Abstract
Background
Bipolar disorder (BD) is associated with cognitive impairment and mitochondrial dysfunction. However, the associations among mitochondrial DNA copy number (MCN), treatment response, and cognitive function remain elusive in BD patients.
Methods
Sixty euthymic BD patients receiving valproate (VPA) and 66 healthy controls from the community were recruited. The indices of metabolic syndrome (MetS) were measured. Quantitative polymerase chain reaction analysis of blood leukocytes was used to measure the MCN. Cognitive function was measured by calculating perseverative errors and completed categories on the Wisconsin Card Sorting Test (WCST). The VPA treatment response was measured using the Alda scale.
Results
BD patients had significantly higher MCN, triglyceride, and C-reactive protein (CRP) levels, waist circumference, and worse performance on the WCST than the controls. Regression models showed that BD itself and the VPA concentration exerted significant effects on increased MCN levels. Moreover, the receiver operating characteristic curve analysis showed that an MCN of 2.05 distinguished VPA responders from nonresponders, with an area under the curve of 0.705 and a sensitivity and specificity of 0.529 and 0.816, respectively. An MCN level ≥2.05 was associated with 5.39 higher odds of being a VPA responder (P = .006). BD patients who were stratified into the high-MCN group had a higher VPA response rate, better WCST performance, lower CRP level, and less MetS.
Conclusions
The study suggests a link between the peripheral MCN and cognitive function in BD patients. As an inflammatory status, MetS might modulate this association.
Keywords: Bipolar disorder, cognitive function, metabolic syndrome, mitochondrial DNA copy number, valproate
Significance Statement.
This study is the first, to our knowledge, to investigate the association between mitochondrial DNA copy number (MCN) and cognitive function in euthymic bipolar disorder (BD) patients receiving valproate (VPA). In the current study, BD patients showed significantly higher MCN than the controls, even after adjustment for age, sex, and metabolic syndrome (MetS). The VPA concentration was positively correlated with the MCN in BD patients. In addition, we evaluated the stratification effect of MCN on the clinical features of BD patients. We found that the MCN significantly distinguished VPA responders from non-responders. Our results revealed a higher percentage of VPA responders and better cognitive performance of BD patients in the high-MCN group than in the low-MCN group. Taken together, the study findings provide better insights into how MCN might serve as a biomarker in BD.
Introduction
Bipolar disorder (BD) is a severe and chronic psychiatric illness characterized by recurrent episodes of mania and depression, which significantly affect the functioning and quality of life of patients. Moreover, accumulating evidence for cognitive impairments has been documented in BD patients (Green, 2006; Berk et al., 2007). Patients with BD show cognitive dysfunction in verbal memory and frontal executive tasks (Martinez-Aran et al., 2004). A more recent meta-analysis also confirmed evident deficits in verbal learning, trail-making, and verbal working tasks (Bourne et al., 2013). These cognitive impairments might be long lasting, even in stable euthymic BD patients (Mur et al., 2008). A review of meta-analyses from longitudinal studies concluded that cognitive deficits did not progress but appeared generally stable (Bora and Ozerdem, 2017). Cognitive dysfunction could partially explain compromised psychosocial and occupational functioning (Zarate et al., 2000; Mur et al., 2008).
Mitochondrial DNA (mtDNA) is a double-stranded, closed circular molecule located in the mitochondria with no introns or histones. Without histone protection, mtDNA is susceptible to oxidative injury because mitochondria can generate reactive oxygen species during ATP synthesis (Furda et al., 2012). An altered mtDNA copy number (MCN) could result from inflammation potentially playing a pathogenic role in mitochondrial dysfunction and disease (Malik and Czajka, 2013). Therefore, the MCN is a potential biomarker of mitochondrial function, and an abnormal MCN has been suggested to be correlated with various psychiatric disorders, such as schizophrenia, BD, and Alzheimer’s disease (Mancuso et al., 2007; Li et al., 2015).
Recent evidence has suggested a role for mitochondrial dysfunction in the possible pathophysiology of BD (Kato and Kato, 2000). Regarding BD, several in vivo studies have examined MCNs but produced conflicting results (Chang et al., 2014; de Sousa et al., 2014; Fries et al., 2017; Rollins et al., 2018; Wang et al., 2018). Fries et al. and Rollins et al. found a significantly increased MCN in BD patients compared with controls (Fries et al., 2017; Rollins et al., 2018), while de Sousa et al. did not observe a significant difference in the MCN between depressed BD patients and healthy controls (de Sousa et al., 2014). Although a meta-analysis failed to identify any significant association between the MCN and BD, a subgroup analysis revealed a markedly lower MCN in Asian patients with BD (Yamaki et al., 2018).
Increased inflammation and prevalent MetS are strongly correlated with mitochondrial dysfunction (Huang et al., 2011; Malik and Czajka, 2013), and both conditions have been reported in BD patients (Kapczinski et al., 2011; Benros et al., 2013). More recently, chronic inflammation has been hypothesized to be one of the imperative underlying mechanisms of brain pathology in BD (Kapczinski et al., 2011; Benros et al., 2013; Benedetti et al., 2020; Giridharan et al., 2020). C-reactive protein (CRP), a sensitive peripheral biomarker of inflammation and metabolic disturbances (Nordestgaard and Zacho, 2009), is associated with mood status and related cognitive deficits in BD patients (Chang et al., 2012; Dickerson et al., 2013).
Although mitochondrial dysfunction and cognitive impairment have been separately observed in BD patients, the link has not been fully explored in BD. Therefore, the aims of our study were to explore whether MCN levels in euthymic BD patients receiving valproate (VPA) are associated with cognitive performance and the treatment response and to identify possible confounding factors, including medications and mood status.
METHODS
Participants
The Institutional Review Board for the Protection of Human Subjects at National Cheng Kung University Hospital approved the research protocol (IRB no. A-ER-104-031). All the participants were recruited from outpatient settings at the National Cheng Kung University Hospital and provided written informed consent regarding their willingness to participate in the research. To assess whether BD affects the MCN, we recruited healthy controls from the community after the exclusion of individuals with mental illnesses by a senior psychiatrist using the Chinese version of the Mini International Neuropsychiatry Interview. All BD patients were initially evaluated in an interview by an attending psychiatrist using the Chinese Version of the Modified Schedule of Affective Disorder and Schizophrenia–Life Time, which has good interrater reliability, to determine diagnoses according to the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). The patients also met the following inclusion criteria: (1) 20–70 years old, (2) diagnosed with BD according to the DSM-5 criteria, and (3) received VPA treatment. The mood of each patient was evaluated using the 17-item Hamilton Depression Rating Scale (HDRS) and the 11-item Young Mania Rating Scale (YMRS). All participants meeting the following criteria were excluded through chart reviews and patient-reported questionnaires to avoid confounding factors that influence mitochondrial function: (1) a serious surgical condition or physical illness, including heart disease, stroke, kidney dialysis, and transplant; (2) cerebrovascular disease; (3) neurodegenerative disorders; and (4) macrovascular disorders. In addition, all the patients with BD were treated with VPA (Depakine, Carbon Blanc, France). Concomitant fluoxetine (Prozac, Bourgoin-Jallieu, France) (20 mg/d) was permitted to treat depressive symptoms, and lorazepam (Ativan, Munster, Germany) (<8 mg) was used for night-time sedation and to treat agitation and insomnia during the study, the dosage of which was adjusted according to the clinical manifestations and the patient’s tolerance.
