Abstract

Electrochemical, surface, and density functional theory (DFT)/Monte Carlo (MC) simulation studies were used in investigating the characteristics of N,N′-(disulfanne-1,2-dicarbonothioyl)bis(N,N′-bis(2,6-dimethylphenyl)formimidamide) (DS1), N,N′-(disulfanne-1,2-dicarbonothioyl)bis(N,N′-bis(2,6-diisopropylphenyl)formimidamide) (DS2), N,N′-(disulfanne-1,2-dicarbonothioyl)bis(N,N′-dimesitylformimidamide) (DS3), and N,N′-(disulfanne-1,2-dicarbonothioyl)bis(N,N′-bis(2,6-dichlorophenyl)formimidamide) (DS4) as inhibitors of acid corrosion of mild steel. The inhibitors were found to effectively reduce the rates of steel dissolution at the anode as well as cathodic hydrogen evolution. The order of inhibition efficiencies of studied compounds is DS1 (PDP/LPR/EIS: 98.60/97.98/96.94%) > DS2 (PDP/LPR/EIS: 98.36/96.86/96.90%) > DS3 (PDP/LPR/EIS: 94.66/87.44/94.30%) > DS4 (PDP/LPR/EIS: 83.57/77.02/75.17%) at 1.00 mM, and the overall efficiencies appeared to depend on the molecular and electronic structures of the compounds. The compounds offered high resistance to charge transfer across the electrode/electrolyte system by forming adsorbed film whose resistance increased with an increase in concentration. Findings suggested that the adsorption process involved combined chemisorption and physisorption. DFT calculations and MC simulations provided theoretical justifications for the experimental results.
1. Introduction
Over the years, carbon steel has been widely utilized in numerous domestic, industrial, and engineering processes, as well as in the construction of geothermal power plants. This attraction has been mainly attributed to the low cost, excellent mechanical properties, and weldability of carbon steel.1 These low-alloyed materials could function well in a nonsaline environment with moderate pH, but undergo severe corrosion in a more aggressive environment. As of 2016, records available in NACE International’s IMPACT report estimated the global cost of corrosion (excluding individual safety and environmental consequences) to be US$ 2.3 trillion, which is approximately 3.4% of the global GDP.2 Similar reports also stated that, using available corrosion control practices, an estimated savings of 15–35% of the cost of corrosion could be realized. As such, the development and use of corrosion protection strategies becomes invaluable, and the use of organic inhibitors has gained prominence in the mitigation of corrosion-related problems.3−5
Organic compounds employed as corrosion inhibitors possess certain properties like the existence of heteroatoms (N, S, O, and P), aromatic rings, and rich electron clouds around the multiple bonds. Research studies have shown that the listed properties enhance the adsorption of these types of chemicals on the surface of the metal by the formation of electrostatic interactions (physical adsorption) and/or coordinate covalent bond (chemical adsorption) between inhibitor molecules and the metal, ensuring the coverage of the active sites (on the metal), thereby, protecting it from aggressive ions.6−8
Thiuram disulfide derivatives have been reported to possess antimicrobial activities,9−11 antioxidant activities,12 and anticancer properties,13,14 respectively. These compounds are also used in vulcanizing synthetic rubber.15 As per corrosion studies, Ousslim et al.16 evaluated the mitigation of mild steel corrosion in 3.9 M HCl using bis(1-benzylpiperazine)thiuram disulfide and achieved an inhibition efficiency of 91.2% at 10–3 M. In a similar report, the team assessed the inhibitory performance of three piperidine compounds including bis(4-benzylpiperidine)thiuram disulfide (P2) for mild steel degradation in 5.5 M H3PO4. At elevated concentration, P2 behaved as a predominant cathodic inhibitor and inhibited corrosion to the tune of 96% at 1 mM concentration at 298 K.17 The inhibitive effect of three disulfides flavoring agents (PPD, DBD, and DAD) on copper in 0.5 M H2SO4 was investigated via electrochemical and computational techniques by Tan et al.18 The study reported excellent inhibition efficacies of 98.5% (PPD), 92.10% (DBD), and 91.50% (DAD) with a maximum concentration of 5 mM at 298 K. Recently, an extensive study was conducted on the corrosion protection of cobalt in alkaline solution using two disulfide compounds, 2,2-dibenzamidodiphenyl disulfide and 3,3-dithiodipropionic acid. The study established that the studied disulfide derivatives excellently impeded galvanic corrosion and their inhibition mechanism complied with the Langmuir adsorption model.19 Another report explored the relationship between the number of heteroatoms in three disulfide compounds and their adsorption potential on copper in sulfuric acid using electrochemical and surface analyses and theoretical calculations.20
Thiuram disulfide compounds are generally synthesized via oxidation of the suitable dithiocarbamate salts using iodine. Generally, the mechanism of reaction involves the oxidation of dithiocarbamates to their conforming radicals, which proceeds to react quickly to yield the corresponding thiuram disulfide.21 There is the availability of multiple conjugated connections with more than two heteroatoms (N and S), imine functional group (−C=N−), multiple pi electrons in the aromatic rings, and multiple bonds in the backbone of the formamidine-based thiuram disulfides in this study. It is, therefore, expected that these properties will promote electrons transfer between the various functionalities in the molecule and the metal, resulting in enhanced adsorption, vis-a-vis corrosion inhibition efficacy. Furthermore, the existence of both electron-donating and -withdrawing groups in the molecular framework of the thiuram disulfides will furnish research information on the effects of these groups on corrosion inhibition capabilities.
We, therefore, report here the corrosion inhibition characteristics of four novel formamidine-based thiuram disulfides namely; N,N′-(disulfanne-1,2-dicarbonothioyl)bis(N,N′-bis(2,6-dimethylphenyl)formimidamide) (DS1), N,N′-(disulfanne-1,2-dicarbonothioyl)bis(N,N′-bis(2,6-diisopropylphenyl)formimidamide) (DS2), N,N′-(disulfanne-1,2-dicarbonothioyl)bis(N,N′-dimesitylformimidamide) (DS3), and N,N′-(disulfanne-1,2-dicarbonothioyl)bis(N,N′-bis(2,6-dichlorophenyl)formimidamide) (DS4) on mild steel in 1 M HCl. The study involved electrochemical [potentiodynamic polarization (PDP), linear polarization resistance (LPR), and electrochemical impedance spectroscopy (EIS)], surface morphological [scanning electron microscopy (SEM) and atomic force microscopy (AFM)], and theoretical [density functional theory (DFT), and Monte Carlo (MC) simulation] analyses. Theoretical approaches were used to investigate a possible correlation between the structures of the molecules and corrosion inhibition efficiencies. Compounds DS1, DS2, DS3, and DS4 were synthesized as represented in Scheme 1, and the molecular structure analysis of DS3 is also presented. Synthesis of these compounds can be carried out with relatively nontoxic reagents considering the advocacy for environmentally benign corrosion inhibitors.
Scheme 1. Synthesis Routes for Inhibitors DS1, DS2, DS3, and DS4; Reproduced with Permission from Ref (9); Copyright 2020 Elsevier.

2. Experimental Section
2.1. Typical Synthesis Protocol of DS1, DS2, DS3, and DS4
The protocol for the synthesis of compounds DS1, DS2, DS3, and DS4 is as presented in Scheme 1. Comprehensive experimental procedures, purification methods, yield (experimental), mass spectrometry, Fourier transform infrared, UV–vis, 1H and 13C NMR, and elemental analysis data had been reported elsewhere.9
2.2. Single-Crystal X-ray Diffraction Analysis of Compounds DS1–4
Single-crystal X-ray diffraction data and analyses for compounds DS1, DS2, and DS4, respectively, had been reported previously.9 For compound DS3, data collection and crystal evaluation were done using similar procedures as reported.9 The crystallographic data and structure refinement parameters for compound DS3 are given in Table SI 1 (Supporting Information).
2.3. Materials for Corrosion Studies
Coupons of mild steel with established compositions (wt %): C (0.076%), P (0.012%), Al (0.023%), Si (0.026%), Mn (0.192%), Cr (0.050%), and Fe (99.621%) were utilized. Mild steel is a multipurpose ferrous metal widely utilized in several commercial industries because it is easily available, 100% recyclable, low cost, and has excellent strength and superior mechanical properties;22−24 hence, it is chosen for the present study. Preparation of samples for electrochemical and surface analyses was undertaken following previously reported methods.25,26 The aggressive media, 1 M HCl was made from 32% grade HCl bought from Merck South Africa which corresponds with the concentration of acid often employed in industrial operations such as etching, acid cleaning, descaling, and pickling. The optimal concentration (1.00 mM) of the inhibitors was prepared by initial dissolution of the respective compounds in 5% analytical grade acetone and a calculated volume of 1 M HCl. Working concentrations; 0.10, 0.25, 0.50, and 0.75 mM, respectively were obtained by serial dilution to gain insight into the inhibitor’s effectiveness at minute concentrations. pH measurement of the corrosive 1 M HCl solution, and solutions containing 1.00 mM concentration of DS1–4 was carried out and presented.
