Abstract
The noncatalytic oxidative desulfurization process of straight-run kerosene and diesel fractions with high sulfur contents due to their treatment with air without using expensive catalysts and strong oxidants is considered. The research was carried out in a periodic mode in a bubble-type reactor with a volume of 1 × 10–3 m3 under the following conditions: 180–200 °C; 2.5–3.0 MPa; the process lasted for 20–30 min; the air/raw material ratio was 1.6–2.2 m3/min per 1 m3 of raw material. The process can be used to obtain industrial jet fuels and diesel fuel components with good lubricating properties. The possibility and expediency of carrying out the process in the presence of water have been studied. The ratio of water/raw materials (vol.) varied from 0:1 to 2:1. It was proven that the water presence in the reaction medium has a positive effect on the studied process and decreases the oxidation intensity of hydrocarbon medium by 2–4 times. This is due to both the slowing of oxidation reactions by water during peroxide decomposition and chain growth and branching and the partial change in chemical oxidation with the formation of phenols and/or tertiary alcohols, which are inhibitors of oxidation reactions. On the other hand, water has almost no effect on the removal degree of sulfur compounds.
1. Introduction
In recent years, SOx emissions from fuel combustion have become a serious environmental problem. Strict environmental policies have been implemented worldwide, limiting the sulfur level in fuels to 10 ppm.1 Diesel fuel (DF) has become one of the main types of transport fuels and, accordingly, one of the largest sources of environmental pollution. To prevent air pollution from exhaust gases, the governments around the world have pledged to reduce sulfur in transport fuels over the past decade. For example, US regulations have made it mandatory to limit the sulfur content to 15 ppm in automotive diesel fuel2 (since July 2006). The European Parliament has adopted a new resolution on the diesel and petrol quality since 2003 (P5_TA-PROV (2003) 0029). According to this norm, the sulfur content in the fuel should not exceed 10 ppm. In general, most countries of the world had an average permissible sulfur content in transport fuels of about 10 ppm from 2003 to 2010.1−7
In Ukraine, the requirements are not so strict, but the permissible content of sulfur compounds has decreased significantly in recent years. According to various DSTU (Ukrainian state regulatory document), it is allowed to produce DF with sulfur contents of 10 and 500–2000 ppm,8 10, 50, and 350 ppm,9 and 10 and 50 ppm.10
Requirements for the environmental properties of jet fuels (JF) are less stringent both in the world and in Ukraine. Therefore, the aviation contribution to environmental pollution is becoming increasingly significant, as the sulfur content in JF is at the level of 0.1–0.4% by weight (1000–4000 ppm); for special types, a sulfur content of up to 1.0% by weight (10,000 ppm) is allowed.11,12 This is due to the problems of flight safety, energy consumption of jet fuels, and their stability during transportation and storage.13,14 This approach is also due to the fact that the main emissions of JF combustion products occur at high altitudes.
Today, the main fuel purification technology for sulfur compounds (hydrotreating, HDS) has a number of shortcomings, the main ones of which are as follows:
As a result of hydrotreating, along with sulfur compounds, all other heteroatomic substances are removed and hydrogenation of some aromatics occurs; as a result, the purified product has a lower content of substances able to sorb on metal surfaces, and the lubricating properties of fuels become worse.
Compounds of the thiophene series and other condensed sulfur compounds that may be present in the raw material are not hydrotreated due to their structure and the difficulties that arise during their contact with catalysts.
In addition, for economic reasons, hydrotreating cannot be organized at low-capacity production facilities of DF or JF (for example, at the disposal of used plastic and rubber products).
Alternative technologies for desulfurization of straight-run fractions have been studied in detail over the past few decades, including extraction, selective adsorption, biodesulfurization, and oxidative desulfurization (ODS). Among these new processes, noncatalytic oxidative desulfurization (NODS) is seen as one of the promising new methods, and now, it has become more popular. The biggest advantage of NODS compared to the usual HDS process is that it can be carried out in the liquid phase, under relatively mild conditions, and at the same time, there is no need for expensive hydrogen.1,15,16
Current industrial oxidation treatment processes are aimed only at removing mercaptan sulfur or increasing jet fuels’ thermal and oxidative stability after hydrotreating. Most of the ODS processes that continue to be developed and improved over the past 20 years are designed to eliminate the latest of the above shortcomings of HDS (reducing the sulfur content to 10–15 ppm and less in hydrotreated fuels).15−28 These processes, as well as HDS, are relatively complex and require the use of expensive catalysts and oxidants.15−28
We are studying the possibility of noncatalytic processes of oxidative desulfurization of straight-run kerosene and diesel fractions with a high sulfur content, as well as hydrogenates due to their air treatment without the use of expensive catalysts and strong oxidizers. This study is based on processes that proved the possibility of relatively selective oxidation of sulfur of organic combustible minerals (coal) without the use of catalysts and in the presence of H2O.29−36 Previously, there were studies on the main process conditions,37,38 prospects for JF industrial production,38 and DF components with good lubricating properties.39 The proposed technology can also be used for refining oil fuels or regeneration of spent mineral motor oils produced on a small scale, especially when hydrotreating is uneconomical or technologically impossible. Finally, the process can also be used for further treatment of hydrogenates and partial desulfurization of straight-run fractions to obtain fuel components with improved lubricating properties.39−43 The chemistry of the oxidation process of sulfur compounds has also been studied in sufficient detail under proposed conditions.44
The results also showed that oxidation can be carried out in the liquid phase and water presence.45 Water is able to increase the oxidation rate due to better transport of oxygen to the reaction zone46,47 and to show an inhibitory effect on the hydrocarbon oxidation.48−52 Therefore, it is important to investigate how significant the water impact is on NODS processes and to establish the nature of this impact.
2. Experimental Section
2.1. Methods of Experiments and Analyses
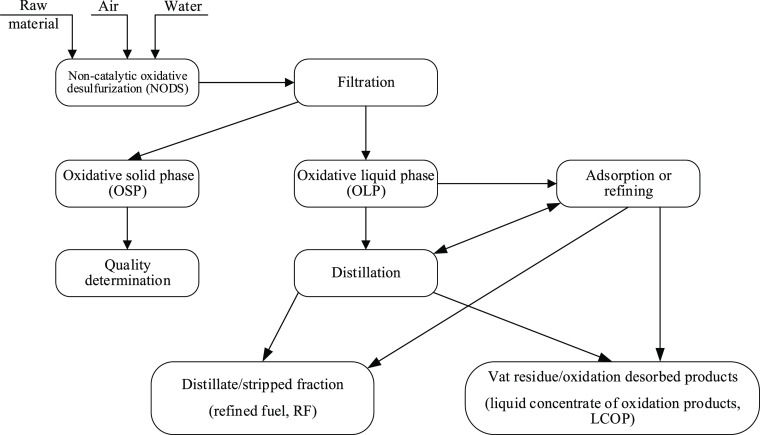
In the case of raw material oxidation in the water presence, first, aqueous phase separation was performed followed by oxidate division and analysis according to the scheme shown in Figure 1.
Figure 1.
Scheme of oxidate division and investigation.
Distillation was carried out by the Engler method. The adsorption process was carried out by the developed method using silica gel as an adsorbent. Benzene was used to desorb the purified product. A mixture of alcohol and benzene was used to desorb the liquid oxidation concentrate.
