Abstract
Background
Adequate maternal vaccination coverage is critical for the prevention and control of infectious disease outbreaks such as pertussis, influenza, and more recently COVID-19. To guide efforts to increase vaccination coverage this study examined the extent of vaccination coverage in pregnant New Zealand women over time by area-level deprivation and ethnicity.
Methods
A retrospective cohort study was used consisting of all pregnant women who delivered between 01 January 2013 and 31 December 2018, using administrative health datasets. Outcomes were defined as receipt of influenza or pertussis vaccination in any one of the relevant data sources (National Immunisation Register, Proclaims, or Pharmaceutical collection) during their eligible pregnancy. Ethnicity was prioritised as Māori (NZ indigenous), Pacific, Asian, and Other or NZ European and deprivation was defined using New Zealand Index of Multiple Deprivation (IMD).
Results
Between 2013 and 2018, Asian women had the highest maternal vaccination coverage (36%) for pertussis, while Māori and Pacific women had the lowest, 13% and 15% respectively. Coverage of pertussis vaccination during pregnancy in low deprivation Māori women was 24% and 28% in Pacific women. This is in comparison to 30% and 25% in high deprivation Asian and European/Other women, respectively. Similar trends were seen for influenza.
Conclusion
Between 2013 and 2018 maternal vaccination coverage increased for pertussis and influenza. Despite this coverage remains suboptimal, and existing ethnic and deprivation inequities increased. There is an urgent need to focus on equity, to engage and support ethic communities by creating genuinely accessible, culturally appropriate health services.
1. Introduction
Globally, vaccination is recognised as a critical to the prevention and control of infectious-disease outbreaks [1]. Pertussis and influenza are highly contagious communicable diseases which are significant public health issues globally and in New Zealand (NZ) [2]. In 2018, greater than150,000 cases of pertussis occurred globally [3] and pertussis remains one of the top 10 causes of infant mortality worldwide with an estimated 24 million cases and 160,000 deaths annually in children younger than 5 years [4], [5]. NZ has a pertussis epidemic cycle every four to five years, the last one in 2017–2018 resulted in over 4,200 cases. The highest burden was in infants less than 1 year old (274 per 100,000), 50% of which required hospitalisation. A disproportionately higher rate was seen Māori (∼400 per 100,000) and Pacific infants (∼550 per 100,000) [6]. The burden from influenza is also substantial, particularly among young children, older adults, pregnant women, and those with underlying conditions [7]. However, both diseases are vaccine preventable and these vaccines are available on the NZ vaccine schedule free to pregnant women. Vaccines for both influenza and pertussis have strong safety data and well-established efficacy profiles in pregnant and non-pregnant populations [8], [9], [10], [11], [12], [13]. Thus, pertussis and influenza vaccines represent a safe and effective way to reduce morbidity and mortality caused by these diseases in pregnant women and their infants.
Immunisation is an important public health measure, but it is only effective if it is being used. Therefore, measuring coverage helps to identify gaps and monitor trends [14]. Maternal vaccination coverage in NZ has increased between 2013 and 2018 for both influenza and pertussis vaccines [14]. Influenza coverage increased from 11% to 31% by 2018 and pertussis coverage from 10% to 44% however, maternal vaccination status remains sub-optimal with evidence of socioeconomic and geographic variation [15]. Moreover, there is recent evidence that childhood coverage may be declining in some parts of NZ [15]. More broadly, low vaccine uptake during pregnancy is a major public health challenge for many high-income countries [16]. Understanding how we could increase vaccination during pregnancy is particularly timely with the current pandemic and the recommendation of the COVID-19 vaccine for pregnant women.
Few international studies have assessed pregnancy vaccination coverage according to ethnicity and socio-economic deprivation. A large cross-sectional population-based study assessing racial disparities in influenza vaccine coverage in the United States, included 131,743 women and found vaccination coverage ranged from 39% in Black women to 55% in Asian women [46]. Overall, Black women were 30% (95% CL 0.65–0.74) less likely to receive maternal influenza vaccination compared with White women [17]. Similarly in the UK a linear gradient of decreasing coverage with increasing deprivation was found after adjusting for ethnicity, with coverage 14% lower in the most deprived quintile compared with the least deprived. Crude coverage was highest in White-British women (63%, 95% CI 62–63) compared with all other ethnicities whose crude coverage ranging from − 0·4% (Chinese) to − 25% (black-other) lower. However, after adjusting for deprivation quintile and local teams, coverage differences according to ethnicity reduced. This was most evident among Indian, Bangladeshi and Chinese ethnicities, who, after adjusting, had higher coverage than white-British women [18]. In NZ, significant inequities exist in childhood vaccination coverage according to ethnicity and socio-economic deprivation [19]. Children living in areas of low deprivation and who are of Māori and Pacific ethnicity have the lowest coverage, both in terms of timeliness and completeness [20]. However, it is unknown if these trends extend to maternal vaccinations. Furthermore, the inequitable burden of pertussis infections is in Māori and Pacific infants [6], so inequities in maternal vaccination coverage may exacerbate this burden. As such there is a need to determine the extent of ethnic and socio-economic inequities in maternal vaccination coverage in NZ to add to an emerging body of international evidence [17], [18].
While maternal coverage in NZ has been previously described [14], the aim of this study was to further explore how maternal vaccination coverage varies according to ethnicity and socio-economic status. Pertussis is endemic worldwide and is still difficult to control, despite decades of universal childhood vaccination [21]. In addition to this, greater than60 national vaccine programs have been disrupted or suspended due to COVID-19 [22]. NZ hasn’t been exempt, with timely childhood coverage at 6 months of age falling over the last 24 months from 78% to 75%, this fall has been particularly stark for Māori infants (62% to 55%) [23]. Consequently, our study aims to first describe how maternal vaccination coverage has changed over time, according to delivery year. Second, we will assess differences in maternal vaccination coverage according to ethnicity. Third, we describe how maternal vaccination coverage varies according to socio-economic deprivation using the New Zealand Deprivation Index 2013 and Indices of Multiple Deprivation. Finally, we will investigate differences between ethnicity by deprivation.
2. Method
2.1. Study design
This was a retrospective cohort study. The study population consisted of all pregnant women with a delivery between 01 January 2013 and 31 December 2018. Women were excluded if the gestational age at delivery was less than 20 weeks or greater than 45 weeks (most women are induced at 43 weeks or earlier), were missing date of last menstrual period or a gestational age at delivery, if maternal age at delivery was less than 12 years of age or greater than 50 years of age, and if they were flagged as being a non-resident.
2.2. Data sources
NZ administrative health data sources were used to undertake this research.
2.2.1. National maternity collection (MAT)
The MAT is a collection of the demographic and clinical features of women in NZ using publicly funded maternity/newborn services from 9 months before birth to 3 months postpartum. Coverage is estimated to be around 80% of births in NZ, as of 2011 [24]. Relevant data fields included National Health Index number (NHI) (encrypted), mother’s date of birth, mother’s prioritised ethnicity, District Health Board, socioeconomic deprivation level, delivery date, date of last menstrual period, gestational age, lead maternity carer, parity, and number of antenatal visits.
2.2.2. National health Index (NHI)
The NHI database contains demographic information for all people born in NZ and for people born outside of NZ who access the healthcare system (note: the NHI database includes records for travellers and other people who do not live in NZ). A person’s unique NHI number, date of birth, date of death, and sex are static; however, the remaining data fields may change over time. Data fields relevant to this study include NHI (encrypted), date of birth, prioritised ethnicity, geographic area of residence (district health board), and socioeconomic deprivation level decile (NZ Deprivation Index 13).
2.2.3. Primary health organisation enrolments (PHO)
The PHO Enrolment Collection provides data on patient enrolment with primary care health care providers. Data fields relevant to this study include NHI (encrypted), 2013 meshblock of residence, PHO registration, and date of last general practice consultation or interaction which includes services provided by practice nurses.
2.2.4. National Immunisation register (NIR)
A register of all immunisation enrolments and events as per the National Immunisation Schedule in NZ. Relevant data fields include NHI (encrypted), vaccine type, vaccination date, and provider type. Providers enter the vaccination details into this register electronically.
