Abstract
Driven by the possibility of precise transformational change in nutrient-enrichment technology to meet global food demand, advanced nutrient delivery strategies have emerged to pave the path toward success for nutrient enrichment in edible parts of crops through bioderived nanocarriers with increased productivity. Slow and controlled release of nutrient carrier materials influences the nutrient delivery rate in soil and in the edible parts of crops with a sluggish nutrient delivery to enhance their availability in roots by minimizing nutrient loss. With a limited understanding of the nutrient delivery mechanism in soil and the edible parts of crops, it is envisaged to introduce nutrient-enrichment technology for nutrient delivery that minimizes environmental impact due to its biodegradable nature. This article attempts to analyze the possible role of the cellulose matrix for nutrient release and the role of cellulose nanocomposites and nanofibers. We have proposed a few cellulose derived biofortificant materials as nutrient carriers, such as (1) nanofibers, (2) polymer–nanocellulose–clay composites, (3) silk-fibroin derived nanocarriers, and (4) carboxymethyl cellulose. An effort is undertaken to describe the research need by linking a biopolymer derived nanocarrier for crop growth regulation and experimental nitrogen release analysis. We have finally provided a perspective on cellulose nanofibers (CNFs) for microcage based nutrient loading ability. This article aims to explain why biopolymer derived nutrient carriers are the alternative candidate for alleviating nutrient deficiency challenges which are involved in focusing the nutrient delivery profile of biopolymers and promising biofortification of crops.
1. Introduction
Conventional agricultural practices may no longer be a sustainable option to meet the increasing food demands for a growing population without damaging the environment.1 Sustainable agricultural techniques need to be adopted in every sector as urgently as possible to mitigate some of the growing hurdles of toxic pesticides, resistant pests, and increasing soil contaminants and more importantly address the challenges of micronutrient deficiency in soil.2 Modern agriculture is seeking alternatives for the use of agrochemicals through implementation of nanotechnology with bioderived nanomaterials to achieve precision farming that aims at increased productivity with minimal resources.3 Two major areas where nanotechnology can contribute to agriculture cropping are improving crop yields and increasing resource utilization efficiency. This is an exciting and promising area that contains a scope of rapid expansion by means of improved understanding of fundamental interactions between plants and engineered nanomaterials.4 Nature derived polymeric nanomaterials can be utilized in varying applications including nanoherbicides, nanodetectors, and nanofertilizers to resolve the conventional challenges of agriculture.5 For instance, nanocarriers are employed to carry and deliver pesticides in a more controlled and slow release profile to achieve “precision farming”, which targets major crop production with nutrients in edible parts without affecting water and soil resources.6 Major global health challenges arise within one-third of the population from micronutrient deficiencies such as zinc (Zn), iron (Fe), iodine(I), selenium (Se), and vitamin A which are simply due to the lack of availability. Low dietary diversity and over-reliance on staple crops have led to a situation where we expect nanotechnology to potentially offer micronutrients more directly into the edible part of a crop.7,8 Micronutrient deficiencies are generally problems in the region where soil contains low plant available micronutrients such as Zn deficiencies in food products.9 Staple cereal grains, such as wheat, are, however, inherently low in micronutrient concentration and bioavailability to adequately attain human nutritional requirements.10 Molecular and genetic research into Zn uptake, transport, and grain deposition in cereals is critically important for identifying “bottlenecks” in the biofortification of food crops with Zn.11 Zinc deficiency in soil potentially affects millions of hectares of cropland globally in cereal-growing regions.9,12 Therefore, increasing the level of Zn uptake by crops needs further investigation.13 Transgenic strategies for the biofortification of cereals with Zn are still in their infancy for enhanced root uptake, transport, and grain accretion capacity for Fe or Zn or both in recent work.14,15 Providing crop plants with sufficient Zn through the soil and foliar fertilizer strategy under field conditions is essential for biofortification efforts.16−18
As compared to traditional fertilizers, not only can cellulosic biopolymer based slow release nanocarriers currently improve the nutrient conversion ratio, but also their degradation by soil microorganisms occurs at a slower rate which creates a controlled release profile.19 Biofortification of staple foods can be achieved through a sequence of conventional breeding, by selecting genotypes with the highest micronutrient content.20,21 For example, in a lignin based slow release system, a lignin coating layer offers hydrophobicity that can slow down dissolution and release of micronutrients in the soil.22 In the case of slow release fertilizers, various natural, synthetic, or biological coatings act as a barrier to restrict or optimize the translocation of fertilizer nutrients into soil solution.23
2. Controlled Release Mechanism of Nutrients
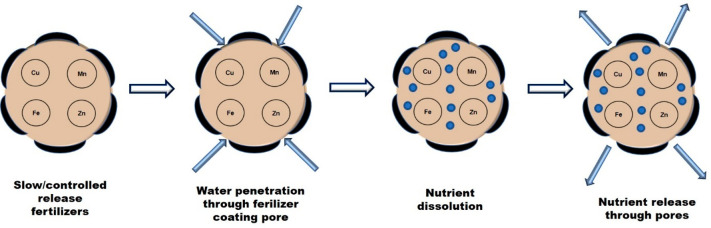
The nutrient release rate is controlled by the preparation method and ratio of the coating layer. For example, the hydroxyl and carbonyl groups of lignin may generate insoluble chelates and complexes with Fe and Zn metal ions.22 However, the slow release mechanism is challenging to visualize.24 To understand the effects of lignin on retarding the dissolution of urea (i.e., release of urea), the properties of lignin obtained from the pulping spent liquor were investigated to realize the water penetration and dissolution of urea from the impregnated straws.25 Conventionally, multimicronutrient slow release fertilizers of zinc, iron, manganese, and copper were introduced.26 The rate curve reveals a multistage process with linear rates at each stage. In recent times, several nanoscale nutrient delivery systems and their interaction mechanisms with active ingredients were found to act through (a) encapsulation, emulsion, entrapment, surface adsorption, (b) crop field application of nanoscale delivery agents, and (c) nutrient release from nanoscale delivery agents. as shown in Figure 1. Generally, micronutrient release from a carrier occurs via water penetration, nutrient dissolution, and nutrient release through porous channels as shown in Figure 2. Toward this pathway, for example, alginate biopolymer rapidly cross-links to divalent cation Ca2+ by forming a rigid shell which entraps small molecules in the core, which enhances the porosity of the granules.27 This incorporation affects the morphological features, which leads to different combinations of the mechanism of nutrient delivery via polymer relaxation and diffusion through porous architectures.
Figure 1.
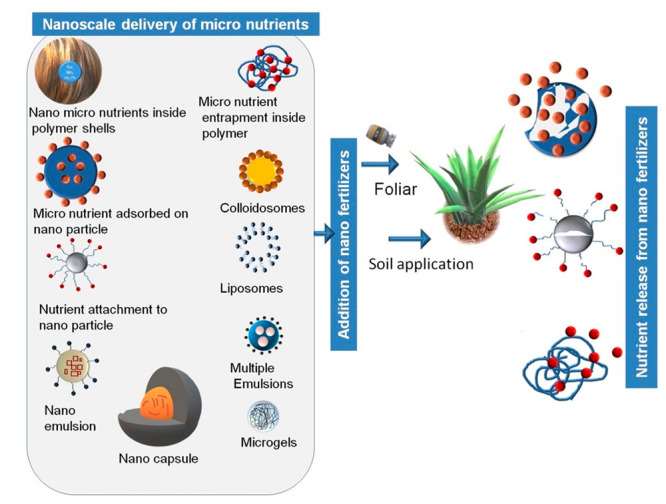
Nanoscale nutrient delivery systems’ encapsulation, emulsion, entrapment, surface adsorption, binding of active ingredients, field application of nanofertilizers, and nutrient release from nanofertilizers.
Figure 2.
Controlled release of micronutrients from slow release fertilizers via multiple steps.
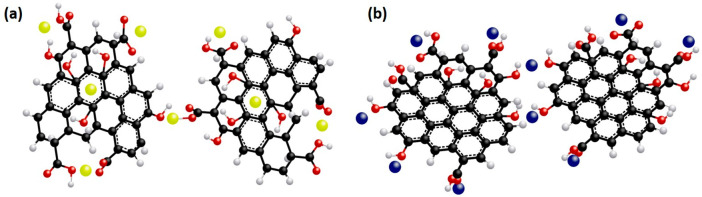
We try to understand the controlled release of micronutrients via the following steps: (a) controlled release of a fertilizer with a porous surface architecture, (b) penetration of soil water through the surface pores of controlled release fertilizers (CRF), (c) dissolution of nutrients through soil water, and (d) release of dissolved nutrients. It was observed that graphene oxide (GO) sheets are loaded with the micronutrients Cu or Zn metal ions attached to the oxygen functional groups on the surface and edges of the sheets.28 This significant difference in the release patterns of the micronutrients from the GO based carriers compared to the ZnSO4 and CuSO4 salts is partly due to tight coordination of the metal ions and oxygen functional groups on the GO surface.29 In the cases of Zn2+ and Cu2+, the Cu2+ tends to bind in a syn conformation with oxygen containing functional groups (e.g., carboxylate groups), whereas Zn2+ ions are more likely to bind in a direct conformation, while they are sharing two oxygen atoms of the same carboxylic group (Figure 3).
Figure 3.
(a) Schematic diagram of GO sheets’ interactions with (a) Zn2+ ions and (b) Cu2+ ions (Originally drawn images following Figure 7a and b from ref (28). Copyright American Chemical Society 2017).
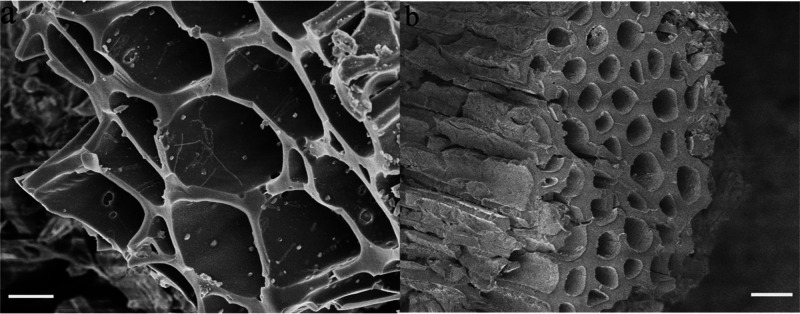
The similar porous surface architecture of biochar based controlled release nitrogen fertilizers (BCRNFs) with water retention was derived via a hydrothermal method and characterized their physicochemical and morphological properties.30 The N-release profile (biochar nitrogen fertilizer (BNF-2), biobased biochar nitrogen fertilizer (BBNF), and BCRNF) following a parabolic diffusion model indicates that the release involves a combination of dissolution, adsorption, and diffusion processes.31 As revealed from SEM based microstructure analysis of biochar+urea (B+U), postnutrient release in water results in a smooth and strongly networked surface morphology with microholes (Figure 4a). However, an undulating and coarse surface was evident for BCRNF including nanoscale rough bulges (Figure 4b).