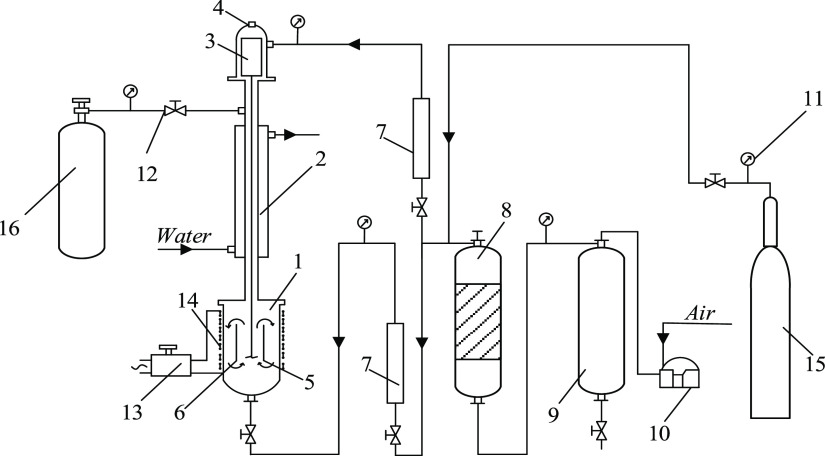
Oxidation was performed in the laboratory. The laboratory installation (see Figure 2) consisted of a reactor, an air compression and purification system, a system for cooling and reduction of gaseous reaction products, and devices for control and measurement of temperature, pressure, and consumption. The main apparatus of the process was a bubble reactor. The reactor design allowed us to maintain a pressure of up to 15 MPa at temperatures of up to 350 °C. Vertical movement of the reaction mixture in the reactor was achieved by installing a cylindrical tube in the reactor vessel. Due to the stirrer operation, the reaction mixture was circulated in the following direction: up along the inner space of the pipe and down into the space between the reactor vessel and the pipe.
Figure 2.
Scheme of laboratory installation of noncatalytic processes of oxidative desulfurization of petroleum fractions: (1), a reactor, (2) a refrigerator, (3) an electric motor, (4) seals, (5) a thermocouple, (6) a pipe, (7) a rotameter, (8,9) receivers, (10) a compressor, (11) manometers, (12) valves, (13) a transformer, (14) an electric heater, (15) a cylinder with nitrogen, and (16) a cylinder for gas collection.
The installation consisted of a reactor section, air compression and purification systems, gaseous reaction products’ cooling and recovery systems, and devices for regulating and measuring process factors. The reaction mixture with a volume of up to 0.7 × 10–3 m3 was loaded into a bubble-type reactor (1) made of stainless-steel X18H10 and equipped with a fitting for air supply down the apparatus and a flange attached to the refrigerator (2). Exhaust air discharged from the reactor passed through the refrigerator (2), in the intertube space of which water was supplied for cooling. The vapor of fuel and water removed from the reactor was mainly condensed in the refrigerator (2). The reactor design allowed us to maintain a pressure of up to 6 MPa at a temperature of 400 °C. Vertical mixing of the reaction mixture in the reactor was achieved due to the cylindrical tube (6) installed in the reactor vessel. If necessary, before the start of the experiment in the reactor (1) from the cylinder (15), nitrogen was fed to remove air and prevent premature oxidation of the reaction mixture. After reaching the set pressure, nitrogen supply was stopped. At the same time, the reaction mixture was heated in the reactor using an electric heater (14). The air in the reactor (1) was forced by the compressor (10) and accumulated in the receiver (9), where it was purified from water and oil. Air was dried in the receiver (8), passing through a layer of silica gel. The air flow rate was measured with a rotameter (7) and regulated by a valve (12). After reaching the set temperature in the reactor (1), air was supplied to the reactor through a valve. The pressure in the reactor (1) was maintained at a given level by selecting part of the oxidation gases through the valve in the cylinder (16), the pressure of which was monitored by a manometer. The temperature of the reaction mixture was measured by a thermocouple (5) and controlled by a laboratory voltage transformer (13).
2.2. Initial Materials
Samples of straight-run kerosene (SRKF) and straight-run diesel (SRDF) fractions with different sulfur contents were taken for research (see Table 1). To increase the concentration of sulfur compounds, SRDF was distilled into narrower ones.
Table 1. Raw Material Characteristics.
| indicator
values for straight-run diesel fractions |
indicator values for the narrow fraction SRDF1 (fraction of >320 °C) | indicator
values for the narrow fraction SRDF2 |
|||||
|---|---|---|---|---|---|---|---|
| indicator name | indicator values for the straight-run kerosene fraction (SRKF) | SRDF1 | SRDF2 | fraction of 165–280 °C | fraction of 280–350 °C | methods of analyses | |
| acidity, mg of KOH per 100 cm3 | 0.7 | 2.8 | 1.8 | 11.5 | 1.6 | 5.9 | GOST ISO 6618-201353 |
| concentration of actual resins, mg per 100 cm3 | 9.0 | 28 | 25 | 49 | 7.5 | 15.0 | GOST 8489-8554 |
| total sulfur content, wt % | 0.151 | 0.250 | 0.671 | 0.671 | 0.323 | 0.869 | DSTU ISO 3012:201855 |
| content of mercaptan sulfur, wt % | 0.0063 | 0.013 | 0.009 | 0.01 | 0.008 | DSTU ISO 3012:201855 | |
| thermooxidative stability (TOS) under static conditions at 150 °C, mg per 100 cm3 | 33.3 | a | a | a | a | a | GOST 11802-8856 |
For diesel fractions, the value of the thermal-ossification stability index is not normalized.
2.3. Methods of Processing Results
The total or mercaptan sulfur removal degree (TRDS or SRDS, respectively) after distillation (adsorption) was determined as changes in the ratio of the sulfur content to its initial content, %:
where Sx0 and Sx are the total sulfur contents (mercaptan), wt %, in the raw material and the purified product, respectively.
To characterize the oxidant movement hydrodynamic parameters and the ability to calculate the main dimensions of the reactor during its simulation (reproduction), the linear rate of oxidant (LRO) and the contact time between the raw material and the oxidant (hereinafter fictitious contact duration) were calculated and recorded. The LRO was calculated as the ratio between the volumetric oxidant flow rate (m3/s) and the reactor cross-sectional area (m2).
To describe the air/raw material ratio, time, and contact area between them, the concept of “volumetric oxidant flow rate” (VOFR, min–1) was used, which is numerically equal to the ratio of oxidant consumption, m3/min, to the volume of raw materials, m3.
IR spectra of the initial kerosene fraction and distillates were obtained on an IR spectrometer brand SPECORD M80. Special KBr cuvettes with absorption layer thicknesses of 0.1 and 1.0 mm were used for analysis.
3. Results and Discussion
Research conditions for studying the water influence on the process of oxidative purification of kerosene and diesel fractions are given in Table 2. The value of process factors is based on previous studies.37−44 Experimental results are presented in Tables 3 and 4.