To collect lifestyle factors, including exercise, alcohol consumption, and smoking, we used self-report questionnaires. Regarding exercise, the categories were none (Group 1), <1 time/mo (Group 2), 1–3 times/mo (Group 3), 1–2 times/wk (Group 4), 3–5 times/wk (Group 5), and almost every day (Group 6). Since the frequency of exercise did not significantly differ between the BD patients and controls, we report the results briefly (exercise: yes [Groups 2–6] or not [Group 1]). In addition, regarding alcohol intake, participants were grouped by current alcohol use or not. They were also grouped by current smoking or not.
Assessment of the Response to VPA Treatment—
The response to VPA treatment was measured using the Alda scale (Manchia et al., 2013), which consists of an A criterion and B criterion. Criterion A was used to assess the clinical improvement and indicated changes in the frequency and severity of mood symptoms. Criterion B was used to assess the causal relationship between VPA treatment and clinical improvement. The total Alda score was calculated by subtracting the total B score from the A score (A − B total). For participants with a total B score greater than the A score, the total score was set to 0. The scores ranged from 0 to 10, and a higher score indicated a greater response. We used the total Alda score as a continuous variable of the VPA treatment response and defined the dichotomous phenotype with an Alda score cutoff ≥5 as a responder and with a score <5 as a nonresponders (Ahn et al., 2017).
Measurements
Fasting blood samples were collected between 8:00 am and 10:00 am. Ten milliliters of whole blood was drawn from the antecubital vein of each patient. Plasma, which was isolated from whole blood after centrifugation at 3000 × g for 15 minutes at 4°C, was immediately stored at −80°C.
Determination of the Copy Number and Oxidative Damage in Human Leukocyte mtDNA—
A FlexiGene DNA Kit (Qiagen, Hilden, Germany) was used to extract genomic DNA from the buffy coat, and all the procedures followed the instructions of the FlexiGene DNA Handbook. The methods of testing leukocyte MCNs and oxidative damage were described in our previous studies (Chang et al., 2014). The leukocyte MCN was assessed using a LightCycler Instrument (Roche, Mannheim, Germany). Briefly, PCR was performed by amplifying the ND1 gene in mtDNA and the β-globin gene in nuclear DNA from sampled DNA, and the calculation of the MCN was interpolated from the linearity of the dose-dependently constructed standard plasmid of the ND1/β-globin gene.
Metabolic Index—
Fasting total cholesterol, low-density lipoprotein, and triglyceride concentrations were measured using enzymatic methods with a TBA-200FR automated analyzer (Toshiba Lab Medical, Tokyo, Japan). In addition, the body mass index (BMI) of each patient was measured at each time point. BMI was calculated as weight (kg) divided by height squared (m2), and waist circumference was measured at the level midway between the lateral lower rib margin and the superior anterior iliac crest. According to the modified National Cholesterol Education Panel, Adult Treatment Panel III (NCEP ATP III) criteria (Grundy et al., 2005), MetS was defined as the presence of 3 or more of the following dysregulations of metabolic components: (1) abdominal obesity: waist circumference ≥90 cm in men or ≥80 cm in women; (2) hypertension: blood pressure ≥130/85 mmHg; (3) hyperglycemia: fasting glucose level ≥100 mg/dL or a diabetes diagnosis; (4) low high-density lipoprotein (HDL-C) concentration: HDL-C < 40 mg/dL in men and <50 mg/dL in women; and (5) hypertriglyceridemia: triglyceride level ≥150 mg/dL.
Serum VPA Concentration—
The serum trough concentration of VPA was assessed using the homogeneous enzyme immunoassay method and measured at the Union Clinical Laboratory (Taipei, Taiwan). The limit of detection was 3 μg/mL.
Plasma High-Sensitivity CRP Level—
Plasma CRP levels were determined using enzyme-linked immunosorbent assay with a human high sensitivity CRP Instant ELISA kit (Bender MedSystem GmbH, Vienna, Austria) according to the manufacturer’s instructions. The limit of detection was 3 pg/mL, and the intra- and interassay CVs were 6.9% and 13.1%, respectively.
Cognitive Performance Task—Wisconsin Card-Sorting Test (WCST)—
The WCST was administered by an experienced clinical neuropsychologist. Sixty-four cards were used for the test. All the definitions of the indices are described in the WCST manual (Heaton et al., 1993). Using a computerized version of the WCST, the patients were required to match response cards to 4 stimulus cards along 1 of 3 dimensions (color, form, or number) based on sign feedback (correct or wrong). The participants were not provided any information about the dimensions. After sorting a series of 10 cards in 1 category, the participant was asked to sort the cards again in a different category. The index of completed categories and perseverative errors were used to assess performance on the WCST.
Statistical Analysis
Statistical analyses were performed using the Statistical Package for Social Sciences, version 23.0 (SPSS Inc., Chicago, IL, USA). Categorical variables are reported as numbers and percentages, and continuous variables are reported as the means ± SD unless specified otherwise. Categorical variables were assessed using chi-square tests, and continuous variables were assessed using t tests. Log transformation was performed to ensure that the MCN and CRP concentration data conformed to a normal distribution. Associations of MCN with sex, MetS diagnosis, and MetS components in univariate analyses were assessed using t tests, and correlation of MCN and age was assessed using Pearson correlation. A linear regression model was applied to examine the relationship between BD disease and MCN after adjusting for age, sex, MetS diagnosis, and MetS components. We used an ANCOVA adjusted for age, sex, and VPA concentration to compare the characteristics of BD patients. In addition, a receiver operating characteristic curve was constructed to distinguish VPA responders from nonresponders based on the MCN level. The odds ratios of the cutoff points for the MCN value and VPA responders were calculated accordingly (Parshall, 2013). The level of significance was set to .05 for 2-sided tests.
