Abstract
Garcinia cambogia (Gaertn.) Desr. (known as Malabar tamarind) is a popular traditional herbal medicine and is one of the well-known folk medicines reported for the treatment of obesity and incorporated in several nutraceuticals worldwide. These effects are mediated by a myriad of bioactive compounds with most effects attributed to its hydroxy citric acid (HCA) content. This review aims to present a holistic overview on novel trends in the production of G. cambogia bioactive components and how extraction optimization is important to ensure best product quality with its reported nanoformulations with particular emphasis on HCA content. Further, an overview of the different analytical approaches used for quality control assessment of G. cambogia plant and its nutraceuticals is presented highlighting both advantages and limitations. Moreover, analytical approaches for detecting G. cambogia metabolites in biological fluids with emphasis on HCA level to determine its pharmacokinetics and proof of efficacy are presented for the first time.
Introduction
Garcinia genus, a member of family Clusiaceae, comprises more than 300 species. They are native to Asia and Africa and are commonly called sap trees, kokum, Garcinias or mangosteens, and monkey fruits. Garcinia plants, besides being ornamental, find industrial pharmaceutical, and culinary applications.1Garcinia cambogia (Gaertn.) Desr., (syn. Garcinia gummi-gutta (L.) Roxb., known as Malabar tamarind, has been used for centuries in Southeastern Asia as an appetite suppressant to make meals more filling and satisfying.2 The sour rind of the fruit is utilized as a condiment, and it is used in folk medicine to manage several ailments, e.g., piles, constipation, edema, rheumatism, intestinal parasites, and irregular menstruation.3G. cambogia rind is considered a rich source of organic acids, amino acids, benzophenones, xanthones, and flavonoids4 as depicted in Figure 1. Polyisoprenylated benzophenones precursors of xanthones were detected in Ceylon fruit rind to include garcinol and guttiferones K, I, J, M, and N. Moreover, xanthones, viz., oxy-guttiferone K,5 and oxy-guttiferones K2, I, and M were also reported from Ceylon fruits.6 With regard to primary metabolites, fruit is enriched in amino acids including arginine, glutamine, proline, and γ-aminobutyric acid.7 In addition to amino acids, organic acids represented by (−)-hydroxycitric acid (HCA) and its lactone are responsible for its sour taste,2 followed by citric and malic acids.8 It contains ca. 10–30% acid calculated as citric acid,9 with HCA as the major one.10
Figure 1.
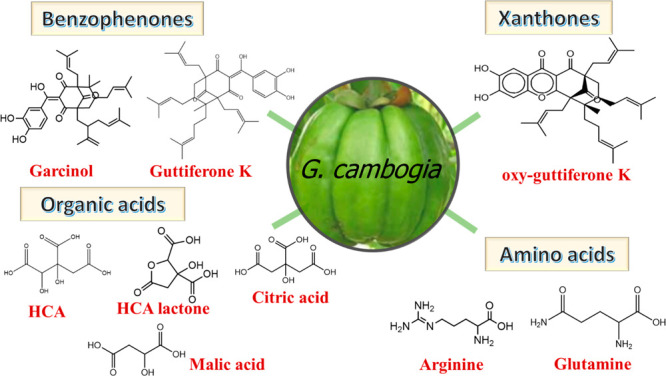
Representative examples of G. cambogia major chemical constituents. HCA, hydroxy citric acid.
Although herbal preparations are gaining more popularity worldwide, they often encounter contamination and adulteration affecting their safety and or efficacy.11 The increasing interest in phytomedicine efficacy along with the increase in legal requirements for safety and reliable contents of active ingredients drives the development of analytical tools for proper quality control of such a complex, multicomponent mixture.12 Targeted and untargeted analyses play a crucial role in the quality control of nutraceuticals. Whereas targeted methods are more selective, sensitive, and often quantitative, the untargeted approach embraces the complexity of natural samples and is more suited for analysis of nutraceuticals.13 Modern techniques comprise hyphenated chromatography with spectroscopy, e.g., gas chromatography–mass spectrometry (GC–MS), high-performance liquid chromatography–diode array detection (HPLC-DAD), HPLC–MS, HPLC–nuclear magnetic resonance (NMR), and capillary electrophoresis-diode array detection (CE-DAD) have been widely used for qualitative and quantitative analysis. Recently, advanced analytical tools such as profiling and fingerprinting are often supplemented by multivariate data analysis, i.e., chemometrics to help analyze and dissipate such rich chemical info data set.14
The present review highlights the current analytical approaches employed for the quality control purposes of G. cambogia extracts and in nutraceuticals. Analysis of G. cambogia bioactivity in biofluids is presented to track its pharmacokinetics and efficacy. Furthermore, it represents an up-to-date comprehensive overview on extraction and optimization approaches together with the nano formulation of G. cambogia bioactives to improve efficacy, with particular emphasis on HCA content.
Garcinia Health Benefits
G. cambogia potential use in body weight loss has been validated through several in vivo animal15−18 and clinical studies17,19 mainly due to (−)-hydroxycitric acid (HCA). Nevertheless, few studies failed to show the same outcome; a randomized clinical trial of 66 subjects to administer 1500 mg HCA/day for 12 weeks, while they were on a high-fiber and low-energy diet, did not reduce body weight or fat mass20 contrary to other studies with positive results using a simple diet rich in carbohydrates.21 Discrepancy may be attributed to the low HCA administered dose or the high-fiber diet that may have inhibited its gastrointestinal absorption.10 Furthermore, treatment with 3 g per day for 3 days did not influence energy expenditure or respiratory quotient, suggesting that HCA does not affect the fasting rate of fat oxidation in the short term.22 In contrast, longer administration of G. cambogia supplementation (45 mg/kg for 6 months) did not affect cats’ body weight.23 Thus, further controlled, long-term clinical studies ought to be conducted on a large scale to assess the efficacy of G. cambogia or HCA with monitoring of its bioavailability. G. cambogia supplementation was found to reduce high-fat-diet-induced obesity by suppressing epididymal and inguinal subcutaneous white adipose tissue mass and adipocyte size.24 Morover, G. cambogia administration resulted in significant blood glucose-lowering effects.25 HCA has attracted attention in Garcinia among its many other bioactives owing to its antiobesity effect and various action mechanisms in obesity management. Chiefly, HCA is a competitive inhibitor of the ATP-citrate lyase enzyme that converts mitochondria-derived citrate into acetyl CoA, a precursor for both fatty acid and mevalonate synthesis pathways. Reduction of acetyl-CoA units leads to less fatty acid biosynthesis and lipogenesis from a high carbohydrate diet. On the other hand, it stimulates liver gluconeogenesis and glycogen storage, which sequentially impacts the liver glucoreceptors bringing about satiety through the vagus nerve. Gluconeogenesis also could contribute to the ATP wasting and calorie consumption.9 HCA-SX, a Ca/K-HCA rich extract, enhanced the release and availability of serotonin and inhibited its uptake in the rat brain cortex like SRRIs, and consequently could control appetite and manage several serotonin-deficient conditions, e.g., insomnia, depression, headaches, and migraine.26 These effects are suggestive for multiple mechanisms through which Garcinia fruit aids in weight loss. Additionally, it upregulated serotonin receptor genes involved in satiety.2 The concurrent use of G. cambogia extract with probiotics further ameliorated weight gain and adiposity, through modifying gut microbiota composition, especially suppressing Clostridium aminophilum, the bacteria highly correlated with obese phenotypes.27 Such an effect is suggestive of a synergistic action of Garcinia and probiotics in weight reduction. Moreover, the effects of HCA extracts from G. cambogia on metabolic, atherogenic, and inflammatory biomarkers were examined in obese women with nonalcoholic fatty liver disease. Results revealed that weight, body mass index, waist circumference, fasting blood sugar, hip circumference, triglyceride, and low-density lipoprotein cholesterol showed a decrease, concurrent with an increase in high-density lipoprotein cholesterol.28 Interestingly, fruit rinds of G. cambogia, G. indica, and G. atroviridis contain (2S,3S)-HCA with a potential to inhibit ATP-citrate lyase,10 while the calyx of Hibiscus subdariffa and H. rosa-sinensis produce (2S,3R)-HCA which suppresses intestinal α-glucosidase and pancreatic α-amylase, reducing carbohydrate metabolism. Likewise, (2S,3R)-HCA is produced by Streptomyces sp. U121 and Bacillus megaterium G45C at trace levels.29