2.2.5. Proclaims
A claims dataset that contains the fee-for-service payments made to general practices for patient visits. Relevant data fields include NHI (encrypted), vaccination type, and date of service (vaccination date). Any claim made for the vaccination administration is automatically captured by this database.
2.2.6. Pharmaceutical collection
Claims dataset for community pharmacy that contains claim and payment information from pharmacists for subsidised dispensing. Relevant data fields include NHI (encrypted), claim type (vaccine type), and dispensing date (vaccination date).
2.3. Outcomes
The outcomes of interest were receipt of influenza or pertussis vaccination during pregnancy, both as binary variables (N/Y). Vaccination status for each woman was determined as having a valid entry for a pertussis vaccine and/or influenza vaccine in any one of the relevant data sources (NIR, Proclaims, or Pharmaceutical Collection), during their eligible pregnancy. Due to the number of data sources available with vaccination information, they were prioritised in the following order: NIR, Proclaims, and Pharmaceutical Collection. If multiple vaccinations events were reported during a woman’s eligible pregnancy, only the first valid entry was selected. A vaccination was considered valid if it occurred between last menstrual period and censoring (delivery date or end of assigned study period whichever came first).
Ethnicity was prioritised (Māori, Pacific, Asian, Other, and NZ European) and area level socioeconomic deprivation (NZ Deprivation Index 13 (NZDep13) and the Indices of Multiple Deprivation (IMD)) were categorised from deciles to quintiles (1 (least deprived) to 5 (most deprived)) and categories (low deprivation = 1–3, medium = 4–7, and high = 8–10). If district health board, ethnicity, NZDep13 were missing in the MAT collection, then the NHI dataset was used to fill-in missing values where available.
2.4. Area-level deprivation
The NZDep13 Index of Deprivation is a small area measure of deprivation that is derived from variables collected at the national census which describe household socioeconomic characteristics. NZDep13 combines nine variables from the 2013 census which reflect eight dimensions of deprivation (communication, income, employment, qualifications, home ownership, support, household crowding, transport) [25]. The NZ IMD 2013 is an alternative to NZDep which uses 28 deprivation indicators to form seven deprivation domains [26]. In contrast to NZDep, the NZ IMD gathers data from numerous providers including MoH, NZ Police, Inland Revenue and MSD and has the additional advantage of being updated regularly, rather than five yearly [26]. The NZ IMD measures deprivation at the neighbourhood level using custom designed data zones, with each zone having a mean of 712 people per zone [26]. The index values are the same as for NZDep − 1 (least deprived), 10 (most deprived). The major advantage of the NZ IMD is that unlike NZDep which provides an overall deprivation rating, the NZ IMD provides an insight into the facets or components of deprivation at a neighbourhood level [26].
In this study, three NZ IMD categories were examined separately to assess how vaccination coverage varied according to different deprivation domains. Health, access, education, income, and housing were the NZ IMD categories chosen to be examined in greater detail. The health domain identifies areas that have a greater level of morbidity and mortality than expected for the age profile of their population [26]. This domain was chosen as maternal vaccination directly influences maternal and infant morbidity and mortality [27]. The access domain is a measure of the cost and convenience involved in accessing basic amenities including General Practice services [26], and was examined as it was highlighted in the literature as an important determinant of vaccination likelihood [18], [28]. The education domain identifies young people who are not in employment, education or training (NEET), as well as the proportion of people without a formal qualification in the working population [26]. The literature review found low educational attainment was a risk factor associated with low maternal vaccine uptake internationally [28], [29]. Thus, education was analysed separately to assess if the trend seen overseas, also applies in NZ. We additionally examined the income and housing domain.
PHO registration data was used to help ascertain a woman’s IMD score by providing the 2013 meshblock of residence. Date of last GP consultation, or PHO registration, was matched to within 5 years of a woman’s delivery date, thus providing their meshblock around the time of pregnancy which in turn linked to an IMD datazone that provided the IMD score for area of residence related to each of a woman’s pregnancies [26]. For the cohort, 7,706 pregnancies were missing IMD data of which 1,744 were missing PHO enrolment data and while the remaining had PHO data they lacked linkable meshblock data.
2.5. Statistical analysis
Demographic variables (deprivation, ethnicity, and district health board) were imputed using the NHI dataset and if gestational age or last menstrual period date were missing then they were imputed using delivery date and if both were missing the woman’s pregnancy was excluded. Descriptive variables are presented as counts and percentages. Rates were calculated per 100 pregnant women, with the population denominator calculated as the total number of women in the cohort. The association between pertussis or influenza receipt and exposures was explored using logistic regression. Specifically, the SAS procedure GENMOD with a binomial distribution and logit link and autoregressive structure accounting for repeated subjects. Main exposures were maternal ethnicity and deprivation. Models were additionally adjusted for: year of delivery, maternal age at last menstrual period, parity, model of antenatal care, and area of residence (DHB). Odds ratios (OR) and 95% confidence intervals are presented. Effect modification was examined with inclusion of an interaction between ethnicity and deprivation. Where a significant Wald test indicated an interaction, separate logistic regression models were run. All tests for assessing statistical significance were two-sided with a p less than 0.05. All statistical analyses were undertaken using SAS Enterprise Guide (9.4) statistical software (SAS Institute Inc., Cary, NC, USA). This study was approved by the University of Auckland Human Participants Ethics Committee (Ref. 022536).
3. Results
3.1. Descriptive statistics
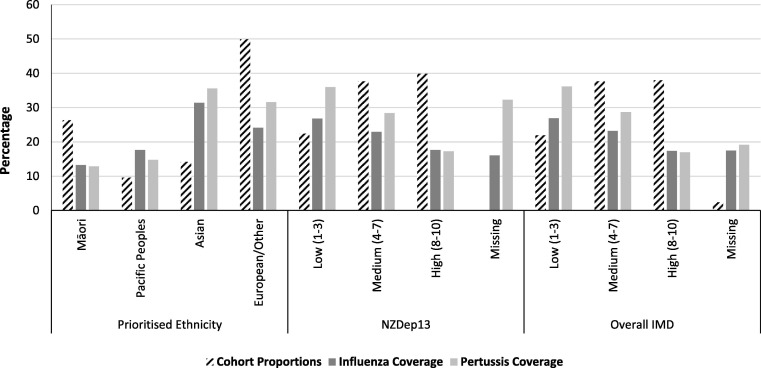
Our cohort of women included 323,622 pregnancies between 2013 and 2018, of which 26.3% were to women who identified as Māori and 9.6% pregnancies to Pacific women (Fig. 1 ). The cohort flowchart and further characteristic descriptors can be found in online supplementary materials Figure S1 and Table S1.
Fig. 1.
Proportion of the cohort and maternal coverage, by ethnicity and deprivation.
3.2. Maternal vaccination coverage has changed over time
Between 2013 and 2018, influenza vaccination coverage has increased from 11.2% to 30.8%, and pertussis 10.2% to 43.6% (Table S1). More detailed has been previously published [14].
3.3. Maternal vaccination coverage according to ethnicity
As shown in Fig. 1, women who identified as Asian or European/Other had the highest maternal coverage with 31.4% and 24.1% for influenza and 35.6% and 31.6% for pertussis, respectively. In comparison, only 13.3% of women who identified as Māori and 17.7% of Pacific women received an influenza vaccination during their pregnancies, and 12.9% and 14.8%, respectively for pertussis vaccination.
3.4. Maternal vaccination coverage according to socio-economic deprivation
Overall, a deprivation gradient was observed over the time period (Table 1 ). Maternal coverage decreased with increasing deprivation for overall indicators and the specific IMD domains, with the exception of the Access domain for pertussis maternal coverage which ranged very little between the deprivation categories (24.3% to 26.4%) (Table 1). No discernible difference was seen between overall area level deprivation between NZDep13 and the IMD (Table 1). The 7,706 pregnancies missing IMD data were more likely to be in the two least deprived quintiles of the NZDep13 and less likely to have received a vaccination during the pregnancy compared to those with IMD data (online supplementary materials: Table S2).