Figure 4.
Drawn pattern from the SEM images of (a) B+U and (b) BCRNF in the postnutrient release phase. (Adapted in part with permission from ref (30). Copyright 2019 Springer Nature.)
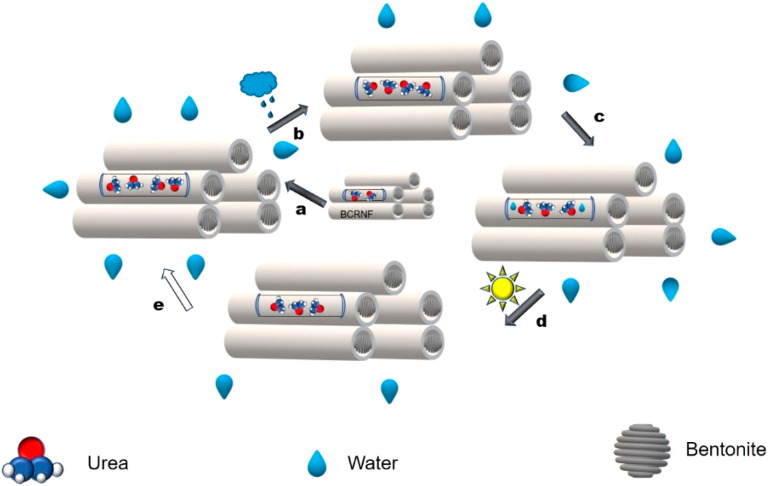
It is proposed that the nutrient release mechanism for BCRNF involves adsorption of biochar and bentonite followed by multistage diffusion as distinct controlled release processes (Figure 5).30 Initially granules adsorb moisture, resulting in swelling of bentonite at the orifice of pores and channels in the biochar. A subsequent buildup of osmotic pressure and irrigation water penetrates the channels of the biochar to condense on the solid fertilizer. This is followed by diffusion based slow release of the nutrient solution under a concentration or pressure gradient.
Figure 5.
Controlled release mechanism of biochar in soil: (a) granules–moisture interaction, (b) adsorption of moisture and swelling of bentonite, (c) nutrient dissolution and release via diffusion, (d) dehydration of bentonite and stored nutrient solution diffusion into soil, (e) adsorption of moisture under higher osmotic pressure. (Adapted in part with permission from ref (30). Copyright 2019 Springer Nature.)
There are a series of chelated micronutrient fertilizers that were employed in the past as conventional fertilizers with a maximum chelation rate of iron (Fe), copper (Cu), and manganese (Mn) under acidic pH and with maximum chelation of zinc (Zn) under alkaline pH.32 Chelated zinc, soluble and coated fertilizers for zinc nutrition of maize, i.e., fertilizers containing either Zn-EDTA or Zn-ligno-sulphonate (Zn-LS) which were fixed over pellets of urea and then coated, had the advantage of adding both Zn and N in a single dose with a coating for the slow release feature for the availability of Zn in soil.33 Zinc and manganese micronutrient applications with an increase in Zn ratio in compound foiler nitrogen phosphorous pottasium (NPK) fertilizer for the growth, yield, and quality of corn plants were reported which are sensitive to the zinc (Zn) deficiency in soil.34
3. Neutraceutical Properties of Crops
Chitosan nanoparticles may be used as a delivery system for micronutrients to crops, and these chitosan nanoparticles possess antimicrobial activity.35 Maize waste mainly consists of lignin and cellulose that can be useful36 and corncob biochar based nanocomposites and can be successfully used as carriers of microelements. On a smaller scale, arabic gum37 and active carbon composites38 can be used as carriers for micronutrients. Slow release fertilizers have been reported to increase the yield and vitamin C content in potatoes and were proven to be an effective source of micronutrients and superior over conventional micronutrient sulfates. The good response to the slow release fertilizer was due to the facts that sufficient Mo is released at the initial stages (20–50% over 3 weeks) and the root nodule activity is higher. Slow release Mo fertilizer increased nodulation by 105–161%.39
Chillies (Capsicum frutescens) grown on a black soil showed excellent response to slow release Fe–Mn fertilizer. At 2 kg/ha Fe and 1 kg/ha Mn, the yield increased by 179% compared to the control (no Fe–Mn fertilization). The average Fe uptake and vitamin C in chillies were observed to be higher in slow release fertilizer treatments.40 Rana et al.41 reported that the growth attributes and quality parameters of cabbage significantly increased with the application of biofertilizers and micronutrients. Fertilizer release control and soil property improvement are two major issues for which multicomponent and multifunctional sustained release zinc fertilizer was derived from lignosulfonic acid waste.42 If we survey the global annual micronutrient production trend in metric tons as per each year of data which were averaged from two previous years from the US Geological Service for the micronutrients (Figure 6),43,44Figure 6 shows that, other than boron, the production of micronutrients has shown a significant increase over the years.43,44Table 1 presents a series of the selected most promising research results of the application of Engineered nanomaterials (ENMs) as nanofertilizers.
Figure 6.
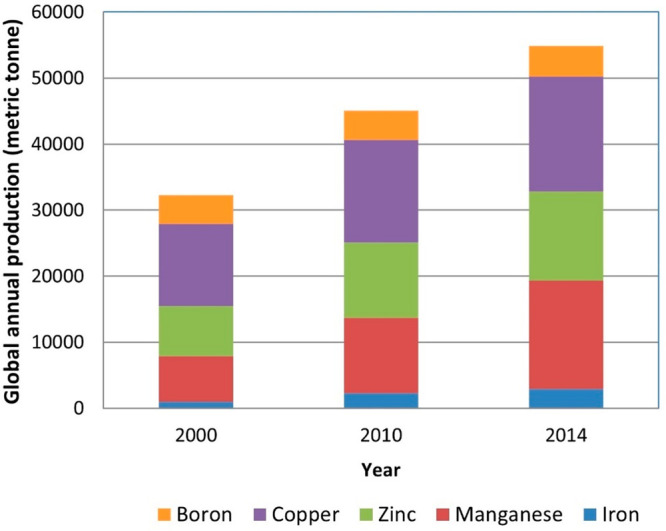
Global annual micronutrient production trend in metric tons against years. (Adapted in part with permission from ref (43 and 44). Copyright 2015 and 2016 Springer Nature.)
Table 1. Series of Different Types of ENMs Exhibiting Nanofertilizer Potential.
| Nanomaterial | Concentration | Plant | Application type | Details | Ref |
|---|---|---|---|---|---|
| ZnO | 6 mg kg–1 | Sorghum | Root and foliar | Improved plant productivity and stimulated grain nutritional values and N use efficiency, compared with untreated control | (45) |
| 2–16 mg L–1 | Tomato | Root | At 8 mg L–1, shoot length (35.8%), root length (28.6%), leaf area (27.9%), antioxidant activities; proline content (65%) and photosynthetic rate increased, compared with control | (46) | |
| 25–200 mg L–1 | Cotton | Root | Significantly increased growth (131%), total biomass (131%), total chlorophyll (139%), carotenoids (139%), total soluble protein contents (179%), compared with untreated control | (47) | |
| Zn–chitosan | 20 mg g–1 (w/w) | Wheat | Foliar | Enhanced Zn uptake; about 27 and ∼42% increase in the two wheat varieties, compared to the control | (48) |
| Fe2O3 | 0.25–1 g L–1 | Soybean | Foliar | Increased grain yield by 48%, compared to control | (49) |
| 100–200 mg L–1 | Spinach | Root | At 200 mg kg–1, the plant biomass and Fe uptake increased in the plant, compared to control | (50) | |
| 50–800 mg L–1 | Tomato | Root | Enhanced seed germination, increased plant growth and total biomass, compared to control | (51) | |
| FeS | 2–10 mg L–1 | Mustard | Foliar | Induced growth and yield of plant and increased antioxidant enzyme activities, compared to control | (52) |
| CuO | 0.02–8 mg L–1 | Maize | Root and foliar | Both solution culture and foliar exposure enhanced maize growth (51%) and regulated different enzyme activities, compared to control | (53) |
| 10–500 mg L–1 | Tomato and cauliflower | Root | Root length (18%), chlorophyll (14%) and sugar (7%) contents increased in tomato plant at 10 mg L–1, compared to control. Concentration dependent increase in antioxidant enzyme activities, and lignin deposition observed. | (54) | |
| Cu–chitosan–PVA | 0.02–10 mg kg–1 | Tomato | Root | At 10 mg kg–1, tomato yield (17%), stem diameter (13%), and dry biomass (30%) increased. At 0.02 mg kg–1, lycopene content, and antioxidant capacity (10%) increased, compared to control | (54) |
| Cu–chitosan | 0.06 g L–1 | Tomato | Root | Enhanced plant growth (21–29%) and yield (30%), stomata conductance (7%), and increased the leaf catalase (462%) and fruit lycopene content (12%), compared to control | (55) |
4. Controlled Release Time Scale of Biopolymers
Zn, ZnO, Cu, and CuO nanoparticles were identified as alternatives for seed coating and foiler application based nutrient delivery.56 Therefore, we got to investigate how immobilization of ZnO NPs on biopolymers will possibly slow down Zn release.57 Tensile properties can be modulated in a formulation with a biopolymer from lignin which offers a barrier to phosphorus diffusion through triple phosphate (TSP) fertilizer.58 Tensile strength can be varied by using PEG, which increases the tensile strength of polysaccharide film composites. In this regard, slow release of dicyandiamide poly(hydroxybutyrate-co-hydroxyvalerate) (DCD-PHBV) pellets was fabricated using a laboratory scale based on the diffusion and biodegradable nature of the PHBV matrix.59 In another strategy, superhydrophobic biopolymer coated slow release fertilizers (SBSFs) display formidable slow release. Nutrient release is influenced by the slow diffusion of water vapor into the internal urea core of the SBSF, which enhances the slow release feature.60 A strategy of using urease inhibitor helps in increasing the efficiency of fertilizers, which is further enhanced by an encapsulation process by an appropriate biopolymer to control the nitrogen release behavior on soil of the biopolymer urea fertilizer, in which case chitosan/starch/Allicin/urea cross-linked biopolymers were prepared.61
5. Cellulose Matrix for Nutrient Release