Table 2. Conditions for Studying the Influence of Water on the Process of Oxidative Purification of Kerosene and Diesel Fractions.
| factor | SRKF | fraction of >320 °C SRDF1 | fraction of 280–350 °C SRDF2 |
|---|---|---|---|
| temperature, °C | 200 | 180 | 180 |
| duration, min | 20 | 30 | 30 |
| VOFR, min–1 | 2.160 | 1.650 | 1.650 |
| LRO, m/s | 0.0026 | 0.0030 | 0.0030 |
| pressure, MPa | 3.0 | 2.5 | 3.0 |
Table 3. Influence of Water on the Yield of Basic Products during Oxidative Purification of Kerosene and Diesel Fractions.
| yield, wt % on raw materials |
||||
|---|---|---|---|---|
| ratio of water/raw materials, vol. | oxidate | OSP | OP (distillate) | LCOP (vat residue) |
| Raw materials—SRKF | ||||
| 0:1 | 98.64 | 2.42 | 92.77 | 3.45 |
| 1:10 | 98.27 | 0.10 | 94.24 | 3.93 |
| Raw materials—fraction of >320 °C SRDF1 | ||||
| 0:1 | 98.49 | 4.21 | 80.95 | 13.33 |
| 1:10 | 97.34 | 0.29 | 85.40 | 11.65 |
| Raw materials—280–350 °C SRDF2 | ||||
| 0:1 | 99.61 | 2.39 | 91.94 | 5.28 |
| 1:10 | 99.17 | 0.64 | 93.38 | 5.15 |
Table 4. Influence of Water on the Quality of Purified Fuel Obtained by Oxidative Purification of Kerosene and Diesel Fractions.
| indicator
name |
||||
|---|---|---|---|---|
| ratio of water/raw materials, vol. | total sulfur content, wt % | CAG, mg per 100 cm3 | acidity, mg of KOH per 100 cm3 | TRDS, % |
| Raw materials—SRKF | ||||
| 0:1 | 0.059 | 3.8 | 75.2 | 60.93 |
| 1:10 | 0.058 | 2.0 | 33.8 | 61.59 |
| Raw materials—fraction of >320 °C SRDF1 | ||||
| 0:1 | 0.394 | 45 | 39.6 | 41.28 |
| 1:10 | 0.383 | 39 | 22.8 | 42.92 |
| Raw materials—280–350 °C SRDF2 | ||||
| 0:1 | 0.654 | 41 | 10.5 | 24.74 |
| 1:10 | 0.617 | 26 | 7.3 | 29.00 |
The obtained results indicate that the process of oxidation purification without water is accompanied by the formation a significant amount of an insoluble solid phase and therefore low distillate yield (see Table 3). At the same time, the addition of water to the reaction mixture increases the oxidative purified fuel (OPF) yield and also sharply (4–24 times) reduces the oxidative solid phase (OSP) amount.
The oxidation degree reduction of the hydrocarbon part is confirmed by the presence of a lower number of oxidation intermediate compounds (substances that form actual gums and organic acids, see Table 4).
During oxidation purification in the presence of water, there is generally some reduction in the oxidate amount. This is explained, apparently, by the fact that more raw materials evaporate (see Figure 3); part of the formed oxidation products, primarily acids, are soluble in the aqueous phase. The latter is confirmed by the experimental data: the aqueous phase obtained by SRKF oxidation at a water/raw material ratio of 1:5 was characterized in an acidic reaction (the pH value was 2; acidity of 330 mg of KOH per 100 cm3).
Figure 3.
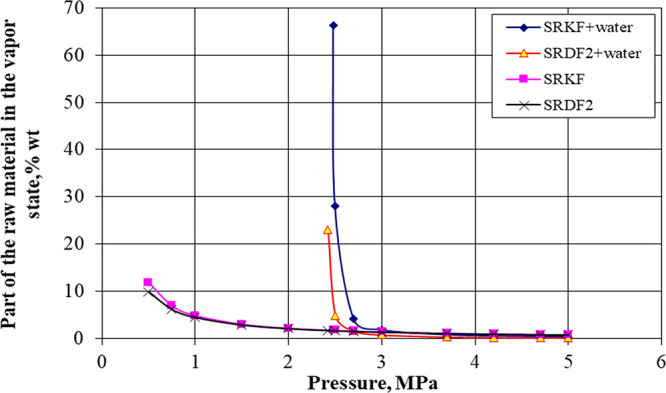
Dependence of the part of raw materials in the vapor state at 200 °C on pressure.
On the other hand, the water presence does not have any negative impact on the conversion intensity and the sulfur compound removal degree: the TRDS even increases slightly, which is due to the increased yield of purified fuel.
All of the above indicates a positive effect of water on the oxidation of middle distillate petroleum fractions (MDPF), as it increases the process selectivity.
The above studies indicate the feasibility of using water during MDPF oxidative purification. At the same time, the influence of water quantity on the main characteristics of oxidative purification of liquid fuels has not been studied. Conditions for studying the influence of the ratio of water/raw materials are given in Table 5. The obtained results are given in Tables 6 and 7 and Figure 4. As a result of the separation of the oxidative liquid phase (OLP) by distillation, the end temperatures of distillation were as follows: for the kerosene fraction and the fr. of 165–280 °C SRDF2, 280 °C; for the fr. of 280–350 °C SRDF2, 350 °C.
Table 5. Conditions for Studying the Influence of the Water/Raw Material Ratio on the Process of Oxidative Purification of Diesel and Kerosene Fractions.
| factor | SRKF | diesel fractions |
|---|---|---|
| temperature, °C | 200 | 180 |
| duration, min | 20 | 30 |
| VOFR, min–1 | 2.160 | 1.650 |
| LRO, m/s | 0.0026 | 0.0030 |
| pressure, MPa | 3.0 | 3.0 |
Table 6. Influence of the Water/Raw Material Ratio on the Yield of Basic Products during Oxidative Purification of Diesel and Kerosene Fractions.
| yield, wt % on raw materials |
||||
|---|---|---|---|---|
| ratio of water/raw materials, vol. | oxidate | OSP | OP (distillate) | LCOP (vat residue) |
| SRKF | ||||
| 0:1 | 98.64 | 2.42 | 92.77 | 3.45 |
| 1:10 | 98.27 | 0.10 | 94.24 | 3.93 |
| 1:5 | 98.96 | 0.10 | 94.84 | 4.02 |
| 1:3 | 99.03 | 0.10 | 94.94 | 3.99 |
| 1:2 | 98.13 | 0.09 | 94.23 | 3.81 |
| 1:1.5 | 97.51 | 0.09 | 93.76 | 3.66 |
| 1:1 | 96.87 | 0.02 | 93.29 | 3.56 |
| 1,5:1 | 96.27 | 0.02 | 92.75 | 3.50 |
| 2:1 | 96.08 | 0.02 | 92.61 | 3.45 |
| Fraction of 165–280 °C SRDF2 | ||||
| 0:1 | 98.66 | 1.61 | 92.90 | 4.15 |
| 1:10 | 99.08 | 0.44 | 95.11 | 3.53 |
| 1:5 | 98.44 | 0.21 | 95.02 | 3.21 |
| 1:2.5 | 97.85 | 0.13 | 94.81 | 2.91 |
| Fraction of 280–350 °C SRDF2 | ||||
| 0:1 | 99.61 | 2.39 | 91.94 | 5.28 |
| 1:10 | 99.17 | 0.64 | 93.38 | 5.15 |
| 1:5 | 98.93 | 0.28 | 93.77 | 4.88 |
| 1:2.5 | 98.44 | 0.23 | 93.64 | 4.57 |
| 1:1 | 98.22 | 0.21 | 93.57 | 4.44 |
| 2:1 | 98.34 | 0.21 | 93.45 | 4.68 |
Table 7. Influence of the Water/Raw Material Ratio on the Quality of Purified Fuel Obtained by Oxidative Purification of Diesel and Kerosene Fractions.