Pharmacological and clinical studies confirm the safety of HCA intake at levels up to 2800 mg/day.9 Nevertheless, several side effects were reported including ocular complications30 and hepatic toxicity31 either upon consumption of G. cambogia with multiple ingredients32−34 or due to its concurrent use with other drugs35 or its administration beyond the recommended doses.30
G. cambogia and HCA derivatives, e.g., calcium, potassium, and sodium salts, are widely available in pharmaceutical preparations used for weight loss, lipid abnormalities, improving exercise endurance, and cardio protection.10 Super CitriMax, a Ca/K salt of 60% HCA extract from G. cambogia, is more soluble and bioavailable than a Ca-based one proven safe and effective in weight control.36 Other health benefits revealed for Garcinia include, e.g., anti-ulcerogenic, antiseptic,37 anti-inflammatory, hepatoprotective, antioxidant, erythropoietic, and cytotoxic effect, though most are based on animal studies and less explored in humans to be conclusive compared to the slimming effect reported before.35 These effects are likely attributed to garcinol, the polyisoprenylated benzophenone that exhibits anti-ulcer, anti-inflammatory, antimicrobial, and anticarcinogenic activities,5 while the hypolipidemic effect of its flavonoid fraction (10 mg/kg BW/day) in rats exceeded that of Myristica fragrance, Cocos nucifera, and Saraka asoka without exerting any toxic effects.38
Analysis and QC Approaches of G. cambogia and in Nutraceuticals
G. cambogia fruit is a valued weight-loss supplement owing to its richness in organic acids, mainly hydroxycitric acid (HCA) and HCA lactone, benzophenones, and polyisoprenylated benzophenones, viz., garcinol (camboginol or guttiferone E) and isogarcinol (cambogin), guttiferone I, guttiferone N, guttiferone J, guttiferone K, and guttiferone M.39 Xanthones were also reported in G. cambogia such as garbogiol, rheediaxanthone A, oxy-guttiferone I, oxy-guttiferone K, oxy-guttiferone K2, and oxy-guttiferone M39 (Figure 1). Xanthones, benzophenones, and HCA as the major constituents in G. cambogia were reported for their biological activities such as antiobesity,40 hypolipidemic,41 and anticancer activity.42
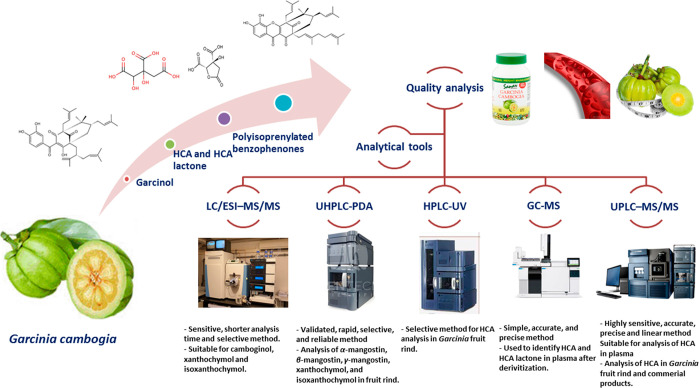
Several analytical methodologies were reported in the literature for the determination and quantification of G. cambogia fruit bioactive metabolites and its commercial products (Table 1, Figure 2). Moreover, the bioavailability and pharmacokinetics of these metabolites in the human body have been monitored to prove drug efficacy and safety inside the body. The next sections shall cover different approaches employed for the analysis of Garcinia major bioactive classes, highlighting both advantages and limitations for each technique.
Table 1. Different Analytical Methodologies Employed for the Analyses of G. cambogia Fruit Bioactive Metabolities.
| Sample | Composition | Methodology | Advantages | Results | Ref |
|---|---|---|---|---|---|
| Dried and smoked Garcinia cambogia fruit rinds | (−)-HCA | UV Spectrophotometry | Reliable, sensitive and specific method for HCA determination | 828 μg/mL of HCA was resulted from 100 g G. cambogia and the absorbance was measured at 467 nm after addition of sodium meta vanadate solution | (44) |
| G. cambogia fruit rinds | Xanthochymol and isoxanthochymol | LC/ESI–MS/MS | Sensitive, short analysis time and selective | The limits of detection for xanthochymol and isoxanthochymol were 1.0 ng/mL and 0.5 ng/mL, and xanthochymol was quantified (4.93 ng/mL) | (54) |
| Dried leaves of G. cambogia, G. indica, G. xanthochymus, and G. morella | (−)-HCA, lactone, and citric acid | HPLC | Rapid and sensitive methods in isolation of HCA and lactones | The amount of (−)-HCA, lactone, and citric acid in G. cambogia were estimated at 7.95%w/w, 3.25%w/w, and 0.13%w/w, respectively | (45) |
| The fruit rinds of G. cambogia | Isoxanthochymol and camboginol | (LC/ESI-MS/MS) | A validated, sensitive and selective, rapid method | The limits of detection for isoxanthochymol and camboginol were 2.0 and 5.0 ng/mL and quantified 11.3 and 57.7 ng/mL, respectively | (55) |
| G. cambogia fruit rinds | HCA | HPLC-UV | Selective method for HCA analysis, validated for levels ranging from 2–10 mg/mL with acceptable reproducibility and accuracy | The method showed good reproducibility and accuracy at concentrations ranging from 2 to 10 μg of HCA. The mean recovery of the HCA from G. cambogia extracts ranged from 98.4–100.5% | (8) |
| Fruit rinds of G. cambogia | Isoxanthochymol and camboginol | HPLC-LC-ESI-MS | Validated in terms of repeatability and precision | LOD and LOQ were 5 and 10 g/mL for isoxanthochymol and 50 and 100 g/mL for camboginol, respectively. The intra- and interday precisions were 2.34% and 3.41% for isoxanthochymol and 3.35% and 3.66% for camboginol. Quantification of isoxanthochymol and camboginol in Garcinia extracts were 16.6 and 88.2 mg/g | (56) |
| Fruit rinds of 8 different Garcinia species | α-mangostin, β-mangostin, γ-mangostin, xanthochymol, and isoxanthochymol | UHPLC-PDA | The method is accurate, precise, linear, and reproducible | xanthochymol, and isoxanthochymol were detected in different Garcinia species extracts and in G. cambogia was 21.42 and 0.89%w/w, respectively | (57) |
| Ten herbal products that included either G. cambogia or G. indica | (−)-hydroxycitric acid and (−)-hydroxycitric acid lactone | 1H NMR | Rapid, no need for additional cleanup of extracts or derivatization, possible to detect of all kind of organic molecules in the same sample, highly reproducible with little instrument-instrument variation, noninvasive, and nondestructive method | (−)-hydroxycitric acid and (−)-hydroxycitric acid lactone content in G. cambogia and G. indica ranged from 1.7% to 16.3%, and 3.5% to 20.7% respectively. | (53) |
| Garcinia commercial formulation, 20 mg/kg in rat plasma | HCA | UPLC–MS/MS | Highly sensitive, accurate, precise and linear method for determination of HCA quality control in rat plasma | the concentration range of precision was 10.5–5000 ng/mL, LLOQ, 31.25 ng/mL | (59) |
| Human plasma | HCA | UPLC-MS/MS | Rapid, selective and sensitive UPLC-MS/MS method | 5.02–12.01% (CV%) 0.29 to 9.20% (RE%). Linearity range 0.05–10 μg/mL | (58) |
| Human plasma | HCA | GC-MS | The derivatizing reagent used was N,O-bis(trimethylsilyl)fluoroacetamide and 10% trimethylchlorosilane | HCA plasma level ranged from 0.8 to 8.4 mg/mL 30 min and 2 h after ingestion of 2 g HCA, respectively | (50) |
Figure 2.
Different analytical methods applied for the analysis and quality control of G. cambogia.