Encapsulation in a chitosan alginate nanocarrier as nanoencapsulation in chitosan nanoparticles to reduce the toxicity of herbicides and chitosan alginate nanocarriers retards the rapid release of the water-soluble insecticide acetamiprid.62 Chitosan and hydroxyapatite (HA) were two naturally occurring forms used for micro- and macronutrient delivery to soil, respectively. The natural cellulose and other biopolymer pores in wood stems can be used for storing nanofertilizers for N and other element release under different soil samples.63 The major focus of the early stage investigation was the process of nitrogen release by fertilizer with cellulose nanofibrils which prevents the fertilizer granules from sticking onto each other.64 It was found that matrix based fertilizer delivery reduced nutrient leaching while maintaining growth of crops.65 For example, matrix based fertilizer (MBF) helps formulations of both anionic and cationic compounds such as in the case of Al(SO4)2·3H2O and/or a Fe2(SO4)3·3H2O–lignin–cellulose matrix. While using these MBFs for addition of pesticides to soil, the ion-exchange matrix will likely bind metochlor and diazinon to the starch–cellulose–lignin matrix, which reduces leaching for both pesticide and nutrients, thereby creating a connection between an effective biofortificant and the cellulose based matrix.66 The porous architecture spans length scales from the micro- (within particles) to the macro- (within the polymeric matrix) levels, leading to tunable patterns of nitrogen release.27
The cellulose matrix has been widely used for either thickening or gelling agent emulsion stabilization.67 It is possible to create nanoscale capsules for cellulose for protection and hosting micronutrients for delivery in soil.68 In a recent study, it was shown that slow and sustained release of nitrogen fertilizer is dependent on a natural cellulose based outer core containing micro-/nanoporous cavities consisting of two sections: (1) an inner nanocore for micronutrient nanoparticles and (2) a natural cellulose based outer core containing micro-/nanoporous cavities.69 Among these combinations, a ZnO NP/alginate composite offers a steady Zn delivery in soil pores while avoiding the early stage Zn toxicity induced by conventional methods of Zn delivery.57 Evidence in favor of the mechanistic side of nutrient release is not easily derivable; however, the limited scope in comparing the nutrient release mechanism is described herein. In this process, the uptake and translocation of nanomaterials or nanocarriers of nutrients within plants develop nanoenabled biofortification.70 The urea loaded hydroxyapatite nanocarrier delivers plant nutrients efficiently for rice production therein. Urea doped hydroxyapatite nanoparticles (Ur@HANP) was also tested in sand columns and agricultural soil for understanding the retention capacity of plant nutrients.71,72 A combination of faster and slow release behavior of nutrient release attains an equilibrium after a well-defined number of days which was witnessed for mesoporous silica nanoparticles (MeSiNPs) with a water-soluble N-polymer.73 The FeII content of ≥37 mg kg–1 in rice plants (on a dry weight basis) at 60 days after sowing was found to be a guide value for monitoring the Fe-nutrition status of rice.74 In a study of the aluminum–organic acids interaction in the rice rhizosphere in acid soil, the major organic acids, total weak acid concentration, and monomeric and polymeric Al were identified and quantified in the rhizosphere and nonrhizosphere of rice.75−77
6. Cellulose Nanocomposite and Nanofiber
The controlled release of micronutrients for biofortification of soil by cellulose nanocomposite and nanofibers was never known until recently.78 Nanocellulose derived hydrogels based on electrostatic interaction result in ionic gelation, molecular assembly, and chemical cross-linking based hydrogels with heterogeneity in linking density.79 Nanocellulose is now used in fertilizers for precision agriculture by developing superadsorbent nanocomposites by using hydrolyzed polyacrylamide and methylcellulose.80 In this context, nanocellulose can promote high water absorption capacity, biodegradability, and slow release of inputs due to the “obstruction effect” and imprisonment of the input in the percolation network or “locking effect”. However, nanocellulose remains an obstacle for agriculture application due to the cost barrier.81
In general, cellulose nanomaterials in the form of cellulose nanofibers (CNFs) and cellulose nanocomposites (CNCs) exhibit high stiffness and specific surface area and low density.82 The inorganic nanoparticle encapsulations of Zn, Cu, Fe, cerium (Ce), and titanium (Ti) are extensively studied both in laboratory and field conditions.83 Biopolymeric nanocarriers of natural polymers, chitosan, and pectin were developed to provide a sustained and controlled release of encapsulated carbendazim with good bioefficacy and inhibition against fungi such as Fusarium oxysporus and Aspergillus parasiticus. Mostly, nanocellulose derived materials as delivery agents remain within certain morphology types such as films/nanofibers and nanoparticles which help in nanoencapsulation in agriculture applications.8485 Drug releasing approaches by cellulose based materials are known of with carboxymethyl cellulose/Cu biocomposite hydrogels with pH sensitive swelling ratios with promising results from drug release tests in vitro.(86)(87) In the process of drug release, entanglement of individual particles in partially fibrillated microcrystalline cellulose led to formation of an elastic network.88 First order and zero order drug release profiles compared to the optimum filament were achieved by using a hydroxypropyl cellulose (HPC) blend with ethyl cellulose (EC).89
7. Cellulose Derived Biofortificants
The effects of a combined spray of Zn and Fe on the grain concentrations of different crops grown on a range of soil types and under different environmental conditions are not known. Slow mineralization of N in T1 (cellulose-g-poly(acrylamide)) MN content and higher C contents is known.64 Similarly, a recycled cellulose fiber and clay saturated with micronutrient copper ions and copper nanoparticles transformed into a mineral cellulose fiber carrier.90 Therefore, we propose a number strategies to obtain cellulose and similar biopolymeric composites.
7.1. Cellulose Nanofiber
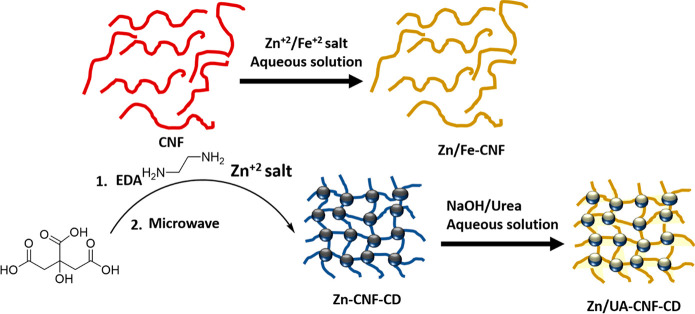
The tool of heterogeneous amphiphilic interactions between materials of biological and synthetic origin will be implemented for deriving a self-assembled cellulose nanofiber (CNF) composite filled with Zn-EDTA or Zn-lignosulfonate with an extension to cellulose nanofiber (CNF) with ethylene diamine (ED) in the presence of Zn2+ salt (Scheme 1).91,92
Scheme 1. UA Coating on CNF Assembly upon Loading Nutrients Zn.
7.2. Polymer–Nanocellulose–Nanoclay
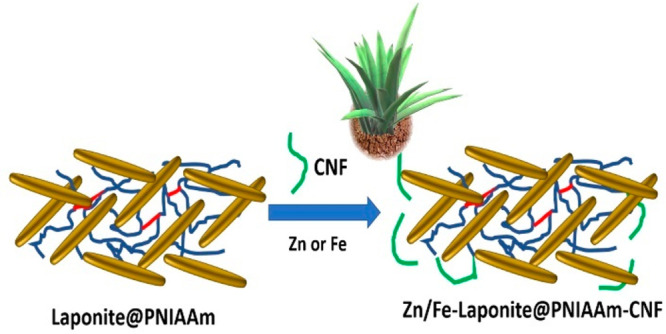
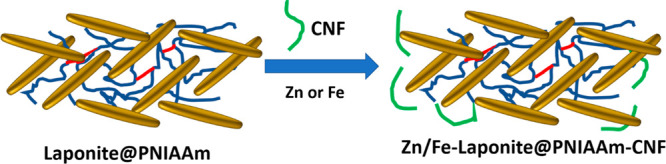
A uniform bonding of hydrophilic poly(N-isopropylacrylamide) (PNIAAm) and CNF occurs through hydrogen bonds, in which PNIAAm would undergo conformational change above the lower critical solution temperature (LCST) where the hydrogel film engages with both hydrophobic PNIAAm and hydrophilic CNF.93 Poly(N-isopropylamide) (PNIPAm)/CNF films can be prepared through a photoinitiated radical polymerization with 1.5 wt % photoinitiator (2-hydroxy-2-methylpropiophenone). PNIPAAm chains are fully extended and locked through interchain hydrogen bonding below the LCST (Scheme 2). This strategy can further be modified by a PNIPAAm/CNF-Laponite composite (Scheme 2).
Scheme 2. Dual-Charged Nanoclay Laponite Scaffold Directed PNIAAM/CNC-Laponite Composite for Fe and Zn Delivery.
7.3. Silk Fibroin Based pH Responsive Composite
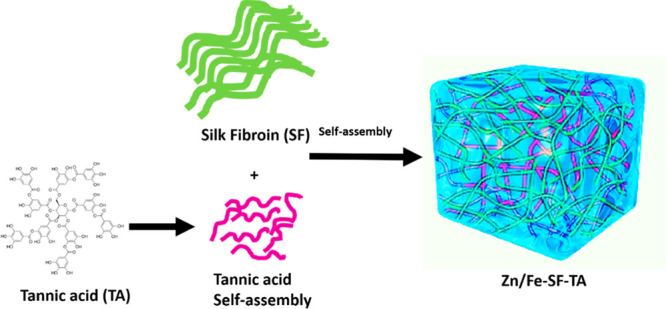
We envisaged construction of a silk fibroin (SF) based self-assembly by using tannic acid (TA), a biocompatible molecular glue to gelation with SF through hydrogen bonding, hydrophobic interactions, and π–π stacking. This process of sol–gel transition of SF under physiological conditions (37 °C, pH 7.4) can generate a supramolecular assembly (Scheme 3).94,95
Scheme 3. Illustration of the Process for Preparing the SF–TA Composite for Loading Zn and Fe.
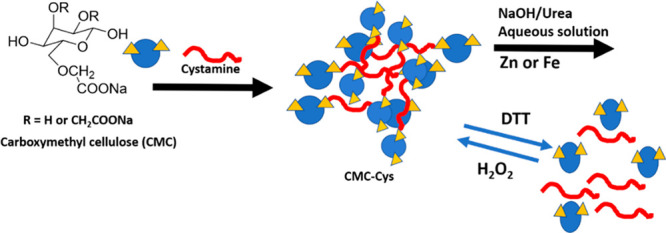
7.4. Redox Responsive Carboxymethyl Cellulose (CMC)
Considering a lower redox potential (equilibrium Eh −20 mV) due to the low oxygen content in the soil, a large amount of reductive substances accumulates in the soil. Reductive substances in soil provide “‘triggers’” for redox responsive hydrogels for controlled release of nutrients.96,97 CMC was cross-linked by cystamine dihydrochloride (CYS_2HCl) in the presence of 1-(3-(dimethylamino)propyl)-3-ethylcarbodiimidehydrochloride (EDC) and N-hydroxysuccinimide (NHS) (Scheme 4).