2.4. Electrochemical Analyses
Electrochemical experiments were performed at 30 ± 1 °C on AutoLab (302N model) potentiostat/galvanostat obtained from Metrohm. This AutoLab is driven by NOVA 2.1 software, where data analyses were performed. The electrochemical set-up and components of the cell were as previously reported.25 The working electrode was allowed to undergo initial free corrosion for 1800 s without application of external potential/current until a stable open circuit potential (OCP) was achieved. Thereafter, at a scan rate of 1 mV s–1 with respect to the OCP, the potential of the mild steel was varied between −250 and +250 mV. Linear portions of the anodic and cathodic Tafel curves were extrapolated until reaching a juncture that afforded important PDP parameters, and inhibition efficacies are (% IEPDP) calculated from eq 1
| 1 |
where icorro and icorr are corrosion current densities in the electrode/electrolyte system in blank and in the presence of inhibitors, respectively.25 In EIS, impedances (i.e., Zreal and Zimaginary) were obtained and plotted following the experimental set-up previously reported.26 Data from the plotted spectra were fitted into an equivalent Randle circuit, and significant parameters, including solution resistance, charge transfer resistance, and so forth, were deducted. Corrosion inhibition efficiencies with respect to EIS measurements (% IEEIS) were calculated using the following equation
| 2 |
where Rct and Rcto are the resistances to charge transfer in the electrode/electrolyte system without and with inhibitors, respectively.25 In addition, polarization resistances (Rp) were determined at a potential range of −10 to +10 mV with respect to the equilibrium potential. The current response was recorded at a scan rate of 0.125 mV s–1. The values of LPR were obtained for the blank and inhibitor-containing solutions. The following equation was employed to obtain the inhibition performance from the LPR method
| 3 |
where Rp and Rpo are the polarization resistances in the inhibitor-containing solutions and blank solution, respectively. All PDP, LPR, and EIS measurements were carried out in triplicate without stirring, and a thermostatic water bath was employed to regulate the temperature of the solutions during measurements.
2.5. Surface Morphology Study
Analyses of the mild steel surface morphology after immersion in the aggressive electrolyte for 24 h and also in the same solution containing 1.00 mM of DS1 were done using SEM (model JEOL JSM-6610 LV). AFM analyses for DS1–4 were done using an Icon Brock instrument, and data analyses and 3D images were obtained using Nanoscope analysis software. Mild steel coupons used were pretreated as described in Section 2.3.
2.6. Computational Details
Molecular structures of the studied compounds (DS1–DS4) were drawn and visualized with GaussView 5.0, which were taken as the starting geometries for the full geometry optimization calculations. Full geometry optimization of the structures was achieved with the DFT method using the generalized gradient approximation function of Perdew, Burke, and Ernzerhof (PBE)27,28 together with Dunning’s correlation consistent basis sets (double), often expressed as cc-pVDZ.29−33 PBE functional offers some improvements upon the local spin density description of atoms, molecules, and solids, while it is a simple and cost-effective functional for obtaining satisfactory geometry of organic molecules.27 More so, cc-pVDZ basis set is defined for all atoms with respective polarization functions. Therefore, PBE/cc-pVDZ is a reasonable model for the calculations, taking into consideration the large size of the molecules without hugely compromising accuracy. Optimized structures were characterized to have minimum energy on the potential energy surface by the absence of imaginary frequency in the force constant calculations. All calculations were carried out in the gas phase using the Gaussian 16 software.34 MC simulations of the adsorption of optimized molecules of the inhibitor on Fe(110) cleaved surface were carried out using Materials Studio 2019 according to the methodology described in previous works.35,36 Fe(110) crystal slab was adopted as a representative of the mild steel sheet used in the experimental studies. The choice of Fe(110) is due to its proven most favorable configuration based on the combined factors of force/energy, distance, and area.35,37 The energy of the cleaved Fe crystal was minimized in a Forcite module optimization step. The optimized Fe(110) was then expanded into a 10-dimension supercell and put in a vacuum slab of 30 Å size. The DFT-optimized structure of each isolated inhibitor molecule was adsorbed on the Fe(110) surface using the adsorption locator module. The simulated annealing optimization of the Fe–inhibitor complex was achieved with a COMPASS27 force field. The “fine” option of accuracy level was adopted for the simulation.
3. Results and Discussion
3.1. Single Crystal X-ray Structural Analysis
Suitable crystals of DS3 used for this analysis were grown out from a mixture of 1,1-dichloromethane and methanol at 3:1 by slowly evaporating the solvent pair. The structure of DS3 is given in Figure 1. The asymmetric unit of DS3 contains one whole molecule of the compound. The crystal structure entails two dithiocarbamate ligands joined by a disulfide bond (S–S) and the ligands being almost perpendicular to each other, with a torsion angle of 88.90° (Table S2 Supporting Information). The two C–S bond lengths are different and correspond to formal single [C–S; C(11)–S(2) = 1.8003(17) and C(21)–S(3) = 1.8015(17)] and double [C=S; C(11)–S(1) = 1.6384(4) and C(21)–S(4) = 1.6330(17)] bonds. The C–N bond in the compound’s dithiocarbamate unit was observed to be shorter when compared to the normal C–N single bond, which is 1.47 Å,38 and this signifies a partial bond character in the C–N bond length that is C(11)–N(2) = 1.364(2) and C(21)–N(3) = 1.368(2).39,40 The C=S, C–S, and C–N bond lengths measured are comparable to those of related structures in literature.41,42
Figure 1.

ORTEP diagram representation of DS3 drawn at 50% thermal ellipsoid probability. Hydrogen atoms are omitted for clarity.
3.2. pH Measurement and OCP Versus Time Profiles
pH measurements of test solution and solution containing optimum concentration of inhibitors was carried out and values obtained are as followed; 1 M HCl (pH = −0.07), DS1 (pH = 0.01), DS2 (pH = 0.02), DS3 (pH = 0.01), and DS4 (pH = 0.00). These findings signified that the inhibitors at optimum concentration did not significantly change the concentration of the corrodent. The OCP of the mild steel (working electrode) in the corrosive electrolyte was monitored over a period of 1800 s. OCP data were documented for 1 M HCl, and for varying concentrations of DS1, DS2, DS3, and DS4, respectively. The resulting plots are given in Figure 2. The fluctuations observed could be attributed to the slow dissolution of metallic oxide films which compete with the formation of the inhibitor films on the metal surfaces.26 No clear pattern was obtained for the OCP of the electrode without or with varying concentrations of the respective inhibitors, looking at the variation of the respective OCP with respect to time. This is an indication that the compounds function as mixed-type corrosion inhibitors.43
Figure 2.
OCP vs time profile of mild steel in 1 M HCl without and with varying concentrations of (a) DS1, (b) DS2, (c) DS3, and (d) DS4 at 30 °C.
Stable OCP was observed for DS2 and DS3 between 500 and 700 s, at higher inhibitor concentrations. The OCP-time profiles showed that 1800 s was enough for the corroding system to reach a quasi-equilibrium. The addition of the respective inhibitor molecules (at all concentrations) resulted in a shift toward a less negative potential compared with the blank, an indication that anodic protection could be the more favored process. The clear difference observed in the OCP plot for DS4 in comparison to DS1-3 could be attributed to differences in the adsorption abilities on the metal surface resulting from the introduction of an electron-withdrawing group (Cl) in the aromatic moiety of DS4.
3.3. PDP Measurements
An external potential from a direct current source was applied to the corrosion system after a stable OCP was attained to monitor the current responses at the cathode and anode simultaneously. As a result, insights were gained into the kinetics of the corrosion processes occurring at the anode as well as the cathode. The polarization curves acquired in 1 M HCl solution, and in solutions containing varying concentrations of DS1, DS2, DS3, and DS4, respectively are presented in Figure 3.
Figure 3.
Tafel polarization plots of mild steel corrosion in 1 M HCl at varying concentrations of (a) DS1, (b) DS2, (c) DS3, and (d) DS4 at 30 °C.
