1; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3), see Tables 1 and 2; HRESIMS m/z 475.3777 [M + H]+ (calcd for C30H51O4, 475.3782).
(24S)-9,19-Cyclolanostane-3β,12α,16β,24-tetrol (6):
White amorphous powder; [α]D25 +26.0 (c 1.0, MeOH); UV (MeOH) λmax 224 nm; IR νmax (film) 3365, 2957, 2925, 2848, and 1036 cm–1; 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3), see Tables 1 and 2; HRESIMS m/z 499.3757 [M + Na]+ (calcd for C30H52NaO4, 499.3758).
X-ray Crystallographic Analysis of Compounds 1–3
Single crystals of compounds 1–3 were recrystallized by an evaporation method with MeOH and analyzed using a Bruker D8 Venture diffractometer equipped with a monochromatic fine-focus Cu Kα (λ = 1.54178 Å) radiation source. Data collection was carried out using a PHOTON 100 CMOS detector at 223(2) K using the APEX2 software (Bruker AXS Inc.). The crystal structure was refined by full-matrix least-squares refinement using the SHELXL-2014 computer program.23 Further analysis of the properties of the single crystals was performed using PLATON.24 Molecular graphics were computed using the Mercury 4.2 software. Crystallographic data for 1–3 were deposited in the Cambridge Crystallographic Data Centre (deposition numbers CCDC 2173493, CCDC 2173494, and CCDC 2173491). These data can be obtained free of charge at www.ccdc.cam.ac.uk.
Crystal Data of Curculigone A (1):
Monoclinic crystal, C30H50O5, Mr = 490.70, size 0.155 × 0.070 × 0.055 mm3, a = 13.4533(4) Å, b = 7.5536(2) Å, c = 13.6495(3) Å, α = 90.00°, β = 92.5160(10)°, γ = 90.00°, V = 1385.74(7) Å3, T = 223(2) K, space group P21, Z = 2, μ = 0.613 mm–1; 29287 collected reflections, 5803 independent reflections (Rint = 0.0275), R1 (all data) = 0.0445, wR2 (all data) = 0.1252. The absolute structure was determined by the Flack parameter x = 0.00(5) and Bijvoet pair analysis by Hooft method (Hooft y = 0.02(4), P2 (true) = 1.000, P3 (true) = 1.000, P3 (racemic twin) = 0.000).
Crystal Data of Curculigone B (2):
Orthorhombic crystal, C30H46O4, Mr = 470.67, size 0.564 × 0.053 × 0.030 mm3, a = 7.9126(2) Å, b = 31.3030(7) Å, c = 31.6212(8) Å, α = 90.00°, β = 90.00°, γ = 90.00°, V = 7832.3(3) Å3, T = 223(2) K, space group P212121,Z = 12, μ = 0.604 mm–1; 91532 collected reflections, 16472 independent reflections (Rint = 0.0714), R1 (all data) = 0.0738, wR2 (all data) = 0.1569. The absolute structure was determined by the Flack parameter x = −0.14 (8) and Bijvoet pair analysis by Hooft method (Hooft y = −0.16(7), P2 (true) = 1.000, P3 (true) = 1.000, P3 (racemic twin) = 0.000).
Crystal Data of Curculigone C (3):
Monoclinic crystal, C31H54O5, Mr = 506.74, size 0.600 × 0.097 × 0.088 mm3, a = 13.4720(15) Å, b = 7.5719(6) Å, c = 13.7835(9) Å, α = 90.00°, β = 91.428 (6)°, γ = 90.00°, V = 1405.6(2) Å3, T = 223(2) K, space group P21,Z = 2, μ = 0.618 mm–1; 25265 collected reflections, 5828 independent reflections (Rint = 0.0281), R1 (all data) = 0.0432, wR2 (all data) = 0.1242. The absolute structure was determined by the Flack parameter x = 0.08 (5) and Bijvoet pair analysis by Hooft method (Hooft y = 0.07(4), P2 (true) = 1.000, P3 (true) = 1.000, P3 (racemic twin) = 0.000).
Measurement of LPS-Induced Nitric Oxide Production and Cell Viability
As an indicator of NO synthesis, the nitrite concentration in the supernatant of RAW264.7 cells was measured according to the Griess reaction following a previously reported protocol. Cell viability of the remaining cells was determined by a MTT (Sigma Chemical Co., St. Louis, MO)-based colorimetric assay.25
Acknowledgments
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MIST) (No. 2020R1A2C1008406). The authors wish to thank the Korea Basic Science Institute for the NMR spectroscopic measurements.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c03243.
The authors declare no competing financial interest.