Scheme 4. Gelation Strategy of CMC-Cyst Formation for Nutrient Delivery to Soil and Redox Responsive Behavior of CMC-Cys via Regeneration under H2O2.
8. Cellulose Based Slow Release Agents
For the cellulose derived nanofiber class of materials as nanoenabled carriers for nutrient release, previous studies of different kinds of nanostructured materials as nutrient carriers such as nanoclays, hydroxyapatite, nanoparticles, mesoporous silica, carbon nanomaterials, and polymeric nanoparticles are more known.98 Recent developments of superadsorbent nanocomposites use hydrolyzed polyacrylamide and methylcellulose reinforced with montmorillonite for the loading and slow release of agricultural nutrients including macronutrients (urea) and micronutrients (sodium octaborate).80 Such strategies have also offered formation of rigid hydrogels due to formation of a percolation network capable of avoiding the conversion of type I CNC into type II, which is more lyophilized.99 The incorporation of nanocellulose into the hydrogel type of nanocarrier formulation promotes improvements in features and a significant improvement in the mechanical properties as a function of particle–particle and particle–polymer interactions.79 The cellulose derived carriers for micronutrient delivery will involve controlled release systems through CNC composites (e.g., CNC-alginate microsphere) with consistent swelling patterns, higher encapsulation efficiency, and promising sustained release profiles of micronutrients which would sort of resemble drug delivery.100 It is claimed that highly ordered mesoporous channels have been created with a pore size of ∼2.9 nm in mesoporous silica nanoparticles (MeSiNPs) and with a source of N-nutrients which involves a water-soluble branched polyethyleneimine (bPEI) attachment.73 In such a case secondary interactions such as hydrogen bonding and van der Waals forces dominate the release mechanism of pesticides.101
9. Perspective on Cellulose Nanofibers (CNFs) as Nanocarriers
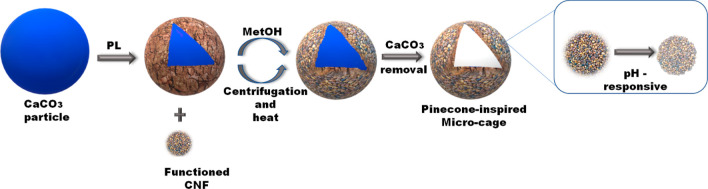
Microcage type tunable porous structures with hydrophilicity, mechanical stability, and pH triggering features can bring required kind of on-demand nutrient loading and release delivery systems.102 This opens a new avenue for such size based micronutrient delivery based on the polymer network of CNFs.103,104 A schematic (Figure 7) shows the formation process of the CNF architecture with microcage for microsized nutrients.102
Figure 7.
Schematic showing the formation process of a CNF architectured microcage for large-sized drug delivery. (Adapted with permission from ref (102). Copyright 2020 Wiley-VCH.)
Nanoencapsulation involves coating of chitosan, liposome, polylactide, and lipids and protein nanoparticle encapsulation.105 Therefore, effective translocation of nutrients is carried by a CNF colloidal dispersion to the shoot and root length and chlorophyll by a significant increase of the protein content with effective translocation.106 To design new generation nanocarriers for the biofortification of nutrients, not only controlled release capabilities for effective nutrient release but also mitigation of harmful effects on the environment and human health is a prerequisite.107 Either for crop growth regulation of pest control, for efficient utilization of fertilizer, a nanoplatform should satisfy several requirements: (1) biodegradability, (2) higher nutrient or fertilizer loading capacity, (3) flexible response to stimuli in the external environment (pH, light, temperature), and (4) a stable delivery system.
10. Research Needs in Specific Areas
Nanoclays, hydroxyapatite, nanoparticles, mesoporous silica, carbon based nanomaterials, polymeric nanoparticles, and similar other nanomaterials offer their nutrient loading capacities and rapid nutrient release profiles.108 The choice of nanoenabled carrier to deliver nutrients for biofortification depends on a few factors, which include the following: (1) holding of a large amount of nutrients, (2) a suitable release rate, and (3) minimizing nutrient conversion to non-bioavailable forms.
The set of data in Table 2 suggests various modes of releasing agricultural chemicals.
Table 2. Nanocarriers for Crop Growth Regulation Based on Macro- and Micronutrient Delivery.
| Entry | Nanocarrier | Fertilizer | Role | Ref |
|---|---|---|---|---|
| 1 | SA@MSN-SS-C10 | Phytohormone SA delivery | An increasing release of SA was observed in the presence of GSH due to the cleavage of disulfide bonds between decanethiol and MSNs. | (109) |
| 2 | [(Ca10(PO4)6(OH)2] NPs (HA NPs) | Slow release of nitrogen to crops | Urea-HA NPs revealed a slow and sustained release behavior, which released 86% urea within 55 min and with a long-term drug release up to 1 week | (63) |
| 3 | Superhydrophobic BPU coated fertilizer (SBPCF) | Nitrogen released | Biobased polyurethane (BPU) coating containing numerous hydrophilic groups and microholes coat urea prills | (110) |
| 4 | Hollow mesoporous carbon NPs (HMCNs) and cationic polymer (polyethylenimine) coated PHMCN | Selenate from PHMCN-Se under alkaline pH | Release of selenate from PHMCN-Se could also be stimulated by anions including PO43+, CO32–, and OH– | (111) |
| 5 | Gibberellin acid (GA3) hormone delivery system based on water-soluble carboxylatopillar [5]arene ammonium (WP[5]A) functionalized Fe3O4 NPs (WP[5]A-Fe3O4) | Bidirectional pH-responsive capability under pH < 4 or pH > 5 conditions | The lengths of stems and roots of cabbages increased obviously after GA3-HMSN/Fe3O4 treatment for 5 d | (112) |
| 6 | Hydrophobic carboxymethyl cellulose (HCMC) and 3,3′- dithiobis(propionohydrazide) | pH- and redox-dual responsive 3D nanogel (HCMC) | Simultaneous release of SA and remediation of soil | (113) |
| 7 | Nano U-NPK | Ca, P, K, urea, and NO3 – from the nano U-NPK | Macronutrients to durum wheat via a controlled manner by slow release behavior | (114) |
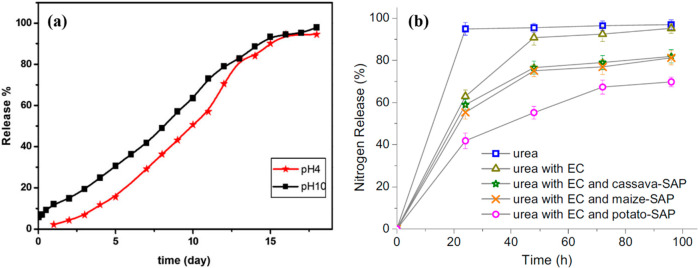
In comparison to urea–HA nanohybrids with slow release behavior with use efficiency of nitrogen ∼48% (Table 2, entry 2), a further increase of nitrogen release up to 80% was achieved via a coating strategy (Table 2, entry 3). The future prospect of micronutrient delivery to the edible parts of crops depends on several factors, as revealed from the results in Table 2. To enhance the effectiveness of fertilizers, core ethyl cellulose (EC) as an inner coating and cellulose based superabsorbent polymer (SAP) with the biochemical inhibitors dicyandiamide (DCD) and thiourea as an outer coating,115 bromoacetylated cellulose cross-linked with urea hydrogel,116 carboxymethyl cellulose-Na+-g-cl-poly(AAm) hydrogel,117 and superabsorbent polymers SAPWS (grafting wheat straw (WS) to poly(acrylic-co-acrylamide))118 are known. In addition, ethyl cellulose (EC) as an inner coating and starch based superabsorbent polymer (starch-SAP) as an outer coating with a stretched 3D network119 and carboxymethyl cellulose-g-poly(acrylamide)/montmorillonite superabsorbent composite were reported.120 A comparison is shown between the release behavior of urea from superabsorbent composite with 2.7% montmorillonite at pH 4 and 10 (Figure 8a))120 and the release behavior of pure urea particles and coated products in soil at ambient temperature (Figure 8b).119 The nutrient release from the fertilizer involves three main stages: (i) water imbibition into the starch-SAP via penetration through the EC layer; (ii) the urea core being gradually dissolved by water; and (iii) nutrient delivery to soil by penetration through the layers of EC and starch-SAP hydrogel.
Figure 8.
(a) Nitrogen release behavior of urea from superabsorbent composite with 2.7% montmorillonite at pH 4 and 10. (b) Release behaviors in oil for pure urea particles, urea particles coated with ethyl cellulose (EC) (Reprinted with permission from ref (120). Copyright 2018 Wiley-VCH), and urea particles coated with EC plus starch based superabsorbent polymer (SAP) (Reprinted with permission from ref (119). Copyright 2016 Elsevier).
12. Summary and Outlook
The nanoenabled technology for crop growth regulation includes pesticide detection, mycotoxins’ detection, phytopathogen inactivation, and pest control for improving agricultural nanotechnology for biofortification.121 The specific agricultural applications include micronutrient delivery for replacement of mesoporous silica with biopolymers for the cargo delivery system. The biopolymeric carrier systems contain the following features: (1) improved utilization efficiency of nutrients from leaves, stems, petioles, and roots and (2) functionalization by gated materials to respond to exogeneous and endogenous stimuli such as pH, light, temperature, and enzymes. In the process of nanocarrier implementation for micronutrient delivery to breeding plants, global agricultural techniques can be integrated which include mutagenesis, genetic modification, organic farming, pesticides, and organic fertilizer management. There are limited studies on toxicity analysis and long-term monitoring of the degradation process of nanomaterials used for biofortification and analysis of the ultimate impact on the environment. There are limited data available on the premature release of micronutrient cargoes during storage in the nanocarriers. Therefore, effective design principles for the surface to lock the micronutrients into the porous network for long-term protection of fertilizers are key challenges.122,123 Therefore, future research must address the following conditions for macro- or micronutrient release: (1) fluctuating temperatures and (2) fertilizer placement.