RESULTS
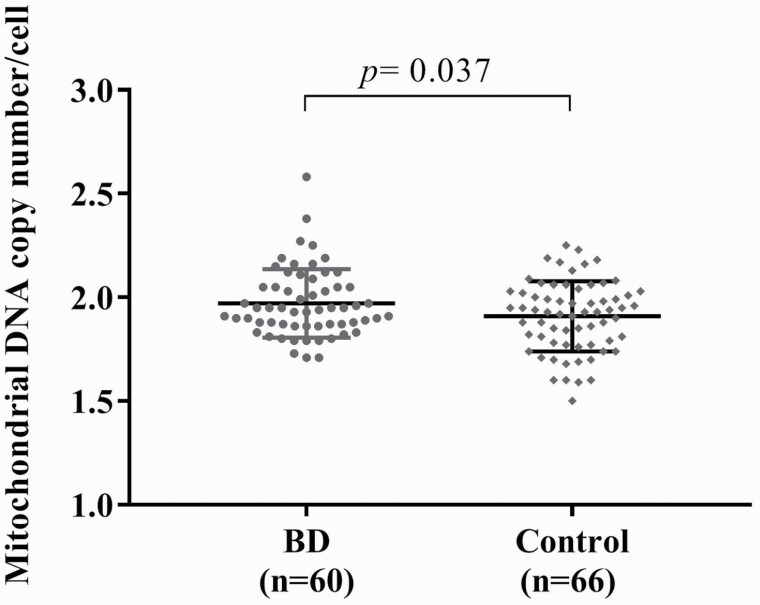
We recruited 66 healthy controls and 60 BD patients receiving VPA. The demographic data and clinical characteristics are shown in Table 1. Age and sex did not differ between the healthy controls and BD patients. BD patients had significantly higher HDRS and YMRS scores, MCN (Fig. 1), and CRP levels than the controls. The indices of MetS also significantly differed between the groups. Regarding cognitive tests, BD patients had worse performance on the WCST cognitive test than the controls. BD patients had more perseverative errors (14.14 ± 11.07 vs 8.97 ± 4.84, t = 3.305, df = 124, P = .001) and fewer completed categories (2.24 ± 1.69 vs 3.38 ± 1.37, t = −4.117, df = 124, P < .001).
Table 1.
Demographic Characteristics and Measurements of the Healthy Controls and BD Patients
| Demographic data | BD | Control | Comparison | ||
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | t/χ 2 | 95% CI | P value | |
| Age, y | 37.9 ± 12.98 | 35.45 ± 10.99 | 1.144 | (−1.78, 6.68) | .255 |
| Sex, female, n (%) | 36 (60%) | 39 (59.1%) | 0.011 | — | 1.000 |
| HDRS score | 4.03 ± 5.04 | 1.15 ± 1.96 | 4.155 | (1.50, 4.26) | <.001* |
| YMRS score | 1.68 ± 3.19 | 0.14 ± 0.58 | 3.686 | (0.70, 2.37) | <.001* |
| Exercise, n (%) | 37 (61.7%) | 44 (66.7%) | 0.342 | — | .581 |
| Smoker, n (%) | 10 (16.9%) | 9 (13.6%) | 0.265 | — | .627 |
| Alcohol intake, n (%) | 8 (13.3%) | 14 (21.2%) | 1.354 | — | .348 |
| MCN | 1.97 ± 0.16 | 1.91 ± 0.17 | 2.108 | (0.01, 0.12) | .037* |
| CRP, ng/mL | 5.22 ± 0.56 | 4.93 ± 0.66 | 2.424 | (0.05, 0.53) | .017* |
| VPA concentration, µg/mL | 62.42 ± 26.51 | — | — | — | — |
| Indices of MetS | |||||
| Waist circumference, cm | 87.13 ± 13.42 | 80.08 ± 13.3 | 2.959 | (2.33, 11.77) | .004* |
| TG, mg/dL | 126.58 ± 81.36 | 94.68 ± 60.03 | 2.484 | (6.45, 57.36) | .015* |
| HDL-C, mg/dL | 53.83 ± 19.62 | 59.64 ± 13.92 | −1.928 | (−11.76, 0.15) | .056 |
| Fasting glucose, mg/dL | 93.09 ± 23.43 | 90.83 ± 10.5 | 0.706 | (−4.06, 8.57) | .482 |
| HbA1c, % | 5.50 ± 0.97 | 5.39 ± 0.36 | 0.786 | (−0.15, 0.36) | .433 |
| SBP, mm Hg | 120.18 ± 15.95 | 120.56 ± 25.05 | −0.100 | (−7.87, 7.11) | .921 |
| DBP, mm Hg | 76.15 ± 11.05 | 78.52 ± 15.94 | −0.959 | (−7.25, 2.52) | .340 |
| MetS diagnosis, n (%) | 16 (26.7%) | 10 (15.2%) | 2.545 | — | .127 |
| MetS components | 1.62 ± 1.46 | 0.91 ± 1.17 | 3.008 | — | .004* |
| Abdominal obesity, n (%) | 36 (60.0%) | 19 (28.8%) | 12.448 | — | .001* |
| Hypertriglyceridemia, n (%) | 15 (25.0%) | 9 (13.6%) | 2.632 | — | .117 |
| Low HDL-C, n (%) | 21 (35.0%) | 9 (13.6%) | 7.907 | — | .006* |
| Hyperglycemia, n (%) | 10 (16.7%) | 10 (15.2%) | 0.054 | — | 1.000 |
| Hypertension, n (%) | 15 (25.0%) | 20 (30.3%) | 0.441 | — | .554 |
| Cognitive function in the WCST | |||||
| Perseverative errors | 14.14 ± 11.07 | 8.97 ± 4.84 | 3.305 | (2.05, 8.28) | .001* |
| Categories completed | 2.24 ± 1.69 | 3.38 ± 1.37 | −4.117 | (−1.68, −0.59) | <.001* |
Abbreviations: BD, bipolar disorder; 95% CI, 95% confidence interval; CRP, C-reactive protein; DBP, diastolic blood pressure; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HDRS, 17-item Hamilton Depression Rating Scale; MetS, metabolic syndrome; MCN, mitochondrial DNA copy number; SBP, systolic blood pressure; TG, triglyceride; VPA, valproate; WCST, Wisconsin Card Sorting Test; YMRS, Young Mania Rating Scale.
*P < .05.
Figure 1.
Comparison of the mitochondrial DNA copy number (MCN) between bipolar disorder (BD) patients receiving valproate (VPA) and controls. The MCN in BD patients treated with VPA was higher than that in controls.
