Abstract

Changes in various physicochemical properties (P(n)) of organic compounds with the number of carbon atoms (n) can be roughly divided into linear and nonlinear changes. To date, there has been no general equation to express nonlinear changes in the properties of organic homologues. This study proposes a general equation expressing nonlinear changes in the physicochemical properties of organic homologues, including boiling point, viscosity, ionization potential, and vapor pressure, named the “NPOH equation”, as follows: P(n) = P(1)αn – 1e∑i=2n(β/(i – 1)) where α and β are adjustable parameters, and P(1) represents the property of the starting compound (pseudo-value at n = 1) of each homologue. The results show that various nonlinear changes in the properties of homologues can be expressed by the NPOH equation. Linear and nonlinear changes in the properties of homologues can all be correlated with n and the “sum of carbon number effects”, ∑i=2n(1/i – 1). Using these two parameters, a quantitative correlation equation can be established between any two properties of each homologue, providing convenient mutual estimation of the properties of a homologue series. The NPOH equation can also be used in property correlation for structures with functionality located elsewhere along a linear alkyl chain as well as for branched organic compounds. This work can provide new perspectives for studying quantitative structure–property relationships.
1. Introduction
In 2021, Kontogeorgis et al.1 investigated the industrial requirements for thermodynamic and transport properties, reporting that: “In terms of models, companies ideally wish for a single universal model for all/many applications, but there is understanding that this is possibly utopian. The second major wish is the need for predictive models validated on extensive experimental databases and not only on just a few available experimental data points.” This shows the importance of molecular modeling. The quantitative structure–property relationship (QSPR) method is important for understanding and predicting compound properties and it has seen rapid development.2,3 Recently, molecular dynamics and machine learning have also been used in drug design.4
The two key steps for developing successful QSPR models are molecular descriptor calculations and equation selection. Generally, the development of validated QSPR models first involves calculating the molecular descriptors and then, based on experimental data, selecting an appropriate number of descriptors from a large number of calculated molecular descriptors to establish the QSPR equation.2,5−9 In this method, descriptor screening and equation optimization are time-consuming, and the physical meaning of the resulting equations is unclear and difficult to interpret. Other methods are also available for predicting the physicochemical properties of compounds. In these methods, theoretical equations are first proposed and molecular characteristic parameters are extracted for specific compounds. Data fitting is then used to form specific application equations, such as in state equation 1(2)
| 1 |
where u and w are characteristics of the equation (u = 1 and w = 0 or u = 2 and w = −1 for Redlich-Kwong10 or Peng–Robinson (PR),11 respectively), and a and b are parameters computed using critical pressure (Pc) and temperature (Tc), as shown in eqs 2 and 3
| 2 |
| 3 |
where Ωa and Ωb have specific values depending on the selected equation, and the temperature function, α(T), is equal to 1 for T = Tc. Similar to eq 1, these theoretical equations have clear physical meanings, allowing molecular descriptors to be calculated in a targeted manner. However, this type of equation is not easy to propose.
The aforementioned methods show that a QSPR equation can be established more easily if the general regularity of changes in compound properties is known in advance and then molecular descriptors are selected. Therefore, we have considered classifying chain organic compounds and discussing the regularity of changes in their properties, which is more conducive to establishing QSPR equations. Accordingly, organic compounds with different functional groups were first divided into homologues and then into isomers. Following the concept of homologue–isomer property correlation, we can establish a structure–property correlation equation for chain organic compounds with different functional groups. Changes in the properties (P) of organic homologues are known to be related to the number of carbon atoms (n), allowing a theoretical equation between P and n, P = f(n), to be established. On this basis, the characteristic parameter of the molecular structure of an isomer (s) can be extracted using the molecular descriptor calculation in the QSPR method, and then the property-change equation of the chain organic compound (P = f(n, s)) can be established. This accounts for the advantages of the two aforementioned methods and reduces the difficulty of establishing structure–property correlation equations. This study aimed to explore a general equation for expressing changes in the physicochemical properties of organic homologues.
Much research has focused on expressing the regularity of property changes in organic homologues, with four types of empirical expression having been proposed. As early as 1961, Kreglewsky12 proposed eq 4 to evaluate the boiling points of n-paraffin compounds in the range of C1–C100. This work pioneered the quantitative correlation of homologue properties
| 4 |
where Y is the boiling point value at carbon number n and Y0 is the boiling point pseudo-value at n = 0, Y∞ = 1078, and β = 4.999 × 10–3.
In 1985, Gasem and Robinson et al.13,14 proposed eq 5 for evaluating the critical properties of n-paraffins, known as the ABC equation
| 5 |
where variables Y, Y∞, and Y0 are property values at carbon number n, the limiting property value as n becomes very large, and the property pseudo-value at n = 1, respectively, and β and α are adjustable parameters.