| indicator
name |
|||||
|---|---|---|---|---|---|
| ratio of water/raw materials, vol. | total sulfur content, wt % | mercaptan sulfur content, wt % | CAG, mg per 100 cm3 | acidity, mg of KOH per 100 cm3 | TOS, mg per 100 cm3 |
| SRKF | |||||
| 0:1 | 0.059 | 0.0012 | 3.8 | 75.2 | 19.6 |
| 1:10 | 0.058 | 0.0017 | 2.0 | 33.8 | 13.4 |
| 1:5 | 0.057 | 0.0017 | 2.0 | 29.5 | 11.5 |
| 1:3 | 0.055 | 0.0017 | 1.9 | 27.8 | 11.4 |
| 1:2 | 0.053 | 0.0016 | 1.8 | 26.1 | 11.3 |
| 1:1.5 | 0.052 | 0.0015 | 1.7 | 24.3 | 11.3 |
| 1:1 | 0.051 | 0.0015 | 1.5 | 22.4 | 11.2 |
| 1,5:1 | 0.050 | 0.0015 | 1.0 | 20.9 | 11.0 |
| 2:1 | 0.052 | 0.0016 | 1.0 | 18.4 | 9.9 |
| Fraction of 165–280 °C SRDF2 | |||||
| 0:1 | 0.175 | 6.3 | 5.4 | ||
| 1:10 | 0.160 | 5.1 | 1.4 | ||
| 1:5 | 0.154 | 4.5 | 1.1 | ||
| 1:2.5 | 0.187 | 5.0 | 1.0 | ||
| Fraction of 280–350 °C SRDF2 | |||||
| 0:1 | 0.654 | 41 | 10.5 | ||
| 1:10 | 0.617 | 26 | 7.3 | ||
| 1:5 | 0.618 | 27 | 5.1 | ||
| 1:2.5 | 0.625 | 22 | 4.9 | ||
| 1:1 | 0.650 | 20 | 4.1 | ||
| 2:1 | 0.671 | 18 | 3.4 | ||
Figure 4.
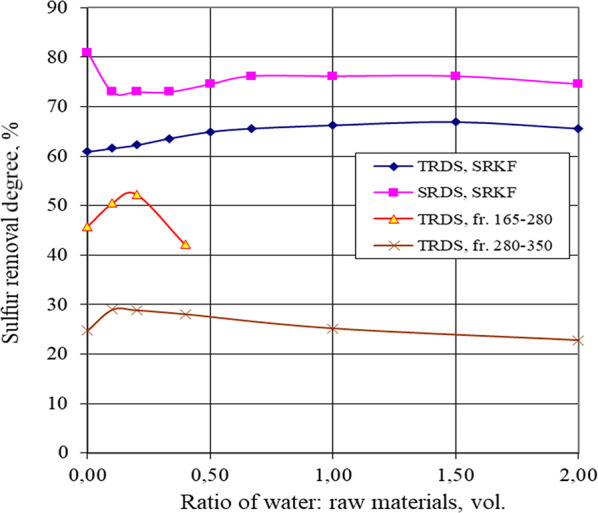
Dependence of TRDS and SRDS on the ratio of water/raw materials.
The obtained results confirm that adding water to the reaction medium and increasing its amount slow down oxidation reactions and hydrocarbon medium condensation in the distillate. The amounts of the OSP and the liquid concentrate of oxidation products (LCOP) and the presence of incomplete oxidation products in the distillate (fuel acidity, actual gums, and TOS) are reduced.
It should be noted that in the distillates obtained by oxidation without water, for several days, the thermal and oxidative stability significantly deteriorated and the actual resin content increased (2–3 times). Purified fuels obtained in the water presence were devoid of this disadvantage. Therefore, if the oxidation was carried out without water, then, in the future, in the purified fuel, there is a continued process of combining their existing intermediate oxidation products.
The yields of oxidate and purified fuel with an increasing amount of water fed into the reaction medium are maximum. This is due to the fact that with the addition of small amounts of water, the yields of these products increase (compared to the case when the purification is carried out without H2O) by reducing the amount of oxidation products. At high water/raw material ratios (1:2.5 and higher), the distillate and OLP yields begin to decrease due to losses of raw materials with water vapor and the dissolution of oxidation products in the aqueous phase.
Small portions of water have almost no effect on the sulfur compound oxidation rate: the sulfur removal degree (SRD), with both total and mercaptan almost unchanged and with the addition of the first portions of water, even slightly increases, apparently due to the increased yield of purified products and improved selectivity of extraction oxidation products. A further increase in the amount of water leads to a slight inhibition of the sulfur compound conversion rate. If raw materials with a lower sulfur content are taken, then more water can be used: the maximum SRD for SRKF (sulfur content in the raw material, St0 = 0.151 wt %) is the water/raw material ratio of 1.5:1; for the fraction of 165–280 °C (St0 = 0.323 wt %), 1:5; for the fraction of 280–350 °C (St0 = 0.869 wt %), 1:10.
A further research task was to establish the inhibition caused by water on the hydrocarbon oxidation reactions and the oxidation degree for sulfur-containing raw materials under the process conditions.
It is well-known that liquid-phase oxidation of hydrocarbons with molecular oxygen occurs via a radical-chain mechanism through the stage of peroxide formation.48,57 The rate of this reaction can be reduced in three ways:
reducing the initiation rate (reducing the rate of peroxide formation);
reducing the intensity of chain branching (reducing the decomposition rate of peroxides into free radicals);
reducing the growth rate of chains (deactivation of free radicals or reducing their reactivity).
The inhibition mechanism of hydrocarbon oxidation reactions with water is explained differently in the literature, but the fact is that in the presence of H2O, there is a decrease in the intensity of hydrocarbon oxidation by reducing the rate of radical formation and their deactivation.
The authors of refs (51) and (52) claim that in the presence of surfactants (sulfur-, oxygen-, and nitrogen-containing compounds) in the hydrocarbon environment, peroxides with surface activity create hydrogen bonds with water and further participate in the micelle formation at the hydrocarbon/water boundary. The peroxides involved in the micelle formation become more stable, the rate of their decomposition with the formation of free radicals decreases, and, as a consequence, the total rate of hydrocarbon oxidation reactions also decreases. In ref (58), it was reported that in the presence of water, the total oxidation rate decreases due to the formation of cyclic hydroperoxide dimers, which decompose at a lower rate than simple peroxides.
In ref (59), it was reported that the effect of water on toluene oxidation with air oxygen can be explained by the formation of free radical/water complexes (C6H5CH2·H2O), reducing the conversion rate of this radical.