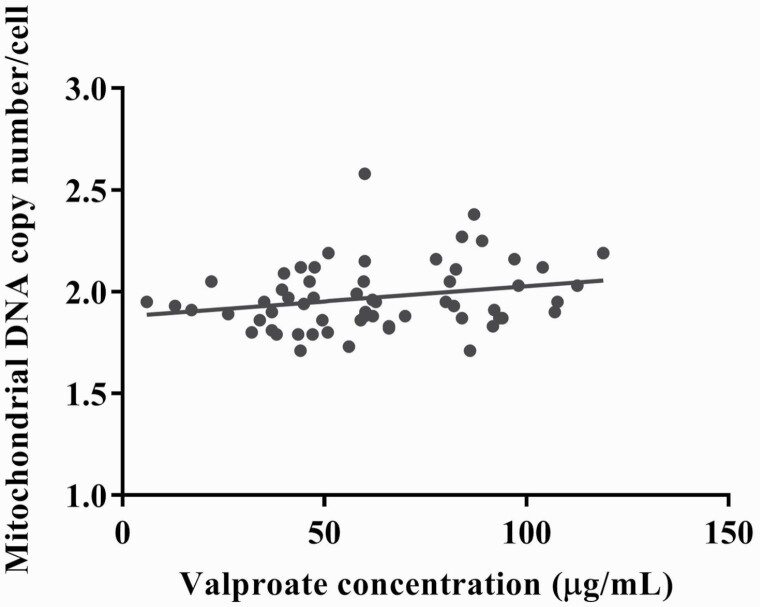
To investigate whether age, sex, MetS diagnosis, and MetS components were associated with the MCN, univariate analyses were performed in BD and controls, respectively. The MCN level was significantly associated with MetS diagnosis and hypertriglyceridemia in BD patients, whereas it was associated with abdominal obesity and hyperglycemia in the control (supplementary Tables 1 and 2). Age was not correlated with MCN in BD patients and the controls (r = −0.088 and P = .502 vs r = 0.200 and P = .108, respectively). To investigate whether the fluoxetine treatment variable was associated with the MCN, univariate analysis was performed. We found that the MCN level did not significantly differ between fluoxetine users (n = 7) and nonusers (n = 53) (1.93 ± 0.06 vs 1.98 ± 0.17, t = −1.478, P = .152). Furthermore, to clarify the association between BD and MCN, regression models predicting MCN among all of the participants were performed. The regression model (Table 2) showed that BD itself exerted a significant effect on increased MCN even after adjusting for age, sex, and MetS. In addition, we identified a significant positive correlation between the VPA concentration and MCN (r = 0.264, P = .041), and the result was consistent even after adjustment for age and sex (partial correlation r = 0.333, P = .011) (Fig. 2).
Table 2.
Linear Regression Models Predicting the Mitochondrial Copy Number Among All Participants (n = 126)
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Beta | P value | Beta | P value | |
| Participant group (patients vs controls) | 0.194 | .031* | 0.188 | .047* |
| Age | 0.108 | .249 | 0.119 | .218 |
| Sex | 0.181 | .048* | 0.220 | .022* |
| MetS (yes vs no) | −0.119 | .209 | N/A | N/A |
| Abdominal obesity (yes vs no) | N/A | N/A | 0.098 | .366 |
| Hypertriglyceridemia (yes vs no) | N/A | N/A | −0.176 | .100 |
| Low HDL-C level (yes vs no) | N/A | N/A | −0.112 | .271 |
| Hyperglycemia (yes vs no) | N/A | N/A | 0.096 | .337 |
| Hypertension (yes vs no) | N/A | N/A | −0.147 | .129 |
Abbreviations: HDL, high-density lipoprotein; MetS, metabolic syndrome; N/A, not applicable. Model 1 was adjusted for demographic characteristics and metabolic syndrome. Model 2 was adjusted for demographic characteristics and 5 components of metabolic syndrome. Beta: standardized coefficients calculated using the linear regression model.
*P < .05.
Figure 2.
The correlation between the valproate (VPA) concentration and mitochondrial DNA copy number (MCN). There was a positive correlation between the MCN and VPA concentration in bipolar patients (correlation coefficients r = 0.264, P = .041; r = 0.333, P = .011 adjusted for age and sex).
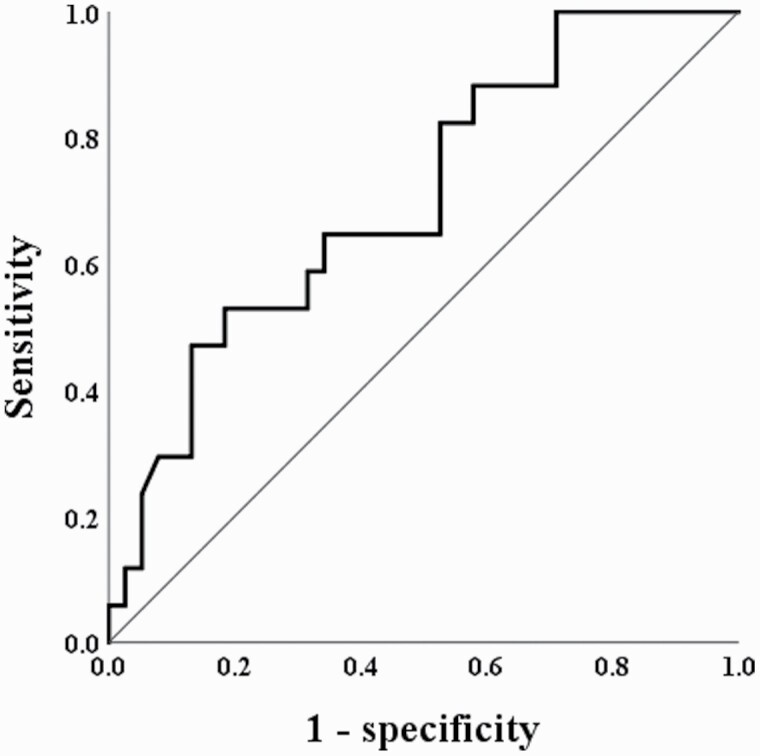
Moreover, the MCN was significantly different between VPA responders and nonresponders (2.06 ± 0.18 vs 1.94 ± 0.14, t = 2.667, df = 53, P = .010). To investigate whether the MCN could be a differential biomarker for VPA responders and nonresponders, we further estimated the cutoff value of the MCN. Receiver operating characteristic curve analysis (Fig. 3) was performed in the 2 groups, and the results showed that an MCN of 2.05 distinguished VPA responders from nonresponders (P = .012), with an area under the curve of 0.705. The sensitivity and specificity were 0.529 and 0.816, respectively.
Figure 3.
Receiver operating characteristic curves for mitochondrial DNA copy number (MCN) cutoff points to determine valproate (VPA) responders and nonresponders in bipolar disorder (BD) patients. A receiver operating characteristic curve analysis showed that an MCN of 2.05 distinguished VPA responders from non-responders, with an area under the curve (AUC) of 0.705 (95% confidence interval = 0.560 to 0.851, P = .012). The sensitivity and specificity were 0.529 and 0.816, respectively. An MCN ≥ 2.05 was associated with 5.39 higher odds of being a VPA responder (P = .006).