Upon extrapolation of the linear parts of the Tafel curves to a juncture with respect to the corrosion potential, important electrochemical variables such as corrosion potential (Ecorr), corrosion current density (icorr), anodic Tafel slope (βa), cathodic Tafel slope (βc), and the calculated corrosion inhibition efficiencies were obtained and presented in Table 1. The icorr value reduced drastically upon the introduction of the respective inhibitor molecules. For instance, icorr of 1 M HCl (378.41 μA/cm–2) reduced significantly to 5.30 μA/cm–2 upon the addition of 1.00 mM of DS1, resulting in 98.60% inhibition efficiency. Similar trends were observed for DS2, DS3, and DS4. The implication here is a commendable drop in the rate of deterioration of the mild steel, an indication that the inhibitor molecules interacted with the metal surfaces, thereby, covering the active sites and preventing attacks from the corrosive electrolyte.44
Table 1. Electrochemical Kinetic Variables Acquired from Tafel Polarization Measurements for Mild Steel in 1 M HCl, and with Varying Concentrations of DS1, DS2, DS3, and DS4 at 30 °C.
| inhibitors | conc. (mM) | –Ecorr (mV, Ag/AgCl) | βa (mV/dec) | –βc (mV/dec) | icorr (μA/cm–2) | % IEPDP | Rp (Ω cm2) | % IELPR |
|---|---|---|---|---|---|---|---|---|
| blank | 0 | 445.37 | 125.79 (±1.24) | 78.80 (±1.24) | 378.41 (±1.88) | 55.59(±1.04) | ||
| DS1 | 0.10 | 445.11 | 112.99 (±1.02) | 47.87 (±0.65) | 38.75 (±1.36) | 89.76 | 376.78(±1.14) | 85.25 |
| 0.25 | 435.85 | 105.74 (±1.04) | 36.45 (±1.12) | 27.58 (±1.02) | 92.71 | 462.76(±1.54) | 87.99 | |
| 0.50 | 444.13 | 107.44 (±1.15) | 48.32 (±0.88) | 24.66 (±0.96) | 93.50 | 586.98(±0.88) | 90.53 | |
| 0.75 | 427.92 | 101.45 (±1.01) | 29.67 (±0.54) | 15.59 (±1.44) | 95.88 | 639.38(±1.24) | 91.31 | |
| 1.00 | 422.90 | 107.25 (±1.12) | 48.95 (±1.21) | 5.30 (±0.98) | 98.60 | 2752.60(±1.16) | 97.98 | |
| DS2 | 0.10 | 432.80 | 111.63 (±1.42) | 61.72 (±0.88) | 31.18 (±0.45) | 91.76 | 553.49(±1.32) | 89.96 |
| 0.25 | 449.15 | 129.19 (±1.24) | 68.81 (±0.65) | 19.70 (±0.66) | 94.79 | 989.59(±1.26) | 94.38 | |
| 0.50 | 449.22 | 121.30 (±1.05) | 73.73 (±0.96) | 15.09 (±1.86) | 96.01 | 1319.53(±0.86) | 95.79 | |
| 0.75 | 441.43 | 127.40 (±1.21) | 65.09 (±0.55) | 10.59 (±0.65) | 97.20 | 1767.10(±0.68) | 96.85 | |
| 1.00 | 438.50 | 60.85 (±1.52) | 43.43 (±1.42) | 6.20 (±0.48) | 98.36 | 1774.60(±0.98) | 96.86 | |
| DS3 | 0.10 | 446.66 | 110.20 (±1.06) | 52.39 (±1.32) | 95.81 (±1.02) | 74.68 | 160.93(±1.14) | 65.46 |
| 0.25 | 436.33 | 103.37 (±0.98) | 32.85 (±1.24) | 45.18 (±1.26) | 88.06 | 239.58(±1.18) | 76.80 | |
| 0.50 | 424.99 | 97.08 (±1.34) | 26.95 (±0.64) | 35.48 (±0.86) | 90.62 | 258.16(±1.32) | 78.47 | |
| 0.75 | 422.25 | 94.18 (±1.32) | 32.75 (±0.94) | 28.29 (±0.98) | 92.52 | 372.97(±0.68) | 85.10 | |
| 1.00 | 410.62 | 72.56 (±0.85) | 28.72 (±0.46) | 20.20 (±0.68) | 94.66 | 442..45(±1.06) | 87.44 | |
| DS4 | 0.10 | 446.52 | 112.48 (±1.26) | 57.21 (±0.84) | 135.98 (±1.32) | 64.07 | 121.09(±1.24) | 54.09 |
| 0.25 | 442.24 | 111.60 (±1.32) | 52.13 (±1.24) | 93.37 (±0.89) | 75.33 | 165.24(±0.14) | 66.36 | |
| 0.50 | 438.64 | 107.52 (±0.98) | 44.22 (±0.97) | 81.12 (±1.42) | 78.56 | 167.72(±1.26) | 66.86 | |
| 0.75 | 434.49 | 103.44 (±1.14) | 41.44 (±1.26) | 73.32 (±0.58) | 80.62 | 175.22(±1.02) | 68.27 | |
| 1.00 | 433.96 | 104.45 (±1.18) | 51.82 (±1.28) | 62.17 (±1.06) | 83.57 | 241.91(±0.24) | 77.02 |
Although a clear pattern was not observed in the changes of the respective Tafel slopes data with respect to the blank (Table 1), Figure 3 revealed that both the cathodic and anodic reactions were affected upon the addition of the inhibitors. This indicates that the molecules prevented corrosion of the metal by surface coverage, resulting in the protection of active sites on the metal surface rather than modifying the anodic and cathodic mechanisms.45 It could also be inferred that inhibitors DS1–4 functioned by controlling the dissolution of the metal and also the activation of hydrogen evolution without changing the dissolution methods. Similarly, no significant variations in Ecorr were observed, but a look at Table 1 reveals a slight shift in the Ecorr to less negative values at an optimum concentration of 1.00 mM for all the studied inhibitors. This observation indicates some preference of the inhibitors toward reduction of anodic dissolution of the mild steel.46 This is in agreement with the argument put forward in the OCP-time plot analysis. Ultimately, the studied molecules inhibited the corrosion process at the anodes and the cathodes concurrently. It could be inferred that the test molecules adsorbed onto the metal surface, thus, impeding the cathodic hydrogen evolution as well as the anodic dissolution of the metal.8 The inhibitory efficiencies (% IEPDP) obtained at 1.00 mM of DS1, DS2, DS3, and DS4 were 98.60, 98.36, 94.66, and 83.57%, respectively. A noticeable difference in the % IEPDP of DS4 could be attributed to its different molecular structure. DS4 has an electron-withdrawing motif (Cl) on the aromatic rings, whereas DS1-3 contains electron-donating motifs in the methyl, isopropyl, and mesityl groups, respectively. As such, electronic and steric contributions could affect the rate of coordination to the metal surface.44,47−49
Polarization resistances derived from LPR assessments of the inhibitory potentials of the four studied formamidine-based thiuram disulfides in acid solution are presented in Table 1. As seen from the data in the table, there was an upsurge in the value of Rp as the inhibitors DS1–4 were introduced into the blank solution. This upsurge trend was observed to correspond to the increase in the dosage of DS1–4 indicating that the inhibitor molecules overspread on the surface of the metal substrate to prevent access to corrosive species. From the table, DS1 afforded a maximum protection of 97.98% to the metal surface in acid, while the polarization resistances of the formamidine-based thiuram disulfides rose steadily from 55.59 Ω cm2 upon increasing the concentration. Furthermore, the inhibition efficacies obtained for DS1–DS4 from LPR and PDP measurements were tallied.
3.4. EIS Analyses
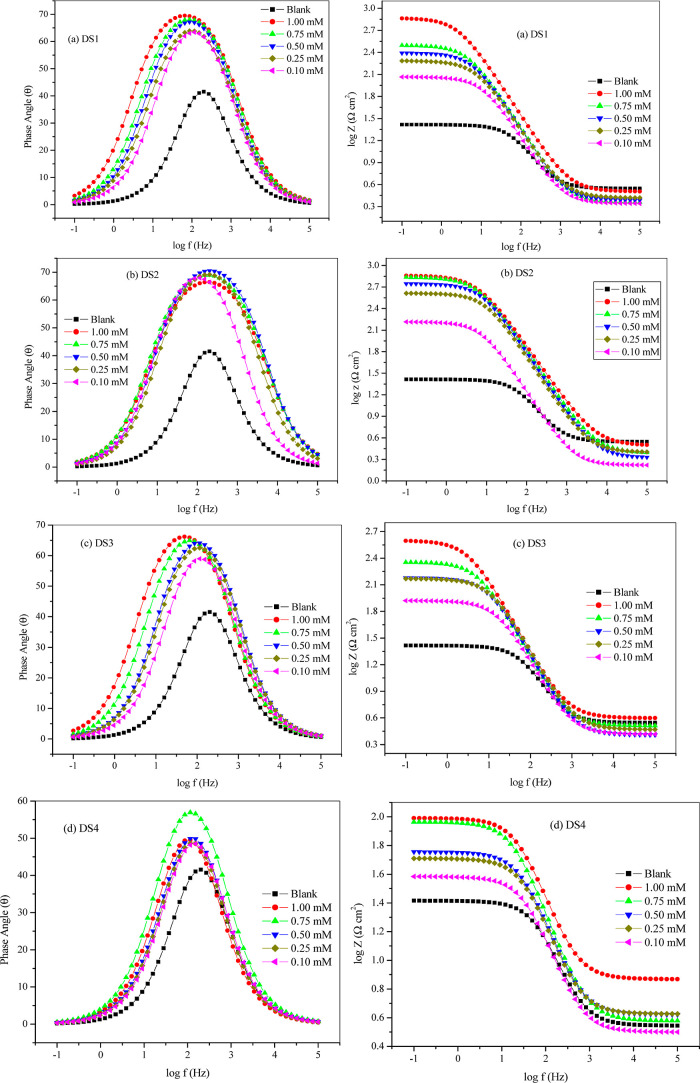
EIS analysis describes the frequency response of metal in 1 M HCl with varying concentrations of DS1, DS2, DS3, and DS4. Impedance is the measured quantity and is the response obtained after the application of external potential with respect to the OCP in the form of alternating current/voltage over a frequency range.50 Data obtained from EIS experiments at varying concentrations of the examined inhibitors are illustrated in Figures 4 and 6. The Nyquist plots (Figure 4) present depressed capacitive loops in the form of semicircles under the real axis. These observations imply that the corrosion mechanism in our system was governed by a definite charge transfer procedure.51,52 Observed depressed semicircular loops point to a nonideal capacitor performance at the corrodent/metal boundary.53 Also, the sizes of the semicircles were directly proportional to the concentration of the inhibitors, entailing a uniform corrosion resistance vis-à-vis decrease in corrosion rate as the concentration of the inhibitors increases.54
Figure 4.