In other words, the abovementioned works indicate that water has an inhibitory effect on the liquid hydrocarbon oxidation due to the formation of complexes with peroxides or hydrocarbon radicals.
It is also known that, depending on the reaction medium pH, the decomposition directions of hydroperoxides and peroxides and, accordingly, the composition of the formed products may be different. In a neutral medium at high temperatures, as already mentioned, the process proceeds by a radical mechanism. Studies based on individual hydrocarbons60,61 show that primary hydroperoxides mainly decompose to form acids; secondary—alcohols, ketones, acids, and hydrocarbons; tertiary—alcohols (phenols) and acetone.
In the oxidation process without water, there is mainly the formation of primary and secondary peroxides, the decomposition of which leads to the formation of acids and unsaturated compounds that are sources of condensation products (solid phase, LCOP, resins, etc.).
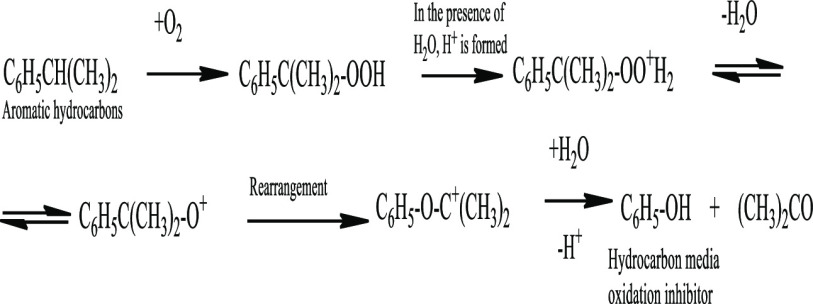
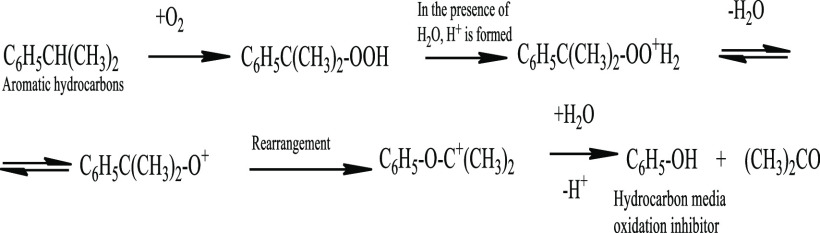
Reactions in an acidic medium can proceed by an ionic mechanism, which may involve the rearrangement of intermediate products and formation of tertiary peroxides, the decomposition of which leads to the formation of alcohols and phenols. Mechanism studies of isopropylbenzene oxidation (see Figure 5) have shown that in an acidic medium, the decomposition of tertiary peroxide leads to the formation of phenol and acetone.61
Figure 5.
Formation of hydrocarbon oxidation inhibitors (phenol) in the presence of water (on the example of isopropylbenzene).
It should be noted that according to ref (61), only strong acids affect the change in the direction of peroxide formation. During oxidation without water, organic acids are formed, which practically do not dissociate in the hydrocarbon medium. Organic acids in alcohols, acids, and aldehydes dissociate rather weakly, and the presence of water significantly increases the degree of dissociation. For example, for benzoic acid, the dissociation constant (pKa) in water is 4.18; in alcoholic solution of cyclohexane, 10.24; in meta-cresol, 10.29; in acetophenone, 9.76.62 It should also be noted that the pKa of sulfur-containing organic acids (possible oxidation products of sulfur compounds) in aqueous solution are very close to the pKa of inorganic acids. For example, for phenolsulfonic acid (HOC6H4SO3H), pKa = 0.39; for HNO3, pKa = −1.64.63
It can be assumed that in the presence of water, intermediate oxidation products (acids, hydroxy acids, and sulfonic acids) obtained at the beginning of the raw material oxidation treatment partially pass from the hydrocarbon medium into the aqueous phase. This fact is confirmed by experimental results: the aqueous phase obtained by oxidation of SRKF at the water/raw material ratio of 1:5 had an acidity of 330 mg of KOH per 100 cm3, which corresponds to a pH value of 2 (the initial distilled water pH was about 6).
Thus, the water addition to the reaction medium leads to the dissociation (“increases” the strength) of organic acids formed at the initial stage of oxidation. As a result, intermediate rearrangement with the tertiary peroxide formation becomes possible; some oxidation reactions proceed according to the scheme shown in Figure 5, with the formation of phenols and/or higher alcohols, the presence of which in the hydrocarbon medium, even in small amounts (0.0005–0.1 wt %), significantly inhibits the oxidation rate of hydrocarbons.63−65 That is, the inhibitory effect of water is shown not only in the stages of growth and chain branching, as described by many authors, but also due to a partial change in the oxidation mechanism.
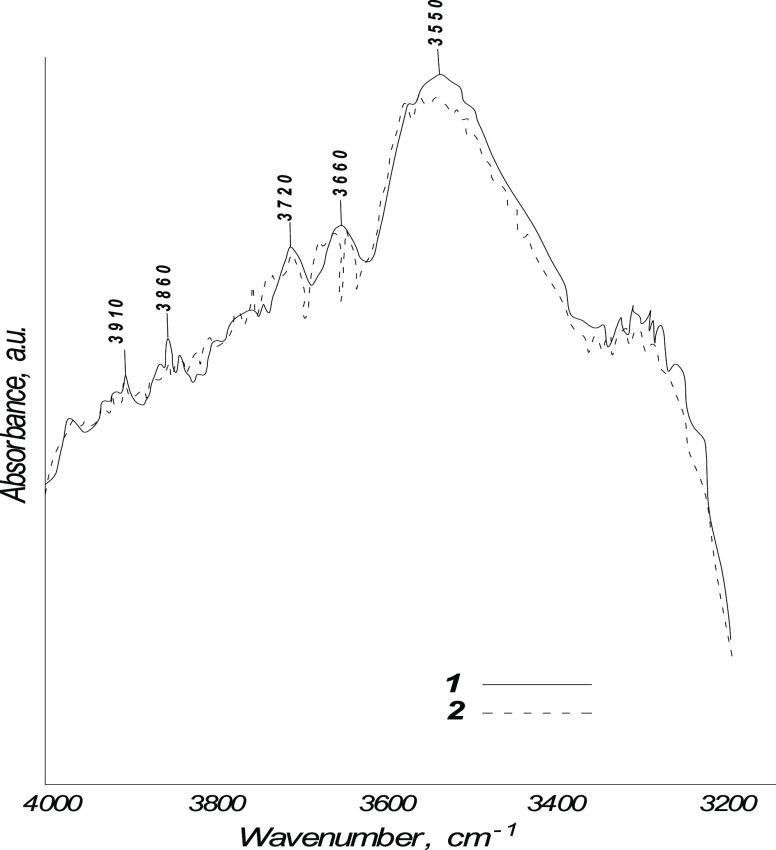
To confirm the formation of phenolic compounds or tertiary alcohols in the presence of water, the IR spectra of distillates (purified fuel) obtained from SRKF with and without water were compared in the absorption range of 4050–3200 cm–1. The IR spectrum of the distillate obtained in the presence of water confirms the presence of phenol, its derivatives, or tertiary alcohols: there are noticeable peaks at 4030, 3910, 3860, 3720, 3660, and about 3550 cm–1 (see Figure 6). The relative peak heights in these regions of the IR spectrum of the distillate obtained without water are much smaller (some peaks are absent).