Hydroxycitric Acid (HCA) and Lactones
HCA is the major organic acid identified in G. cambogia fruit and the main chemical used in weight loss programs due to its antiobesity action and appetite suppressant effect.39,43 HCA content in G. cambogia fruits was analyzed spectrophotometrically by forming a unique, specific, and stable colored complex with sodium meta vanadate and measuring its absorbance at 467 nm.44 HCA amounted for ca. 3–5% of G. cambogia fruits. The average concentration versus absorbance plot showed positive correlation at a concentration range of HCA (5–50 μg/mL).44
In another study, HCA and other organic acid levels in G. cambogia were estimated compared to other species in that genus to include G. indica, G. xanthochymus, and G. morella using HPLC-UV. Results revealed that G. cambogia encompassed (−)-HCA, HCA lactone, and citric acid at 7.9, 3.2, and 0.13% w/w, respectively. Additionally, G. cambogia and G. indica were found to contain the highest (−)-HCA and its lactone levels,45 and suggestive that G. indica can be used instead of G. cambogia especially if targeting its HCA. Likewise, (−)hydroxycitric acid was analyzed in G. cambogia commercial samples using HPLC/UV.46,47 The mean recovery of HCA from G. cambogia extracts ranged 98.4–100.5% with coefficients of variation of 0.25–0.63% and accuracy range of 2–10 μg, reflecting that the method is validated for the routine analysis of HCA in commercial G. cambogia extracts.46 The method also offered selectivity compared to traditional acid base titration methods which quantify for total acids, not only targeting HCA.46 HCA quantification in two G. cambogia marketed extracts containing 50% of HCA was performed using HPLC-DAD.48 HCA quantification in samples ranged 36.1–41.5%, and in agreement with results specified by the distributors. Another LC-based method employing capillary electrophoresis coupled to UV detection at 200 nm was reported for the qualitative determination of HCA in the presence of other organic acids, viz., citric, malic, and tartaric in G. cambogia fruits.49 Lactone and nonlactone from HCA can also be identified and quantified in the fruit rind of G. cambogia using gas chromatography coupled with mass spectrometry (GC/MS) post derivatization.29,50 The derivatization step in GC/MS though complicates the procedure and can affect HCA recovery from its matrix and should be accounted for during absolute quantification.51
Compared to chromatographic techniques extensively reported for HCA determination, direct spectroscopic techniques such as NMR were reported for the quantification of (−)-HCA and its lactone in raw herbal drugs and Garcinia food supplements. Compared to hyphenated techniques, NMR is more robust and can quantify for targeted metabolites with no need for standard curve preparation being normalized to internal standard spiked in sample such as hexamethyldisiloxane (HMDS). Nevertheless, NMR suffers from low sensitivity and signal overlap hampering quantification in certain signals.52 The NMR method was used with DNA barcoding for the authentication of Garcinia fruit rinds and food supplements,53 with 1H NMR spectroscopy found effective for distinguishing between both diasteriomers (2S,3S) and (2S,3R) HCA.29
Polyisoprenylated Benzophenones
Xanthochymol and isoxanthochymol were identified and quantified in G. cambogia fruit rind using the LC/ESI–MS/MS technique, found more sensitive and accurate than classical HPLC-UV methods.54 Two polyisoprenylated benzophenones known as isoxanthochymol and camboginol were identified and quantified in G. cambogia fruit rinds using a sensitive LC/ESI-MS/MS method.55,56 The method was validated in terms of linearity, accuracy, specificity, and precision, posing it to be used for screening of these benzophenones in different G. cambogia extracts including commercial products.55,56 The LC-ESI/MS/MS method was shown to be more sensitive than the classical HPLC-UV method, with the lowest limit of quantification (LLOQ) of 2–4 ng/mL for isoxanthochymol and 5–10 ng/mL for camboginol, employed for the assay of these two compounds in other Garcinia species.55,56 Isoxanthochymol and camboginol were detected in G. cambogia fruit rind at levels of 11.3 and 57.7 ng/mL, respectively, while in the study of Kumar et al. (2009) they were quantified at 16.6 and 88.2 mg/g, respectively.55,56 Three xanthones and two polyisoprenylated benzophenones were identified and quantified in 8 Indian Garcinia fruit rinds using a validated ultra-HPLC (UHPLC)-photodiode array (PDA) method. Metabolites included α-mangostin, β-mangostin, γ-mangostin, xanthochymol, and isoxanthochymol.57 Quantification of each metabolite was reported in extracts of different polarities, viz., n-hexane, chloroform, ethyl acetate, and methanol. α-Mangostin was quantified in G. mangostana detected only in nonpolar extract, i.e., n-hexane at (5.56%, w/w) and absent from polar ones, i.e., chloroform, ethyl acetate, and methanol, whereas β-mangostin was detected at the highest level in chloroform (0.29%, w/w).57 γ-Mangostin was detected in all tested Garcinia species excluding G. pedunculata with the highest level detected in G. mangostana n-hexane extract (0.76%, w/w).57 Among the four examined Garcinia species, G. xanthochymus n-hexane extract showed the maximum level of xanthochymol at 30.38% w/w.57 In addition, isoxanthochymol was quantified in all extracts, with the highest level detected in the chloroform extract of G. indica 3.07%, w/w.57 Ultimately, results revealed that the n-hexane extract is most rich in xanthones and polyisoprenylated benzophenones posing n-hexane as the optimum solvent for higher recovery of these active metabolites from Garcinia.
Pharmacokinetics Determination of G. cambogia Metabolites in Biofluids
Analysis of G. cambogia active constituents and their metabolites in body fluids is performed typically using most sensitive hyphenated analytical techniques, i.e., MS detection to assess active metabolites bioavailability and pharmacokinetics. The first determination of HCA in plasma has been reported using ion chromatography with a linear gradient elution of NaOH ranging 0.5–38.25 mM in water.43 Plasma HCA level was determined after the ingestion of a single bolus dose of HCA solution (4.4 g) over a 3.5 h period revealing maximal plasma level at 60–90 min of 0.12 mmol/L.43 Later, Loe et al. (2001) developed a GC/MS method for the determination of HCA as a marker for G. cambogia administration in human plasma post derivatization, and verified for the determination of HCA bioavailability after ingestion of 2 g HCA in 4 subjects, with plasma HCA level ranging from 0.8 to 8.4 mg/mL 30 min and 2 h after ingestion, respectively.50 The method efficiency was aided by the high separation power of the GC column with improved resolution than typical LC for minimal sample preparation. The advances of high resolution GC and LC coupled to time-of-flight (TOF) mass spectrometers has yet to be reported for profiling of G. cambogia or determination of its biotransformed products inside the body.
The quantification of HCA in humans and rats was evaluated using a rapid, selective, and sensitive UPLC-MS/MS method.58,59 HCA was analyzed in 16 healthy subjects after oral administration of G. cambogia extract revealing an excellent mean peak area ratio of 0.98 and coefficient of variation CV% of 7.17 from normal, lipemic, and hemolyzed plasma samples.58 Excellent intra-assay and inter-assay precision were achieved, ranging from 5.02% to 12.01% (CV%) as well as intra- and interassay accuracy ranging from 0.29% to 9.20% relative errors (RE%). UPLC-MS/MS is highly efficient to be applied for HCA pharmacokinetic study on a large scale, as it can analyze more than 670 plasma samples.58 Likewise, HCA was analyzed in rat plasma after i.v. administration of a dose of 1 mg/kg, where the half-life and the apparent volume of distribution of HCA reached 2.1 ± 0.4 h and 0.6 ± 0.15 L/kg, respectively, whereas with oral administration of 20 mg/kg, HCA showed maximum plasma concentration Cmax at 14.4 μg/mL reached at 1 h. The method was reproducible, sensitive, accurate, precise, and linear in the concentration range of 10–5000 ng/mL.59 Further, the method was successfully used to determine HCA pharmacokinetics (PKs) in rat plasma after the administration of pure HCA and a commercial Garcinia preparation. The absolute bioavailability of HCA after the administration of commercial preparation was at 61.3%, after correcting the dose for the actual content of HCA (i.e., 12 mg/kg). The method was successfully used to determine in vivo PKs of HCA revealing that it is rapidly absorbed with moderate apparent volume of distribution and a good bioavailability. The method reported can be further used routinely for the analysis of HCA and could be helpful for further toxicological evaluation of Garcinia products alone or in a mixture.59 A validated analytical method for quantification of HCA in rat plasma and fetal homogenate using LC-MS/MS analysis was reported. Results revealed that at a range of 20–800 ng/mL the method was linear with limits of quantitation (LOQ) and detection (LOD) equal to 20.0 and 3.9 ng/mL plasma, respectively. The accuracy and precision using quality control standards were ±7.5% and 9.5%, respectively.60
Effect of administration route on blood G. cambogia/HCA level and antioxidative activity was examined in a randomized study on healthy women compared between fasted and fed conditions post-ingestion of 1500/750 mg of G. cambogia/HCA under 8 h of fasting.61 In the fed period a high calorie breakfast (∼600 cal) was given after dosing revealing reduced HCA plasma concentrations in the fed state with a higher volume of distribution and clearance and large interindividual variations observed.61 HCA was found to be affected by the fed state indicating a possible adsorption between HCA and food, so administration is preferable on fasted stomach or at least 2 h before a meal, avoiding against food/HCA interaction.61
In another study, GC/MS was utilized to analyze serum metabolite changes in broiler chickens after the administration of (−)-HCA at 0, 1000, 2000, and 3000 mg/kg diets for 28 days. Results showed that 20 metabolites in the 1000 mg/kg (−)-HCA treatment group and 16 metabolites in the 3000 mg/kg (−)-HCA treatment group were significantly altered. The data indicated that (−)-HCA promoted protein biosynthesis by regulating the metabolic directions of amino acids, to depend on the dose of administered (−)-HCA. Likewise, (−)-HCA decreased body weight gain by reducing the abdominal fat deposition in broiler chickens and inhibited fatty acid synthesis by promoting the citric acid cycle.62 Reduction in cytosolic acetyl-CoA level is associated with decreased lipids biosynthesis and is likely to account for the Garcinia slimming effect.