Nyquist plots of mild steel corrosion in 1 M HCl and at varying concentrations of (a) DS1, (b) DS2, (c) DS3, and (d) DS4. (e) Simulated plot for DS1 in 1 mM HCl at 30 °C.
Figure 6.
Bode plots of mild steel corrosion in 1 M HCl and varying concentrations of (a) DS1, (b) DS2, (c) DS3, and (d) DS4 at 30 °C.
Figure 5 shows the equivalent Randle circuit utilized for the simulation of the EIS data through which the electrochemical parameters in Table 2 were obtained. In the circuit represented in Figure 5, the constant phase element (CPE) replaces the double layer capacitance (Cdl), primarily to account for the depressed capacitive loops observed in the Nyquist plots. The replacement also accounts for the fact that impedance measurements entail microscopic electrode properties like surface defects, inhomogeneity of the local charge, and other complex electrochemical processes.6,50 At low frequencies, absolute impedance was found to increase gradually, indicating good inhibitory effects from the studied inhibitors. The values of the goodness of fit ranging from 0.14991 to 0.74196 (Table 2), and the simulated Nyquist plot presented in Figure 4e show that the proposed equivalent electrical circuit appropriately fits the impedance data obtained for DS1–4 and that the fitted data agreed perfectly with experimental data.
Figure 5.

Equivalent Randle circuit used in the analyses of EIS data.
Table 2. EIS Variables for Mild Steel in 1 M HCl and with Varying Concentrations of DS1, DS2, DS3, and DS4 at 30 °C.
| inhibitors | conc. (mM) | Rs (Ω cm2) | Rct (Ω cm2) | n | Yo (μΩ sn cm–2) | Cdl (μF cm–2) | χ2 | –α (deg) | –S | % IEEIS |
|---|---|---|---|---|---|---|---|---|---|---|
| blank | 0 | 3.50 (±0.06) | 22.5 (±0.4) | 0.873 | 243.0 | 113.90 | 0.17431 | 41.54 | 0.64 | |
| DS1 | 0.10 | 2.18 (±0.02) | 114.0 (±0.2) | 0.869 | 213.0 | 121.61 | 0.43123 | 80.26 | ||
| 0.25 | 2.61 (±0.01) | 245.0 (±0.2) | 0.867 | 168.0 | 102.98 | 0.48832 | 90.81 | |||
| 0.50 | 2.15 (±0.02) | 265.0 (±0.3) | 0.844 | 148.0 | 81.34 | 0.50142 | 91.51 | |||
| 0.75 | 2.27 (±0.02) | 313.0 (±0.1) | 0.860 | 170.0 | 105.45 | 0.65431 | 92.81 | |||
| 1.00 | 3.18 (±0.03) | 736.0 (±0.1) | 0.852 | 120.0 | 78.72 | 0.60828 | 69.49 | 0.77 | 96.94 | |
| DS2 | 0.10 | 1.65 (±0.01) | 163.0 (±0.2) | 0.877 | 200.0 | 123.74 | 0.53373 | 86.20 | ||
| 0.25 | 2.51 (±0.03) | 409.0 (±0.1) | 0.864 | 69.9 | 39.95 | 0.42672 | 94.50 | |||
| 0.50 | 2.10 (±0.03) | 558.0 (±0.3) | 0.858 | 62.4 | 35.80 | 0.74196 | 95.97 | |||
| 0.75 | 2.44 (±0.02) | 688.0 (±0.3) | 0.838 | 68.1 | 37.69 | 0.63103 | 96.73 | |||
| 1.00 | 3.08 (±0.01) | 725.0 (±0.2) | 0.816 | 67.7 | 34.31 | 0.49849 | 66.42 | 0.69 | 96.90 | |
| DS3 | 0.10 | 2.60 (±0.02) | 81.0 (±0.4) | 0.865 | 220.0 | 117.34 | 0.35852 | 72.06 | ||
| 0.25 | 2.94 (±0.01) | 145.0 (±0.6) | 0.864 | 172.0 | 96.20 | 0.36617 | 84.48 | |||
| 0.50 | 2.55 (±0.02) | 149.0 (±0.2) | 0.870 | 180.0 | 104.82 | 0.36722 | 84.90 | |||
| 0.75 | 3.25 (±0.02) | 225.0 (±0.2) | 0.866 | 200.0 | 123.78 | 0.39842 | 90.00 | |||
| 1.00 | 3.95 (±0.02) | 395.0 (±0.3) | 0.856 | 175.0 | 111.64 | 0.41334 | 66.18 | 0.74 | 94.30 | |
| DS4 | 0.10 | 3.16 (±0.04) | 35.3 (±0.2) | 0.869 | 269.0 | 133.31 | 0.24624 | 36.26 | ||
| 0.25 | 4.22 (±0.04) | 47.2 (±0.3) | 0.873 | 210.0 | 107.33 | 0.24244 | 52.33 | |||
| 0.50 | 4.18 (±0.04) | 52.8 (±0.4) | 0.869 | 205.0 | 112.03 | 0.24399 | 57.39 | |||
| 0.75 | 3.78 (±0.02) | 88.6 (±0.3) | 0.873 | 173.0 | 94.21 | 0.23661 | 74.60 | |||
| 1.00 | 7.38 (±0.01) | 90.6 (±0.4) | 0.868 | 144.0 | 74.44 | 0.14991 | 49.54 | 0.58 | 75.17 |
Data presented in Table 2 revealed a significant increase in charge transfer resistance (Rct) upon the introduction of the inhibitor molecules. For example, Rct of blank (22.5 Ω cm2) increased drastically to 736.0 Ω cm2 when 1.00 mM of DS1 was added to the corrosive medium. Similar increments were observed for DS2, DS3, and DS4, respectively. The observed increase in the values of Rct is attributed to the adsorbed inhibitor molecules forming a protective cover on the surface of the metal, restricting access to the corrosive electrolyte. The double layer capacitance (Cdl) for the dissolution of the mild steel in HCl with and without the studied disulfides is computed using the following equation
| 4 |
where Yo represents the CPE constant, Rct represents the resistance of charge transfer, and n represents the phase shift and was used in calculating the double layer capacitance. Values of double layer capacitance (Cdl) decrease with the increasing concentration of the respective inhibitor. This could largely be due to a lower local dielectric constant resulting from the adsorption of inhibitor molecules on the steel surface and concurrent displacement of water molecules from the surface, resulting in an increase in the thickness of the double layer.50 The values of phase shift (n) describe the state of homogeneity of the surface of the working electrode. CPE is known to represent resistance when the value of n = 0 and represents Warburg impedance when n = 0.5. For n = 1, CPE represents capacitance, and n = −1 it represents inductance.55 Phase shift values in this study (Table 2) approach unity, indicating that CPE was getting close to the ideal capacitor behavior. The logarithm of the impedance modulus together with phase angles as a function of the logarithm of the frequency is depicted using Bode and phase angle plots (Figure 6). Phase angle values for all inhibitors at an optimal concentration of 1.00 mM were higher than that of blank (−41.54°) but less than −90° with corresponding values of slope between 0.150 and 0.608, which depicts nonideal capacitor characteristics and better inhibitive behavior.56,57 Observed increases in the impedance modulus and the phase angle maxima at the intermediate frequency when concentrations of inhibitor increase could be attributed to adsorption of more inhibitor molecules on the surface of the working electrode. Single phase peaks at the middle of the frequency range suggest that the formation of respective double layer capacitance at the metal/corrosive solution interface was controlled by a single time constant charge transfer.46,58 Furthermore, single peaks observed from the Bode phase plots for all tested inhibitors and in the blank within the considered frequency range reveal that impedance measurements were proper to be fitted in a one-time constant equivalent model. The results obtained from the employed electrochemical techniques are tallied.
Available literature revealed that some disulfide derivatives have been previously reported to minimize corrosion rates of different metal substrates in various concentrations of electrolytic solutions. In order to appraise the effectiveness of the formamidine thiuram disulfides reported in this study, we compared our results with reports found in the literature, and the summary is presented in Table 3. The table shows that DS1–4 shows excellent inhibitory performance in acid at a relatively very minute concentration, compared with other reported studies.