Figure 6.
IR spectra of purified fuels (distillates) obtained from SRKF with and without water, in the absorption range of 4050–3200 cm–1: (1) water/raw material ratio (vol.) of 1:10; (2) without water.
It should also be noted that the oxidation products of the hydrocarbon medium dissolved in water also help to reduce their amount in the oxidate and subsequently in the distillate.
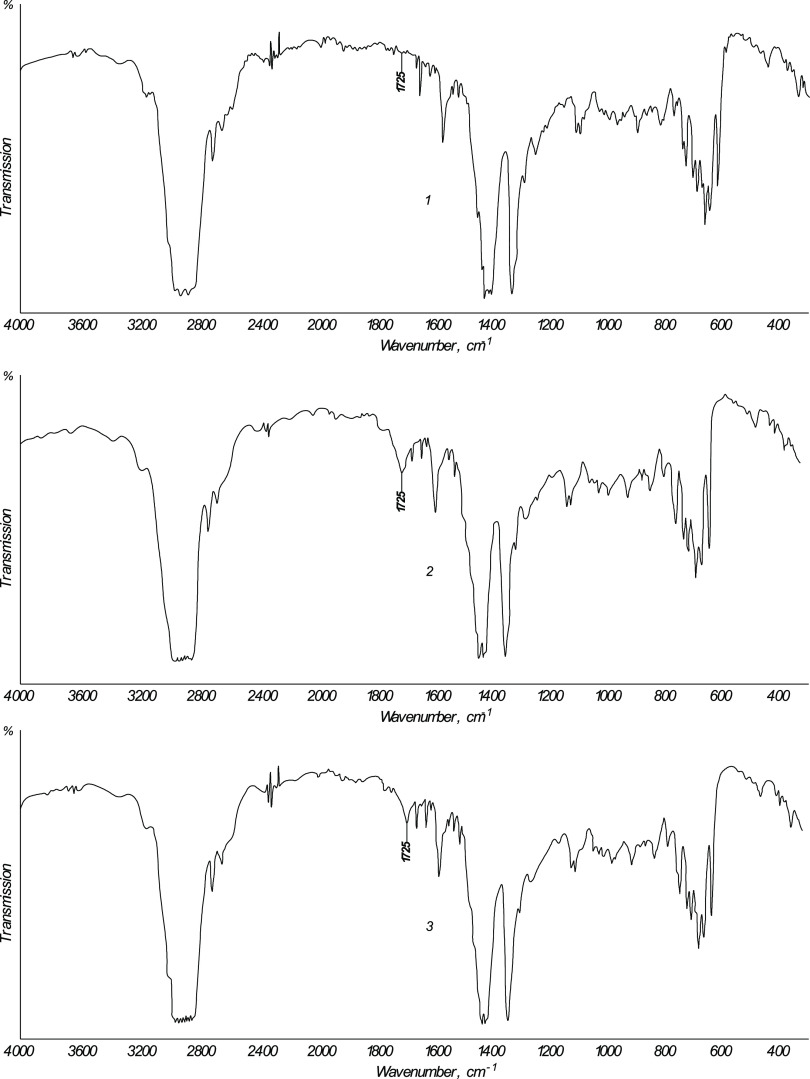
The decrease in the content of intermediate oxidation products of the hydrocarbon medium with the water addition is confirmed by IR spectroscopy of two distillates obtained by SRKF purification without water and in its presence (the volume ratio of water/raw material was 1:10), which are presented in Figure 7. The IR spectrum of the raw material (SRKF) is also presented there. IR spectra in all three cases confirm the presence of characteristic groups for kerosene fractions, in almost the same quantities: C–H–arene (primary and secondary), C–H–aliphatic, C=C–arene, −CH3, and so on. The IR spectrum of the distillate obtained by oxidation without water has a peak at 1725 cm–1, which characterizes carbonyl or carboxyl groups. In the IR spectrum of the distillate obtained by SRKF oxidation in the water presence, in the same absorption region, the peak value of the corresponding carbonyl and carboxyl groups is 2–2.5 times lower, which undoubtedly indicates a sharp decrease in the amount of oxidate compounds in the distillate.
Figure 7.
IR spectra of the initial SRKF and purified fuels (distillates) obtained from it with and without water: (1) SRKF; (2) without water; (3) water/raw material ratio (vol.) of 1:10.
4. Conclusions
The water presence in the reaction medium has a positive effect on the process and reduces the oxidation intensity of the hydrocarbon medium. This is due to both the slowing down of water oxidation reactions in the stages of peroxide decomposition and chain growth and branching and a partial change in the chemistry of oxidation with the formation of phenols and/or tertiary alcohols, which are inhibitors of oxidation reactions.
Since increasing the amount of water fed to the reactor reduces its productivity by raw materials or needs to increase its size, the optimal volume ratio of water/raw materials can be considered to be 1:10–1:2.5.
Glossary
Abbreviations
- DF
diesel fuel
- JF
jet fuel
- DSTU, GOST
State standard of Ukraine
- HDS
hydrotreating
- (N)ODS
(noncatalytic) oxidative desulfurization
- SRKF
straight-run kerosene fraction (fuel)
- SRDF
straight-run diesel fraction (fuel)
- LRO
linear rate of the oxidant
- VOFR
volumetric oxidant flow rate
- TRDS
total sulfur removal degree
- SRDS
sour sulfur removal degree
- OPF
oxidative purified fuel
- OSP
oxidative solid phase
- MDPF
middle distillate petroleum fraction
- OLP
oxidative liquid phase
- LCOP
liquid concentrate of oxidation products
- TOS
thermooxidative stability
- CAG
concentration of actual gums
The authors declare no competing financial interest.
References
- Ismagilov Z.; Yashnik S.; Kerzhentsev M.; Parmon V.; Bourane A.; Al-Shahrani F. M. Oxidative Desulfurization of Hydrocarbon Fuels. Cat. Rev. Sci. Eng. 2011, 53, 199. 10.1080/01614940.2011.596426. [DOI] [Google Scholar]
- U.S.EPA . 2000. Regulatory Impact Analysis: Heavy-Duty Engine and Vehicle Standards and Highway Diesel Fuel Sulfur Control Requirements. EPA 420-R-00-026. December.
- Directive 98/70/EC of the European Parliament and of the Council of 13 October 1998 relating to the quality of petrol and diesel fuels and amending Council Directive 93/12/EEC.
- Directive 2009/30/EC of the European Parliament and of the Council of 23rd April 2009 amending Directive 98/70/EC as regards the specification of petrol, diesel and gas-oil and introducing a mechanism to monitor and reduce greenhouse gas emissions and amending Council Directive 1999/32/EC as regards the specification of fuel used by inland waterway vessels and repealing Directive 93/12/EEC.
- EN 590:2009. Automotive fuels. Diesel. Requirements and test methods.
- https://www.epa.gov/sites/production/files/2015-08/documents/peg.pdf
- Worldwide Fuel Charter. Fourth Edition. September 2006. https://www.oica.net/wp-content/uploads/2007/06/wwfc-fourth-edition-sep-2006.pdf .