Supplementary Material
References
- Lakshmi V.; Pandey K.; Puri A.; Saxena R. P.; Saxena K. C. Immunostimulant principles from Curculigo orchioides. J. Ethnopharmacol. 2003, 89, 181–184. 10.1016/S0378-8741(03)00160-0. [DOI] [PubMed] [Google Scholar]
- Nie Y.; Dong X.; He Y.; Yuan T.; Han T.; Rahman K.; Qin L.; Zhang Q. Medicinal plants of genus Curculigo: Traditional uses and a phytochemical and ethnopharmacological review. J. Ethnopharmacol. 2013, 147, 547–563. 10.1016/j.jep.2013.03.066. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Li J.; Li N. Phytochemistry and pharmacological activity of plants of genus Curculigo: An updated review since 2013. Molecules 2021, 26, 3396. 10.3390/molecules26113396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. H.; Huang J.; Ma X. C.; Li G. Y.; Ma Y. P.; Li N.; Wang J. H. Phenolic glycosides from Curculigo orchioides Gaertn. Fitoterapia 2013, 86, 64–69. 10.1016/j.fitote.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Wang Z. H.; Gong X. Y.; Zhou D. J.; Xu P. F.; Huang M.; Zhang Q. L.; Meng Y. L.; Niu C.; Zhang Y. R. Three new chlorophenolic glucosides from Curculigo orchioides Gaertn. Phytochem. Lett. 2018, 26, 9–11. 10.1016/j.phytol.2018.05.005. [DOI] [Google Scholar]
- Yan J.; Guo W.; Zhou L.; Guo D.; Pei J.; Deng Y.; Zheng H.; Liu D.; Xie X.; Peng C. Three previously undescribed chlorophenyl glycosides from the bulbs of Lilium regale. Chem. Biodiver. 2021, 18, e2100403 10.1002/cbdv.202100403. [DOI] [PubMed] [Google Scholar]
- Wang K. J.; Li N. Norlignan derivatives from Curculigo crassifolia and their DPPH radical scavenging activity. Arch. Pharm. Res. 2008, 31, 1313–1316. 10.1007/s12272-001-2111-4. [DOI] [PubMed] [Google Scholar]
- He Y.; Dong X.; Jia X.; Li M.; Yuan T.; Xu H.; Qin L.; Han T.; Zhang Q. Qualitative and quantitative analysis on chemical constituents from Curculigo orchioides using ultra high performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Anal. 2015, 102, 236–245. 10.1016/j.jpba.2014.09.024. [DOI] [PubMed] [Google Scholar]
- Xu J. P.; Xu R. S.; Li X. Y. Glycosides of a cycloartane sapogenin from Curculigo orchioides. Phytochemistry 1992, 31, 233–236. 10.1016/0031-9422(91)83043-K. [DOI] [Google Scholar]
- Xu J. P.; Xu R. S.; Li X. Y. Four new cycloartane saponins from Curculigo orchioides. Planta Med. 1992, 58, 208–210. 10.1055/s-2006-961431. [DOI] [PubMed] [Google Scholar]
- Yokosuka A.; Sato K.; Yamori T.; Mimaki Y. Triterpene glycosides from Curculigo orchioides and their cytotoxic activity. J. Nat. Prod. 2010, 73, 1102–1106. 10.1021/np100111s. [DOI] [PubMed] [Google Scholar]
- Yokosuka A.; Sato K.; Mimaki Y. Cycloartane glycosides from the rhizomes of Curculigo orchioides. Phytochemistry 2010, 71, 2174–2181. 10.1016/j.phytochem.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Jiao L.; Cao D. P.; Qin L. P.; Han T.; Zhang Q. Y.; Zhu Z.; Yan F. Antiosteoporotic activity of phenolic compounds from Curculigo orchioides. Phytomedicine 2009, 16, 874–881. 10.1016/j.phymed.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Wang M.; Carver J. J.; Phelan V. V.; Sanchez L. M.; Garg N.; Peng Y.; Nguyen D. D.; Watrous J.; Kapono C. A.; Luzzatto-Knaan T.; Porto C.; Bouslimani A.; Melnik A. V.; Meehan M. J.; Liu W.-T.; Crüsemann M.; Boudreau P. D.; Esquenazi E.; Sandoval-Calderón M.; Kersten R. D.; Pace L. A.; Quinn R. A.; Duncan K. R.; Hsu C.-C.; Floros D. J.; Gavilan R. G.; Kleigrewe K.; Northen T.; Dutton R. J.; Parrot D.; Carlson E. E.; Aigle B.; Michelsen C. F.; Jelsbak L.; Sohlenkamp C.; Pevzner P.; Edlund A.; McLean J.; Piel J.; Murphy B. T.; Gerwick L.; Liaw C.-C.; Yang Y.-L.; Humpf H.