In 1997, Marano and Holder15 proposed eq 6 to correlate the thermodynamic and transport properties of n-paraffins and other homologous series, which can be used for a wide range of different thermophysical properties
| 6 |
where five adjustable parameters, n0, Y0, Y∞, β, and γ, are employed.
In 2009, Zenkevich16 employed a recurrent function to correlate the properties of homologous series, as shown in eq 7
| 7 |
where A(n + 1) is the physicochemical property value for homologues with (n + 1) carbon atoms, A(n) is the property value for homologues with n carbon atoms, and coefficients a and b are calculated by the least squares method. Equation 7 is different from eqs 4–6 because the parameter Y∞ is not required.
Notably, eq 4 was proposed more than 60 years ago, and the inherent change regularity of homologue properties with carbon number n remains unknown. The equations proposed previously provide great insight, but two problems remain: (i) Their physical meaning is unclear, making them difficult to explain; and (ii) they cannot correlate one property to another property for a series of homologues. Therefore, this study investigated the change regularity of homologue properties with carbon atom number n to explore the relationship between the properties of homologues.
2. Results and Discussion
2.1. Theoretical Analysis
Changes in the physicochemical properties (P) of organic homologues with carbon atom number n can be roughly divided into linear and nonlinear changes. The linear change of a property is relatively simple and will not be discussed in detail here. This study focuses on the complex nonlinear change of a property. Using n-paraffins (H–(CH2)n–H) as an example, some P values increase with an increasing n value, while some P values decrease with an increasing n value, The P changes show significant differences, as shown in Figure 1. We investigated how these different changes are dominantly affected by n, and whether all of these changes follow a common rule, through theoretical analysis. Usually, organic homologues can be represented as X–(A)n–Y, where A is a repeating structural unit, n is the number of repeating structural units, and X and Y are end groups. For n-paraffins, A is CH2, and both X and Y are H atoms, represented by H–(CH2)n–H. In a series of organic homologues, the property of compound X–(A)n–Y containing n repeating structural units is represented by P(n), and the property of another compound containing n – 1 repeating structural units X–(A)n−1–Y is represented by P(n–1). The molecular structure of X–(A)n–Y is known to be formed by adding repeating structural unit A to molecule X–(A)n−1–Y, namely, X–(A)n−1–Y becomes X–(A)n−1–A–Y. The addition of unit A causes a change of property P(n–1) of X–(A)n−1–Y to property P(n) of X–(A)n–Y. Therefore, property P(n) of X–(A)n–Y can be considered a perturbation based on property P(n–1) of X–(A)n−1–Y. This perturbation effect must be related to the original molecular chain length (A)n−1. Assuming that the perturbation effect acts in the form of an exponential, exp(β/(n – 1)), then, the relationship between P(n) and P(n–1) can be expressed by eq 8
| 8 |
where α is the proportional coefficient of the property change and β is an adjustable parameter of the perturbation effect. Based on eq 8, expressions for P(2), P(3), P(4), ..., can be deduced as follows
| 9 |
| 10 |
| 11 |
From eqs 9–11, eq 8 can also be expressed as eq 12
| 12 |
Equation 12 expresses the relationship between the property of organic homologues X–(A)n–Y and the number of repeating structural units, n. If the logarithm is taken, eq 12 can be expressed as eq 13
| 13 |
where a = ln(P(1)), b = ln(α), and c = β. These are characteristic coefficients related to the
properties of each homologue and can be obtained by regression analysis.
For convenience, term 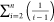 in eqs 12 and 13 is subsequently denoted
as SCNE (abbreviation of “sum of
carbon number effects”). Accordingly, eqs 12 and 13 can be written
in compact forms of eqs 14 and 15, respectively, named the “NPOH
equation” (that is, the abbreviation of “Nonlinear Properties
of Organic Homologues equation”)
in eqs 12 and 13 is subsequently denoted
as SCNE (abbreviation of “sum of
carbon number effects”). Accordingly, eqs 12 and 13 can be written
in compact forms of eqs 14 and 15, respectively, named the “NPOH
equation” (that is, the abbreviation of “Nonlinear Properties
of Organic Homologues equation”)
| 14 |
| 15 |
In contrast to eqs 4–7, eqs 12 and 14 show that property P(n) of homologue X–(A)n–Y is related to the starting compound property P(1) (property pseudo-value at n = 1) of each homologue, as well as the number of repeating structural units, n, and the cumulative perturbation effect, SCNE, of repeating structural units.
Figure 1.

(a) Relationship of boiling point (Tb), (b) viscosity (μ), and (c) ionization potential (Ip) with the carbon atom number (n) of n-paraffins.