Furthermore, we stratified the BD patients into high (≥2.05) and low (<2.05) MCN groups using this cutoff point for the MCN value in BD patients to compare the clinical features (Table 3). Demographic characteristics, such as age, sex, and illness duration, did not differ between the groups (supplementary Table 3). The CRP level was significantly lower in the high-MCN group than in the low-MCN group (5.02 ± 0.55 vs 5.43 ± 0.50 ng/mL, t = −3.006, df = 58, P = .004), even after adjustment for age, sex, and VPA concentration (P = .010). In addition, the percentage of patients with a MetS diagnosis and the number of MetS components were significantly lower in the high-MCN group than in the low-MCN group (5.9 vs 34.9%, χ2 = 5.240, df = 58, P = .025; 0.94 ± 1.14 vs 1.88 ± 1.50, t = −2.333, df = 58, P = .023, respectively). In terms of the treatment response, more BD patients in the high-MCN group were VAP responders than in the low-MCN group (58.8 vs 20.9%, χ2 = 6.785, df = 58, P = .022). To evaluate the odds ratio of the cutoff point for the MCN value and VPA response, 10 patients were VPA responders in the high-MCN group, and 9 patients were VPA responders in the low-MCN group. We found that BD patients with MCN ≥ 2.05 had 5.39 higher odds of being VPA responders (z = 2.723, P = .006, 95% CI = 1.60 to 18.16). In terms of cognitive function, BD patients in the high-MCN group exhibited better cognitive performance. The perseverative errors on the WCST were significantly lower in the high-MCN group than in the low-MCN group (9.77 ± 5.23 vs 15.91 ± 12.31, t = −2.688, df = 58, P = .009), while the number of categories completed did not significantly differ (2.77 ± 1.64 vs 2.02 ± 1.67, t = 1.548, df = 58, P = .127).
Table 3.
Characteristics and Measurements of BD Patients Receiving VPA Sub-grouped by the Cut-off Point of MCN
| MCN ≥ 2.05(n = 17) | MCN < 2.05(n = 43) | 95% CI | Comparison | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | t/χ 2 | (Lower, Upper) | P value | P 1 value | |
| mtDNA copy no. | 2.18 ± 0.13 | 1.89 ± 0.08 | 10.226 | (0.23, 0.35) | <.001* | <.001* |
| CRP, ng/mL | 5.02 ± 0.55 | 5.43 ± 0.5 | −3.006 | (−0.69, −0.14) | .004* | .010* |
| MetS diagnosis, n (%) | 1 (5.9) | 15 (34.9) | 5.240 | — | .025* | — |
| MetS components, n | 0.94 ± 1.14 | 1.88 ± 1.50 | −2.333 | (−1.75, −0.13) | .023* | — |
| Alda scale (A-B total) | 4.31 ± 3.09 | 2.74 ± 2.05 | 0.677 | (−1.18, 2.32) | .506 | .392 |
| Responder, n (%) | 10 (58.8) | 9 (20.9) | 6.785 | — | .022* | — |
| Cognitive function WCST | ||||||
| Perseverative errors | 9.77 ± 5.23 | 15.91 ± 12.31 | −2.688 | (−10.71, −1.57) | .009* | .020* |
| Completed categories | 2.77 ± 1.64 | 2.02 ± 1.67 | 1.548 | (−0.22, 1.7) | .127 | .175 |
Abbreviations: 95% CI, 95% confidence interval; CRP, C-reactive protein; MCN, mitochondrial DNA copy number; MetS, metabolic syndrome; VPA, valproate; WCST, Wisconsin card sorting test.
1The P value was obtained using ANCOVA after adjustment for age, sex and VPA concentration. In ANCOVA analysis, age and VPA concentration were continuous variables, and sex and groups (using the cutoff point of 2.05 for the MCN value: MCN ≥ 2.05 or MCN < 2.05) were categorical variables. Clinical measurements, including mtDNA copy number, CRP, Alda scale, and cognitive function, were dependent variables (outcome variables).
*P < .05.
Discussion
To the best of our knowledge, this study is the first to investigate the association between the MCN and cognitive function in euthymic BD patients receiving VPA. BD patients showed significantly worse cognitive performance and a higher MCN than the controls, and the results remained consistent even after adjustment for important clinical confounders, such as age, sex, and MetS. The VPA concentration was positively correlated with the MCN in BD patients. In addition, we evaluated the stratification effect of MCN on the clinical features of BD patients. We found that the MCN significantly distinguished VPA responders from nonresponders. Our results also revealed a higher percentage of VPA responders and better cognitive performance of BD patients in the high-MCN group than those in the low-MCN group. Regarding metabolic effects, BD patients in the high-MCN group also had lower plasma CRP levels and fewer MetS components than in the low-MCN group. The strengths of the study are that we adjusted for potential confounding factors related to MCN, such as age, sex, MetS, and components of MetS, and considered their effects. In addition, to control the influence of mood status and medication, we recruited euthymic BD patients treated with VPA. Taken together, the inclusion of controls provides better insights into how MCN might serve as a disease biomarker in BD, and our study is the first to suggest positive associations among MCN, the VPA treatment response, and cognitive function in euthymic BD patients treated with VPA.
CRP, a biomarker of systemic inflammation and the risk of inflammatory disease, was negatively correlated with the MCN in our study. Previous studies have reported that BD is associated with severe inflammatory dysregulation and that CRP potentially represents a marker of mood state (Bai et al., 2015). This finding is consistent with our study; BD patients had higher CRP levels than the controls. The association between MetS and inflammation has been emphasized, and chronic inflammation is an integral process in MetS (Lopez-Candales et al., 2017). This result might partially explain why several MetS components, such as abdominal obesity and low HDL-C levels, were significantly increased in our BD patients.
MCN alterations are considered indicators of mitochondrial dysfunction; however, only a few studies have examined whether changes in the MCN occur in BD patients, and the results have been inconclusive (Chang et al., 2014; de Sousa et al., 2014; Fries et al., 2017; Rollins et al., 2018; Wang et al., 2018). These inconsistencies in previous studies might be attributed to differences in age, clinical features, tissue samples, medication use, or ethnicity. Factors such as age (Hartmann et al., 2011), smoking (Wu et al., 2019), alcohol use, and MetS (Fazzini et al., 2021) affect the MCN to a certain degree. For example, aging was reported to be associated with the downregulation of mtDNA-associated genes and a decrease in the MCN (Hartmann et al., 2011). However, previous studies of BD have rarely simultaneously elucidated these factors. These studies investigated patients with euthymic (Chang et al., 2014), depressive (de Sousa et al., 2014), three-mood (Wang et al., 2018), and nonclassified states (Fries et al., 2017; Yamaki et al., 2018). BD has been proposed to be caused by aberrant mitochondrial bioenergetics, and mitochondrial respiration and ATP production have been reported to be increased in the manic state and decreased in the euthymic/depressive state (Bai et al., 2015; Morris et al., 2017). Therefore, further investigations are needed to specify each mood state and to evaluate mitochondrial function.