-U.; Maansson M.; Keyzers R. A.; Sims A. C.; Johnson A. R.; Sidebottom A. M.; Sedio B. E.; Klitgaard A.; Larson C. B.; Boya P C. A.; Torres-Mendoza D.; Gonzalez D. J.; Silva D. B.; Marques L. M.; Demarque D. P.; Pociute E.; O’Neill E. C.; Briand E.; Helfrich E. J. N.; Granatosky E. A.; Glukhov E.; Ryffel F.; Houson H.; Mohimani H.; Kharbush J. J.; Zeng Y.; Vorholt J. A.; Kurita K. L.; Charusanti P.; McPhail K. L.; Nielsen K. F.; Vuong L.; Elfeki M.; Traxler M. F.; Engene N.; Koyama N.; Vining O. B.; Baric R.; Silva R. R.; Mascuch S. J.; Tomasi S.; Jenkins S.; Macherla V.; Hoffman T.; Agarwal V.; Williams P. G.; Dai J.; Neupane R.; Gurr J.; Rodríguez A. M. C.; Lamsa A.; Zhang C.; Dorrestein K.; Duggan B. M.; Almaliti J.; Allard P.-M.; Phapale P.; Nothias L.-F.; Alexandrov T.; Litaudon M.; Wolfender J.-L.; Kyle J. E.; Metz T. O.; Peryea T.; Nguyen D.-T.; VanLeer D.; Shinn P.; Jadhav A.; Müller R.; Waters K. M.; Shi W.; Liu X.; Zhang L.; Knight R.; Jensen P. R.; Palsson B. Ø.; Pogliano K.; Linington R. G.; Gutiérrez M.; Lopes N. P.; Gerwick W. H.; Moore B. S.; Dorrestein P. C.; Bandeira N. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang K. B.; Park E. J.; da Silva R. R.; Kim H. W.; Dorrestein P. C.; Sung S. H. Targeted isolation of neuroprotective dicoumaroyl neolignans and lignans from Sageretia theezans using in silico molecular network annotation propagation-based dereplication. J. Nat. Prod. 2018, 81, 1819–1828. 10.1021/acs.jnatprod.8b00292. [DOI] [PubMed] [Google Scholar]
- Mad Nasir N.; Ezam Shah N. S.; Zainal N. Z.; Kassim N. K.; Faudzi S. M. M.; Hasan H. Combination of molecular networking and LC-MS/MS profiling in investigating the interrelationships between the antioxidant and antimicrobial properties of Curculigo latifolia. Plants 2021, 10, 1488–1506. 10.3390/plants10081488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M.; Kang K. B.; Caraballo-Rodríguez A. M.; Nothias L.-F.; Wandy J.; Chen C.; Wang M.; Rogers S.; Medema M. H.; Dorrestein P. C.; van der Hooft J. J. J. MolNetEnhancer: Enhanced molecular networks by integrating metabolome mining and annotation tools. Metabolites 2019, 9, 144–169. 10.3390/metabo9070144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P.; Erehman J.; Gohlke B. O.; Wilhelm T.; Preissner R.; Dunkel M. Super Natural II - a database of natural products. Nucleic Acids Res. 2015, 43, D935–D939. 10.1093/nar/gku886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao W.; Chen X.; Wang H.; Lu R.; Shao H. A new hepatotoxic triterpenoid ketone from Curculigo orchioides. Fitoterapia 2013, 84, 1–5. 10.1016/j.fitote.2012.10.016. [DOI] [PubMed] [Google Scholar]
- Bifulco G.; Dambruoso P.; Gomez-Paloma L.; Riccio R. Determination of relative configuration in organic compounds by NMR spectroscopy and computational methods. Chem. Rev. 2007, 107, 3744–3779. 10.1021/cr030733c. [DOI] [PubMed] [Google Scholar]
- Jiao W.; Blunt J. W.; Cole A. L. J.; Munro M. H. G. Fumagiringillin, a new fumagillin derivative from a strain of the fungus Aspergillus fumigatus. J. Nat. Prod. 2004, 67, 1434–1437. 10.1021/np049893p. [DOI] [PubMed] [Google Scholar]
- Shannon P.; Markiel A.; Ozier O.; Baliga N. S.; Wang J. T.; Ramage D.; Amin N.; Schwikowski B.; Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spek A. L. PLATON SQUEEZE: a tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 9–18. 10.1107/S2053229614024929. [DOI] [PubMed] [Google Scholar]
- Kim J. G.; Lee J. W.; Le T. P. L.; Han J. S.; Cho Y. B.; Kwon H.; Lee D.; Lee M. K.; Hwang B. Y. Sesquiterpenoids from Chrysanthemum indicum with inhibitory effects on NO production. J. Nat. Prod. 2021, 84, 562–569. 10.1021/acs.jnatprod.0c01121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.