- DSTU 8705:2017. Diesel fuel for long-term storage. Specification.
- DSTU 7688:2015. Diesel fuel EURO. Specification.
- DSTU 4840:2007. Diesel fuel of improved quality. Specification.
- Link D. D.; Baltrus J. P.; Rothenberger K. S.; Zandhuis P.; Minus D. K.; Striebich R. C. Class- and Structure-Specific Separation, Analysis, and Identification Techniques for the Characterization of the Sulfur Components of JP-8 Aviation Fuel. Energy Fuels 2003, 17, 1292–1302. 10.1021/ef0300747. [DOI] [Google Scholar]
- http://large.stanford.edu/courses/2017/ph240/chhoa1/docs/exxon-2008.pdf
- Peckham J. FT jet fuel study may aid lower emissions, Avoid Performance Problems. Gas-to-Liquids News. 2000, 3, 20. [Google Scholar]
- Peckham J. Low-sulfur jet fuel study aims to avoid problems. Diesel Fuel News. 2001, 5, 1. [Google Scholar]
- Timko M. T.; Schmois E.; Patwardhan P.; Kida Y.; Class C. A.; Green W. H.; Nelson R. K.; Reddy C. M. Response of Different Types of Sulfur Compounds to Oxidative Desulfurization of Jet Fuel. Energy Fuels 2014, 28, 2977–2983. 10.1021/ef500216p. [DOI] [Google Scholar]
- Samaniego M. L.; De Luna M. G.; Ong D. C.; Wan M.; Lu M. Isotherm and Thermodynamic Studies on the Removal of Sulfur from Diesel Fuel by Mixing-Assisted Oxidative–Adsorptive Desulfurization Technology. Energy Fuels 2019, 33, 1098–1105. 10.1021/acs.energyfuels.8b04242. [DOI] [Google Scholar]
- Zou J.; Lin Y.; Wu S.; Zhong Y.; Yang C. Molybdenum Dioxide Nanoparticles Anchored on Nitrogen-Doped Carbon Nanotubes as Oxidative Desulfurization Catalysts: Role of Electron Transfer in Activity and Reusability. Adv. Funct. Mater. 2021, 31, 2100442. 10.1002/adfm.202100442. [DOI] [Google Scholar]
- Zou J.; Lin Y.; Wu S.; Wu M.; Yang C. Construction of bifunctional 3-D ordered mesoporous catalyst for oxidative desulfurization. Sep. Purif. Technol. 2021, 264, 118434. 10.1016/j.seppur.2021.118434. [DOI] [Google Scholar]
- Mohumed H.; Rahman S.; Imtiaz S. A.; Zhang Y. Oxidative-Extractive Desulfurization of Model Fuels Using a Pyridinium Ionic Liquid. ACS Omega 2020, 5, 8023–8031. 10.1021/acsomega.0c00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.-J.; Li F.-T. Oxidative Desulfurization of Model Gasoline Over Modified Titanium Silicalite. Pet. Sci. Technol. 2015, 33, 196. 10.1080/10916466.2014.974817. [DOI] [Google Scholar]
- Zhang T.; Chen X.; Ding B.; Ding Z.; Wang Y.; Qiu H.; Jiang Y.; Dai S.; Hou Z. Deep Oxidative Desulfurization of Model Fuels Catalyzed by Subnanosized Ti Oxoclusters. Energy Fuels 2022, 36, 1402–1416. 10.1021/acs.energyfuels.1c03766. [DOI] [Google Scholar]
- Tian Y.; Yao Y.; Zhi Y.; Yan L.; Lu S. Combined Extraction–Oxidation System for Oxidative Desulfurization (ODS) of a Model Fuel. Energy Fuels 2015, 29, 618–625. 10.1021/ef502396b. [DOI] [Google Scholar]
- Mirante F.; Alves A.; Julião D.; Almeida P. L.; Gago S.; Valença R.; Ribeiro J. C.; De Castro B.; Granadeiro C. V.; Balula S. S. Large-pore silica spheres as support for samarium-coordinated undecamolybdophosphate: Oxidative desulfurization of diesels. Fuel 2020, 259, 116213. 10.1016/j.fuel.2019.116213. [DOI] [Google Scholar]
- Jatav S.; Srivastava V. C. Ce/Al2O3 as an Efficient Catalyst for Oxidative Desulfurization of Liquid Fuel. Pet. Sci. Technol. 2019, 37, 633. 10.1080/10916466.2018.1560323. [DOI] [Google Scholar]
- Mokhtar W. N. A. W.; Bakar W. A. W. A.; Abdullah W. N. W.; Toeman S.; Rosid S. J. M. Role of Mn/Al2O3 Catalyst in Deep Oxidative Desulfurization of Diesel. Pet. Sci. Technol. 2018, 36, 1741. 10.1080/10916466.2018.1511581. [DOI] [Google Scholar]
- Wang B.; Dai B.; Kang L.; Zhu M. Synthesis of three-dimensional ordered mesoporous W-doped KIT-6 for oxidative desulfurization catalyst of fuels. Fuel 2020, 265, 117029. 10.1016/j.fuel.2020.117029. [DOI] [Google Scholar]
- Akopyan A. V.; Shlenova A. O.; Cherednichenko K. A.; Polikarpova P. D. Immobilized Multifunctional Ionic Liquids for Highly Efficient Oxidation of Sulfur-Containing Compounds in Model Fuels. Energy Fuels 2021, 35, 6755–6764. 10.1021/acs.energyfuels.1c00172. [DOI] [Google Scholar]
- Du X.; Liu J.; Chen H.; Zhang Z. Study on the Electrochemical Oxidation Desulfurization Behavior of Model Diesel on Anodic Alumina Oxide and Ceria Nanotubes. Energy Fuels 2018, 32, 2612–2621. 10.1021/acs.energyfuels.7b03629. [DOI] [Google Scholar]
- Hayvanovych V.; Pysh’yev S. Desulfurization of low-rank coal with high sulfur content is the first stage of coal burning at heat electric stations. Energy Fuels 2003, 17, 1186–1190. 10.1021/ef0202945. [DOI] [Google Scholar]
- Pysh’yev S.; Shevchuk K.; Chmielarz L.; Kuśtrowski P.; Pattek-Janczyk A. Effect of the water-vapor content on the oxidative desulfurization of sulfur-rich coal. Energy Fuels 2007, 21, 216–221. 10.1021/ef060251e. [DOI] [Google Scholar]
- Pysh’yev S.; Gunka V.; Astakhova A.; Prysiazhnyi P.; Bratychak M. Effect of coal quality on its desulphurization 1. Influence of the organic matter. Chem. Chem. Technol. 2012, 6, 443–450. 10.23939/chcht06.04.443. [DOI] [Google Scholar]
- Pysh’yev S.; Gunka V.; Prysiazhnyi Y.; Shevchuk K.; Pattek-Janczyk A. Study of oxidative desulphurization process of coal with different metamorphism degrees. Ranliao Huaxue Xuebao 2012, 40, 129–137. 10.1016/S1872-5813(12)60009-7. [DOI] [Google Scholar]
- Pyshyev S.; Prysiazhnyi Y.; Miroshnichenko D.; Bilushchak H.; Pyshyeva R. Desulphurization and usage of medium-metamorphized black coal. 1. Determination of the optimal conditions for oxidative desulphurization. Chem. Chem. Technol. 2014, 8, 225–234. 10.23939/chcht08.02.225. [DOI] [Google Scholar]