Table 1.
Vaccination status by different domains of deprivation, as measured by the IMD, in New Zealand pregnant women (2013–2018) (n = 323,622).
| Total Cohort | Influenza Vaccinated | Pertussis Vaccinated | ||||||
|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | |||
| Access Deprivation | ||||||||
| Low (1–3) | 102,488 | (31.7) | 23,780 | (23.2) | 27,019 | (26.3) | ||
| Medium (4–7) | 131,528 | (40.6) | 28,958 | (22.0) | 34,823 | (26.4) | ||
| High (8–10) | 81,900 | (25.3) | 16,256 | (19.8) | 19,907 | (24.3) | ||
| Missing | 7,706 | (2.4) | 1,349 | (17.5) | 1,481 | (19.2) | ||
| Education Deprivation | ||||||||
| Low (1–3) | 73,436 | (22.7) | 20,677 | (28.1) | 27,728 | (37.7) | ||
| Medium (4–7) | 123,701 | (38.2) | 27,979 | (22.6) | 33,749 | (27.2) | ||
| High (8–10) | 118,779 | (36.7) | 20,338 | (17.1) | 20,272 | (17.0) | ||
| Missing | 7,706 | (2.4) | 1,349 | (17.5) | 1,481 | (19.2) | ||
| Health Deprivation | ||||||||
| Low (1–3) | 72,248 | (22.3) | 18,725 | (25.9) | 24,722 | (34.2) | ||
| Medium (4–7) | 121,836 | (37.6) | 28,236 | (23.1) | 34,977 | (28.7) | ||
| High (8–10) | 121,832 | (37.6) | 22,033 | (18.0) | 22,050 | (18.0) | ||
| Missing | 7,706 | (2.4) | 1,349 | (17.5) | 1,481 | (19.2) | ||
| Housing Deprivation | ||||||||
| Low (1–3) | 71,668 | (22.1) | 17,690 | (24.6) | 23,227 | (32.4) | ||
| Medium (4–7) | 124,350 | (38.4) | 27,706 | (22.2) | 34,171 | (27.4) | ||
| High (8–10) | 119,898 | (37.0) | 23,598 | (19.6) | 24,351 | (20.3) | ||
| Missing | 7,706 | (2.4) | 1,349 | (17.5) | 1,481 | (19.2) | ||
| Income Deprivation | ||||||||
| Low (1–3) | 70,835 | (21.9) | 19,262 | (27.1) | 25,724 | (36.3) | ||
| Medium (4–7) | 121,377 | (37.5) | 27,980 | (23.0) | 34,771 | (28.6) | ||
| High (8–10) | 123,704 | (38.2) | 21,752 | (17.5) | 21,254 | (17.1) | ||
| Missing | 7,706 | (2.4) | 1,349 | (17.5) | 1,481 | (19.2) | ||
3.5. Differences between ethnicity by deprivation
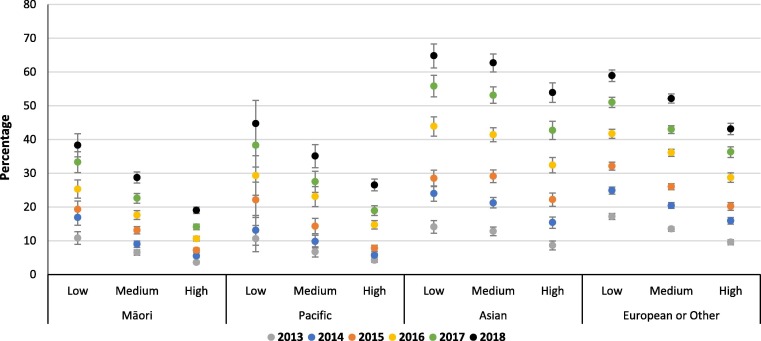
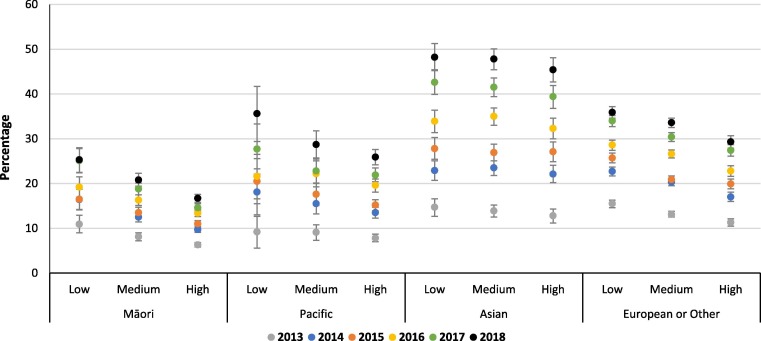
An ethnicity by deprivation interaction was found, with women who identified as Māori or Pacific living in areas of low overall deprivation (IMD) having similar rates of maternal vaccination coverage as Asian or European women living in areas of high deprivation (Fig. 2, Fig. 3 ). Coverage of pertussis vaccination in low deprivation Māori women was 24.4% (95% CI: 23.3, 25.5) and in Pacific women 27.8% (25.4, 30.2) compared to 29.9% (29.0, 30.9) and 25.0% (24.5, 25.5) in high deprivation Asian and European/Other women, respectively (Fig. 2). Similar trends were seen for influenza vaccination (Fig. 3). Ethnicity by deprivation results by year are presented in online supplementary materials Tables S3 and S4
Fig. 2.
Annual maternal pertussis vaccination coverage, by ethnicity and overall IMD deprivation.
Fig. 3.
Annual maternal influenza vaccination coverage, by ethnicity and overall IMD deprivation.
Table 2, Table 3 show trends in coverage rates by ethnicity and the health, education, and income domains of deprivation. Coverage for Māori and Pacific women fell with increasing deprivation in these domains, with coverage rates in the areas of lowest deprivation for these women similar to women of European/Other ethnicity in areas of high deprivation (Table 2, Table 3).
Table 2.
Examination of association between maternal pertussis vaccination coverage and ethnicity by deprivation category, for New Zealand pregnant women (2013 – 2018).