Regarding the effects of VPA on the MCN, possible underlying mechanisms have been suggested in previous studies (Bachmann et al., 2009; Sitarz et al., 2014; Beltrán-Sarmiento et al., 2018). In a cell line study, VPA administration increased the MCN by regulating mitochondrial biogenesis in BD patients (Sitarz et al., 2014). In cell and animal studies, Bcl-2 was the target of long-term VPA use, providing protection against methamphetamine-induced neurotoxicity and mitochondrial damage in the brain (Bachmann et al., 2009). VPA could enhance mitochondrial function and protect against mitochondrially mediated toxicity (Bachmann et al., 2009). This result is consistent with our study showing that the VPA concentration is positively correlated with the MCN (r = 0.264, P = .041) in BD patients. In addition, 12 months of VPA treatment in epileptic children showed a significant antioxidant effect of decreasing seizure activity by increasing the activities of plasmatic antioxidant enzymes (such as glutathione reductase, superoxide dismutase, and catalase) and decreasing oxidant markers (such as hydrogen peroxide) (Beltrán-Sarmiento et al., 2018). Although the mechanism underlying the association between MCN and VPA is unclear, the anti-inflammatory and antioxidant effects of VPA in the brain might play an important role (Ximenes et al., 2013).
Considering BD patients alone, after adjusting for age, sex, and VPA use, the high-MCN group had significantly lower CRP levels and fewer MetS components and perseverative errors than the low-MCN group (Table 3). In a population-based longitudinal study, the MCN level was negatively correlated with CRP levels in older adults (Wu et al., 2017). The leukocyte MCN level was lower among postmenopausal women with MetS than among those without MetS (Kim et al., 2012). However, in contrast to our findings, increased oxidative stress or inflammation led to enhanced mitochondrial biogenesis and increased MCN (Malik and Czajka, 2013; Morris et al., 2017). Obesity-related MetS is possibly associated with deregulated lipid and carbohydrate metabolism. An increase in either of these substrates will increase the demand on the mitochondria and the utilization of the electron transport chain (Rudich et al., 2007).
The broad consensus is that even euthymic BD patients present cognitive deficits compared with controls, such as deficits in attention, verbal and nonverbal memory, and executive function (Zarate et al., 2000; Lima et al., 2018). Regarding the association between cognitive performance and the MCN, BD patients who had a higher MCN performed better regarding perseverative errors on the WCST. In a general population, elderly Korean women with higher MCNs performed better on cognitive tests after adjusting for age and education level (Lee et al., 2010). In addition, the MCN in peripheral blood cells is associated with cognition in patients with mild cognitive impairment or dementia (Lee et al., 2010; Delbarba et al., 2016), and a reduction in the mtDNA amount is a feature of the early pathogenesis occurring in patients with Alzheimer’s disease. Until now, it has not been totally clear how changes in mitochondrial dysfunction could cause cognitive impairment in BD. In an animal study, antioxidant treatment resolved mitochondrial integrity, cognitive behavior, and cortical connectivity (Fernandez et al., 2019). mtDNA mutations or polymorphisms result in altered mitochondrial calcium regulation (Kato, 2007), and the therapeutic mechanism of lithium is involved in intracellular calcium signaling (Vawter et al., 2000). In addition, cognitive impairment in BD patients might reflect the disease process, and vice versa. Therefore, this fact implicates mitochondria-related oxidative stress as a therapeutic target in BD or in cognitive impairment due to BD. Moreover, BD patients with impaired insulin sensitivity perform worse on neurocognitive tests (Chang et al., 2021); although the underlying mechanism remains unclear, insulin resistance might lead to neuroprogression through blood-brain barrier leakage and proinflammatory cytokine activation in the brain (Craft and Watson, 2004). Further studies are necessary to investigate the mechanism underlying the association between the MCN and cognitive function and to provide possible therapeutic targets in BD.
Our study has some limitations, including those inherent to a case-control, observational analysis in which no causal relationship could be deduced. The mean HDRS and YMRS scores of our patients with BD were within the normal range, indicating that our patients were clinically euthymic; therefore, the results of our study should not be generalized to other disease states. Furthermore, the MCN was measured in peripheral leukocytes instead of brain tissues, but Feng and colleagues reported a positive association between the MCN in peripheral leukocytes and brain tissues (Feng et al., 2013). Using peripheral blood as a proxy for brain tissues to measure MCN levels is more feasible and less invasive.
In summary, euthymic BD patients receiving VPA had significantly higher MCN levels than controls, even after adjustment for confounding factors, and the VPA concentration was positively correlated with the MCN. In addition, the MCN distinguished VPA responders from nonresponders. Furthermore, we identified a positive correlation among the leukocyte MCN treatment response and cognitive function in euthymic BD patients. More studies are needed to assess longitudinal changes in the MCN and cognition in BD patients and to explore the mechanisms underlying the association between the MCN and cognition.
Supplementary Material
Acknowledgments
We thank all the participants of this study for their exceptional cooperation as well as valuable contributions. We also thank Chih-Ying Lin for administrative support.
This work was supported by the Ministry of Science and Technology of Taiwan (MOST 105-2320-B-006-014, MOST 106-2320-B-006-040, MOST 107-2320-B-006-071, MOST 108-2320-B-006-047-MY3); the National Cheng Kung University Hospital (NCKUH-10802013, NCKUH-10902014, NCKUH-11002026); and the Changhua Christian Hospital (108-CCH-IRP-109).
Interest Statement
None.
Contributor Information
Cheng-Chen Chang, School of Medicine, Chung Shan Medical University, Taichung, Taiwan; Department of Psychiatry, Chung Shan Medical University Hospital, Taichung, Taiwan.
Po See Chen, Department of Psychiatry and Institute of Behavioral Medicine College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Jhih-Rong Lin, Institute of Clinical Pharmacy and Pharmaceutical Sciences College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Yi-An Chen, Institute of Clinical Pharmacy and Pharmaceutical Sciences College of Medicine, National Cheng Kung University, Tainan, Taiwan.
Chin-San Liu, Vascular and Genomic Research Center, Changhua Christian Hospital, Changhua, Taiwan; Graduate Institute of Integrated Medicine, College of Chinese Medicine, China Medical University, Taichung, Taiwan.
Ta-Tsung Lin, Vascular and Genomic Research Center, Changhua Christian Hospital, Changhua, Taiwan.
Hui Hua Chang, Institute of Clinical Pharmacy and Pharmaceutical Sciences College of Medicine, National Cheng Kung University, Tainan, Taiwan; School of Pharmacy, College of Medicine, National Cheng Kung University, Tainan, Taiwan; Department of Pharmacy, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan; Department of Pharmacy, National Cheng Kung University Hospital, Dou-Liou Branch, Yunlin, Taiwan.