Nanonutrient carrier based delivery of micronutrients may also affect the inhibition of chlorophyll synthesis and photosynthetic efficiency. Micronutrient fertilizer may offer an inhibitive effect on the dissolution rate of nutrient nanoparticles such as ZnO nanoparticles. Therefore, controlled decrease of the nanoparticle size depends on precise surface engineering of nanoparticles, which offers better control of dissolution; however, sometimes it increases the toxicity of the system in the case of micronutrient formation. Even though engineered nanoparticles can generally enhance plant growth and be effective for suppressing disease, however, regarding crop damage for root fungal pathogens and other similar kinds of sudden death syndrome, nanoenabled modulation of the nutrition and transport systems of plants has not been developed by using engineered nanoparticles. Besides nanoscale metal particle induced disease suppression and plant growth, however, effective delivery of critical micronutrients at the early stages of plant growth is further challenging.124 The feature of bioaccumulation of metal nanoparticles within the plants and crops is one of the major concerns that involves particle translocation to edible tissues. The particle size and shape dependent phytotoxicity can cause a potential risk to the environment unless pursued under caution. Even when zinc micronutrient plays a significant role, however, Zn phytotoxicity in plants results from Zn interference in chlorophyll biosynthesis and additional biochemical reactions by causing iron deficiency chlorosis as a result of excessive Zn in soil. An adverse modification of protein, lipis, and nuceic acid content by generation of unwanted radical species cannot be ruled out as a result of Zn-NPs accumulation due to the uncontrolled delivery pattern of Zn micronutrients.125 Apart from bioaccumulation and phytotoxicity related issues, reversal of photosynthesis parameters as a results of the antioxidant activity level change due to accumulation of nanoparticles as a result of nutrient delivery is a matter of consideration as a coexposure effect.
This review attempts to foresee where the current situation is heading and possible solutions if we rely on natural polymeric nanostructural carriers for the delivery of micronutrients. In this context, we revisited the origin of slow release techniques of micronutrients and recollected knowledge of using advanced slow release carriers. We have proposed a few strategies and derived a perspective on cellulose nanofiber based nutrient carriers for biofortification.126
Acknowledgments
S.D. acknowledges research funding support by the Department of Biotechnology, Ministry of Science & Technology, Government of India, for grant number BT/RLF/Re-entry/41/2017 under DBT Ramalingaswami Re-entry Fellowship (2019–2024) and a DBT-Public Health Food and Nutrition research grant (2022–2025) BT/PR36226/PFN/20/1478/2020.
The authors declare no competing financial interest.
References
- Jie C.; Jing-zhang C.; Man-zhi T.; Zi-tong G. Soil degradation: a global problem endangering sustainable development. J. Geograph. Sci. 2002, 12 (2), 243–252. 10.1007/BF02837480. [DOI] [Google Scholar]
- Sampathkumar K.; Tan K. X.; Loo S. C. J. Developing Nano-Delivery Systems for Agriculture and Food Applications with Nature-Derived Polymers. iScience 2020, 23 (5), 101055. 10.1016/j.isci.2020.101055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Lü S.; Zhang Z.; Zhao X.; Li X.; Ning P.; Liu M. Environmentally friendly fertilizers: A review of materials used and their effects on the environment. Sci. Total Environ. 2018, 613–614, 829–839. 10.1016/j.scitotenv.2017.09.186. [DOI] [PubMed] [Google Scholar]
- Pulizzi F. Nano in the future of crops. Nat. Nanotechnol. 2019, 14 (6), 507–507. 10.1038/s41565-019-0475-1. [DOI] [PubMed] [Google Scholar]
- Valencia G. A.; Zare E. N.; Makvandi P.; Gutierrez T. J. Self-Assembled Carbohydrate Polymers for Food Applications: A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18 (6), 2009–2024. 10.1111/1541-4337.12499. [DOI] [PubMed] [Google Scholar]
- Duhan J. S.; Kumar R.; Kumar N.; Kaur P.; Nehra K.; Duhan S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. 10.1016/j.btre.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen A. Expectations from nano in agriculture. Nat. Nanotechnol. 2019, 14 (6), 515–516. 10.1038/s41565-019-0471-5. [DOI] [PubMed] [Google Scholar]
- Gödecke T.; Stein A. J.; Qaim M. The global burden of chronic and hidden hunger: Trends and determinants. Global Food Security 2018, 17, 21–29. 10.1016/j.gfs.2018.03.004. [DOI] [Google Scholar]
- Cakmak I.; McLaughlin M. J.; White P. Zinc for better crop production and human health. Plant and Soil 2017, 411 (1), 1–4. 10.1007/s11104-016-3166-9. [DOI] [Google Scholar]
- Cakmak I.; Kutman U. B. Agronomic biofortification of cereals with zinc: a review. Eur. J. Soil Sci. 2018, 69 (1), 172–180. 10.1111/ejss.12437. [DOI] [Google Scholar]
- Nguyen T. D.; Cavagnaro T. R.; Watts-Williams S. J. The effects of soil phosphorus and zinc availability on plant responses to mycorrhizal fungi: a physiological and molecular assessment. Sci. Rep 2019, 9 (1), 14880. 10.1038/s41598-019-51369-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humayan Kabir A.; Swaraz A. M.; Stangoulis J. Zinc-deficiency resistance and biofortification in plants. J. Plant Nutri. Soil Sci. 2014, 177 (3), 311–319. 10.1002/jpln.201300326. [DOI] [Google Scholar]
- Kah M.; Tufenkji N.; White J. C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14 (6), 532–540. 10.1038/s41565-019-0439-5. [DOI] [PubMed] [Google Scholar]
- Trijatmiko K. R.; Dueñas C.; Tsakirpaloglou N.; Torrizo L.; Arines F. M.; Adeva C.; Balindong J.; Oliva N.; Sapasap M. V.; Borrero J.; Rey J.; Francisco P.; Nelson A.; Nakanishi H.; Lombi E.; Tako E.; Glahn R. P.; Stangoulis J.; Chadha-Mohanty P.; Johnson A. A. T.; Tohme J.; Barry G.; Slamet-Loedin I. H. Biofortified indica rice attains iron and zinc nutrition dietary targets in the field. Sci. Rep. 2016, 6, 19792–19792. 10.1038/srep19792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alloway B. J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem Health 2009, 31 (5), 537–48. 10.1007/s10653-009-9255-4. [DOI] [PubMed] [Google Scholar]
- Ludwig Y.; Slamet-Loedin I. H. Genetic Biofortification to Enrich Rice and Wheat Grain Iron: From Genes to Product. Front. plant Sci. 2019, 10, 833–833. 10.3389/fpls.2019.00833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar M.; Bhatnagar-Mathur P.; Dumbala S.; Anjaiah V.; Sharma K.. Crop Biofortification Through Genetic Engineering: Present Status and Future Directions. Conference: Genomics and Crop Improvement: Relevance and Reservations, Institute of Biotechnology, Acharya NG Ranga Agricultural University, Hyderabad 500 030 India, 2011.
- Kumar J.; Gupta D. S.; Kumar S.; Gupta S.; Singh N. P. Current Knowledge on Genetic Biofortification in Lentil. J. Agricul. Food Chem. 2016, 64 (33), 6383–6396. 10.1021/acs.jafc.6b02171. [DOI] [PubMed] [Google Scholar]
- Hess S. Y.Zinc: Deficiency Disorders and Prevention Programs. In Encyclopedia of Human Nutrition, 3rd ed.; Caballero B., Ed.; Academic Press: Waltham, 2013; pp 431–436. [Google Scholar]
- Zhang H.; Demirer G. S.; Zhang H.; Ye T.; Goh N. S.; Aditham A. J.; Cunningham F. J.; Fan C.; Landry M. P. DNA nanostructures coordinate gene silencing in mature plants. Proc. Nat. Acad. Sci. 2019, 116 (15), 7543–7548. 10.1073/pnas.1818290116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirer G.; Zhang H.; Goh N.; Chang R.; Landry M.. Nanotubes Effectively Deliver siRNA to Intact Plant Cells and Protect siRNA Against Nuclease Degradation. SSRN Electronic Journal 2019, 10.2139/ssrn.3352632. [DOI] [Google Scholar]
- Chen J.; Fan X.; Zhang L.; Chen X.; Sun S.; Sun R.-C. Research Progress in Lignin-Based Slow/Controlled Release Fertilizer. ChemSusChem 2020, 13 (17), 4356–4366. 10.1002/cssc.202000455. [DOI] [PubMed] [Google Scholar]
- Li Y.; Sun Y.; Liao S.; Zou G.; Zhao T.; Chen Y.; Yang J.; Zhang L. Effects of two slowrelease nitrogen fertilizers and irrigation on yield, quality, and water-fertilizer productivity of greenhouse tomato. Agric. Water Manag. 2017, 186, 139–146. 10.1016/j.agwat.2017.02.006. [DOI] [Google Scholar]
- Sipponen M. H.; Rojas O. J.; Pihlajaniemi V.; Lintinen K.; Österberg M. Calcium Chelation of Lignin from Pulping Spent Liquor for Water-Resistant Slow-Release Urea Fertilizer Systems. ACS Sustain. Chem. Eng. 2017, 5 (1), 1054–1061. 10.1021/acssuschemeng.6b02348. [DOI] [Google Scholar]
- Sipponen M. H.; Pastinen O. A.; Strengell R.; Hyötyläinen J. M. I.; Heiskanen I. T.; Laakso S. Increased Water Resistance of CTMP Fibers by Oat (Avena sativa L.) Husk Lignin. Biomacromolecules 2010, 11 (12), 3511–3518. 10.1021/bm101007u. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S.; Ghosh K.; Varadachari C. Multimicronutrient Slow-Release Fertilizer of Zinc, Iron, Manganese, and Copper. Int. J. Chem. Eng. 2014, 2014, 327153. 10.1155/2014/327153. [DOI] [Google Scholar]
- de Matos M.; Mattos B. D.; Tardy B. L.; Rojas O. J.; Magalhães W. L. E. Use of Biogenic Silica in Porous Alginate Matrices for Sustainable Fertilization with Tailored Nutrient Delivery. ACS Sustainable Chem. Eng. 2018, 6 (2), 2716–2723. 10.1021/acssuschemeng.7b04331. [DOI] [Google Scholar]
- Kabiri S.; Degryse F.; Tran D.; da Silva R.; McLaughlin M.; Losic D. Graphene Oxide: A New Carrier for Slow Release of Plant Micronutrients. ACS Appl. Mater. Interfaces 2017, 9, 43325–43335. 10.1021/acsami.7b07890. [DOI] [PubMed] [Google Scholar]
- Sun P.; Zhu M.; Wang K.; Zhong M.; Wei J.; Wu D.; Xu Z.; Zhu H. Selective Ion Penetration of Graphene Oxide Membranes. ACS Nano 2013, 7 (1), 428–437. 10.1021/nn304471w. [DOI] [PubMed] [Google Scholar]
- Liu X.; Liao J.; Song H.; Yang Y.; Guan C.; Zhang Z. A Biochar-Based Route for Environmentally Friendly Controlled Release of Nitrogen: Urea-Loaded Biochar and Bentonite Composite. Sci. Rep. 2019, 9 (1), 9548. 10.1038/s41598-019-46065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.; Du C.; Li T.; Shen Y.; Zhou J. Thermal post-treatment alters nutrient release from a controlled-release fertilizer coated with a waterborne polymer. Sci. Rep. 2015, 5 (1), 13820. 10.1038/srep13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie M.; Raza W.; Xu Y. C.; Shen Q.-R. Preparation and Optimization of Amino Acid Chelated Micronutrient Fertilizer by Hydrolyzation of Chicken Waste Feathers and the Effects on Growth of Rice. J. Plant Nutrition 2008, 31 (3), 571–582. 10.1080/01904160801895092. [DOI] [Google Scholar]