| Cases | Population | Rate1 | (95 %CI) | OR2 | (95 %CI) | |||
|---|---|---|---|---|---|---|---|---|
| Overall Deprivation | ||||||||
| Māori | ||||||||
| Low | 1,826 | 7,475 | 24.4 | (23.3, 25.5) | 0.67 | (0.63, 0.71) | ||
| Medium | 3,980 | 24,443 | 16.3 | (15.8, 16.8) | 0.51 | (0.49, 0.53) | ||
| High | 5,046 | 51,074 | 9.9 | (9.6, 10.2) | 0.37 | (0.36, 0.39) | ||
| Pacific | ||||||||
| Low | 520 | 1,872 | 27.8 | (25.4, 30.2) | 0.68 | (0.61, 0.76) | ||
| Medium | 1,262 | 6,518 | 19.4 | (18.3, 20.4) | 0.54 | (0.50, 0.58) | ||
| High | 2,790 | 22,220 | 12.6 | (12.1, 13.0) | 0.43 | (0.41, 0.45) | ||
| Asian | ||||||||
| Low | 4,543 | 11,306 | 40.2 | (39.0, 41.4) | 1.01 | (0.97, 1.06) | ||
| Medium | 7,421 | 19,822 | 37.4 | (36.6, 38.3) | 0.99 | (0.95, 1.03) | ||
| High | 4,040 | 13,499 | 29.9 | (29.0, 30.9) | 0.77 | (0.74, 0.81) | ||
| European or Other | ||||||||
| Low | 18,798 | 50,132 | 37.5 | (37.0, 38.0) | ref | – | ||
| Medium | 22,455 | 71,232 | 31.5 | (31.1, 31.9) | 0.89 | (0.86, 0.91) | ||
| High | 9,068 | 36,322 | 25.0 | (24.5, 25.5) | 0.74 | (0.72, 0.77) | ||
| Access Deprivation | ||||||||
| Māori | ||||||||
| Low | 3,404 | 24,962 | 13.6 | (13.2, 14.1) | 0.56 | (0.53, 0.58) | ||
| Medium | 4,569 | 35,446 | 12.9 | (12.5, 13.3) | 0.51 | (0.49, 0.53) | ||
| High | 2,879 | 22,584 | 12.7 | (12.3, 13.2) | 0.42 | (0.40, 0.44) | ||
| Pacific | ||||||||
| Low | 2,403 | 16,586 | 14.5 | (13.9, 15.1) | 0.61 | (0.58, 0.64) | ||
| Medium | 1,812 | 12,015 | 15.1 | (14.4, 15.8) | 0.53 | (0.50, 0.56) | ||
| High | 357 | 2,009 | 17.8 | (15.9, 19.6) | 0.43 | (0.38, 0.49) | ||
| Asian | ||||||||
| Low | 7,405 | 20,522 | 36.1 | (35.3, 36.9) | 1.05 | (1.01, 1.09) | ||
| Medium | 7,198 | 20,058 | 35.9 | (35.1, 36.7) | 0.91 | (0.87, 0.95) | ||
| High | 1,401 | 4,047 | 34.6 | (32.8, 36.4) | 0.68 | (0.63, 0.74) | ||
| European or Other | ||||||||
| Low | 13,807 | 40,417 | 34.2 | (33.6, 34.7) | ref | – | ||
| Medium | 21,244 | 64,009 | 33.2 | (32.7, 33.6) | 0.94 | (0.91, 0.97) | ||
| High | 15,270 | 53,260 | 28.7 | (28.2, 29.1) | 0.68 | (0.66, 0.70) | ||
| Education Deprivation | ||||||||
| Māori | ||||||||
| Low | 1,829 | 7,225 | 25.3 | (24.2, 26.5) | 0.66 | (0.62, 0.70) | ||
| Medium | 3,958 | 25,214 | 15.7 | (15.2, 16.2) | 0.48 | (0.46, 0.51) | ||
| High | 5,065 | 50,553 | 10.0 | (9.7, 10.3) | 0.39 | (0.37, 0.41) | ||
| Pacific | ||||||||
| Low | 763 | 3,002 | 25.4 | (23.6, 27.2) | 0.64 | (0.59, 0.70) | ||
| Medium | 1,601 | 9,464 | 16.9 | (16.1, 17.7) | 0.52 | (0.49, 0.56) | ||
| High | 2,208 | 18,144 | 12.2 | (11.7, 12.7) | 0.44 | (0.41, 0.46) | ||
| Asian | ||||||||
| Low | 6,928 | 17,311 | 40.0 | (39.1, 41.0) | 1.01 | (0.97, 1.05) | ||
| Medium | 6,429 | 18,368 | 35.0 | (34.1, 35.9) | 0.92 | (0.88, 0.96) | ||
| High | 2,647 | 8,948 | 29.6 | (28.5, 30.7) | 0.78 | (0.74, 0.83) | ||
| European or Other | ||||||||
| Low | 18,208 | 45,898 | 39.7 | (39.1, 40.2) | ref | – | ||
| Medium | 21,761 | 70,655 | 30.8 | (30.4, 31.2) | 0.83 | (0.80, 0.85) | ||
| High | 10,352 | 41,133 | 25.2 | (24.7, 25.7) | 0.76 | (0.73, 0.79) | ||
| Health Deprivation | ||||||||
| Māori | ||||||||
| Low | 2,093 | 9,664 | 21.7 | (20.7, 22.6) | 0.67 | (0.63, 0.71) | ||
| Medium | 4,071 | 26,052 | 15.6 | (15.1, 16.1) | 0.63 | (0.60, 0.66) | ||
| High | 4,688 | 47,276 | 9.9 | (9.6, 10.2) | 0.55 | (0.52, 0.57) | ||
| Pacific | ||||||||
| Low | 514 | 1,989 | 25.8 | (23.6, 28.1) | 0.71 | (0.63, 0.79) | ||
| Medium | 1,302 | 6,601 | 19.7 | (18.7, 20.8) | 0.68 | (0.64, 0.73) | ||
| High | 2,756 | 22,020 | 12.5 | (12.0, 13.0) | 0.62 | (0.59, 0.66) | ||
| Asian | ||||||||
| Low | 4,433 | 11,016 | 40.2 | (39.1, 41.4) | 1.09 | (1.04, 1.14) | ||
| Medium | 7,031 | 19,004 | 37.0 | (36.1, 37.9) | 1.17 | (1.12, 1.22) | ||
| High | 4,540 | 14,607 | 31.1 | (30.2, 32.0) | 1.12 | (1.07, 1.18) | ||
| European or Other | ||||||||
| Low | 17,682 | 49,579 | 35.7 | (35.1, 36.2) | ref | – | ||
| Medium | 22,573 | 70,179 | 32.2 | (31.7, 32.6) | 1.08 | (1.04, 1.11) | ||
| High | 10,066 | 37,928 | 26.5 | (26.0, 27.1) | 1.10 | (1.06, 1.15) | ||
| Housing Deprivation | ||||||||
| Māori | ||||||||
| Low | 2,088 | 10,247 | 20.4 | (19.5, 21.3) | 0.66 | (0.62, 0.69) | ||
| Medium | 4,310 | 29,799 | 14.5 | (14.0, 14.9) | 0.69 | (0.66, 0.72) | ||
| High | 4,454 | 42,946 | 10.4 | (10.1, 10.7) | 0.71 | (0.67, 0.75) | ||
| Pacific | ||||||||
| Low | 473 | 1,910 | 24.8 | (22.5, 27.0) | 0.69 | (0.61, 0.77) | ||
| Medium | 1,084 | 5,584 | 19.4 | (18.3, 20.6) | 0.73 | (0.68, 0.79) | ||
| High | 3,015 | 23,116 | 13.0 | (12.6, 13.5) | 0.81 | (0.76, 0.85) | ||
| Asian | ||||||||
| Low | 3,012 | 7,734 | 38.9 | (37.6, 40.3) | 1.01 | (0.96, 1.07) | ||
| Medium | 6,048 | 16,483 | 36.7 | (35.8, 37.6) | 1.24 | (1.18, 1.29) | ||
| High | 6,944 | 20,410 | 34.0 | (33.2, 34.8) | 1.46 | (1.39, 1.53) | ||
| European or Other | ||||||||
| Low | 17,654 | 51,777 | 34.1 | (33.6, 34.6) | ref | – | ||
| Medium | 22,729 | 72,484 | 31.4 | (30.9, 31.8) | 1.21 | (1.17, 1.25) | ||
| High | 9,938 | 33,425 | 29.7 | (29.1, 30.3) | 1.40 | (1.34, 1.45) | ||
| Income Deprivation | ||||||||
| Māori | ||||||||
| Low | 1,879 | 7,917 | 23.7 | (22.7, 24.8) | 0.67 | (0.63, 0.71) | ||
| Medium | 3,869 | 24,152 | 16.0 | (15.5, 16.5) | 0.56 | (0.53, 0.59) | ||
| High | 5,104 | 50,923 | 10.0 | (9.7, 10.3) | 0.46 | (0.44, 0.49) | ||
| Pacific | ||||||||
| Low | 577 | 2,068 | 27.9 | (25.6, 30.2) | 0.71 | (0.64, 0.80) | ||
| Medium | 1,287 | 6,751 | 19.1 | (18.0, 20.1) | 0.60 | (0.56, 0.65) | ||
| High | 2,708 | 21,791 | 12.4 | (12.0, 12.9) | 0.52 | (0.49, 0.56) | ||
| Asian | ||||||||
| Low | 4,834 | 11,909 | 40.6 | (39.4, 41.7) | 1.03 | (0.98, 1.07) | ||
| Medium | 7,380 | 20,024 | 36.9 | (36.0, 37.7) | 1.08 | (1.03, 1.12) | ||
| High | 3,790 | 12,694 | 29.9 | (28.9, 30.8) | 0.95 | (0.89, 1.00) | ||
| European or Other | ||||||||
| Low | 18,434 | 48,941 | 37.7 | (37.1, 38.2) | ref | – | ||
| Medium | 22,235 | 70,450 | 31.6 | (31.1, 32.0) | 0.97 | (0.94, 1.00) | ||
| High | 9,652 | 38,295 | 25.2 | (24.7, 25.7) | 0.91 | (0.87, 0.95) | ||
Rate per 100 pregnant women.