Extraction Optimization and Nanoformulation of G. cambogia Bioactives
Extraction Optimization
Several methods have been described for the extraction and isolation of HCA from G. cambogia. First methods reported on (−) HCA isolation in the form of its lactone on a large scale from dried rinds, used either acetone followed by aqueous extraction of the acid with a yield of ca. 15%63 or hot water for extraction, followed by alcohol precipitation of pectin; then KOH is added to obtain ca. 20% of HCA potassium salt after purification.64 A more recent method used an anion exchange column for the adsorption of (−)-HCA from a salt-free water extract of Garcinia rinds, eluted with sodium/potassium hydroxide, and then loaded on a cation exchange column to yield concentrate containing 23–54% free HCA and 6–20% HCA lactone.65
Although fewer techniques were reported for G. cambogia extraction, several methods including novel extraction techniques have been described for the better utilization and recovery of other Garcinia species, of potential to be applied in the future to G. cambogia considering its close chemical composition. Microwave-assisted extraction followed by microencapsulation was applied to G. cowa to reduce the lactonization of HCA, and to produce higher HCA yield with an overall improved antioxidant activity compared to that extracted using autoclave.66 Moreover, response surface methodology was adopted for G. mangostana rind to optimize for the extraction of antioxidant pectic-polysaccharide (ca. 12.0–12.4%) with higher uronic acid content (ca. 20.2–21.1 mg/g) and antioxidant activity 225–252% DPPHsc/g,67 total phenolics,68 and xanthone-rich extract.69 Applying response surface methodology for G. mangostana revealed that using 0.05 solid to solvent ratio and methanol (69.77%) for 2 h extraction yielded the maximum total phenolic content (140.66 mg gallic acid equivalent (GAE)/g). While optimization of microwave-assisted extraction revealed that using 25 mL/g (solvent to solid ratio), ethanol (71%) and irradiation time of 2.24 min were found optimum for obtaining antioxidant-rich xanthone extract from G. mangostana. Likewise, and from another Garcinia sp., response surface methodology was employed to optimize for the aqueous two-phase system extraction of G. indica fruit rind bioactives, viz. anthocyanins, garcinol, isogarcinol, and hydroxycitric acid. To clarify, an aqueous two-phase system made up of 1-propanol (15.202% w/w) and (NH4)2SO4 (10.242% w/w) with 2.5 g crude load achieved the maximum yield of garcinol (97.39%) and isogarcinol (92.38%) in the top phase, anthocyanins (99.19%) and HCA (99.83%) in the bottom one with a purity higher than 99% by applying secondary aqueous two-phase system.70 Furthermore, high-intensity ultrasound-assisted extraction for G. madruno increased the yield of bioflavonoids by ca. 3-fold (370 mg/100 mL of the total BF),71 while cellulase-assisted extraction was applied for the direct and enhanced recovery of mangostins (potential xanthone compounds) from G. mangostana pericarps72 which possess promising biological activities.73 Additionally, α-mangostin was extracted from G. mangostana using natural deep eutectic solvents designed as green solvents leaving no solvent residue in the final product.74 Such success in improving the extraction output in other Garcinia species should encourage its application for extraction optimization in G. cambogia especially for inclusion in nutraceuticals.
Nanotechnology and Nanoformulations
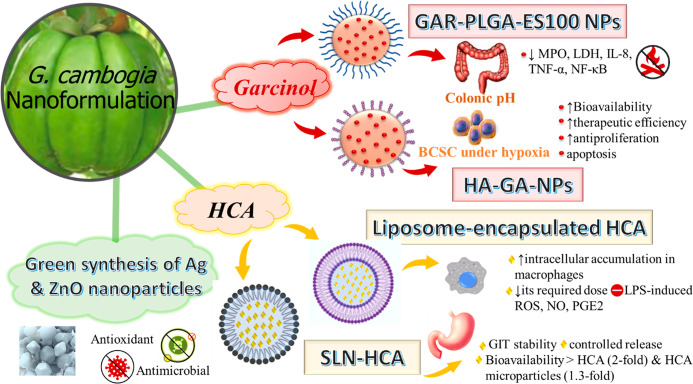
The use of natural products for the treatment of metabolic diseases is limited by their low bioavailability. Formulation of these phytochemicals such as polyphenols and flavonoids by nanotechnology has the potential to improve their hydrophilicity, stability, bioavailability, and safe and controlled delivery to target sites. Nanoformulations such as nanoemulsion, nanoliposomes, solid lipid nanoparticles, nanostructured lipid carriers, and polymeric nanoparticles are the most common reported in the literature.75 Nanotechnology has been rapidly developing in the past decade in nutraceuticals.76 Nanotechnology is increasingly utilized to design novel formulations of nutraceuticals for the following objectives: to protect degradation of compounds during storage or processing, mask undesirable tastes, enhance their solubility and dispersion, control their release, and improve their oral bioavailability.77 Nanocarriers, e.g., nanodispersion, nanoemulsion, liposoms, and polymer micelles, enclose and deliver bioactive compounds to increase their gastrointestinal tract uptake through active endocytosis or to enhance their bioavailability.78G. cambogia has been employed for the green synthesis of silver nanoparticles with strong antimicrobial potential compared to the original plant extract.79 Likewise, it was used in the synthesis of multifunctional ZnO nanoparticles with antioxidant properties (DPPH radical scavenging activity with IC50 93.16 μg/mL), cytotoxic activity (IC50 27.5 μg/mL and 68.8 μg/mL for MCF 7 and HeLA cell lines, respectively using MTT assay), and antimicrobial activity against Staphylococcus aureus, Escherichia coli, and Candida albicans with 19, 13, and 14 mm zones of inhibition at a concentration of 500 μg/mL (Figure 3).80
Figure 3.
Nanoformulations prepared from G. cambogia to improve bioavailability of selective metabolites and efficacy. BCSC, breast cancer stem cells; GAR-PLGA-ES100 NPs, garcinol-poly(lactic-co-glycolic acid)-Eudragit S100; HA-GA-NPs, garcinol-hyaluronic acid-poly(lactic-co-glycolic acid); IL-8, interleukin-8; LDH, lactate dehydrogenase; MPO, Myeloperoxidase; NF-κB, nuclear factor kappa light chain enhancer of activated B-cells; NO, nitric oxide; PGE2, Prostaglandin E2; ROS, Reactive oxygen species; SLN-HCA, hydroxy citric acid loaded nanoparticles; TNF-α, tumor necrosis factor-α.