- Alvarez J. M.; Obrador A.; Rico M. I. Effects of chelated zinc, soluble and coated fertilizers, on soil zinc status and zinc nutrition of maize. Communications in Soil Sci. and Plant Analy. 1996, 27 (1–2), 7–19. 10.1080/00103629609369539. [DOI] [Google Scholar]
- Kumar R.; Kumawat N.; Kumar S.; Singh A. K.; Bohra J. S. Effect of NPKS and Zn Fertilization on, Growth, Yield and Quality of Baby Corn-A Review. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1392–1428. 10.20546/ijcmas.2017.603.161. [DOI] [Google Scholar]
- Malerba M.; Cerana R. Chitosan Effects on Plant Systems. Int. J. molecul..sci. 2016, 17 (7), 996. 10.3390/ijms17070996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurfadila; Maddu A.; Maddu A; Winarti C; Kurniati M Cellulose-based nano hydrogel from corncob by gamma irradiation. IOP Conference Series: Earth and Environ. Sci. 2019, 299, 012003. 10.1088/1755-1315/299/1/012003. [DOI] [Google Scholar]
- El-Batal A. I.; Gharib F. A. E.-L.; Ghazi S. M.; Hegazi A. Z.; Hafz A. G. M. A. E. Physiological Responses of Two Varieties of Common Bean (Phaseolus Vulgaris L.) to Foliar Application of Silver Nanoparticles. Nanomater. Nanotechnol. 2016, 6, 13. 10.5772/62202. [DOI] [Google Scholar]
- Singh P.; Singh R.; Borthakur A.; Srivastava P.; Srivastava N.; Tiwary D.; Mishra P. K. Effect of nanoscale TiO2- activated carbon composite on Solanum lycopersicum (L.) and Vigna radiata (L.) seeds germination. Energy, Ecology and Environment 2016, 1 (3), 131–140. 10.1007/s40974-016-0009-8. [DOI] [Google Scholar]
- Bandyopadhyay S.; Bhattacharya I.; Ghosh K.; Varadachari C. New Slow-Releasing Molybdenum Fertilizer. J. Agri. Food Chem. 2008, 56 (4), 1343–1349. 10.1021/jf072878g. [DOI] [PubMed] [Google Scholar]
- Bhattacharya I.; Bandyopadhyay S.; Varadachari C.; Ghosh K. Development of a Novel Slow-Releasing Iron-Manganese Fertilizer Compound. Indust. Eng.Chem. Res. 2007, 46 (9), 2870–2876. 10.1021/ie060787n. [DOI] [Google Scholar]
- Rana S.; Thakur K. S.; Bhardwaj R. K.; Kansal S.; Sharma R. Effect of biofertilizers and micronutrients on growth and quality attributes of cabbage (Brassica oleracea var. capitata L.). Int. J. Chem. Studies 2020, 8 (1), 1656–1660. 10.22271/chemi.2020.v8.i1x.8501. [DOI] [Google Scholar]
- Wang L.; Liu X. Sustained Release Technology and Its Application in Environmental Remediation: A Review. Int. J. Environ. Res. Public Health 2019, 16 (12), 2153. 10.3390/ijerph16122153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindraban P. S.; Dimkpa C.; Nagarajan L.; Roy A.; Rabbinge R. Revisiting fertilisers and fertilisation strategies for improved nutrient uptake by plants. Biology and Fertility of Soils 2015, 51 (8), 897–911. 10.1007/s00374-015-1039-7. [DOI] [Google Scholar]
- Dimkpa C. O.; Bindraban P. Fortification of micronutrients for efficient agronomic production: a review. Agron. Sustainable Dev. 2016, 36, 7. 10.1007/s13593-015-0346-6. [DOI] [Google Scholar]
- Dimkpa C. O.; White J. C.; Elmer W. H.; Gardea-Torresdey J. Nanoparticle and Ionic Zn Promote Nutrient Loading of Sorghum Grain under Low NPK Fertilization. J. Agri.Food Chem. 2017, 65 (39), 8552–8559. 10.1021/acs.jafc.7b02961. [DOI] [PubMed] [Google Scholar]
- Ding H.; Liu D.; Liu X.; Li Y.; Kang J.; Lv J.; Wang G. Photosynthetic and stomatal traits of spike and flag leaf of winter wheat (Triticum aestivum L.) under water deficit. Photosynthetica 2018, 56 (2), 687–697. 10.1007/s11099-017-0718-z. [DOI] [Google Scholar]
- Venkatachalam P.; Priyanka N.; Manikandan K.; Ganeshbabu I.; Indiraarulselvi P.; Geetha N.; Muralikrishna K.; Bhattacharya R. C.; Tiwari M.; Sharma N.; Sahi S. V. Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2017, 110, 118–127. 10.1016/j.plaphy.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Deshpande P.; Dapkekar A.; Oak M. D.; Paknikar K. M.; Rajwade J. M. Zinc complexed chitosan/TPP nanoparticles: A promising micronutrient nanocarrier suited for foliar application. Carbohyd. Polym. 2017, 165, 394–401. 10.1016/j.carbpol.2017.02.061. [DOI] [PubMed] [Google Scholar]
- Sheykhbaglou R.; Sedghi M.; Shishevan M. T.; Sharifi R. S. Effects of Nano-Iron Oxide Particles on Agronomic Traits of Soybean. Notulae Scientia Biologicae 2010, 2 (2), 112–113. 10.15835/nsb224667. [DOI] [Google Scholar]
- Jeyasubramanian K.; Gopalakrishnan Thoppey U. U.; Hikku G. S.; Selvakumar N.; Subramania A.; Krishnamoorthy K. Enhancement in growth rate and productivity of spinach grown in hydroponics with iron oxide nanoparticles. RSC Adv. 2016, 6 (19), 15451–15459. 10.1039/C5RA23425E. [DOI] [Google Scholar]
- Shankramma K.; Yallappa S.; Shivanna M. B.; Manjanna J. Fe2O3 magnetic nanoparticles to enhance S. lycopersicum (tomato) plant growth and their biomineralization. Appl. Nanosci. 2016, 6 (7), 983–990. 10.1007/s13204-015-0510-y. [DOI] [Google Scholar]
- Rawat M.; Nayan R.; Negi B.; Zaidi M. G. H.; Arora S. Physio-biochemical basis of iron-sulfide nanoparticle induced growth and seed yield enhancement in B. juncea. Plant Physiol. Biochem. 2017, 118, 274–284. 10.1016/j.plaphy.2017.06.021. [DOI] [PubMed] [Google Scholar]
- Adhikari T.; Sarkar D.; Mashayekhi H.; Xing B. Growth and enzymatic activity of maize (Zea mays L.) plant: Solution culture test for copper dioxide nano particles. J. Plant Nutrition 2016, 39 (1), 99–115. 10.1080/01904167.2015.1044012. [DOI] [Google Scholar]
- Singh A.; Singh N. B.; Hussain I.; Singh H. Effect of biologically synthesized copper oxide nanoparticles on metabolism and antioxidant activity to the crop plants Solanum lycopersicum and Brassica oleracea var. botrytis. J. Biotechnol. 2017, 262, 11–27. 10.1016/j.jbiotec.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Juárez Maldonado A.; Ortega-Ortíz H.; Pérez-Labrada F.; cadenas-pliego G.; Benavides-Mendoza A. Cu Nanoparticles absorbed on chitosan hydrogels positively alter morphological, production, and quality characteristics of tomato. J. Appl. Botany Food Quality 2016, 89, 183–189. 10.5073/JABFQ.2016.089.023. [DOI] [Google Scholar]
- Gilbertson L. M.; Pourzahedi L.; Laughton S.; Gao X.; Zimmerman J. B.; Theis T. L.; Westerhoff P.; Lowry G. V. Guiding the design space for nanotechnology to advance sustainable crop production. Nat. Nanotechnol. 2020, 15 (9), 801–810. 10.1038/s41565-020-0706-5. [DOI] [PubMed] [Google Scholar]
- Martins N. C. T.; Avellan A.; Rodrigues S.; Salvador D.; Rodrigues S. M.; Trindade T. Composites of Biopolymers and ZnO NPs for Controlled Release of Zinc in Agricultural Soils and Timed Delivery for Maize. ACS Appl. Nano Mater. 2020, 3 (3), 2134–2148. 10.1021/acsanm.9b01492. [DOI] [Google Scholar]
- Fertahi S.; Bertrand I.; Amjoud M. B.; Oukarroum A.; Arji M.; Barakat A. Properties of Coated Slow-Release Triple Superphosphate (TSP) Fertilizers Based on Lignin and Carrageenan Formulations. ACS Sustain. Chem. Eng. 2019, 7 (12), 10371–10382. 10.1021/acssuschemeng.9b00433. [DOI] [Google Scholar]
- Levett I.; Pratt S.; Donose B. C.; Brackin R.; Pratt C.; Redding M.; Laycock B. Understanding the Mobilization of a Nitrification Inhibitor from Novel Slow Release Pellets, Fabricated through Extrusion Processing with PHBV Biopolymer. J. Agric. Food Chem. 2019, 67 (9), 2449–2458. 10.1021/acs.jafc.8b05709. [DOI] [PubMed] [Google Scholar]
- Xie J.; Yang Y.; Gao B.; Wan Y.; Li Y. C.; Cheng D.; Xiao T.; Li K.; Fu Y.; Xu J.; Zhao Q.; Zhang Y.; Tang Y.; Yao Y.; Wang Z.; Liu L. Magnetic-Sensitive Nanoparticle Self-Assembled Superhydrophobic Biopolymer-Coated Slow-Release Fertilizer: Fabrication, Enhanced Performance, and Mechanism. ACS Nano 2019, 13 (3), 3320–3333. 10.1021/acsnano.8b09197. [DOI] [PubMed] [Google Scholar]
- Ee Huey C.; Zaireen Nisa Yahya W.; Mansor N. Allicin incorporation as urease inhibitor in a chitosan/starch based biopolymer for fertilizer application. Mater. Today: Proc. 2019, 16, 2187–2196. 10.1016/j.matpr.2019.06.109. [DOI] [Google Scholar]
- Kumar S.; Chauhan N.; Gopal M.; Kumar R.; Dilbaghi N. Development and evaluation of alginate-chitosan nanocapsules for controlled release of acetamiprid. International J. Biolog. Macromolecules 2015, 81, 631–637. 10.1016/j.ijbiomac.2015.08.062. [DOI] [PubMed] [Google Scholar]
- Kottegoda N.; Sandaruwan C.; Priyadarshana G.; Siriwardhana A.; Rathnayake U. A.; Berugoda Arachchige D. M.; Kumarasinghe A. R.; Dahanayake D.; Karunaratne V.; Amaratunga G. A. J. Urea-Hydroxyapatite Nanohybrids for Slow Release of Nitrogen. ACS Nano 2017, 11 (2), 1214–1221. 10.1021/acsnano.6b07781. [DOI] [PubMed] [Google Scholar]
- Rop K.; Karuku G. N.; Mbui D.; Michira I.; Njomo N. Formulation of slow release NPK fertilizer (cellulose-graft-poly(acrylamide)/nano-hydroxyapatite/soluble fertilizer) composite and evaluating its N mineralization potential. Ann. Agri. Sci. 2018, 63 (2), 163–172. 10.1016/j.aoas.2018.11.001. [DOI] [Google Scholar]
- Entry J. A.; Sojka R. E. Matrix-Based Fertilizers Reduce Nutrient Leaching While Maintaining Kentucky Bluegrass Growth. Water, Air, and Soil Pollution 2010, 207 (1), 181–193. 10.1007/s11270-009-0127-4. [DOI] [Google Scholar]
- Entry J. A.; Sojka R. E. Matrix-Based Fertilizers Reduce Pesticide Leaching in Soil. Water, Air, & Soil Pollution 2012, 223 (3), 1295–1302. 10.1007/s11270-011-0945-z. [DOI] [Google Scholar]
- Perez J.; Mardare C.; Hassel A. W.; Brüggemann O. Self-assembled cellulose particles for agrochemical applications. Eur. Polym. J. 2017, 93, 706–716. 10.1016/j.eurpolymj.2017.02.023. [DOI] [Google Scholar]
- Luo Y.; Hu Q.. 7 - Food-derived biopolymers for nutrient delivery. In Nutrient Delivery; Grumezescu A. M., Ed.; Academic Press, 2017; pp 251–291. [Google Scholar]
- Kottegoda N.; Munaweera I.; Madusanka N.; Sandaruwan C.; Sirisena D.; Disanayake N.; Ismail M.; de Alwis A.; Karunaratne V.. Plant nutrient nanoparticles encapsulated cellulose matrix for slow and sustained release of nitrogen. Conferences and Workshops of Sri Lanka, 2012, http://dl.nsf.gov.lk/handle/1/9345.