Adjusted for delivery year, age at last menstrual period, parity, lead maternity carer, district health board, and deprivation.
Table 3.
Examination of association between maternal influenza vaccination coverage and ethnicity by deprivation category, for New Zealand pregnant women (2013 – 2018).
| Cases | Population | Rate1 | (95 %CI) | OR2 | (95 %CI) | |||
|---|---|---|---|---|---|---|---|---|
| Overall Deprivation | ||||||||
| Māori | ||||||||
| Low | 1,430 | 7,475 | 19.1 | (18.1, 20.1) | 0.76 | (0.71, 0.81) | ||
| Medium | 3,677 | 24,443 | 15.0 | (14.6, 15.5) | 0.67 | (0.64, 0.70) | ||
| High | 6,060 | 51,074 | 11.9 | (11.6, 12.2) | 0.60 | (0.58, 0.63) | ||
| Pacific | ||||||||
| Low | 429 | 1,872 | 22.9 | (20.7, 25.1) | 0.87 | (0.78, 0.97) | ||
| Medium | 1,256 | 6,518 | 19.3 | (18.2, 20.3) | 0.82 | (0.77, 0.88) | ||
| High | 3,774 | 22,220 | 17.0 | (16.4, 17.5) | 0.84 | (0.80, 0.87) | ||
| Asian | ||||||||
| Low | 3,705 | 11,306 | 32.8 | (31.7, 33.8) | 1.24 | (1.18, 1.30) | ||
| Medium | 6,326 | 19,822 | 31.9 | (31.1, 32.7) | 1.27 | (1.22, 1.32) | ||
| High | 4,100 | 13,499 | 30.4 | (29.4, 31.3) | 1.27 | (1.21, 1.33) | ||
| European or Other | ||||||||
| Low | 13,543 | 50,132 | 27.0 | (26.6, 27.5) | ref | – | ||
| Medium | 17,089 | 71,232 | 24.0 | (23.6, 24.4) | 0.95 | (0.92, 0.97) | ||
| High | 7,605 | 36,322 | 20.9 | (20.5, 21.4) | 0.89 | (0.86, 0.92) | ||
| Access Deprivation | ||||||||
| Māori | ||||||||
| Low | 3,568 | 24,962 | 14.3 | (13.8, 14.8) | 0.71 | (0.68, 0.74) | ||
| Medium | 4,783 | 35,446 | 13.5 | (13.1, 13.9) | 0.66 | (0.64, 0.69) | ||
| High | 2,816 | 22,584 | 12.5 | (12.0, 12.9) | 0.56 | (0.53, 0.59) | ||
| Pacific | ||||||||
| Low | 3,048 | 16,586 | 18.4 | (17.7, 19.0) | 0.94 | (0.90, 0.99) | ||
| Medium | 2,075 | 12,015 | 17.3 | (16.5, 18.0) | 0.81 | (0.77, 0.85) | ||
| High | 336 | 2,009 | 16.7 | (14.9, 18.5) | 0.63 | (0.56, 0.72) | ||
| Asian | ||||||||
| Low | 6,653 | 20,522 | 32.4 | (31.6, 33.2) | 1.33 | (1.28, 1.38) | ||
| Medium | 6,226 | 20,058 | 31.0 | (30.3, 31.8) | 1.17 | (1.13, 1.22) | ||
| High | 1,252 | 4,047 | 30.9 | (29.2, 32.7) | 1.06 | (0.98, 1.14) | ||
| European or Other | ||||||||
| Low | 10,511 | 40,417 | 26.0 | (25.5, 26.5) | ref | – | ||
| Medium | 15,874 | 64,009 | 24.8 | (24.4, 25.2) | 0.94 | (0.91, 0.97) | ||
| High | 11,852 | 53,260 | 22.3 | (21.9, 22.7) | 0.79 | (0.77, 0.82) | ||
| Education Deprivation | ||||||||
| Māori | ||||||||
| Low | 1,402 | 7,225 | 19.4 | (18.4, 20.4) | 0.74 | (0.70, 0.79) | ||
| Medium | 3,775 | 25,214 | 15.0 | (14.5, 15.4) | 0.66 | (0.63, 0.69) | ||
| High | 5,990 | 50,553 | 11.8 | (11.5, 12.1) | 0.59 | (0.56, 0.61) | ||
| Pacific | ||||||||
| Low | 709 | 3,002 | 23.6 | (21.9, 25.4) | 0.94 | (0.86, 1.02) | ||
| Medium | 1,725 | 9,464 | 18.2 | (17.4, 19.1) | 0.81 | (0.76, 0.86) | ||
| High | 3,025 | 18,144 | 16.7 | (16.1, 17.3) | 0.80 | (0.76, 0.85) | ||
| Asian | ||||||||
| Low | 5,717 | 17,311 | 33.0 | (32.2, 33.9) | 1.26 | (1.21, 1.31) | ||
| Medium | 5,739 | 18,368 | 31.2 | (30.4, 32.1) | 1.23 | (1.18, 1.28) | ||
| High | 2,675 | 8,948 | 29.9 | (28.8, 31.0) | 1.21 | (1.14, 1.28) | ||
| European or Other | ||||||||
| Low | 12,849 | 45,898 | 28.0 | (27.5, 28.5) | ref | – | ||
| Medium | 16,740 | 70,655 | 23.7 | (23.3, 24.1) | 0.91 | (0.88, 0.94) | ||
| High | 8,648 | 41,133 | 21.0 | (20.6, 21.5) | 0.87 | (0.83, 0.90) | ||
| Health Deprivation | ||||||||
| Māori | ||||||||
| Low | 1,705 | 9,664 | 17.6 | (16.8, 18.5) | 0.76 | (0.72, 0.80) | ||
| Medium | 3,840 | 26,052 | 14.7 | (14.3, 15.2) | 0.73 | (0.70, 0.76) | ||
| High | 5,622 | 47,276 | 11.9 | (11.6, 12.2) | 0.70 | (0.66, 0.73) | ||
| Pacific | ||||||||
| Low | 467 | 1,989 | 23.5 | (21.3, 25.6) | 0.97 | (0.87, 1.08) | ||
| Medium | 1,272 | 6,601 | 19.3 | (18.2, 20.3) | 0.89 | (0.83, 0.95) | ||
| High | 3,720 | 22,020 | 16.9 | (16.4, 17.4) | 0.95 | (0.90, 1.00) | ||
| Asian | ||||||||
| Low | 3,673 | 11,016 | 33.3 | (32.3, 34.4) | 1.32 | (1.26, 1.39) | ||
| Medium | 6,074 | 19,004 | 32.0 | (31.2, 32.8) | 1.37 | (1.32, 1.43) | ||
| High | 4,384 | 14,607 | 30.0 | (29.1, 30.9) | 1.39 | (1.32, 1.46) | ||
| European or Other | ||||||||
| Low | 12,880 | 49,579 | 26.0 | (25.5, 26.4) | ref | – | ||
| Medium | 17,050 | 70,179 | 24.3 | (23.9, 24.7) | 1.02 | (0.99, 1.05) | ||
| High | 8,307 | 37,928 | 21.9 | (21.4, 22.4) | 1.04 | (1.00, 1.08) | ||
| Housing Deprivation | ||||||||
| Māori | ||||||||
| Low | 1,711 | 10,247 | 16.7 | (15.9, 17.5) | 0.73 | (0.69, 0.77) | ||
| Medium | 4,147 | 29,799 | 13.9 | (13.5, 14.3) | 0.76 | (0.73, 0.79) | ||
| High | 5,309 | 42,946 | 12.4 | (12.0, 12.7) | 0.82 | (0.78, 0.86) | ||
| Pacific | ||||||||
| Low | 407 | 1,910 | 21.3 | (19.2, 23.4) | 0.88 | (0.79, 0.99) | ||
| Medium | 1,022 | 5,584 | 18.3 | (17.2, 19.4) | 0.88 | (0.81, 0.95) | ||
| High | 4,030 | 23,116 | 17.4 | (16.9, 18.0) | 1.12 | (1.06, 1.18) | ||
| Asian | ||||||||
| Low | 2,486 | 7,734 | 32.1 | (30.9, 33.4) | 1.25 | (1.18, 1.32) | ||
| Medium | 5,197 | 16,483 | 31.5 | (30.7, 32.4) | 1.40 | (1.35, 1.46) | ||
| High | 6,448 | 20,410 | 31.6 | (30.8, 32.4) | 1.61 | (1.53, 1.68) | ||
| European or Other | ||||||||
| Low | 13,086 | 51,777 | 25.3 | (24.8, 25.7) | ref | – | ||
| Medium | 17,340 | 72,484 | 23.9 | (23.6, 24.3) | 1.09 | (1.06, 1.12) | ||
| High | 7,811 | 33,425 | 23.4 | (22.9, 23.9) | 1.17 | (1.12, 1.22) | ||
| Income Deprivation | ||||||||
| Māori | ||||||||
| Low | 1,479 | 7,917 | 18.7 | (17.7, 19.6) | 0.75 | (0.70, 0.80) | ||
| Medium | 3,548 | 24,152 | 14.7 | (14.2, 15.2) | 0.67 | (0.64, 0.70) | ||
| High | 6,140 | 50,923 | 12.1 | (11.8, 12.4) | 0.64 | (0.61, 0.68) | ||
| Pacific | ||||||||
| Low | 488 | 2,068 | 23.6 | (21.5, 25.7) | 0.91 | (0.82, 1.01) | ||
| Medium | 1,272 | 6,751 | 18.8 | (17.8, 19.9) | 0.82 | (0.76, 0.88) | ||
| High | 3,699 | 21,791 | 17.0 | (16.4, 17.5) | 0.88 | (0.83, 0.93) | ||
| Asian | ||||||||
| Low | 3,957 | 11,909 | 33.2 | (32.2, 34.3) | 1.25 | (1.19, 1.31) | ||
| Medium | 6,320 | 20,024 | 31.6 | (30.8, 32.3) | 1.28 | (1.22, 1.33) | ||
| High | 3,854 | 12,694 | 30.4 | (29.4, 31.3) | 1.33 | (1.25, 1.41) | ||
| European or Other | ||||||||
| Low | 13,338 | 48,941 | 27.3 | (26.8, 27.7) | ref | – | ||
| Medium | 16,840 | 70,450 | 23.9 | (23.5, 24.3) | 0.95 | (0.92, 0.98) | ||
| High | 8,059 | 38,295 | 21.0 | (20.6, 21.5) | 0.93 | (0.88, 0.97) | ||
Rate per 100 pregnant women.
Adjusted for delivery year, age at last menstrual period, parity, lead maternity carer, district health board, and deprivation.
Very little difference in maternal vaccination rates were seen by deprivation for the ‘Access to Services’ domain, with Asian or European/Other women having nearly twice the rate of coverage as women who identified as Māori and Pacific (Table 2, Table 3). Annual rates between 2013 and 2018 by ethnicity and deprivation showed only a slight increase for Māori and Pacific women with no difference between levels of Access deprivation. For example, Māori women with low Access deprivation in 2013 had 7.6% influenza vaccination coverage compared to 7.1% for those with high Access deprivation, this increased to 20.8% and 16.5% by 2018, respectively (Table S4).
4. Discussion