With regard to nanoformulation employment as a drug delivery system imparting higher clinical efficacy, several assembled drug nanoparticles have been developed, e.g., liposomes, polymeric nanoparticles, niosomes, dendrimers, metallic nanoparticles, and nanostructured lipid carriers.81 A nanodelivery system encompassing garcinol, hyaluronic acid, and poly(lactic-co-glycolic acid) (HA-GA-NPs) has been developed as a nanocarrier for garcinol targeting breast cancer stem cells under hypoxia (Figure 3). The HA-GA-NPs nanodelivery system improved the bioavailability, and consequently the therapeutic efficiency of garcinol, the aqueous insoluble polyisoprenylated benzophenone derivative. HA-GA-NPs exhibited higher antiproliferative activity using WST-1 cell proliferation assay (IC50 5.54, 4.03, and 0.81 μg/mL at 24, 48, 72 h, respectively) compared to that of pure garcinol (IC50 21.16, 17.06, 10.22 μg/mL, respectively). It also showed enhanced cellular uptake via receptor-mediated endocytosis, and apoptosis potential confirmed by caspase-3/7 activation.82 Moreover, pH-sensitive biodegradable nanoparticles has been formulated using garcinol, poly(lactic-co-glycolic acid), and Eudragit S100 (GAR-PLGA-ES100 NPs) as illustrated in Figure 3. It specifically targeted the colonic pH where it releases the drug to exert its anti-inflammatory effect of potential for application in ulcerative colitis. GAR-PLGA-ES100 NPs decreased myeloperoxidase (MPO) and lactate dehydrogenase (LDH) activity, downregulated interleukin (IL)-8, tumor necrosis factor (TNF-α), and nuclear factor kappa light chain enhancer of activated B-cells (NF-κB) expression suggestive that it could be beneficial in inflammatory bowel disease.83
On the other hand, HCA loaded nanoparticles (SLN-HCA) demonstrated better HCA gastrointestinal stability (ca. 88%) with controlled release (Figure 3). They showed superior bioavailability compared to unencapsulated HCA and microparticles (SLM-HCA) by 2- and 1.3-fold, respectively.84 Furthermore, liposome-encapsulated HCA improved HCA intracellular accumulation in macrophages (ca. 4 times) and decreased the required dose to combat LPS-induced ROS, NO, and PGE2 (ca. 10 times), suggestive of its possible use in inflammatory conditions.85
Although the G. cambogia whole extract has not yet been nanoformulated to the best of our knowledge, an interesting formula has been prepared from G. mangostana extract. The extract as well as α-mangostin were individually encapsulated into the mucoadhesive, relatively acid stable nanocarriers ethylcellulose and methylcellulose (ECMC). The G. mangostana loaded naonocarrier showed sustained release of the extract at pH 2.0 and 7.4. The encapsulated extracts exhibited a strong in vitro activity against Helicobacter pylori nearly similar to the free ones, where α-mangostin showed nearly half MIC values (31.3–62.5 μg/mL) compared to G. mangostana extract against 5 H. pylori strains (62.5–125 μg/mL). Nevertheless, α-mangostin exhibited nearly the same activity as metronidazole; however it was weaker than both clarithromycin (higher MIC by 130–4170-fold) and amoxicillin (higher MIC by 100–7800-fold). The encapsulated forms not only retained the in vitro anti-Helicobacter pylori effect but also exhibited anti-adhesion potential even stronger than that of free extracts. In vivo study revealed the ability of orally administrated encapsulated forms to tackle H. pylori in mice, contrary to the unencapsulated forms which may be attributed to the mucoadhesive properties of ECMC nanospheres and the sustained release,86 and to be considered for inclusion in future clinical trials.
Conclusion
The current review capitalizes on the increasing application of G. cambogia fruit in nutraceuticals mainly for its antiobesity effects. Nevertheless, its effectiveness as an antiobesity agent remains contradictory. On the other hand, it exerts other promising biological activities, viz., anti-ulcerogenic, antiseptic, anti-inflammatory, hepatoprotective, antioxidant, erythropoietic, and cytotoxic effects which are clinically less well explored. Henceforth, further controlled, large-scale clinical trials are recommended to unravel its effectiveness related to its bioavailability. Moreover, the pharmacological effects of its individual compounds should be further studied and their synergistic effects should be assessed. In addition, its interaction with gut microbiota should be further explored and related to its antiobesity effect. Several analytical procedures employed for the quality control as well as pharmacokinetics evaluation of G. cambogia phytochemicals were reviewed. Analytical techniques targeted either qualitative and quantitative analyses of G. cambogia bioactive metabolites in fruit rind and further commercially available products. UPLC coupled to high-resolution MS appeared to be the most suitable tool offering high sensitivity and accuracy for the analysis of HCA in fruit rind, commercial products, and human plasma, while GC-MS analysis post derivatization enabled the characterization of HCA and its metabolites in plasma to be considered for detection of HCA metabolic fate post administration. The application of NMR analysis and other metabolome tools is still limited, and coupling with multivariate data analysis has yet to be considered for the analysis and quality control of G. cambogia metabolites in comparison to other Garcinia species. Based on the published data, it is evident that traditional methods of extraction are still widely applied for G. cambogia. On the other hand, recent strategies for extraction optimization are less explored in G. cambogia, while the success of using optimization methodologies for other Garcinia species should be considered as a sign to be examined for G. cambogia. Few nanoformulations have been prepared from G. cambogia bioactive constituents, i.e., garcinol and HCA, and successfully induced their targeted activities. Future research should focus now on the development of nanoformulations for different bioactive compounds of G. cambogia, as well as its whole extract to benefit from their synergistic effects. Also, such nanoformulations should be biologically assessed compared to the original extracts, and their safety should be addressed prior to their clinical and safety assessments being investigated.
Acknowledgments
Dr. Mohamed Farag acknowledges the Alexander von Humboldt foundation, Germany for purchasing of GCMS analysis.
Author Present Address
Dr. Mohamed A. Farag current affiliation is Pharmacognosy Department, College of Pharmacy, Cairo University, Kasr El-Aini St., Cairo P.B. 11562, Egypt
The authors declare no competing financial interest.
References
- Hemshekhar M.; Sunitha K.; Santhosh M. S.; Devaraja S.; Kemparaju K.; Vishwanath B.; Niranjana S.; Girish K. An overview on genus Garcinia: phytochemical and therapeutical aspects. Phytochem. Rev. 2011, 10 (3), 325–351. 10.1007/s11101-011-9207-3. [DOI] [Google Scholar]
- Roy S.; Rink C.; Khanna S.; Phillips C.; Bagchi D.; Bagchi M.; Sen C. K. Body weight and abdominal fat gene expression profile in response to a novel hydroxycitric acid-based dietary supplement. Gene Expr. 2003, 11 (5–6), 251–262. 10.3727/000000003783992289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham Z.; Malik S.; Rao G. E.; Narayanan S. L.; Biju S. Collection and characterisation of Malabar tamarind [Garcinia cambogia (Gaertn.) Desr.]. Genet. Resour. Crop Evol. 2006, 53 (2), 401–406. 10.1007/s10722-004-0584-y. [DOI] [Google Scholar]