- Usman M.; Farooq M.; Wakeel A.; Nawaz A.; Cheema S. A.; Rehman H. u.; Ashraf I.; Sanaullah M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. 10.1016/j.scitotenv.2020.137778. [DOI] [PubMed] [Google Scholar]
- Pradhan S.; Durgam M.; Mailapalli D. R. Urea loaded hydroxyapatite nanocarrier for efficient delivery of plant nutrients in rice. Archives Agro. Soil Sci. 2021, 67 (3), 371–382. 10.1080/03650340.2020.1732940. [DOI] [Google Scholar]
- Vishwakarma K.; Kumar N.; Shandilya C.; Mohapatra S.; Bhayana S.; Varma A. Revisiting Plant-Microbe Interactions and Microbial Consortia Application for Enhancing Sustainable Agriculture: A Review. Front. Microbiol. 2020, 11, 560406. 10.3389/fmicb.2020.560406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plohl O.; Gyergyek S.; Zemljič L. F. Mesoporous silica nanoparticles modified with N-rich polymer as a potentially environmentally-friendly delivery system for pesticides. Microporous Mesoporous Mater. 2021, 310, 110663. 10.1016/j.micromeso.2020.110663. [DOI] [Google Scholar]
- Pal S.; Datta S. P.; Rattan R. K.; Singh A. K. Diagnosis and Amelioration of Iron Deficiency under Aerobic Rice. J. Plant Nutrition 2008, 31 (5), 919–940. 10.1080/01904160802043262. [DOI] [Google Scholar]
- Pal S.; Datta S. C.; Reza S. K. Interrelationship of Organic Acids and Aluminum Concentrations in Rhizosphere and Nonrhizosphere Soil Solution of Rice in Acidic Soil. Communications in Soil Sci. Plant Analy. 2011, 42 (8), 932–944. 10.1080/00103624.2011.558962. [DOI] [Google Scholar]
- Pal S.; Panwar P. Interrelationship of Carbon Sequestration, Soil Fertility, and Microbial Indices as Influenced by Long-Term Land Uses in Lower Himalayan Region, India. Communications in Soil Sci. Plant Analy. 2013, 44 (5), 869–883. 10.1080/00103624.2012.747608. [DOI] [Google Scholar]
- Pal S.; Panwar P.; Bhardwaj D. Soil quality under forest compared to other land-uses in acid soil of North Western Himalaya, India. Ann. Forest Res. 2013, 56 (1), 187–198. [Google Scholar]
- Thomas B.; Raj M.; B A.; H R.; Joy J.; Moores A.; Drisko G.; Sanchez C. Nanocellulose, a Versatile Green Platform: From Biosources to Materials and Their Applications. Chem. Rev. 2018, 118, 11575. 10.1021/acs.chemrev.7b00627. [DOI] [PubMed] [Google Scholar]
- Nascimento D. M.; Nunes Y. L.; Figueirêdo M. C. B.; de Azeredo H. M. C.; Aouada F. A.; Feitosa J. P. A.; Rosa M. F.; Dufresne A. Nanocellulose nanocomposite hydrogels: technological and environmental issues. Green Chem. 2018, 20 (11), 2428–2448. 10.1039/C8GC00205C. [DOI] [Google Scholar]
- Bortolin A.; Serafim A. R.; Aouada F. A.; Mattoso L. H. C.; Ribeiro C. Macro- and Micronutrient Simultaneous Slow Release from Highly Swellable Nanocomposite Hydrogels. J. Agri. Food Chem. 2016, 64 (16), 3133–3140. 10.1021/acs.jafc.6b00190. [DOI] [PubMed] [Google Scholar]
- Zhou Y.; Fu S.; Zhang L.; Zhan H. Superabsorbent nanocomposite hydrogels made of carboxylated cellulose nanofibrils and CMC-g-p(AA-co-AM). Carbohydr. Polym. 2013, 97 (2), 429–35. 10.1016/j.carbpol.2013.04.088. [DOI] [PubMed] [Google Scholar]
- Dufresne A. Cellulose nanomaterials as green nanoreinforcements for polymer nanocomposites. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2018, 376, 20170040. 10.1098/rsta.2017.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhya; Kumar S.; Kumar D.; Dilbaghi N. Preparation, characterization, and bio-efficacy evaluation of controlled release carbendazim-loaded polymeric nanoparticles. Environ. Sci. Pollu. Res. 2017, 24 (1), 926–937. 10.1007/s11356-016-7774-y. [DOI] [PubMed] [Google Scholar]
- Khaledian Y.; Pajohi-Alamoti M.; Bazargani-Gilani B. Development of cellulose nanofibers coating incorporated with ginger essential oil and citric acid to extend the shelf life of ready-to-cook barbecue chicken. J. Food Process. Preservation 2019, 43 (10), e14114 10.1111/jfpp.14114. [DOI] [Google Scholar]
- Davidson D.; Verma M.; Gu F. Controlled root targeted delivery of fertilizer using an ionically crosslinked carboxymethyl cellulose hydrogel matrix. SpringerPlus 2013, 2, 318. 10.1186/2193-1801-2-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholamali I. Facile Preparation of Carboxymethyl Cellulose/Cu Bio-Nanocomposite Hydrogels for Controlled Release of Ibuprofen. Regenerative Eng. Transl. Med. 2019, 6, 115–124. 10.1007/s40883-019-00133-2. [DOI] [Google Scholar]
- Yang H.-Y.; Bao B.-L.; Liu J.; Qin Y.; Wang Y.-R.; Su K.-Z.; Han J.-C.; Mu Y. Temperature dependence of bioelectrochemical CO2 conversion and methane production with a mixed-culture biocathode. Bioelectrochem 2018, 119, 180–188. 10.1016/j.bioelechem.2017.10.002. [DOI] [PubMed] [Google Scholar]
- Dong Y.; Paukkonen H.; Fang W.; Kontturi E.; Laaksonen T.; Laaksonen P. Entangled and colloidally stable microcrystalline cellulose matrices in controlled drug release. Int. J. Pharm. 2018, 548 (1), 113–119. 10.1016/j.ijpharm.2018.06.022. [DOI] [PubMed] [Google Scholar]
- Homaee Borujeni S.; Mirdamadian S. Z.; Varshosaz J.; Taheri A. Three-dimensional (3D) printed tablets using ethyl cellulose and hydroxypropyl cellulose to achieve zero order sustained release profile. Cellulose 2020, 27 (3), 1573–1589. 10.1007/s10570-019-02881-4. [DOI] [Google Scholar]
- Nyenhuis J.; Drelich J. Essential micronutrient biofortification of sprouts grown on mineral fortified fiber mats. Int. J. Biological, Biomolecular, Agri. Food.Biotechnol.Eng. 2015, 9, 943–946. 10.5281/zenodo.1110189. [DOI] [Google Scholar]
- Laitinen O.; Suopajärvi T.; Liimatainen H. Enhancing packaging board properties using micro- and nanofibers prepared from recycled board. Cellulose 2020, 27, 7215. 10.1007/s10570-020-03264-w. [DOI] [Google Scholar]
- Zhao S.-W.; Zheng M.; Zou X.-H.; Guo Y.; Pan Q.-J. Self-Assembly of Hierarchically Structured Cellulose@ZnO Composite in Solid-Liquid Homogeneous Phase: Synthesis, DFT, Calculations, and Enhanced Antibacterial Activities. ACS Sustain. Chem. & Eng. 2017, 5 (8), 6585–6596. 10.1021/acssuschemeng.7b00842. [DOI] [Google Scholar]
- Jin Y.; Shen Y.; Yin J.; Qian J.; Huang Y. Nanoclay-Based Self-Supporting Responsive Nanocomposite Hydrogels for Printing Applications. ACS Appl. Mater. & Interfaces 2018, 10 (12), 10461–10470. 10.1021/acsami.8b00806. [DOI] [PubMed] [Google Scholar]
- Janpetch N.; Saito N.; Rujiravanit R. Fabrication of bacterial cellulose-ZnO composite via solution plasma process for antibacterial applications. Carbohy. Polym. 2016, 148, 335–344. 10.1016/j.carbpol.2016.04.066. [DOI] [PubMed] [Google Scholar]
- Jing J.; Liang S.; Yan Y.; Tian X.; Li X. Fabrication of Hybrid Hydrogels from Silk Fibroin and Tannic Acid with Enhanced Gelation and Antibacterial Activities. ACS Biomater. Sci. & Eng. 2019, 5 (9), 4601–4611. 10.1021/acsbiomaterials.9b00604. [DOI] [PubMed] [Google Scholar]
- Jantrawut P.; Bunrueangtha J.; Suerthong J.; Kantrong N. Fabrication and Characterization of Low Methoxyl Pectin/Gelatin/Carboxymethyl Cellulose Absorbent Hydrogel Film for Wound Dressing Applications. Materials (Basel) 2019, 12 (10), 1628. 10.3390/ma12101628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamel S.; El-Gendy A. A.; Hassan M. A.; El-Sakhawy M.; Kelnar I. Carboxymethyl cellulose-hydrogel embedded with modified magnetite nanoparticles and porous carbon: Effective environmental adsorbent. Carbohyd. Poly. 2020, 242, 116402. 10.1016/j.carbpol.2020.116402. [DOI] [PubMed] [Google Scholar]