This nationwide retrospective cohort study aimed to examine the extent to which maternal vaccination coverage in pregnancy varies according to ethnicity and socio-economic status in NZ from 2013 to 2018. It extends evidence by illustrating a widening inequity in maternal vaccination coverage over time despite notable increases over time. Our study found that maternal vaccination has increased for all ethnic groups between 2013 and 2018 however, over the same period socioeconomic and ethnic inequities have increased. Asian and European women had the highest rates and odds of receiving pregnancy vaccines, while Māori and Pacific women had lowest.
Our study found significant disparities in coverage by ethnicity. While coverage was found to be uniformly low, the relative difference did not change overtime between the groups with the lowest and highest coverage (∼a factor of 3), while the absolute margin did change. We found Māori living in low deprivation areas had a coverage increase of only 15 percentage points between 2013 and 2018 compared to 50 percentage points for those who identify as Asian living in low deprivation areas. The causes of ethnic inequities in maternal vaccination coverage are multifactorial. Causes can include barriers to accessing primary and maternity care, geographical access [15], and systems barriers [20]. There is often a tendency to explain health inequities by focusing on the role of the broader socioeconomic status or to take a victim blaming approach [30]. Socioeconomic status does undoubtedly have a role in determining the financial and physical access a person has to vaccination and therefore does contribute to ethnic disparities [31]. However, this study found ethnic inequities in maternal vaccination coverage persisted across the social gradient, with Māori and Pacific women living in the least deprived areas having lower odds of vaccination compared with other non-Māori women living in the least deprived areas. Consequently, wider systemic factors such as racism and the ongoing impacts of the colonisation process, must be considered as causes for ethnic inequities [20], [32]. Consideration of appropriate delivery of information to Māori and Pacific women, for example kanohi ki te kanohi kōrero (face-to-face discussion) may be more acceptable or meaningful than provision of written material as commonly occurs . Vaccinating outside of general practice, e.g. in schools, community outreach [33], [34], or pharmacy [35] aids access by Māori.
The lack of culturally appropriate antenatal engagement and care are important factors contributing to health inequities for pregnant Māori and Pacific women [30]. Qualitative research exploring the lived realities of pregnant Māori women found the majority of participants were pro-active and engaged early with primary health services to confirm their pregnancy [30]. Thus, dispelling the victim blaming discourse of Māori women booking late in pregnancy as a reason for inequities [30]. Makowharemahihi, et al., [30] found the health system failed participants as they transitioned to an LMC. During this transition, women experienced a lack of information regarding the antenatal process, were not given assistance to find an LMC and struggled to find LMCs with availability, let alone an LMC who provided culturally sensitive care [30]. This led to a long lag time between confirmation of pregnancy and first LMC visit. When women do not receive early antenatal care (defined as having LMC care during the first trimester) or have less than five antenatal visits, they are less likely to discuss maternal vaccination with their LMC and are consequently less likely to be vaccinated [28]. Thus, the health system’s failure to help Māori (and Pacific) women navigate the transition to LMC care, the lack of culturally appropriate maternity care and LMC availability, is likely to underpin ethnic inequities in maternal vaccination coverage.
Internationally, and in New Zealand, health-care provider recommendation to vaccinate has been identified as a key predictor of vaccination uptake amongst pregnant women [36], [37], [38]. The importance of health-care provider recommendation is illustrated by the findings of a study where recommendation to vaccinate was associated with higher odds of maternal vaccination (OR:41.89 95% CI: 20.68, 84.86) compared with women who did not receive a vaccination recommendation [37]. Alongside this, several studies have found health-care provider recommendation to vaccinate varies according to patient ethnicity and socioeconomic status [29], [39]. For example, in an American study, offers or referrals for maternal vaccination were lowest amongst Black, unmarried and uninsured women, corresponding with these women having the lowest vaccination coverage [29]. An Australian study found Indigenous women were open to vaccination during pregnancy, however ethnic bias among healthcare providers was identified as a possible cause for low maternal vaccine coverage in Indigenous women [39]. Thus, health-care provider bias and failure to provide vaccination recommendations to Māori and Pacific women could be a reason for ethnic inequities in maternal vaccination coverage in NZ. This point is highlighted by the almost non-existent gradient in coverage we found for the ‘Access’ domain of deprivation, a measure of proximity to basic amenities that includes General Practice [26]. This indicates that physical access to a GP has little effect on increasing maternal coverage, despite GP’s being the almost exclusive providers of maternal pertussis immunisation and the lead provider of maternal influenza vaccinations with pharmacy only providing funded maternal influenza vaccination since 2017. Within the NZ context, while health-care provider recommendations have aided maternal vaccination uptake [38], [40], no specific research has identified the impact of health-care provider recommendations and bias on ethnic inequities in maternal vaccination coverage. However, the impact of racism and unconscious bias within the health sector on Māori health is well documented, with Harris and Cormack explaining a person’s socially-assigned ethnicity is linked to real health advantage for those who are perceived as European and with tangible health risk for people socially-assigned as Māori [41]. This is illustrated by research that has found Māori experience a much slower and longer pathway through the healthcare system compared with non-Māori [42] and receive care which is of different quality to non-Māori [43]. Examples of differential care include Māori patients being less likely to receive pain relief than non-Māori during childbirth, Māori receiving less screening for and treatment of cardiovascular disease despite having increased rates of disease and Māori being less likely to be diagnosed and treated for depression [43]. These findings, coupled with international evidence of Indigenous women and women from minority groups being less likely to be recommended maternal vaccination, suggest racism and unconscious bias likely to contribute to who does and does not receive a recommendation for maternal vaccination in NZ.