Table 3. Comparison of the Inhibition Efficacies of Studied Thiuram Disulfides for Metals Obtained in the Present Study with Similar Studies in the Literature.
| S/N | disulfides | metal/electrolyte | conc. (mM) | max. % IE | refs |
|---|---|---|---|---|---|
| 1. | bis(1-benzylpiperazine)thiuram disulfide (P2) | mild steel/3.9 M HCl | 1 | 90.00 | (16) |
| 2. | bis(4-benzyl piperidine)thiuram disulfide (P3) | mild steel/5.5 M H3PO4 | 1 | 93.00 | (17) |
| 3. | diallyl disulfide (DAD) | copper/0.5 M H2SO4 | 5 | 91.50 | (18) |
| 4. | propyl disulfide (PPD) | copper/0.5 M H2SO4 | 5 | 92.10 | (18) |
| 5. | dibenzyl disulfide (DBD) | copper/0.5 M H2SO4 | 5 | 98.50 | (18) |
| 6. | phenyl disulfide (PDF) | copper/0.5 M H2SO4 | 5 | 97.70 | (20) |
| 7. | 2,2′-dithiodipyridine (DDP) | copper/0.5 M H2SO4 | 5 | 99.20 | (20) |
| 8. | 5,5-dithiobis(1-phenyl-1H-tetrazole) (DPT) | copper/0.5 M H2SO4 | 5 | 99.70 | (20) |
| 9. | bis(2-benzothiazolyl)-disulfide | mild steel/1 M HCl | 0.20 | 68.20 | (59) |
| 10. | bis(1-benzyl piperazine)thiuram disulfide (C5) | mild steel/3.2 M H2SO4 | 1 | 95.00 | (60) |
| 11. | 2,2′ benzothiazolyl disulfide | mild steel/1 M HCl | 0.15 | 98.34 | (61) |
| 12. | 2,2′ benzothiazolyl disulfide | mild steel/0.5 M H2SO4 | 0.15 | 99.29 | (61) |
| 13. | N,N′-(disulfanne-1,2-dicarbonothioyl)bis(N,N′-bis(2,6-dimethylphenyl)formimidamide) (DS1) | mild steel/1 M HCl | 1 | 98.60 | present study |
| 14. | N,N′-(disulfanne-1,2-dicarbonothioyl)bis(N,N′-bis(2,6-diisopropylphenyl)formimidamide) (DS2) | mild steel/1 M HCl | 1 | 98.36 | present study |
| 15. | N,N′-(disulfanne-1,2-dicarbonothioyl)bis(N,N′-dimesitylformimidamide) (DS3) | mild steel/1 M HCl | 1 | 94.66 | present study |
| 16. | N,N′-(disulfanne-1,2-dicarbonothioyl)bis(N,N′-bis(2,6-dichlorophenyl)formimidamide) (DS4) | mild steel/1 M HCl | 1 | 83.57 | present study |
3.5. Adsorption Study
Adsorption isotherms give insights into the extent and mode of interactions between inhibitor molecules and the metal surface. This is described in terms of the ratio of the sites occupied by the inhibitor per unit of the surface, reported as the surface coverage (θ). The linear form of the Langmuir adsorption isotherm model (eq 5) best fitted our experimental data, when values of coefficient of regression (R2) and slopes were considered. This indicates the probable development of a monolayer of the inhibitor molecules on the surface of the working electrode.62
| 5 |
In eq 5, the equilibrium constant associated with inhibitors adsorbing on the surface of the mild steel is Kads, and Cinh represents the concentration of the inhibitor. Plots of the Langmuir isotherms for DS1, DS2, DS3, and DS4 are presented in Figure 7, and the corresponding thermodynamic variables are shown in Table 4. High values of Kads obtained in this study represent favorable and strong adsorption of inhibitor molecules on the metal surfaces.63
Figure 7.

Langmuir adsorption plots for the corrosion of metal in the presence of DS1, DS2, DS3, and DS4 at 303 K in acidic media.
Table 4. Adsorption Variables for the Inhibition of Mild Steel Corrosion Using DS1, DS2, DS3, and DS4 at 303 K in Acidic Media.
| inhibitors | method | R2 | slope | intercept | Kads (M–1) × 104 | ΔGads (kJ mol–1) |
|---|---|---|---|---|---|---|
| DS1 | PDP | 0.9997 | 1.0067 | 0.0177 | 5.65 | –37.69 |
| EIS | 0.9994 | 1.0153 | 0.0317 | 3.15 | –36.21 | |
| DS2 | PDP | 0.9999 | 1.0184 | 0.0088 | 11.36 | –39.45 |
| EIS | 0.9999 | 1.0191 | 0.0119 | 8.40 | –38.69 | |
| DS3 | PDP | 0.9999 | 1.0495 | 0.0257 | 3.89 | –36.75 |
| EIS | 0.9988 | 1.0332 | 0.0462 | 2.16 | –35.26 | |
| DS4 | PDP | 0.9997 | 1.1641 | 0.0450 | 2.22 | –35.33 |
| EIS | 0.9898 | 1.1399 | 0.1994 | 5.02 | –37.39 |
Calculated values of the standard Gibbs free energy of adsorption (ΔGads) presented in Table 4 suggest the possibility of a combination of the formation of coordinate bond through electron transfer between the inhibitors and metal, and also electrostatic interactions by the charged molecules and the charged metal surface.64 Also, negative values of ΔGads in this study as well as the values tending toward −40 kJ mol–1 is a strong indication that the chemisorption mechanism was favored and the adsorption of DS1, DS2, DS3, and DS4 molecules on the metal surfaces suggest spontaneous adsorption.54
3.6. Surface Morphology Studies
3.6.1. Scanning Electron Microscopy
Mild steel samples were submerged in the aggressive electrolyte and a solution containing an optimum concentration of DS1 at 30 °C, respectively. The coupons were retrieved after 24 h, cleaned with double distilled water, dried, and stored in a desiccator prior to analyses. Information on the relative ability and effectiveness of the inhibitors to adsorb on the metal surfaces, thereby creating a protective barrier from the corrosive electrolyte was obtained from the SEM images (Figure 8). Evidently, a smoother surface of the metal coupon in the presence of 1.00 mM of DS1 (Figure 8b) indicates efficient corrosion inhibition, in relation to the mild steel recovered from the blank solution (Figure 8a).
Figure 8.

SEM images of metal specimens submerged in (a) 1 M HCl and (b) a solution of HCl—1.00 mM DS1 for 24 h.
3.6.2. Atomic Force Microscopy
Surface roughness and topography of mild steel coupons without and in the presence of optimum concentrations of DS1–4 inhibitors retrieved after 24 h immersion are depicted in Figure 9. The 3D AFM micrograph of the coupon retrieved from the blank solution shows a rough surface with a roughness height of 149 nm (Figure 9a). However, upon the addition of 1.00 mM of the inhibitors, the maximum roughness height reduces to 68.1, 71.5, 80.6, and 96.9 nm for DS1, DS2, DS3, and DS4 (Figure 9b–e), respectively. The marked decrease in roughness height observed confirms the adsorption of inhibitor molecules on the steel surface, resulting in the formation of a protective barrier between the corrosive electrolyte and the metal surface.
Figure 9.
3D AFM micrographs of mild steel in (a) 1 M HCl and in the presence of 1.00 mM concentration of (b) DS1, (c) DS2, (d) DS3, and (e) DS4 at 30 °C.
3.7. DFT Based Quantum Chemical Calculations
Optimized structures and charge density distributions of the frontier molecular orbitals (FMOs) of the inhibitor molecules are shown in Figure 10, as visualized in GaussView 5.0. The four molecules assume similar configurations, having the atoms of the thioamide–dithioperoxy group in planar orientation. The molecules also adopt a near symmetric trans-orientation. The four molecules also display similar distributions of electron density for the FMOs. The electron density distribution of the highest occupied molecular orbital (HOMO) of each molecule involves the thioamide–dithioperoxy group with slight extension to the adjoining atoms. DS4 has the most delocalized HOMO density. Similarly, the electron density distribution of the lowest unoccupied molecular orbital (LUMO) spread on the thioamide–dithioperoxy group and extended to neighboring atoms. It appears that the essential active site in the molecules for the donor–acceptor interaction is the thioamide–dithioperoxy group. FMOs’ electron density is not extended to aromatic rings. These observations suggest that a possible donor–acceptor interaction between the inhibitor molecules and the Fe atom (in the mild steel) would essentially involve the lone pair and π-electrons in the thioamide–dithioperoxy group and unoccupied atomic orbitals of Fe.
Figure 10.
Optimized molecular structures and FMOs’ charge density for the studied inhibitor molecules.
Reactivity parameters derived from the DFT calculations are listed in Table 5. Results in Table 5 reveal that the salient orbital energies, that is the energies of the HOMO and LUMO of the four molecules are not significantly different. As a result, the reactivity indices derived from these orbital energies are quite close. It is difficult to derive a definite pattern for the quantum chemical parameters in a way that would be correlated with the observed inhibition efficiencies. However, DS4 has the lowest EHOMO value, which suggests that it has the least propensity to donate electrons to vacant orbitals of Fe. DS1 has a lesser value of ELUMO than DS3, which agrees with higher inhibition efficiencies of the former and might relate to the ability to accept electrons from occupied orbitals of Fe during back-bonding.