References
- Ahn SW, Baek JH, Yang SY, Kim Y, Cho Y, Choi Y, Lee K, Park T, Hong KS (2017) Long-term response to mood stabilizer treatment and its clinical correlates in patients with bipolar disorders: a retrospective observational study. Int J Bipolar Disord 5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann RF, Wang Y, Yuan P, Zhou R, Li X, Alesci S, Du J, Manji HK (2009) Common effects of lithium and valproate on mitochondrial functions: protection against methamphetamine-induced mitochondrial damage. Int J Neuropsychopharmacol 12:805–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai YM, Su TP, Li CT, Tsai SJ, Chen MH, Tu PC, Chiou WF (2015) Comparison of pro-inflammatory cytokines among patients with bipolar disorder and unipolar depression and normal controls. Bipolar Disord 17:269–277. [DOI] [PubMed] [Google Scholar]
- Beltrán-Sarmiento E, Arregoitia-Sarabia CK, Floriano-Sánchez E, Sandoval-Pacheco R, Galván-Hernández DE, Coballase-Urrutia E, Carmona-Aparicio L, Ramos-Reyna E, Rodríguez-Silverio J, Cárdenas-Rodríguez N (2018) Effects of valproate monotherapy on the oxidant-antioxidant status in Mexican epileptic children: a longitudinal study. Oxid Med Cell Longev 2018:7954371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetti F, Aggio V, Pratesi ML, Greco G, Furlan R (2020) Neuroinflammation in bipolar depression. Front Psychiatry 11:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benros ME, Waltoft BL, Nordentoft M, Ostergaard SD, Eaton WW, Krogh J, Mortensen PB (2013) Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry 70:812–820. [DOI] [PubMed] [Google Scholar]
- Berk M, Conus P, Lucas N, Hallam K, Malhi GS, Dodd S, Yatham LN, Yung A, McGorry P (2007) Setting the stage: from prodrome to treatment resistance in bipolar disorder. Bipolar Disord 9:671–678. [DOI] [PubMed] [Google Scholar]
- Bora E, Özerdem A (2017) Meta-analysis of longitudinal studies of cognition in bipolar disorder: comparison with healthy controls and schizophrenia. Psychol Med 47:2753–2766. [DOI] [PubMed] [Google Scholar]
- Bourne C, et al. (2013) Neuropsychological testing of cognitive impairment in euthymic bipolar disorder: an individual patient data meta-analysis. Acta Psychiatr Scand 128:149–162. [DOI] [PubMed] [Google Scholar]
- Chang CC, Jou SH, Lin TT, Liu CS (2014) Mitochondrial DNA variation and increased oxidative damage in euthymic patients with bipolar disorder. Psychiatry Clin Neurosci 68:551–557. [DOI] [PubMed] [Google Scholar]
- Chang HH, Lee IH, Gean PW, Lee SY, Chi MH, Yang YK, Lu RB, Chen PS (2012) Treatment response and cognitive impairment in major depression: association with C-reactive protein. Brain Behav Immun 26:90–95. [DOI] [PubMed] [Google Scholar]
- Chang HH, Tseng HH, Chang WH, Huang KC, Lu TH, Yang YK, Chen PS (2021) Peripheral insulin sensitivity predicting cognitive function in euthymic bipolar disorder patients. CNS Spectr 1–6. doi: 10.1017/S1092852921000158. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Craft S, Watson GS (2004) Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol 3:169–178. [DOI] [PubMed] [Google Scholar]
- de Sousa RT, Uno M, Zanetti MV, Shinjo SM, Busatto GF, Gattaz WF, Marie SK, Machado-Vieira R (2014) Leukocyte mitochondrial DNA copy number in bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry 48:32–35. [DOI] [PubMed] [Google Scholar]
- Delbarba A, Abate G, Prandelli C, Marziano M, Buizza L, Arce Varas N, Novelli A, Cuetos F, Martinez C, Lanni C, Memo M, Uberti D (2016) Mitochondrial alterations in peripheral mononuclear blood cells from Alzheimer’s disease and mild cognitive impairment patients. Oxid Med Cell Longev 2016:5923938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R (2013) Elevated C-reactive protein and cognitive deficits in individuals with bipolar disorder. J Affect Disord 150:456–459. [DOI] [PubMed] [Google Scholar]
- Fazzini F, Lamina C, Raftopoulou A, Koller A, Fuchsberger C, Pattaro C, Del Greco FM, Döttelmayer P, Fendt L, Fritz J, Meiselbach H, Schönherr S, Forer L, Weissensteiner H, Pramstaller PP, Eckardt KU, Hicks AA, Kronenberg F; GCKD Investigators (2021) Association of mitochondrial DNA copy number with metabolic syndrome and type 2 diabetes in 14 176 individuals. J Intern Med 290:190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng YM, Jia YF, Su LY, Wang D, Lv L, Xu L, Yao YG (2013) Decreased mitochondrial DNA copy number in the hippocampus and peripheral blood during opiate addiction is mediated by autophagy and can be salvaged by melatonin. Autophagy 9:1395–1406. [DOI] [PubMed] [Google Scholar]
- Fernandez A, Meechan DW, Karpinski BA, Paronett EM, Bryan CA, Rutz HL, Radin EA, Lubin N, Bonner ER, Popratiloff A, Rothblat LA, Maynard TM, LaMantia AS (2019) Mitochondrial dysfunction leads to cortical under-connectivity and cognitive impairment. Neuron 102:1127–1142.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries GR, Bauer IE, Scaini G, Wu MJ, Kazimi IF, Valvassori SS, Zunta-Soares G, Walss-Bass C, Soares JC, Quevedo J (2017) Accelerated epigenetic aging and mitochondrial DNA copy number in bipolar disorder. Transl Psychiatry 7:1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furda AM, Marrangoni AM, Lokshin A, Van Houten B (2012) Oxidants and not alkylating agents induce rapid mtDNA loss and mitochondrial dysfunction. DNA Repair (Amst) 11:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giridharan VV, Sayana P, Pinjari OF, Ahmad N, da Rosa MI, Quevedo J, Barichello T (2020) Postmortem evidence of brain inflammatory markers in bipolar disorder: a systematic review. Mol Psychiatry 25:94–113. [DOI] [PubMed] [Google Scholar]
- Green MF (2006) Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry 67:e12. [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F; American Heart Association; National Heart, Lung, and Blood Institute (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752. [DOI] [PubMed] [Google Scholar]