This study has a number of limitations. Firstly, influenza vaccinations delivered in workplaces are not usually captured by governmental claims data or registered in the National Immunisation Register (NIR). Consequently, it is possible pregnant women who received their influenza vaccination through an occupational scheme have not been included in this study and therefore maternal influenza coverage may have been underestimated. A second limitation of this study is that area level deprivation has been analysed, as opposed to individual deprivation. Individual-level deprivation is associated with worse health-related quality of life and results in greater health inequities compared with area level deprivation [44]. Therefore, the impact of deprivation on maternal vaccination is likely to have been underestimated in this study. Due to the limitations of administrative data, changes to specific enablers and barriers to maternal vaccination, such as health-seeking or health practitioner behaviours, have not been reported on. Lastly, this study does not interpret the odds as risk, it is worth noting that odds ratios calculated using logistic regression can over-estimate the relative risk [45].
5. Conclusion
This nationwide retrospective cohort study is one of the first to investigate variation in maternal vaccination coverage in pregnancy women by ethnicity and specific components of deprivation over time. While maternal vaccination coverage increased during 2013 to 2018 it is still suboptimal and inequities by ethnicity and socio-economic deprivation increased. Given the inequitable burden of pertussis infections is in Māori and Pacific infants [46], NZ must commit to solutions that increase maternal vaccination coverage for Māori and Pacific women. This is also crucial in order for NZ to meet obligations to uphold Māori rights under te Tiriti o Waitangi and the United Nations Declaration on the Rights of Indigenous Peoples.
The current model of vaccination in pregnancy relies on women mobilising a range of resources including their own knowledge of antenatal care and finances and overcoming physical and inconvenience access barriers, as well as healthcare bias. The ability to mobilise such resource and overcome such barriers, is less likely to be within reach of Indigenous, ethnic minority and socially deprived women. Understanding how to increase uptake for maternal vaccinations is critical for the health of our mothers and their infants, even more so in this COVID-19 era with unvaccinated pregnant women being at high risk of severe COVID-19 infection. The roll out of COVID-19 vaccinations and the novel solutions being sought to get coverage in hard-to-reach populations provides an opportunity to create a more responsive service with a greater focus on equity. Solutions generated from within Māori and Pacific communities with engaged and responsive health services have been successful in addressing inequities in other public health issues and are needed again to address the inequities found here.
CRediT authorship contribution statement
Leah Pointon: Conceptualization, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization. Anna S Howe: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration. Matthew Hobbs: Conceptualization, Writing – original draft, Writing – review & editing, Visualization. Janine Paynter: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Writing – review & editing, Supervision. Natalie Gauld: Conceptualization, Formal analysis, Writing – review & editing. Nikki Turner: Conceptualization, Writing – review & editing. Esther Willing: Conceptualization, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. We would like to thank Barbara McArdle, National Influenza Coordinator, Immunisation Advisory Centre, for her invaluable knowledge and advice.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: NG has received funding from Green Cross Health (a provider of primary health care services including pharmacy and general practice), the Pharmacy Guild of New Zealand and the Pharmaceutical Society of New Zealand for reclassifying vaccinations to allow pharmacist administration. NG has led an investigator-led study funded by GSK. JP and ASH have been investigators on GSK funded studies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.02.079.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.World Health Organization. Vaccines and Immunization [Available from: https://www.who.int/health-topics/vaccines-and-immunization#tab=tab_1.
- 2.Kiedrzynski T., Bissielo A., Suryaprakash M., Bandaranayake D. Whooping cough—where are we now? A review. NZ Med J. 2015;128(1416):21–27. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Pertussis (Whooping Cough) [updated 18/11/2019. Available from: https://www.cdc.gov/pertussis/index.html.
- 4.Wood N., McIntyre P. Pertussis: review of epidemiology, diagnosis, management and prevention. Paediatr Respir Rev. 2008;9(3):201–212. doi: 10.1016/j.prrv.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Yeung K.H.T., Duclos P., Nelson E.A.S., Hutubessy R.C.W. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis. 2017;17(9):974–980. doi: 10.1016/S1473-3099(17)30390-0. [DOI] [PubMed] [Google Scholar]
- 6.ESR (Institute of Environmental Science and Research). Pertussis Report 2019 [Available from: https://surv.esr.cri.nz/surveillance/PertussisRpt.php.
- 7.Lafond KE, Porter RM, Whaley MJ, Suizan Z, Ran Z, Aleem MA, et al. Global burden of influenza-associated lower respiratory tract infections and hospitalizations among adults: A systematic review and meta-analysis. PLoS Med. 2021;18(3):e1003550. [DOI] [PMC free article] [PubMed]
- 8.Griffin J.B., Yu L., Watson D., Turner N., Walls T., Howe A.S., et al. Pertussis Immunisation in Pregnancy Safety (PIPS) Study: a retrospective cohort study of safety outcomes in pregnant women vaccinated with Tdap vaccine. Vaccine. 2018;36(34):5173–5179. doi: 10.1016/j.vaccine.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Donegan K., King B., Bryan P. Safety of pertussis vaccination in pregnant women in UK: observational study. BMJ. 2014;349 doi: 10.1136/bmj.g4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kharbanda E.O., Vazquez-Benitez G., Lipkind H.S., Klein N.P., Cheetham T.C., Naleway A., et al. Evaluation of the association of maternal pertussis vaccination with obstetric events and birth outcomes. JAMA. 2014;312(18):1897. doi: 10.1001/jama.2014.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petousis-Harris H., Jiang Y., Yu L., Watson D., Walls T., Turner N., et al. A retrospective cohort study of safety outcomes in New Zealand infants exposed to Tdap vaccine in utero. Vaccines. 2019;7(4):147. doi: 10.3390/vaccines7040147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pool V., Iskander J. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol. 2006;194(4):1200. doi: 10.1016/j.ajog.2005.07.091. [DOI] [PubMed] [Google Scholar]
- 13.Moro PL, Broder K, Zheteyeva Y, Walton K, Rohan P, Sutherland A, et al. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009. American journal of obstetrics and gynecology. 2011;204(2):146. e1-. e7. [DOI] [PubMed]
- 14.Howe A.S., Pointon L, Gauld N, Paynter J, Willing E, Turner N. Pertussis and Influenza immunisation coverage of pregnant women in New Zealand. Vaccine. 2020;38(43):6766-76. [DOI] [PubMed]
- 15.Marek L., Hobbs M., McCarthy J., Wiki J., Tomintz M., Campbell M., et al. Investigating spatial variation and change (2006–2017) in childhood immunisation coverage in New Zealand. Soc Sci Med. 2020;264:113292. doi: 10.1016/j.socscimed.2020.113292. [DOI] [PubMed] [Google Scholar]
- 16.Wiley K.E., Leask J. Respiratory vaccine uptake during pregnancy. Lancet Respir Med. 2013;1(1):9–11. doi: 10.1016/S2213-2600(13)70024-9. [DOI] [PubMed] [Google Scholar]
- 17.Arnold L.D., Luong L., Rebmann T., Chang J.J. Racial disparities in US maternal influenza vaccine uptake: results from analysis of Pregnancy Risk Assessment Monitoring System (PRAMS) data, 2012–2015. Vaccine. 2019;37(18):2520–2526. doi: 10.1016/j.vaccine.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Byrne L., Ward C., White J.M., Amirthalingam G., Edelstein M. Predictors of coverage of the national maternal pertussis and infant rotavirus vaccination programmes in England. Epidemiol Infect. 2018;146(2):197–206. doi: 10.1017/S0950268817002497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nowlan M., Willing E., Turner N. Influences and policies that affect immunisation coverage-a summary review of literature. The New Zealand Medical Journal. 2019;132(1501):79–88. [PubMed] [Google Scholar]
- 20.Sinclair O., Grant C. New Zealand's immunisation policy fails again and entrenches ethnic disparities. The New Zealand Medical Journal (Online). 2021;134(1542):92–95. [PubMed] [Google Scholar]
- 21.Kandeil W., van den Ende C., Bunge E.M., Jenkins V.A., Ceregido M.A., Guignard A. A systematic review of the burden of pertussis disease in infants and the effectiveness of maternal immunization against pertussis. Expert Rev Vaccines. 2020;19(7):621–638. doi: 10.1080/14760584.2020.1791092. [DOI] [PubMed] [Google Scholar]
- 22.Feldman A.G., O’Leary S.T., Danziger-Isakov L. The Risk of Resurgence in Vaccine-Preventable Infections Due to Coronavirus Disease 2019—Related Gaps in Immunization. Clin Infect Dis. 2021;73(10):1920–1923. doi: 10.1093/cid/ciab127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ministry of Health. National and DHB immunisation data 2021 [updated 12/11/2021. Available from: https://www.health.govt.nz/our-work/preventative-health-wellness/immunisation/immunisation-coverage/national-and-dhb-immunisation-data.