Table 5. Quantum Chemical Variables for the Studied Compounds.
| compound | EHOMO (eV) | ELUMO (eV) | ΔE (eV) | η (eV) | χ (eV) | ΔN | dipole moment (debye) |
|---|---|---|---|---|---|---|---|
| DS1 | –4.918 | –2.918 | 1.999 | 1.000 | 3.918 | 0.451 | 0.000 |
| DS2 | –4.944 | –2.875 | 2.069 | 1.034 | 3.910 | 0.440 | 0.272 |
| DS3 | –4.796 | –2.804 | 1.992 | 0.996 | 3.800 | 0.512 | 0.002 |
| DS4 | –5.004 | –2.981 | 2.023 | 1.012 | 3.992 | 0.409 | 0.001 |
3.8. MC Simulations Studies
The simulated adsorption of DS1–4 on Fe(110) surface as obtained from MC simulation is presented in Figure 11. The equilibrium configurations in Figure 11 are displayed together with the predicted adsorption energies (Eads). The results showed an extremely high magnitude of adsorption energy for DS1 compared to the other compounds. This might be due to its near zero dipole moment (as shown in Table 5) that favors the accumulation of nonpolar inhibitor molecules on the surface layer of the metal.65
Figure 11.

Simulated adsorption of the studied inhibitor molecules on the Fe(110) surface.
3.9. Mechanism of Inhibition
Experimental results obtained from PDP, EIS, LPR, surface studies, and theoretical analyses indicate that the corrosion inhibition mechanism of DS1–4 depends mainly on adsorption on the metal surface, influenced largely by the structural properties of the inhibitor molecules. On adsorption onto the metal surface, a protective layer is formed in the electrode/electrolyte interface. In the aqueous 1 M HCl solution, the formamidine-based thiuram disulfides compounds exist in the protonated forms, and the presence of hydrated Cl– ions on the metal surface provides a strong negative charge, thereby attracting the charged inhibitor molecules. As presented in Figure 12, there is a possibility of multiple interactions, including coordination with the unpaired electron on the metal, electrostatic attraction, and/or interaction between the charged metal surface (negative) and the inhibitor molecule (positive). These processes could be due to the presence of electron-rich aromatic rings and the thioamide–dithioperoxy group. Upon the release of H2 gas during the corrosion process, there is a competition between the aqueous H+ and the protonated species, causing the protonated compounds to revert to the neutral state and, hence, transfer the unpaired electron into the unoccupied orbital of the metal. This process is governed by back-bonding; transferring electrons to the antibonding orbital of the formamidine-based thiuram disulfides compounds.66 Data from adsorption isotherm analysis confirms that the mechanism of adsorption was mainly chemisorption; formation of coordination bond with metal through the thioamide–dithioperoxy group and pi electrons of the aromatic rings.
Figure 12.
Pictorial representation of the adsorption mechanism of the inhibitors.
4. Conclusions
Formamidine-based thiuram disulfide compounds have shown excellent performance in mitigating the corrosion of mild steel in 1 M HCl at 30 °C, and findings are enumerated;
-
1.
Inhibition properties of DS1, DS2, DS3, and DS4 increased with increasing concentration of the respective inhibitors, with DS1 having the best inhibition efficacy of 98.60% at 1.00 mM concentration.
-
2.
Electrochemical studies established that all inhibitors investigated effectively minimized both the cathodic hydrogen evolution and the dissolution of the metal at the anode, with a slight preference for anodic protection, and hence, they are classified as mixed-type inhibitors.
-
3.
Electronic and steric differences in the molecular structures of the molecules exhibited certain effects on the adsorption powers of the compounds.
-
4.
The relationship established between the surface coverage and the concentrations of the respective inhibitors was consistent with the Langmuir adsorption isotherm. The Calculated Gibbs free energy (ΔGads) ranging between −35.26 and −39.45 kJ mol–1 confirmed that the adsorption process was both chemical and physical, with a preference toward chemisorption.
-
5.
SEM and AFM micrographs showed surface coverage and protection from corrosive electrolytes.
-
6.
Molecular calculations and simulation on Fe(110) depicted a near zero dipole moment that favors adsorption of nonpolar inhibitor molecules on the metal surface.
-
7.
Finally, significant savings in terms of cost, and general mitigation of problems associated with corrosion of mild steel in acidic media will be achieved by the application of these new formamidine-based thiuram disulfide compounds as candidate inhibitors in commercial industries.
Acknowledgments
E.D.A. acknowledges the College of Science, Engineering and Technology, the University of South Africa for the postdoctoral research fellowship. S.D.O. and B.O. acknowledge ESKOM TESP. The Centre for High Performance Computing (CHPC), Cape Town, South Africa is hereby acknowledged for granting access to the facilities used for computational studies.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00985.
Single-crystal X-ray diffraction data of compound DS3 (PDF)
Author Contributions
E.D.A., S.D.O., T.W.Q., and L.O.O.: Investigation, Data curation, and Writing-Original draft. E.E.N., B.O., and E.E.E.: Conceptualization, Review and Editing, and Supervision.
The authors declare no competing financial interest.
Supplementary Material
References
- Dwivedi D.; Lepková K.; Becker T. Carbon steel corrosion: a review of key surface properties and characterization methods. RSC Adv. 2017, 7, 4580–4610. 10.1039/c6ra25094g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G.; Thompson N.; Moghissi O.; Gould M.; Payer J.. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study; NACE International: Houston Texas, 2016. [Google Scholar]
- Yang H.-M. Role of Organic and Eco-Friendly Inhibitors on the Corrosion Mitigation of Steel in Acidic Environments-A State-of-Art Review. Molecules 2021, 26, 3473. 10.3390/molecules26113473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rani B. E. A.; Basu B. B. J. Green Inhibitors for Corrosion Protection of Metals and Alloys: An Overview. Int. J. Corros. 2012, 2012, 380217. 10.1155/2012/380217. [DOI] [Google Scholar]
- Marinescu M. Recent advances in the use of benzimidazoles as corrosion inhibitors. BMC Chem. 2019, 13, 136. 10.1186/s13065-019-0655-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkous H.; Djellali S.; Sahraoui R.; Benguerba Y.; Behloul H.; Çukurovali A. Corrosion inhibition of mild steel by 2-(2-methoxybenzylidene) hydrazine-1-carbothioamide in hydrochloric acid solution: Experimental measurements and quantum chemical calculations. J. Mol. Liq. 2020, 307, 112957. 10.1016/j.molliq.2020.112957. [DOI] [Google Scholar]
- Ashassi-Sorkhabi H.; Moradi-Alavian S.; Esrafili M. D.; Kazempour A. Hybrid sol-gel coatings based on silanes-amino acids for corrosion protection of AZ91 magnesium alloy: Electrochemical and DFT insights. Prog. Org. Coat. 2019, 131, 191–202. 10.1016/j.porgcoat.2019.01.052. [DOI] [Google Scholar]