- Hartmann N, Reichwald K, Wittig I, Dröse S, Schmeisser S, Lück C, Hahn C, Graf M, Gausmann U, Terzibasi E, Cellerino A, Ristow M, Brandt U, Platzer M, Englert C (2011) Mitochondrial DNA copy number and function decrease with age in the short-lived fish Nothobranchius furzeri. Aging Cell 10:824–831. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G (1993) Wisconsin Card Sorting Test manual: Revised and expanded. Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Huang CH, Su SL, Hsieh MC, Cheng WL, Chang CC, Wu HL, Kuo CL, Lin TT, Liu CS (2011) Depleted leukocyte mitochondrial DNA copy number in metabolic syndrome. J Atheroscler Thromb 18:867–873. [DOI] [PubMed] [Google Scholar]
- Kapczinski F, Dal-Pizzol F, Teixeira AL, Magalhaes PV, Kauer-Sant’Anna M, Klamt F, Moreira JC, de Bittencourt Pasquali MA, Fries GR, Quevedo J, Gama CS, Post R (2011) Peripheral biomarkers and illness activity in bipolar disorder. J Psychiatr Res 45:156–161. [DOI] [PubMed] [Google Scholar]
- Kato T (2007) Mitochondrial dysfunction as the molecular basis of bipolar disorder: therapeutic implications. CNS Drugs 21:1–11. [DOI] [PubMed] [Google Scholar]
- Kato T, Kato N (2000) Mitochondrial dysfunction in bipolar disorder. Bipolar Disord 2:180–190. [DOI] [PubMed] [Google Scholar]
- Kim JH, Im JA, Lee DC (2012) The relationship between leukocyte mitochondrial DNA contents and metabolic syndrome in postmenopausal women. Menopause 19:582–587. [DOI] [PubMed] [Google Scholar]
- Lee JW, Park KD, Im JA, Kim MY, Lee DC (2010) Mitochondrial DNA copy number in peripheral blood is associated with cognitive function in apparently healthy elderly women. Clin Chim Acta 411:592–596. [DOI] [PubMed] [Google Scholar]
- Li Z, Hu M, Zong X, He Y, Wang D, Dai L, Dong M, Zhou J, Cao H, Lv L, Chen X, Tang J (2015) Association of telomere length and mitochondrial DNA copy number with risperidone treatment response in first-episode antipsychotic-naïve schizophrenia. Sci Rep 5:18553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima IMM, Peckham AD, Johnson SL (2018) Cognitive deficits in bipolar disorders: implications for emotion. Clin Psychol Rev 59:126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Candales A, Hernandez Burgos PM, Hernandez-Suarez DF, Harris D (2017) Linking chronic inflammation with cardiovascular disease: from normal aging to the metabolic syndrome. J Nat Sci 3:e341. [PMC free article] [PubMed] [Google Scholar]
- Malik AN, Czajka A (2013) Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion 13:481–492. [DOI] [PubMed] [Google Scholar]
- Manchia M, et al. (2013) Assessment of response to lithium maintenance treatment in bipolar disorder: a consortium on lithium genetics (ConLiGen) report. Plos One 8:e65636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso C, Scapagini G, Currò D, Giuffrida Stella AM, De Marco C, Butterfield DA, Calabrese V (2007) Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front Biosci 12:1107–1123. [DOI] [PubMed] [Google Scholar]
- Martínez-Arán A, Vieta E, Reinares M, Colom F, Torrent C, Sánchez-Moreno J, Benabarre A, Goikolea JM, Comes M, Salamero M (2004) Cognitive function across manic or hypomanic, depressed, and euthymic states in bipolar disorder. Am J Psychiatry 161:262–270. [DOI] [PubMed] [Google Scholar]
- Morris G, Walder K, McGee SL, Dean OM, Tye SJ, Maes M, Berk M (2017) A model of the mitochondrial basis of bipolar disorder. Neurosci Biobehav Rev 74:1–20. [DOI] [PubMed] [Google Scholar]
- Mur M, Portella MJ, Martínez-Arán A, Pifarré J, Vieta E (2008) Long-term stability of cognitive impairment in bipolar disorder: a 2-year follow-up study of lithium-treated euthymic bipolar patients. J Clin Psychiatry 69:712–719. [PubMed] [Google Scholar]
- Nordestgaard BG, Zacho J (2009) Lipids, atherosclerosis and CVD risk: is CRP an innocent bystander? Nutr Metab Cardiovasc Dis 19:521–524. [DOI] [PubMed] [Google Scholar]
- Parshall MB (2013) Unpacking the 2 × 2 table. Heart and Lung 42:221–226. [DOI] [PubMed] [Google Scholar]
- Rollins BL, Morgan L, Hjelm BE, Sequeira A, Schatzberg AF, Barchas JD, Lee FS, Myers RM, Watson SJ, Akil H, Potkin SG, Bunney WE, Vawter MP (2018) Mitochondrial complex I deficiency in schizophrenia and bipolar disorder and medication influence. Mol Neuropsychiatry 3:157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudich A, Kanety H, Bashan N (2007) Adipose stress-sensing kinases: linking obesity to malfunction. Trends Endocrinol Metab 18:291–299. [DOI] [PubMed] [Google Scholar]
- Sitarz KS, Elliott HR, Karaman BS, Relton C, Chinnery PF, Horvath R (2014) Valproic acid triggers increased mitochondrial biogenesis in POLG-deficient fibroblasts. Mol Genet Metab 112:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vawter MP, Freed WJ, Kleinman JE (2000) Neuropathology of bipolar disorder. Biol Psychiatry 48:486–504. [DOI] [PubMed] [Google Scholar]
- Wang D, Li Z, Liu W, Zhou J, Ma X, Tang J, Chen X (2018) Differential mitochondrial DNA copy number in three mood states of bipolar disorder. BMC Psychiatry 18:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu IC, Lin CC, Liu CS, Hsu CC, Chen CY, Hsiung CA (2017) Interrelations between mitochondrial DNA copy number and inflammation in older adults. J Gerontol A Biol Sci Med Sci 72:937–944. [DOI] [PubMed] [Google Scholar]
- Wu S, Li X, Meng S, Fung T, Chan AT, Liang G, Giovannucci E, De Vivo I, Lee JH, Nan H (2019) Fruit and vegetable consumption, cigarette smoke, and leukocyte mitochondrial DNA copy number. Am J Clin Nutr 109:424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ximenes JC, de Oliveira Gonçalves D, Siqueira RM, Neves KR, Santos Cerqueira G, Correia AO, Félix FH, Leal LK, de Castro Brito GA, da Graça Naffah-Mazzacorati M, Viana GS (2013) Valproic acid: an anticonvulsant drug with potent antinociceptive and anti-inflammatory properties. Naunyn Schmiedebergs Arch Pharmacol 386:575–587. [DOI] [PubMed] [Google Scholar]
- Yamaki N, Otsuka I, Numata S, Yanagi M, Mouri K, Okazaki S, Boku S, Horai T, Ohmori T, Shirakawa O, Sora I, Hishimoto A (2018) Mitochondrial DNA copy number of peripheral blood in bipolar disorder: the present study and a meta-analysis. Psychiatry Res 269:115–117. [DOI] [PubMed] [Google Scholar]
- Zarate CA Jr, Tohen M, Land M, Cavanagh S (2000) Functional impairment and cognition in bipolar disorder. Psychiatr Q 71:309–329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.