- Pyshyev S.; Prysiazhnyi Y.; Shved M.; Kulazynski M.; Miroshnichenko D. Effect of hydrodynamic parameters on the oxidative desulphurization of low rank coal. Int. J. Coal Sci. Technol. 2018, 5, 213–229. 10.1007/s40789-018-0205-6. [DOI] [Google Scholar]
- Gunka V.; Shved M.; Prysiazhnyi Y.; Pyshyev S.; Miroshnichenko D. Lignite oxidative desulphurization: notice 3 – process technological aspects and application of products. Int. J. Coal Sci. Technol. 2019, 6, 63–73. 10.1007/s40789-018-0228-z. [DOI] [Google Scholar]
- Miroshnichenko D. V.; Nazarov V. N.; Nikolaichuk Y. V. Influence of an Oxidant on the Ignition of Coals. Solid Fuel Chem. 2020, 54, 318–325. 10.3103/S0361521920020093. [DOI] [Google Scholar]
- Pysh’yev S.; Lazorko O.; Bratychak M. Oxidative Processing of Light Oil Fractions. A Review. Chem. Chem. Technol. 2009a, 3, 77. 10.23939/chcht03.01.077. [DOI] [Google Scholar]
- Lazorko O.; Pysh’yev S.; Bratychak M. Investigation of straight-run diesel oil fractions with sulfur high content oxidative desulphurization. Chem. Chem. Technol. 2008, 2, 309. 10.23939/chcht02.04.309. [DOI] [Google Scholar]
- Paniv P.; Pysh’yev S.; Gaivanovych V.; Lazorko O. Noncatalytic oxidation desulfurization of the kerosene cut. Chem. Tech. Fuels Oils. 2006, 42, 159–166. 10.1007/s10553-006-0049-4. [DOI] [Google Scholar]
- Pysh’yev S. Application of Non-catalytic Oxidative Desulphurization Process for Obtaining Diesel Fuels with Improved Lubricity. Chem. Chem. Technol. 2012, 6, 229–235. 10.23939/chcht06.02.229. [DOI] [Google Scholar]
- Korchak B.; Grynyshyn O.; Chervinskyy T.; Shapoval P.; Nagurskyy A. Thermooxidative Regeneration of Used Mineral Motor Oils. Chem. Chem. Technol. 2020, 14, 129–134. 10.23939/chcht14.01.129. [DOI] [Google Scholar]
- Pysh’yev S.; Bratychak M. Study on Hydrodynamic Parameters of the Oxidative Desulfurization of High Sulfur Straight-Run Oil Fractions. Chem. Chem. Technol. 2020, 14, 403–411. 10.23939/chcht14.03.403. [DOI] [Google Scholar]
- Pyshyev S.; Korchak B.; Miroshnichenko D.; Nyakuma B. B. Study on Chemistry of Oxidative Desulfurization Process of High Sulfur Straight-Run Oil Fraction. Chem. Chem. Technol. 2021, 15, 414–422. 10.23939/chcht15.03.414. [DOI] [Google Scholar]
- Pysh’yev S.; Lazorko O.; Bratychak M. Temperature and Water Effect on the Oxidative Desulphurization of Straight-run Diesel Fraction with a High Sulphur Content. Chem. Chem. Technol. 2009b, 3, 163–168. 10.23939/chcht03.02.163. [DOI] [Google Scholar]
- Gaivanovych V.Investigation of the oxidation of high-molecular oil compounds by atmospheric oxygen in a water-emulsion medium and in a benzene solution. Dissertation of candidate of technical sciences 05.17.07. Lviv, 1980, p 225.
- Antonyshyn V.I.; Humeneckyj V.V.. Chemistry and Chemical Technology. LPI:Lviv, 1974, pp. 94–100.
- Denisov E.T.; Afanas’ev I.B.. Oxidation and Antioxidants in Organic Chemistry and Biology. CRC Press, Boca Raton, FL: 33487, 2005. [Google Scholar]
- Frankel E.N.Lipid Oxidation, 2nd Ed. Glasgow, The Oily Press, 2005. [Google Scholar]
- Kamal-Eldin A.Lipid Oxidation Pathways. Champain, Illinois: AOCS PRESS, 2003. [Google Scholar]
- Yasuhiro S.; Tomoko N.; Takayuki H.; Isao K. A novel methodology towards deep desulfurization of light oil effected by sulfimides formation. Chem. Commun. 2001, 14, 1256. [Google Scholar]
- Csanyi L. J.; Jaky K. Characteristics of cationic phase-transfer catalysts in the oxidation of hydrocarbons by O2. Phys. Chem. Chem. Phys. 2001, 3, 2018. 10.1039/b009145f. [DOI] [Google Scholar]
- Kellerby S. S.; McClements D. J.; Decker E. A. Role of proteins in oil-in-water emulsions on the stability of lipid hydroperoxides. J. Agric. Food Chem. 2006, 54, 7879–7884. 10.1021/jf061340s. [DOI] [PubMed] [Google Scholar]
- GOST ISO 6618-2013. Petroleum products and lubricants. Determination of acid and base numbers by titration with a color indicator.
- GOST 8489–85. Engine fuel. Method for determining actual resins (according to Budarov).
- DSTU ISO 3012:2018. Petroleum products. Determination of thiol (mercaptan) sulfur in light and middle distillate fuels. Potentiometric method.
- GOST 11802–88. Fuel for jet engines. Method for determining thermal-oxidative stability under static conditions.
- Emannuel N.M.; Denisov E.T.; Maizus Z.K.. Chain reactions of hydrocarbon oxidation in the liquid phase. Moskow, Science, 1965, p 291.
- Csanyi L. J.; Jaky K.; Kiss J. T. Effects of water and onium ion phase-transfer catalyst on the rate of liquid-phase oxidation of hydrocarbons. J. Mol. Cat. 1993, 80, 353–364. 10.1016/0304-5102(93)85008-H. [DOI] [Google Scholar]
- Crytko M. P.; Bub G. K. Oxidation of toluene by cobalt (III) acetate in acetic acid solution. Influence of water. Ind. Eng. Chem. Prod. Res. Dev. 1981, 20, 481–486. [Google Scholar]
- Rao T. S. S.; Awasthi E.-J. Oxidation of alkylaromatics. Chem. Jan. 2007, 4, 1–13. [Google Scholar]
- Kruzhalov B.D.; Golovanenko B.I.. Co-production of phenol and acetone. Moscow, State Scientific and Technical Publishing House of Chemical Literature, 1963, p 197. [Google Scholar]
- Chemist’s Handbook. Leningrad, Chemistry, 1968, pp. 1–6. [Google Scholar]
- Kuliev A.M.Chemistry and technology of additives to oils and fuels. Moskow, Science, 1972, p 358. [Google Scholar]
- Neiland O.Y.Organic chemistry. Moscow, High school, 1990, p 750.
- Gureev A.A.; Fuchs I.H.; Lashchi V.L.. Chemotology. Moskow, Science, 1986, p 366. [Google Scholar]