- Daoud D.; Douadi T.; Hamani H.; Chafaa S.; Al-Noaimi M. Corrosion inhibition of mild steel by two new S-heterocyclic compounds in 1 M HCl: Experimental and computational study. Corros. Sci. 2015, 94, 21–37. 10.1016/j.corsci.2015.01.025. [DOI] [Google Scholar]
- Oladipo S. D.; Olotu F. A.; Soliman M.; Mocktar C.; Omondi B. Formamidine-based thiuram disulfides: Synthesis, structural characterization, biological studies, and preliminary cheminformatics evaluation. J. Mol. Struct. 2020, 1219, 128553. 10.1016/j.molstruc.2020.128553. [DOI] [Google Scholar]
- Horita Y.; Takii T.; Yagi T.; Ogawa K.; Fujiwara N.; Inagaki E.; Kremer L.; Sato Y.; Kuroishi R.; Lee Y.; Makino T.; Mizukami H.; Hasegawa T.; Yamamoto R.; Onozaki K. Antitubercular activity of disulfiram, an antialcoholism drug, against multidrug-and extensively drug-resistant Mycobacterium tuberculosis isolates. Antimicrob. Agents Chemother. 2012, 56, 4140–4145. 10.1128/aac.06445-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.; Malloy G.; Nedunchezian D.; Lukrec A.; Howard R. Disulfiram inhibits the in vitro growth of methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 1991, 35, 785–787. 10.1128/aac.35.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M.; Izakovic M.; Mazur M.; Rhodes C. J.; Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol. Cell. Biochem. 2004, 266, 37–56. 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- Wickström M.; Danielsson K.; Rickardson L.; Gullbo J.; Nygren P.; Isaksson A.; Larsson R.; Lövborg H. Pharmacological profiling of disulfiram using human tumor cell lines and human tumor cells from patients. Biochem. Pharmacol. 2007, 73, 25–33. 10.1016/j.bcp.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Sauna Z. E.; Shukla S.; Ambudkar S. V. Disulfiram, an old drug with new potential therapeutic uses for human cancers and fungal infections. Mol. BioSyst. 2005, 1, 127–134. 10.1039/b504392a. [DOI] [PubMed] [Google Scholar]
- De S. k.; White J. R.. Rubber Technologist’s Handbook; iSmithers Rapra Publishing, 2001; Vol. 1. [Google Scholar]
- Ousslim A.; Bekkouch K.; Hammouti B.; Elidrissi A.; Aouniti A. Piperazine derivatives as inhibitors of the corrosion of mild steel in 3.9 M HCl. J. Appl. Electrochem. 2009, 39, 1075–1079. 10.1007/s10800-008-9759-0. [DOI] [Google Scholar]
- Ousslim A.; Bekkouch K.; Chetouani A.; Abbaoui E.; Hammouti B.; Aouniti A.; Elidrissi A.; Bentiss F. Adsorption and corrosion inhibitive properties of piperidine derivatives on mild steel in phosphoric acid medium. Res. Chem. Intermed. 2014, 40, 1201–1221. 10.1007/s11164-013-1033-3. [DOI] [Google Scholar]
- Tan B.; Zhang S.; Qiang Y.; Guo L.; Feng L.; Liao C.; Xu Y.; Chen S. A combined experimental and theoretical study of the inhibition effect of three disulfide-based flavouring agents for copper corrosion in 0.5 M sulfuric acid. J. Colloid Interface Sci. 2018, 526, 268–280. 10.1016/j.jcis.2018.04.092. [DOI] [PubMed] [Google Scholar]
- Ma T.; Tan B.; Guo L.; Wang W.; Li W.; Ji J.; Yan M.; Kaya S. Experimental and theoretical investigation on the inhibition performance of disulfide derivatives on cobalt corrosion in alkaline medium. J. Mol. Liq. 2021, 341, 116907. 10.1016/j.molliq.2021.116907. [DOI] [Google Scholar]
- Tan B.; Zhang S.; Li W.; Zuo X.; Qiang Y.; Xu L.; Hao J.; Chen S. Experimental and theoretical studies on inhibition performance of Cu corrosion in 0.5 M H2SO4 by three disulfide derivatives. J. Ind. Eng. Chem. 2019, 77, 449–460. 10.1016/j.jiec.2019.05.011. [DOI] [Google Scholar]
- Plyusnin V. F.; Kuznetzova E. P.; Bogdanchikov G. A.; Grivin V. P.; Kirichenko V. N.; Larionov S. V. Dithiocarbamate radicals in laser flash photolysis of thiuram disulphide and dithiocarbamate anion: calculation of optical spectra. J. Photochem. Photobiol., A 1992, 68, 299–308. 10.1016/1010-6030(92)85239-q. [DOI] [Google Scholar]
- Tao Z.; Zhang S.; Li W.; Hou B. Corrosion inhibition of mild steel in acidic solution by some oxo-triazole derivatives. Corros. Sci. 2009, 51, 2588–2595. 10.1016/j.corsci.2009.06.042. [DOI] [Google Scholar]
- Ahmed S. K.; Ali W. B.; Khadom A. A. Synthesis and investigations of heterocyclic compounds as corrosion inhibitors for mild steel in hydrochloric acid. Int. J. Ind. Chem. 2019, 10, 159–173. 10.1007/s40090-019-0181-8. [DOI] [Google Scholar]
- Kairi N. I.; Kassim J. The effect of temperature on the corrosion inhibition of mild steel in 1 M HCl solution by Curcuma longa extract. Int. J. Electrochem. Sci. 2013, 8, 7138–7155. [Google Scholar]
- Akpan E. D.; Isaac I. O.; Olasunkanmi L. O.; Ebenso E. E.; Sherif E.-S. M. Acridine-based thiosemicarbazones as novel inhibitors of mild steel corrosion in 1 M HCl: synthesis, electrochemical, DFT and Monte Carlo simulation studies. RSC Adv. 2019, 9, 29590–29599. 10.1039/c9ra04778f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri T. W.; Olasunkanmi L. O.; Akpan E. D.; Alfantazi A.; Obot I. B.; Verma C.; Al-Mohaimeed A. M.; Ebenso E. E.; Quraishi M. A. Chromeno-carbonitriles as corrosion inhibitors for mild steel in acidic solution: electrochemical, surface and computational studies. RSC Adv. 2021, 11, 2462–2475. 10.1039/d0ra07595g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdew J. P.; Burke K.; Ernzerhof M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. 10.1103/physrevlett.77.3865. [DOI] [PubMed] [Google Scholar]
- Perdew J. P.; Burke K.; Ernzerhof M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1997, 78, 1396. 10.1103/physrevlett.78.1396. [DOI] [PubMed] [Google Scholar]
- Dunning T. H. Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. 10.1063/1.456153. [DOI] [Google Scholar]
- Kendall R. A.; Dunning R. J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. 10.1063/1.462569. [DOI] [Google Scholar]
- Woon D. E. Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J. Chem. Phys. 1993, 98, 1358–1371. 10.1063/1.464303. [DOI] [Google Scholar]
- Peterson K. A.; Woon D. E.; Dunning T. H. Benchmark calculations with correlated molecular wave functions. IV. The classical barrier height of the H+H2→H2+H reaction. J. Chem. Phys. 1994, 100, 7410–7415. 10.1063/1.466884. [DOI] [Google Scholar]
- Wilson A. K.; van Mourik T.; Dunning T. H. Gaussian basis sets for use in correlated molecular calculations. VI. Sextuple zeta correlation consistent basis sets for boron through neon. J. Mol. Struct.: THEOCHEM 1996, 388, 339–349. 10.1016/s0166-1280(96)80048-0. [DOI] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H.; Li X.; Caricato M.; Marenich A. V.; Bloino J.; Janesko B. G.; Gomperts R.; Mennucci B.; Hratchian H. P.; Ortiz J. V.; Izmaylov A. F.; Sonnenberg J. L.; Williams; Ding F.; Lipparini F.; Egidi F.; Goings J.; Peng B.; Petrone A.; Henderson T.; Ranasinghe D.; Zakrzewski V. G.; Gao J.; Rega N.; Zheng G.; Liang W.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Throssell K.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M. J.; Heyd J. J.; Brothers E. N.; Kudin K. N.; Staroverov V. N.; Keith T. A.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A. P.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Millam J. M.; Klene M.; Adamo C.; Cammi R.; Ochterski J. W.; Martin R. L.; Morokuma K.; Farkas O.; Foresman J. B.; Fox D. J.. Gaussian 16 Rev. C.01: Wallingford, CT, 2016.