- Sripradha R.; Sridhar M. G.; Maithilikarpagaselvi N. Antihyperlipidemic and antioxidant activities of the ethanolic extract of Garcinia cambogia on high fat diet-fed rats. J. Complementary Integr. Med. 2016, 13 (1), 9–16. 10.1515/jcim-2015-0020. [DOI] [PubMed] [Google Scholar]
- Masullo M.; Bassarello C.; Suzuki H.; Pizza C.; Piacente S. Polyisoprenylated benzophenones and an unusual polyisoprenylated tetracyclic xanthone from the fruits of Garcinia cambogia. J. Agric. Food Chem. 2008, 56 (13), 5205–5210. 10.1021/jf800416j. [DOI] [PubMed] [Google Scholar]
- Masullo M.; Bassarello C.; Bifulco G.; Piacente S. Polyisoprenylated benzophenone derivatives from the fruits of Garcinia cambogia and their absolute configuration by quantum chemical circular dichroism calculations. Tetrahedron 2010, 66 (1), 139–145. 10.1016/j.tet.2009.11.034. [DOI] [Google Scholar]
- Carratu B.; Boniglia C.; Giammarioli S.; Mosca M.; Sanzini E. Free amino acids in botanicals and botanical preparations. J. Food Sci. 2008, 73 (5), C323–C328. 10.1111/j.1750-3841.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha G.; Sakariah K. Determination of organic acids in Garcinia cambogia (Desr.) by high-performance liquid chromatography. J. Chromatogr. A 1998, 806 (2), 337–339. 10.1016/S0021-9673(98)00054-5. [DOI] [Google Scholar]
- Soni M.; Burdock G.; Preuss H.; Stohs S.; Ohia S.; Bagchi D. Safety assessment of (−)-hydroxycitric acid and Super CitriMax®, a novel calcium/potassium salt. Food Chem. Toxicol. 2004, 42 (9), 1513–1529. 10.1016/j.fct.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Jena B.; Jayaprakasha G.; Singh R.; Sakariah K. Chemistry and biochemistry of (−)-hydroxycitric acid from Garcinia. J. Agric. Food Chem. 2002, 50 (1), 10–22. 10.1021/jf010753k. [DOI] [PubMed] [Google Scholar]
- Posadzki P.; Watson L.; Ernst E. Contamination and adulteration of herbal medicinal products (HMPs): an overview of systematic reviews. Eur. J. Clin. Pharmacol. 2013, 69 (3), 295–307. 10.1007/s00228-012-1353-z. [DOI] [PubMed] [Google Scholar]
- Farag M. A.; Wessjohann L. A. Metabolome classification of commercial Hypericum perforatum (St. John’s Wort) preparations via UPLC-qTOF-MS and chemometrics. Planta Med. 2012, 78 (05), 488–496. 10.1055/s-0031-1298170. [DOI] [PubMed] [Google Scholar]
- Ballin N. Z.; Laursen K. H. To target or not to target? Definitions and nomenclature for targeted versus non-targeted analytical food authentication. Trends Food Sci. Technol. 2019, 86, 537–543. 10.1016/j.tifs.2018.09.025. [DOI] [Google Scholar]
- Bansal A.; Chhabra V.; Rawal R. K.; Sharma S. Chemometrics: a new scenario in herbal drug standardization. J. Pharm. Anal. 2014, 4 (4), 223–233. 10.1016/j.jpha.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa N. Garcinia extract inhibits lipid droplet accumulation without affecting adipose conversion in 3T3-L1 cells. Phytother. Res. 2001, 15 (2), 172–173. 10.1002/ptr.689. [DOI] [PubMed] [Google Scholar]
- Oluyemi K. A.; Omotuyi I. O.; Jimoh O. R.; Adesanya O. A.; Saalu C. L.; Josiah S. J. Erythropoietic and anti-obesity effects of Garcinia cambogia (bitter kola) in Wistar rats. Biotechnol. Appl. Biochem. 2007, 46 (1), 69–72. 10.1042/BA20060105. [DOI] [PubMed] [Google Scholar]
- Ranjith D.; Prakash S. S.; Karunakara A.; Diwakar L.; Reddy G. C. Issue of testicular toxicity of hydroxycitric acid lactone. Curr. Sci. 2011, 100 (1), 24–27. [Google Scholar]
- Kang E. S.; Hwang J. S.; Kim M.-H.; Kim H. J.; Lee C.-K.; Seo H. G. Effect of Garcinia cambogia extract-containing dip-sauce for meat on lipid accumulation and body weight reduction in rats fed high-fat diet. Food Sci. Anim. Resour. 2013, 33 (2), 276–280. 10.5851/kosfa.2013.33.2.276. [DOI] [Google Scholar]
- Kovacs E. M.; Westerterp-Plantenga M. S. Effects of (−)-hydroxycitrate on net fat synthesis as de novo lipogenesis. Physiol. Behav. 2006, 88 (4–5), 371–381. 10.1016/j.physbeh.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Heymsfield S. B.; Allison D. B.; Vasselli J. R.; Pietrobelli A.; Greenfield D.; Nunez C. Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent: a randomized controlled trial. Jama 1998, 280 (18), 1596–1600. 10.1001/jama.280.18.1596. [DOI] [PubMed] [Google Scholar]
- Vasselli J.; Shane E.; Boozer C.; Heymsfield S. Garcinia cambogia extract inhibits body weight gain via increased energy expenditure (EE) in rats. Faseb Journal 1998, A505. [Google Scholar]
- Kriketos A.; Thompson H.; Greene H.; Hill J. (−)-hydroxycitric acid does not affect energy expenditure and substrate oxidation in adult males in a post-absorptive state. Int. J. Obes. 1999, 23 (8), 867–873. 10.1038/sj.ijo.0800965. [DOI] [PubMed] [Google Scholar]
- Leray V.; Dumon H.; Martin L.; Siliart B.; Sergheraert R.; Biourge V.; Nguyen P. No effect of conjugated linoleic acid or Garcinia cambogia on fat-free mass, and energy expenditure in normal cats. J. Nutr. 2006, 136 (7), 1982S–1984S. 10.1093/jn/136.7.1982S. [DOI] [PubMed] [Google Scholar]
- Han J.-H.; Jang K.-W.; Myung C.-S. Garcinia cambogia attenuates adipogenesis by affecting CEBPB and SQSTM1/p62-mediated selective autophagic degradation of KLF3 through RPS6KA1 and STAT3 suppression. Autophagy 2022, 18 (3), 518–539. 10.1080/15548627.2021.1936356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. H.; Kim M. T.; Myung C. S. Garcinia Cambogia Improves High-Fat Diet-Induced Glucose Imbalance by Enhancing Calcium/CaMKII/AMPK/GLUT4-Mediated Glucose Uptake in Skeletal Muscle. Mol. Nutr. Food Res. 2022, 66, 2100669. 10.1002/mnfr.202100669. [DOI] [PubMed] [Google Scholar]
- Ohia S. E.; Opere C. A.; LeDay A. M.; Bagchi M.; Bagchi D.; Stohs S. J. Safety and mechanism of appetite suppression by a novel hydroxycitric acid extract (HCA-SX). Mol. Cell. Biochem. 2002, 238 (1), 89–103. 10.1023/A:1019911205672. [DOI] [PubMed] [Google Scholar]
- Heo J.; Seo M.; Park H.; Lee W. K.; Yoon J.; Caetano-Anolles K.; Ahn H.; Kim S.-Y.; Kang Y.-M.; Cho S. Gut microbiota modulated by probiotics and Garcinia cambogia extract correlate with weight gain and adipocyte sizes in high fat-fed mice. Sci. Rep. 2016, 6 (1), 1–10. 10.1038/srep33566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arefhosseini S.; Tutunchi H.; Golzar S.; Mahboob S.; Pouretedal Z.; Ebrahimi-Mameghani M. The Effect of Hydroxy Citric Acid Supplementation with Calorie-restricted Diet on Metabolic, Atherogenic and Inflammatory Biomarkers in Women with Non-alcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial. Food Funct. 2022, 13, 5124. 10.1039/D1FO03685H. [DOI] [PubMed] [Google Scholar]
- Yamada T.; Hida H.; Yamada Y. Chemistry, physiological properties, and microbial production of hydroxycitric acid. Appl. Microbiol. Biotechnol. 2007, 75 (5), 977–982. 10.1007/s00253-007-0962-4. [DOI] [PubMed] [Google Scholar]
- Cho H.-k.; Han Y. S.; Park J. M. Ocular complications of Garcinia cambogia extract diet pills: Case report. Eur. J. Ophthalmol. 2020, 30 (6), NP21–NP26. 10.1177/1120672119872364. [DOI] [PubMed] [Google Scholar]
- Crescioli G.; Lombardi N.; Bettiol A.; Marconi E.; Risaliti F.; Bertoni M.; Ippolito F. M.; Maggini V.; Gallo E.; Firenzuoli F. Acute liver injury following Garcinia cambogia weight-loss supplementation: case series and literature review. Int. Emerg. Med. 2018, 13 (6), 857–872. 10.1007/s11739-018-1880-4. [DOI] [PubMed] [Google Scholar]