- Guo H.; White J.; Zhenyu W.; Xing B. Nano-enabled fertilizers to control the release and use efficiency of nutrients. Curr.Opin. Environ. Sci. Health 2018, 6, 77–83. 10.1016/j.coesh.2018.07.009. [DOI] [Google Scholar]
- Lourdin D.; Peixinho J.; Bréard J.; Cathala B.; Leroy E.; Duchemin B. Concentration driven cocrystallisation and percolation in all-cellulose nanocomposites. Cellulose 2016, 23 (1), 529–543. 10.1007/s10570-015-0805-x. [DOI] [Google Scholar]
- Lin N.; Huang J.; Chang P. R.; Feng L.; Yu J. Effect of polysaccharide nanocrystals on structure, properties, and drug release kinetics of alginate-based microspheres. Coll. Surf. B Biointerfaces 2011, 85 (2), 270–9. 10.1016/j.colsurfb.2011.02.039. [DOI] [PubMed] [Google Scholar]
- Kose O.; Tran A.; Lewis L.; Hamad W. Y.; MacLachlan M. J. Unwinding a spiral of cellulose nanocrystals for stimuli-responsive stretchable optics. Nat. Commun. 2019, 10 (1), 510. 10.1038/s41467-019-08351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.; Zhou S.; Liu H.; Xing M.; Ding B.; Li B. Pinecone-Inspired Nanoarchitectured Smart Microcages Enable Nano/Microparticle Drug Delivery. Adv. Functl. Mater. 2020, 30 (28), 2002434. 10.1002/adfm.202002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S.; Liu H.; Tang N.; Ge J.; Yu J.; Ding B. Direct electronetting of high-performance membranes based on self-assembled 2D nanoarchitectured networks. Nat. Commun. 2019, 10, 1458. 10.1038/s41467-019-09444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven E.; Saleh W. R.; Lebedev V.; Acquah S. F. A.; Laukhin V.; Alamo R. G.; Brooks J. S. Carbon nanotubes on a spider silk scaffold. Nat. Commun. 2013, 4 (1), 2435. 10.1038/ncomms3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyarathna I. R.; Nedra Karunaratne D. Use of chickpea protein for encapsulation of folate to enhance nutritional potency and stability. Food Bioprod. Proce. 2015, 95, 76–82. 10.1016/j.fbp.2015.04.004. [DOI] [Google Scholar]
- Ashfaq M.; Verma N.; Khan S. Carbon nanofibers as a micronutrient carrier in plants: efficient translocation and controlled release of Cu nanoparticles. Environmental Science: Nano 2017, 4 (1), 138–148. 10.1039/C6EN00385K. [DOI] [Google Scholar]
- Wang C.-Y.; Yang J.; Qin J.-C.; Yang Y.-W. Eco-Friendly Nanoplatforms for Crop Quality Control, Protection, and Nutrition. Adv. Sci. 2021, 8 (9), 2004525. 10.1002/advs.202004525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H.; White J. C.; Wang Z.; Xing B. Nano-enabled fertilizers to control the release and use efficiency of nutrients. Curr. Opin. Environ. Sci. & Health 2018, 6, 77–83. 10.1016/j.coesh.2018.07.009. [DOI] [Google Scholar]
- Yi Z.; Hussain H. I.; Feng C.; Sun D.; She F.; Rookes J. E.; Cahill D. M.; Kong L. Functionalized Mesoporous Silica Nanoparticles with Redox-Responsive Short-Chain Gatekeepers for Agrochemical Delivery. ACS Appl. Mater. Interfaces 2015, 7 (18), 9937–9946. 10.1021/acsami.5b02131. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Yang Y.; Gao B.; Li Y. C.; Liu Z. Superhydrophobic controlled-release fertilizers coated with bio-based polymers with organosilicon and nano-silica modifications. J. Mater. Chem. A 2017, 5 (37), 19943–19953. 10.1039/C7TA06014A. [DOI] [Google Scholar]
- Zhang G.; Zhou L.; Cai D.; Wu Z. Anion-responsive carbon nanosystem for controlling selenium fertilizer release and improving selenium utilization efficiency in vegetables. Carbon 2018, 129, 711–719. 10.1016/j.carbon.2017.12.062. [DOI] [Google Scholar]
- Li X.; Han J.; Wang X.; Zhang Y.; Jia C.; Qin J.; Wang C.; Wu J.-R.; Fang W.; Yang Y.-W. A triple-stimuli responsive hormone delivery system equipped with pillararene magnetic nanovalves. Mater. Chem. Frontiers 2019, 3 (1), 103–110. 10.1039/C8QM00509E. [DOI] [Google Scholar]
- Hou X.; Pan Y.; Xiao H.; Liu J. Controlled Release of Agrochemicals Using pH and Redox Dual-Responsive Cellulose Nanogels. J. Agric. Food Chem. 2019, 67 (24), 6700–6707. 10.1021/acs.jafc.9b00536. [DOI] [PubMed] [Google Scholar]
- Ramírez-Rodríguez G. B.; Dal Sasso G.; Carmona F. J.; Miguel-Rojas C.; Pérez-de-Luque A.; Masciocchi N.; Guagliardi A.; Delgado-López J. M. Engineering Biomimetic Calcium Phosphate Nanoparticles: A Green Synthesis of Slow-Release Multinutrient (NPK) Nanofertilizers. ACS Appl. Bio. Mater. 2020, 3 (3), 1344–1353. 10.1021/acsabm.9b00937. [DOI] [PubMed] [Google Scholar]
- Zhang M.; Yang J. Preparation and characterization of multifunctional slow release fertilizer coated with cellulose derivatives. Int. J. Polym. Mater. Polym. Biomater. 2020, 70, 774–781. 10.1080/00914037.2020.1765352. [DOI] [Google Scholar]
- Mohammadi-Khoo S.; Moghadam P. N.; Fareghi A. R.; Movagharnezhad N., Synthesis of a cellulose-based hydrogel network: Characterization and study of urea fertilizer slow release. J. Appl. Polym. Sci. 2016, 133 ( (5), ), 10.1002/app.42935 [DOI] [Google Scholar]
- Singh A.; Sarkar D. J.; Mittal S.; Dhaka R.; Maiti P.; Singh A.; Raghav T.; Solanki D.; Ahmed N.; Singh S. B. Zeolite reinforced carboxymethyl cellulose-Na+-g-cl-poly(AAm) hydrogel composites with pH responsive phosphate release behavior. J. Appl. Polym. Sci. 2019, 136 (15), 47332. 10.1002/app.47332. [DOI] [Google Scholar]
- Zhao H.; Song J.; Zhao G.; Xiang Y.; Liu Y. Novel Semi-IPN Nanocomposites with Functions of both Nutrient Slow-Release and Water Retention. 2. Effects on Soil Fertility and Tomato Quality. J. Agri. Food Chem. 2019, 67 (27), 7598–7608. 10.1021/acs.jafc.9b00889. [DOI] [PubMed] [Google Scholar]
- Qiao D.; Liu H.; Yu L.; Bao X.; Simon G. P.; Petinakis E.; Chen L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohyd. Polym. 2016, 147, 146–154. 10.1016/j.carbpol.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Kenawy E.-R.; Azaam M. M.; El-nshar E. M. Preparation of carboxymethyl cellulose-g-poly (acrylamide)/montmorillonite superabsorbent composite as a slow-release urea fertilizer. Polymers Adv. Technol. 2018, 29 (7), 2072–2079. 10.1002/pat.4315. [DOI] [Google Scholar]
- Wang C.-Y.; Yang J.; Qin J.-C.; Yang Y.-W. Eco-Friendly Nanoplatforms for Crop Quality Control, Protection, and Nutrition. Adv. Sci. 2021, 8, 2004525. 10.1002/advs.202004525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C.; Zhou X.; Liu Q.; Peng J. W.; Wang W. M.; Zhang Z. H.; Yang Y.; Song H. X.; Guan C. Y. Effects of a controlled-release fertilizer on yield, nutrient uptake, and fertilizer usage efficiency in early ripening rapeseed (Brassica napus L.). J. Zhejiang Univ. Sci. B 2016, 17 (10), 775–786. 10.1631/jzus.B1500216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom C. J.; Jolley V. D.; Blair T. A.; Sutton L. E.; Hopkins B. G. Nitrogen release rates from slow- and controlled-release fertilizers influenced by placement and temperature. PLoS One 2020, 15 (6), e0234544 10.1371/journal.pone.0234544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z. P. Material Nanotechnology Is Sustaining Modern Agriculture. ACS Agri. Sci. Technol. 2022, 2 (2), 232–239. 10.1021/acsagscitech.1c00204. [DOI] [Google Scholar]
- Rajput V.; Minkina T.; Mazarji M.; Shende S.; Sushkova S.; Mandzhieva S.; Burachevskaya M.; Chaplygin V.; Singh A.; Jatav H. Accumulation of nanoparticles in the soil-plant systems and their effects on human health. Ann. Agri. Sci. 2020, 65 (2), 137–143. 10.1016/j.aoas.2020.08.001. [DOI] [Google Scholar]
- Sordo F.; Janecek E.-R.; Qu Y.; Michaud V.; Stellacci F.; Engmann J.; Wooster T. J.; Sorin F. Microstructured Fibers for the Production of Food. Adv. Mater. 2019, 31 (14), 1807282. 10.1002/adma.201807282. [DOI] [PubMed] [Google Scholar]