- Olasunkanmi L. O.; Ebenso E. E. Experimental and computational studies onpropanone derivatives of quinoxalin-6-yl-4,5-dihydropyrazole as inhibitors of mild steel corrosion in hydrochloric acid. J. Colloid Interface Sci. 2020, 561, 104–116. 10.1016/j.jcis.2019.11.097. [DOI] [PubMed] [Google Scholar]
- Olasunkanmi L. O.; Idris A. O.; Adewole A. H.; Wahab O. O.; Ebenso E. E. Adsorption and Corrosion Inhibition Potentials of Salicylaldehyde-based Schiff Bases of Semicarbazide and p-Toluidine on Mild Steel in Acidic Medium: Experimental and Computational Studies. Surf. Interface 2020, 21, 100782. 10.1016/j.surfin.2020.100782. [DOI] [Google Scholar]
- Guo L.; Qi C.; Zheng X.; Zhang R.; Shen X.; Kaya S. Toward understanding the adsorption mechanism of large size organic corrosion inhibitors on an Fe (110) surface using the DFTB method. RSC Adv. 2017, 7, 29042–29050. 10.1039/c7ra04120a. [DOI] [Google Scholar]
- Oladipo S. D.; Omondi B.; Mocktar C. Synthesis and structural studies of nickel (II)-and copper (II)-N, N′-diarylformamidine dithiocarbamate complexes as antimicrobial and antioxidant agents. Polyhedron 2019, 170, 712–722. 10.1016/j.poly.2019.06.038. [DOI] [Google Scholar]
- Oladipo S. D.; Omondi B.; Mocktar C. Co (III) N, N′diarylformamidine dithiocarbamate complexes: Synthesis, characterization, crystal structures and biological studies. Appl. Organomet. Chem. 2020, 34, e5610 10.1002/aoc.5610. [DOI] [Google Scholar]
- Oladipo S. D.; Omondi B. Mercury (II) N, N′-diarylformamidine dithiocarbamates as single-source precursors for the preparation of oleylamine-capped HgS nanoparticles. Transition Met. Chem. 2020, 45, 391–402. 10.1007/s11243-020-00391-y. [DOI] [Google Scholar]
- Jian F.; Jiang L.; Fun H.-K.; Chinnakali K.; Razak I.; You X. Bis (dipropylthiocarbamoyl) disulfide. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. 1999, 55, 573–574. 10.1107/s010827019801573x. [DOI] [Google Scholar]
- Zhai J.; Yin H.-D.; Li F.; Chen S.-W.; Wang D.-Q. Bis(N,N-dibenzylthiocarbamoyl) disulfide. Acta Crystallogr., Sect. E: Struct. Rep. Online 2007, 63, o1969–o1970. 10.1107/s1600536807013293. [DOI] [Google Scholar]
- Farhadian A.; Rahimi A.; Safaei N.; Shaabani A.; Abdouss M.; Alavi A. A theoretical and experimental study of castor oil-based inhibitor for corrosion inhibition of mild steel in acidic medium at elevated temperatures. Corros. Sci. 2020, 175, 108871. 10.1016/j.corsci.2020.108871. [DOI] [Google Scholar]
- Chaouiki A.; Chafiq M.; Lgaz H.; Al-Hadeethi M. R.; Ali I. H.; Masroor S.; Chung I.-M. Green Corrosion Inhibition of Mild Steel by Hydrazone Derivatives in 1.0 M HCl. Coatings 2020, 10, 640. 10.3390/coatings10070640. [DOI] [Google Scholar]
- Liang C.; Liu Z.; Liang Q.; Han G.-C.; Han J.; Zhang S.; Feng X.-Z. Synthesis of 2-aminofluorene bis-Schiff base and corrosion inhibition performance for carbon steel in HCl. J. Mol. Liq. 2019, 277, 330–340. 10.1016/j.molliq.2018.12.095. [DOI] [Google Scholar]
- Qiao K.; Zeng Y. Comparative study on two imidazolium-based ionic liquid surfactants as corrosion inhibitors for N80 steel in 15% hydrochloric acid solution. Mater. Corros. 2020, 71, 1913–1926. 10.1002/maco.202011775. [DOI] [Google Scholar]
- Nazir U.; Akhter Z.; Janjua N. K.; Adeel Asghar M.; Kanwal S.; Butt T. M.; Sani A.; Liaqat F.; Hussain R.; Shah F. U. Biferrocenyl Schiff bases as efficient corrosion inhibitors for an aluminium alloy in HCl solution: a combined experimental and theoretical study. RSC Adv. 2020, 10, 7585–7599. 10.1039/c9ra10692h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. A.; El-Shareef A.; Al-Ghamdi R.; Saeed M. The isoxazolidines: the effects of steric factor and hydrophobic chain length on the corrosion inhibition of mild steel in acidic medium. Corros. Sci. 2005, 47, 2659–2678. 10.1016/j.corsci.2004.11.007. [DOI] [Google Scholar]
- Ahmed M. H. O.; Al-Amiery A. A.; Al-Majedy Y. K.; Kadhum A. A. H.; Mohamad A. B.; Gaaz T. S. Synthesis and characterization of a novel organic corrosion inhibitor for mild steel in 1 M hydrochloric acid. Results Phys. 2018, 8, 728–733. 10.1016/j.rinp.2017.12.039. [DOI] [Google Scholar]
- Encinas-Sánchez V.; de Miguel M. T.; Lasanta M. I.; García-Martín G.; Pérez F. J. Electrochemical impedance spectroscopy (EIS): An efficient technique for monitoring corrosion processes in molten salt environments in CSP applications. Sol. Energy Mater. Sol. Cells 2019, 191, 157–163. 10.1016/j.solmat.2018.11.007. [DOI] [Google Scholar]
- Shukla S. K.; Singh A. K.; Ahamad I.; Quraishi M. Streptomycin A commercially available drug as corrosion inhibitor for mild steel in hydrochloric acid solution. Mater. Lett. 2009, 63, 819–822. 10.1016/j.matlet.2009.01.020. [DOI] [Google Scholar]
- Almzarzie K.; Falah A.; Massri A.; Kellawi H. Electrochemical impedance spectroscopy (EIS) and study of iron corrosion inhibition by turmeric roots extract (TRE) in hydrochloric acid solution. Egypt. J. Chem. 2019, 62, 501–512. 10.21608/EJCHEM.2018.5295.1476. [DOI] [Google Scholar]
- Fouda A. E.-A. S.; El-Askalany A. H.; Molouk A. F. S.; Elsheikh N. S.; Abousalem A. S. Experimental and computational chemical studies on the corrosion inhibitive properties of carbonitrile compounds for carbon steel in aqueous solutions. Sci. Rep. 2021, 11, 21672. 10.1038/s41598-021-00701-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Haddad M. A. M.; Bahgat Radwan A.; Sliem M. H.; Hassan W. M. I.; Abdullah A. M. Highly efficient eco-friendly corrosion inhibitor for mild steel in 5 M HCl at elevated temperatures: experimental & molecular dynamics study. Sci. Rep. 2019, 9, 3695. 10.1038/s41598-019-40149-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M.; Guo L.; He Z.; Marzouki R.; Zhang R.; Berdimurodov E. Insights into the newly synthesized N-doped carbon dots for Q235 steel corrosion retardation in acidizing media: A detailed multidimensional study. J. Colloid Interface Sci. 2022, 608, 2039–2049. 10.1016/j.jcis.2021.10.160. [DOI] [PubMed] [Google Scholar]
- Rosero-Navarro N.; Curioni M.; Bingham R.; Durán A.; Aparicio M.; Cottis R.; Thompson G. Electrochemical techniques for practical evaluation of corrosion inhibitor effectiveness. Performance of cerium nitrate as corrosion inhibitor for AA2024T3 alloy. Corros. Sci. 2010, 52, 3356–3366. 10.1016/j.corsci.2010.06.012. [DOI] [Google Scholar]
- Ji G.; Dwivedi P.; Sundaram S.; Prakash R. Aqueous extract of Argemone mexicana roots for effective protection of mild steel in an HCl environment. Res. Chem. Intermed. 2016, 42, 439–459. 10.1007/s11164-015-2029-y. [DOI] [Google Scholar]
- Tan B.; Zhang S.; Liu H.; Guo Y.; Qiang Y.; Li W.; Guo L.; Xu C.; Chen S. Corrosion inhibition of X65 steel in sulfuric acid by two food flavorants 2-isobutylthiazole and 1-(1,3-Thiazol-2-yl) ethanone as the green environmental corrosion inhibitors: Combination of experimental and theoretical researches. J. Colloid Interface Sci. 2019, 538, 519–529. 10.1016/j.jcis.2018.12.020. [DOI] [PubMed] [Google Scholar]
- Abdeli M.; Parvini Ahmadi N.; Azari Khosroshahi R. Influence of bis-(2-benzothiazolyl)-disulfide on corrosion inhibition of mild steel in hydrochloric acid media. J. Solid State Electrochem. 2011, 15, 1867–1873. 10.1007/s10008-010-1162-1. [DOI] [Google Scholar]
- Ousslim A.; Chetouani A.; Hammouti B.; Bekkouch K.; Al-Deyab S.; Aouniti A.; Elidrissi A. Thermodynamics, quantum and electrochemical studies of corrosion of iron by piperazine compounds in sulphuric acid. Int. J. Electrochem. Sci. 2013, 8, 5980–6004. [Google Scholar]
- Singh A. K.; Quraishi M. Effect of 2, 2′ benzothiazolyl disulfide on the corrosion of mild steel in acid media. Corros. Sci. 2009, 51, 2752–2760. 10.1016/j.corsci.2009.07.011. [DOI] [Google Scholar]
- Solmaz R. Investigation of adsorption and corrosion inhibition of mild steel in hydrochloric acid solution by 5-(4-Dimethylaminobenzylidene)rhodanine. Corros. Sci. 2014, 79, 169–176. 10.1016/j.corsci.2013.11.001. [DOI] [Google Scholar]
- Singh A.; Ansari K. R.; Quraishi M. A.; Kaya S.; Banerjee P. The effect of an N-heterocyclic compound on corrosion inhibition of J55 steel in sweet corrosive medium. New J. Chem. 2019, 43, 6303–6313. 10.1039/c9nj00356h. [DOI] [Google Scholar]
- Okey N. C.; Obasi N. L.; Ejikeme P. M.; Ndinteh D. T.; Ramasami P.; Sherif E.-S. M.; Akpan E. D.; Ebenso E. E. Evaluation of some amino benzoic acid and 4-aminoantipyrine derived Schiff bases as corrosion inhibitors for mild steel in acidic medium: Synthesis, experimental and computational studies. J. Mol. Liq. 2020, 315, 113773. 10.1016/j.molliq.2020.113773. [DOI] [Google Scholar]
- Olasunkanmi L. O.; Obot I. B.; Kabanda M. M.; Ebenso E. E. Some Quinoxalin-6-yl Derivatives as Corrosion Inhibitors for Mild Steel in Hydrochloric Acid: Experimental and Theoretical Studies. J. Phys. Chem. C 2015, 119, 16004–16019. 10.1021/acs.jpcc.5b03285. [DOI] [Google Scholar]
- Lazrak J.; Ech-chihbi E.; El Ibrahimi B.; El Hajjaji F.; Rais Z.; Tachihante M.; Taleb M. Detailed DFT/MD simulation, surface analysis and electrochemical computer explorations of aldehyde derivatives for mild steel in 1.0 M HCl. Colloids Surf., A 2022, 632, 127822. 10.1016/j.colsurfa.2021.127822. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.