- Vitalone A.; Menniti-Ippolito F.; Moro P. A.; Firenzuoli F.; Raschetti R.; Mazzanti G. Suspected adverse reactions associated with herbal products used for weight loss: a case series reported to the Italian National Institute of Health. Eur. J. Clin. Pharmacol. 2011, 67 (3), 215–224. 10.1007/s00228-010-0981-4. [DOI] [PubMed] [Google Scholar]
- Mullin G. E. The use of complementary and alternative medicine for liver disease: part II. Nutr. Clin. Pract. 2013, 28 (2), 277–279. 10.1177/0884533612475133. [DOI] [PubMed] [Google Scholar]
- Narasimha A.; Shetty P. H.; Nanjundaswamy M. H.; Viswanath B.; Bada Math S. Hydroxycut–Dietary supplements for weight loss: Can they induce mania?. Aust. N. Z. J. Psychiatry. 2013, 47 (12), 1205–1206. 10.1177/0004867413493522. [DOI] [PubMed] [Google Scholar]
- Raina R.; Verma P. K.; Taku I.; Malik J. K.; Gupta R. C., Garcinia cambogia. In Nutraceuticals; Elsevier: 2021; pp 975–990. [Google Scholar]
- Roy S.; Shah H.; Rink C.; Khanna S.; Bagchi D.; Bagchi M.; Sen C. K. Transcriptome of Primary Adipocytes from Obese Women in Response to a Novel Hydroxycitric Acid–Based Dietary Supplement. DNA Cell Biol. 2007, 26 (9), 627–639. 10.1089/dna.2007.0617. [DOI] [PubMed] [Google Scholar]
- Mahendran P.; Vanisree A.; Shyamala Devi C. The antiulcer activity of Garcinia cambogia extract against indomethacin-induced gastric ulcer in rats. Phytother. Res. 2002, 16 (1), 80–83. 10.1002/ptr.946. [DOI] [PubMed] [Google Scholar]
- Koshy A. S.; Vijayalakshmi N. Impact of certain flavonoids on lipid profiles—potential action of Garcinia cambogia flavonoids. Phytother. Res. 2001, 15 (5), 395–400. 10.1002/ptr.725. [DOI] [PubMed] [Google Scholar]
- Semwal R. B.; Semwal D. K.; Vermaak I.; Viljoen A. A comprehensive scientific overview of Garcinia cambogia. Fitoterapia 2015, 102, 134–148. 10.1016/j.fitote.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Kim K.-Y.; Lee H. N.; Kim Y. J.; Park T. Garcinia cambogia extract ameliorates visceral adiposity in C57BL/6J mice fed on a high-fat diet. Biosci., Biotechnol., Biochem. 2008, 1772–1780. 10.1271/bbb.80072. [DOI] [PubMed] [Google Scholar]
- Altiner A.; Ates A.; Gursel F. E.; Bilal T. Effect of the antiobesity agent Garcinia cambogia extract on serum lipoprotein (a), apolipoproteins a1 and b, and total cholesterol levels in female rats fed atherogenic diet. J. Anim. Plant. Sci. 2012, 22, 872–877. [Google Scholar]
- Mazzio E. A.; Soliman K. F. In vitro screening for the tumoricidal properties of international medicinal herbs. Phytother. Res. 2009, 23 (3), 385–398. 10.1002/ptr.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon L. J.; van Rooijen J. J.; Niesen B.; Verhagen H.; Saris W. H.; Wagenmakers A. J. Effects of acute (−)-hydroxycitrate supplementation on substrate metabolism at rest and during exercise in humans. Am. J. Clin. Nutr. 2000, 72 (6), 1445–1450. 10.1093/ajcn/72.6.1445. [DOI] [PubMed] [Google Scholar]
- Antony B.; Varghese W.; Elias M. Spectrophotometric determination of hydroxy citric acid. Indian J. Pharm. Sci. 1999, 61 (5), 316. [Google Scholar]
- Bheemaiah M. M.; Kushalappa B. A. Estimation and comparison of amount of organic acids from dried leaves of Garcinia cambogia, Garcinia indica, Garcinia xanthochymus, and Garcinia morella by high-performance liquid chromatography. Pharmacogn. Res. 2019, 11 (1), 86. 10.4103/pr.pr_159_18. [DOI] [Google Scholar]
- Jayaprakasha G.; Sakariah K. Determination of (−) hydroxycitric acid in commercial samples of Garcinia cambogia extract by liquid chromatography with ultraviolet detection. Journal of Liquid Chromatography & Related Technologies 2000, 23, 915. 10.1081/JLC-100101498. [DOI] [Google Scholar]
- Lee H.-J.; Na Y.-G.; Han M.; Pham T. M. A.; Lee H.; Lee H.-K.; Myung C.-S.; Han J.-H.; Kang J.-S.; Kim K.-T. Statistical Design of Sustained-Release Tablet Garcinia cambogia Extract and Bioconverted Mulberry Leaf Extract for Anti-Obesity. Pharmaceutics 2020, 12 (10), 932. 10.3390/pharmaceutics12100932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein Junior L. C.; Antunes M. V.; Linden R.; Vasques C. A. Quantification of (−) hydroxycitric acid in marketed extracts of Garcinia cambogia by high performance liquid chromatography. Lat. Am. J. Pharm. 2010, 29 (5), 835–838. [Google Scholar]
- Vorarat S.; Aromdee C.; Podokmai Y. Determination of alpha hydroxy acids in fruits by capillary electrophoresis. Anal. Sci. 2002, 18 (8), 893–896. 10.2116/analsci.18.893. [DOI] [PubMed] [Google Scholar]
- Loe Y.-c. C; Bergeron N.; Rodriguez N.; Schwarz J.-M. Gas chromatography/mass spectrometry method to quantify blood hydroxycitrate concentration. Anal. Biochem. 2001, 292 (1), 148–154. 10.1006/abio.2001.5046. [DOI] [PubMed] [Google Scholar]
- Farag M. A.; Khattab A. R.; Shamma S.; Afifi S. M. Profiling of Primary Metabolites and Volatile Determinants in Mahlab Cherry (Prunus mahaleb L.) Seeds in the Context of Its Different Varieties and Roasting as Analyzed Using Chemometric Tools. Foods 2021, 10 (4), 728. 10.3390/foods10040728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag M. A.; Sharaf El-Din M. G.; Selim M. A.; Owis A. I.; Abouzid S. F.; Porzel A.; Wessjohann L. A.; Otify A. Nuclear Magnetic Resonance Metabolomics Approach for the Analysis of Major Legume Sprouts Coupled to Chemometrics. Molecules 2021, 26 (3), 761. 10.3390/molecules26030761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seethapathy G. S.; Tadesse M.; Urumarudappa S. K. J.; Gunaga S. V.; Vasudeva R.; Malterud K. E.; Shaanker R. U.; de Boer H. J.; Ravikanth G.; Wangensteen H. Authentication of Garcinia fruits and food supplements using DNA barcoding and NMR spectroscopy. Sci. Rep. 2018, 8 (1), 1–12. 10.1038/s41598-018-28635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K.; Kumar S. Identification and quantification of two biologically active polyisoprenylated benzophenones xanthochymol and isoxanthochymol in Garcinia species using liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2006, 844 (1), 67–83. 10.1016/j.jchromb.2006.07.045. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K.; Kumar S. Liquid chromatography–tandem mass spectrometry method for identification and quantification of two biologically active polyisoprenylated benzophenones, isoxanthochymol and camboginol, in Garcinia species. Biomed. Chromatogr. 2007, 21 (11), 1159–1165. 10.1002/bmc.868. [DOI] [PubMed] [Google Scholar]
- Kumar S.; Sharma S.; Chattopadhyay S. K. High-performance liquid chromatography and LC-ESI-MS method for identification and quantification of two isomeric polyisoprenylated benzophenones isoxanthochymol and camboginol in different extracts of Garcinia species. Biomed. Chromatogr. 2009, 23 (8), 888–907. 10.1002/bmc.1202. [DOI] [PubMed] [Google Scholar]
- Kureshi A. A.; Dholakiya C.; Hussain T.; Mirgal A.; Salvi S. P.; Barua P. C.; Talukdar M.; Beena C.; Kar A.; Zachariah T. J. Simultaneous Identification and Quantification of Three Xanthones and Two Polyisoprenylated Benzophenones in Eight Indian Garcinia Species Using a Validated UHPLC-PDA Method. J. AOAC Int. 2019, 102 (5), 1423–1434. 10.5740/jaoacint.18-0335. [DOI] [PubMed] [Google Scholar]
- de Carvalho Cruz A.; Suenaga E. M.; Prova S. S.; de Oliveira Neto J. R.; Ifa D. R.; da Cunha L. C. New bioanalytical method for the quantification of (−)–hydroxycitric acid in human plasma using UPLC-MS/MS and its application in a Garcinia cambogia pharmacokinetic study. J. Pharm. Biomed. Anal. 2020, 188, 113385. 10.1016/j.jpba.2020.113385. [DOI] [PubMed] [Google Scholar]