- 24.National Health Board Business Unit. National Maternity Collection Data Mart Data Dictionary. Wellington: Ministry of Health; 2011.
- 25.Atkinson J, Salmond C, Crampton P. NZDep2013 Index of Deprivation. Wellington: University of Otago; 2014.
- 26.Exeter DJ, Zhao J, Crengle S, Lee A, M B. The New Zealand Indices of Multiple Deprivation (IMD): A new suite of indicators for social and health research in Aotearoa, New Zealand PLoS ONE. 2017;12(8):e0181260. [DOI] [PMC free article] [PubMed]
- 27.Walls T., Graham P., Petousis-Harris H., Hill L., Austin N. Infant outcomes after exposure to Tdap vaccine in pregnancy: an observational study. BMJ open. 2016;6(1):e009536. doi: 10.1136/bmjopen-2015-009536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barber A., Muscoplat M.H., Fedorowicz A. Coverage with tetanus, diphtheria, and acellular pertussis vaccine and influenza vaccine among pregnant women—Minnesota, March 2013–December 2014. MMWR Morb Mortal Wkly Rep. 2017;66(02):56–59. doi: 10.15585/mmwr.mm6602a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindley M.C., Kahn K.E., Bardenheier B.H., D’Angelo D.V., Dawood F.S., Fink R.V., et al. Vital signs: burden and prevention of influenza and pertussis among pregnant women and infants—United States. Morb Mortal Wkly Rep. 2019;68(40):885–892. doi: 10.15585/mmwr.mm6840e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makowharemahihi C., Lawton B.A., Cram F., Ngata T., Brown S., Robson B. Initiation of maternity care for young Māori women under 20 years of age. N Z Med J. 2014;127(1393):2010–2019. [PubMed] [Google Scholar]
- 31.Willing E. Hitting the target without missing the point: New Zealand’s immunisation health target for two year olds. Policy Studies. 2016;37(6):535–550. [Google Scholar]
- 32.Hobbs M., Ahuriri-Driscoll A., Marek L., Campbell M., Tomintz M., Kingham S. Reducing health inequity for Māori people in New Zealand. The Lancet. 2019;394(10209):1613–1614. doi: 10.1016/S0140-6736(19)30044-3. [DOI] [PubMed] [Google Scholar]
- 33.Mills C., Penney L. The Northland emergency meningococcal C vaccination programme. NZ Med J. 2013;126:30–39. [PubMed] [Google Scholar]
- 34.Poole T., Goodyear-Smith F., Petousis-Harris H., Desmond N., Exeter D., Pointon L., et al. Human papillomavirus vaccination in Auckland: Reducing ethnic and socioeconomic inequities. Vaccine. 2012;31(1):84–88. doi: 10.1016/j.vaccine.2012.10.099. [DOI] [PubMed] [Google Scholar]
- 35.Howe A.S., Gauld N.J., Cavadino A.Y., Petousis-Harris H., Dumble F., Sinclair O., et al. Increasing Uptake of Maternal Pertussis Vaccinations through Funded Administration in Community Pharmacies. Vaccines. 2022;10(2):150. doi: 10.3390/vaccines10020150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mak D.B., Regan A.K., Vo D.T., Effler P.V. Antenatal influenza and pertussis vaccination in Western Australia: a cross-sectional survey of vaccine uptake and influencing factors. BMC pregnancy and childbirth. 2018;18(1):1–10. doi: 10.1186/s12884-018-2051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maher L., Hope K., Torvaldsen S., Lawrence G., Dawson A., Wiley K., et al. Influenza vaccination during pregnancy: coverage rates and influencing factors in two urban districts in Sydney. Vaccine. 2013;31(47):5557–5564. doi: 10.1016/j.vaccine.2013.08.081. [DOI] [PubMed] [Google Scholar]
- 38.Gauld N., Martin S., Sinclair O., Petousis-Harris H., Dumble F., Grant C.C. Influences on Pregnant Women's and Health Care Professionals' Behaviour Regarding Maternal Vaccinations: A Qualitative Interview Study. Vaccines. 2022;10(1):76. doi: 10.3390/vaccines10010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moberley S.A., Lawrence J., Johnston V., Andrews R.M. Influenza vaccination coverage among pregnant Indigenous women in the Northern Territory of Australia. Communicable diseases intelligence quarterly report. 2016;40(3):E340–E346. doi: 10.33321/cdi.2016.40.34. [DOI] [PubMed] [Google Scholar]
- 40.Hill L., Burrell B., Walls T. Factors influencing women’s decisions about having the pertussis-containing vaccine during pregnancy. J Prim Health Care. 2018;10(1):62. doi: 10.1071/HC17040. [DOI] [PubMed] [Google Scholar]
- 41.Harris R., Tobias M., Jeffreys M., Waldegrave K., Karlsen S., Nazroo J. Effects of self-reported racial discrimination and deprivation on Māori health and inequalities in New Zealand: cross-sectional study. The Lancet. 2006;367(9527):2005–2009. doi: 10.1016/S0140-6736(06)68890-9. [DOI] [PubMed] [Google Scholar]
- 42.Sadler L.V., Priest P., Peters J., Crengle S., Jackson R. Cervical cancer audit report: Screening of women with cervical cancer, 2000–2002: Ministry of. Health. 2004 [Google Scholar]
- 43.Robson B., Hauora H.R. Màori Standards of Health IV. A study of the years 2000–2005. Wellington: Te Ropu Rangahau Hauora a Eru Pomare. 2007 [Google Scholar]
- 44.Siegel M., Mielck A., Maier W. Individual income, area deprivation, and health: Do income-related health inequalities vary by small area deprivation? Health Econ. 2015;24(11):1523–1530. doi: 10.1002/hec.3102. [DOI] [PubMed] [Google Scholar]
- 45.McNutt L.-A., Wu C., Xue X., Hafner J.P. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 46.Statistics New Zealand. 2018 Census Place Summaries 2019 [Available from: https://www.stats.govt.nz/tools/2018-census-place-summaries/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.