- Bhutani P.; U. R.; H. N. S.; Ranjanna P. K.; Paul A. T. Rapid and cost-effective LC–MS/MS method for determination of hydroxycitric acid in plasma: Application in the determination of pharmacokinetics in commercial Garcinia preparations. Biomed. Chromatogr. 2020, 34 (10), e4902. 10.1002/bmc.4902. [DOI] [PubMed] [Google Scholar]
- Mutlu E.; Pierfelice J.; Cao Y.; Djonabaye A.; Gleason S.; Burback B.; Waidyanatha S. Development and Validation of an Analytical Method to Quantitate Hydroxycitric Acid, the Key Constituent in Garcinia cambogia Extract, in Rodent Plasma and Fetus. Anal. Lett. 2022, 55 (9), 1382–1397. 10.1080/00032719.2021.2005618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz A. C.; Pinto A. H.; Costa C. D.; Oliveira L. P.; Oliveira-Neto J. R.; Cunha L. C. Food-Effect on (−)–Hydroxycitric Acid Absorption After Oral Administration of Garcinia cambogia Extract Formulation: a Phase I, Randomized, Cross-Over Study. J. Pharm. Sci. 2021, 110 (2), 693–697. 10.1016/j.xphs.2020.10.035. [DOI] [PubMed] [Google Scholar]
- Peng M.; Han J.; Li L.; Ma H. Metabolomics reveals the mechanism of (−)-hydroxycitric acid promotion of protein synthesis and inhibition of fatty acid synthesis in broiler chickens. animal 2018, 12 (4), 774–783. 10.1017/S175173111700221X. [DOI] [PubMed] [Google Scholar]
- Lewis Y. [77] Isolation and properties of hydroxycitric acid. Methods in enzymology 1969, 13, 613–619. 10.1016/0076-6879(69)13085-2. [DOI] [Google Scholar]
- Lewis Y.; Neelakantan S. (−)-Hydroxycitric acid—the principal acid in the fruits of Garcinia cambogia desr. Phytochemistry 1965, 4 (4), 619–625. 10.1016/S0031-9422(00)86224-X. [DOI] [Google Scholar]
- Moffett S. A.; Bhandari A. K.; Ravindranath B. Hydroxycitric acid concentrate and food products prepared therefrom. Biotechnol. Adv. 1997, 15 (1), 276–276. 10.1016/S0734-9750(97)88562-X. [DOI] [Google Scholar]
- Parthasarathi S.; Ezhilarasi P.; Jena B.; Anandharamakrishnan C. A comparative study on conventional and microwave-assisted extraction for microencapsulation of Garcinia fruit extract. Food Bioprod. Process. 2013, 91 (2), 103–110. 10.1016/j.fbp.2012.10.004. [DOI] [Google Scholar]
- Gan C.-Y.; Latiff A. A. Extraction of antioxidant pectic-polysaccharide from mangosteen (Garcinia mangostana) rind: Optimization using response surface methodology. Carbohydr. Polym. 2011, 83 (2), 600–607. 10.1016/j.carbpol.2010.08.025. [DOI] [Google Scholar]
- Cheok C. Y.; Chin N. L.; Yusof Y. A.; Talib R. A.; Law C. L. Optimization of total phenolic content extracted from Garcinia mangostana Linn. hull using response surface methodology versus artificial neural network. Ind. Crops Prod. 2012, 40, 247–253. 10.1016/j.indcrop.2012.03.019. [DOI] [Google Scholar]
- Mohammad N. A.; Zaidel D. N. A.; Muhamad I. I.; Hamid M. A.; Yaakob H.; Jusoh Y. M. M. Optimization of the antioxidant-rich xanthone extract from mangosteen (Garcinia mangostana L.) pericarp via microwave-assisted extraction. Heliyon 2019, 5 (10), e02571. 10.1016/j.heliyon.2019.e02571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nainegali B. S.; Iyyaswami R.; Belur P. D. Partitioning of bio-active compounds from rinds of garcinia indica using aqueous two-phase system: Process evaluation and optimization. Sep. Purif. Technol. 2020, 253, 117520. 10.1016/j.seppur.2020.117520. [DOI] [Google Scholar]
- Carrillo-Hormaza L.; Duque L.; López-Parra S.; Osorio E. High-intensity ultrasound-assisted extraction of Garcinia madruno biflavonoids: Mechanism, kinetics, and productivity. Biochem. Eng. J. 2020, 161, 107676. 10.1016/j.bej.2020.107676. [DOI] [Google Scholar]
- Ng H.-S.; Tan G. Y. T.; Lee K.-H.; Zimmermann W.; Yim H. S.; Lan J. C.-W. Direct recovery of mangostins from Garcinia mangostana pericarps using cellulase-assisted aqueous micellar biphasic system with recyclable surfactant. J. Biosci. Bioeng. 2018, 126 (4), 507–513. 10.1016/j.jbiosc.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Ibrahim M. Y.; Hashim N. M.; Mariod A. A.; Mohan S.; Abdulla M. A.; Abdelwahab S. I.; Arbab I. A. α-Mangostin from Garcinia mangostana Linn: an updated review of its pharmacological properties. Arabian J. Chem. 2016, 9 (3), 317–329. 10.1016/j.arabjc.2014.02.011. [DOI] [Google Scholar]
- Mulia K.; Krisanti E.; Terahadi F.; Putri S. Selected natural deep eutectic solvents for the extraction of α-mangostin from mangosteen (Garcinia mangostana L.) pericarp. Int. J. Technol. 2015, 6 (7), 1211–1220. 10.14716/ijtech.v6i7.1984. [DOI] [Google Scholar]
- Adetunji C. O.; Michael O. S.; Rathee S.; Singh K. R.; Ajayi O. O.; Adetunji J. B.; Ojha A.; Singh J.; Singh R. P. Potentialities of nanomaterials for the management and treatment of metabolic syndrome: A new insight. Mater. Today Adv. 2022, 13, 100198. 10.1016/j.mtadv.2021.100198. [DOI] [Google Scholar]
- Rafique M.; Sadaf I.; Rafique M. S.; Tahir M. B. A review on green synthesis of silver nanoparticles and their applications. Artif. Cells, Nanomed., Biotechnol. 2017, 45 (7), 1272–1291. 10.1080/21691401.2016.1241792. [DOI] [PubMed] [Google Scholar]
- Kumar B.; Smita K., Scope of nanotechnology in nutraceuticals. In Nanotechnology Applications in Food; Elsevier: 2017; pp 43–63. [Google Scholar]
- Müller R. H.; Mäder K.; Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery–a review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50 (1), 161–177. 10.1016/S0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- Aishwarya S.; Gayathri R. Synthesis of silver nanoparticles from Garcinia cambogia extract and its antimicrobial efficacy. Int. J. Res. Pharm. Sci. 2018, 9 (2), 263–267. 10.26452/IJRPS.V9I2.1435. [DOI] [Google Scholar]
- Mannarmannan M.; Biswas K. Biological Activity of ZnO Nanoparticles Synthesized from the Dried Rinds of Garcinia Gummi Gutta. ChemistrySelect 2019, 4 (43), 12739–12742. 10.1002/slct.201903159. [DOI] [Google Scholar]
- Dewanjee S.; Chakraborty P.; Mukherjee B.; De Feo V. Plant-based antidiabetic nanoformulations: the emerging paradigm for effective therapy. Int. J. Mol. Sci. 2020, 21 (6), 2217. 10.3390/ijms21062217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulangamuwa A. C.; Ediriweera M. K.; Rajagopalan U.; Karunaratne D. N.; Tennekoon K. H.; Samarakoon S. R. Development of a New Nanocarrier for Dietary Garcinol: Characterization and In Vitro Efficacy Evaluation Using Breast Cancer Stem Cells Grown in Hypoxia. J. Food Quality 2021, 2021, 6654211. 10.1155/2021/6654211. [DOI] [Google Scholar]
- Jacob E. M.; Borah A.; Pillai S. C.; Kumar D. S. Garcinol Encapsulated Ph-Sensitive Biodegradable Nanoparticles: A Novel Therapeutic Strategy for the Treatment of Inflammatory Bowel Disease. Polymers 2021, 13 (6), 862. 10.3390/polym13060862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezhilarasi P.; Muthukumar S.; Anandharamakrishnan C. Solid lipid nanoparticle enhances bioavailability of hydroxycitric acid compared to a microparticle delivery system. RSC Adv. 2016, 6 (59), 53784–53793. 10.1039/C6RA04312G. [DOI] [Google Scholar]
- Vassallo A.; Santoro V.; Pappalardo I.; Santarsiero A.; Convertini P.; De Luca M.; Martelli G.; Infantino V.; Caddeo C. Liposome-mediated inhibition of inflammation by hydroxycitrate. Nanomaterials 2020, 10 (10), 2080. 10.3390/nano10102080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan-In P.; Tachapruetinun A.; Chaichanawongsaroj N.; Banlunara W.; Suksamrarn S.; Wanichwecharungruang S. Combating Helicobacter pylori infections with mucoadhesive nanoparticles loaded with Garcinia mangostana extract. Nanomedicine 2014, 9 (3), 457–468. 10.2217/nnm.13.30. [DOI] [PubMed] [Google Scholar]