2.2. Applicability of NPOH Equation
2.2.1. Correlation with the Properties of n-Paraffins
To test the applicability of NPOH equations 14 or 15, quantitative correlation analysis was conducted using various physicochemical properties (nonlinear changes) of n-paraffins. First, the SCNE value was calculated, as listed in Table 1, and then eq 15 was employed as a model to perform regression analysis of properties P(n) of n-paraffins. The results are listed in Table 2.
Table 1. Number of Repeating Structural Units, n, and SCNE Values.
| n | n – 1 | SCNE | n | n – 1 | SCNE | n | n – 1 | SCNE | n | n – 1 | SCNE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0 | 26 | 25 | 3.8160 | 51 | 50 | 4.4992 | 76 | 75 | 4.9014 | |
| 2 | 1 | 1.0000 | 27 | 26 | 3.8544 | 52 | 51 | 4.5188 | 77 | 76 | 4.9145 |
| 3 | 2 | 1.5000 | 28 | 27 | 3.8915 | 53 | 52 | 4.5380 | 78 | 77 | 4.9275 |
| 4 | 3 | 1.8333 | 29 | 28 | 3.9272 | 54 | 53 | 4.5569 | 79 | 78 | 4.9403 |
| 5 | 4 | 2.0833 | 30 | 29 | 3.9617 | 55 | 54 | 4.5754 | 80 | 79 | 4.9530 |
| 6 | 5 | 2.2833 | 31 | 30 | 3.9950 | 56 | 55 | 4.5936 | 81 | 80 | 4.9655 |
| 7 | 6 | 2.4500 | 32 | 31 | 4.0272 | 57 | 56 | 4.6115 | 82 | 81 | 4.9778 |
| 8 | 7 | 2.5929 | 33 | 32 | 4.0585 | 58 | 57 | 4.6290 | 83 | 82 | 4.9900 |
| 9 | 8 | 2.7179 | 34 | 33 | 4.0888 | 59 | 58 | 4.6463 | 84 | 83 | 5.0021 |
| 10 | 9 | 2.8290 | 35 | 34 | 4.1182 | 60 | 59 | 4.6632 | 85 | 84 | 5.0140 |
| 11 | 10 | 2.9290 | 36 | 35 | 4.1468 | 61 | 60 | 4.6799 | 86 | 85 | 5.0257 |
| 12 | 11 | 3.0199 | 37 | 36 | 4.1746 | 62 | 61 | 4.6963 | 87 | 86 | 5.0374 |
| 13 | 12 | 3.1032 | 38 | 37 | 4.2016 | 63 | 62 | 4.7124 | 88 | 87 | 5.0489 |
| 14 | 13 | 3.1801 | 39 | 38 | 4.2279 | 64 | 63 | 4.7283 | 89 | 88 | 5.0602 |
| 15 | 14 | 3.2516 | 40 | 39 | 4.2535 | 65 | 64 | 4.7439 | 90 | 89 | 5.0715 |
| 16 | 15 | 3.3182 | 41 | 40 | 4.2785 | 66 | 65 | 4.7593 | 91 | 90 | 5.0826 |
| 17 | 16 | 3.3807 | 42 | 41 | 4.3029 | 67 | 66 | 4.7744 | 92 | 91 | 5.0936 |
| 18 | 17 | 3.4396 | 43 | 42 | 4.3267 | 68 | 67 | 4.7894 | 93 | 92 | 5.1044 |
| 19 | 18 | 3.4951 | 44 | 43 | 4.3500 | 69 | 68 | 4.8041 | 94 | 93 | 5.1152 |
| 20 | 19 | 3.5477 | 45 | 44 | 4.3727 | 70 | 69 | 4.8186 | 95 | 94 | 5.1258 |
| 21 | 20 | 3.5977 | 46 | 45 | 4.3949 | 71 | 70 | 4.8328 | 96 | 95 | 5.1363 |
| 22 | 21 | 3.6454 | 47 | 46 | 4.4167 | 72 | 71 | 4.8469 | 97 | 96 | 5.1468 |
| 23 | 22 | 3.6908 | 48 | 47 | 4.4380 | 73 | 72 | 4.8608 | 98 | 97 | 5.1571 |
| 24 | 23 | 3.7343 | 49 | 48 | 4.4588 | 74 | 73 | 4.8745 | 99 | 98 | 5.1673 |
| 25 | 24 | 3.7760 | 50 | 49 | 4.4792 | 75 | 74 | 4.8880 | 100 | 99 | 5.1774 |
Table 2. Correlation Equations of Properties for n-Paraffins H–(CH2)n–H (Model Equation: ln(P(n)) = a + b(n – 1) + cSCNE).
| no. | propertya | range of nb | ref | a | b | c | R | S | Nc | F |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Tb | 2–100 | (17) | 4.6973 | –0.004569 | 0.51040 | 0.9999 | 0.0057 | 34 | 73 691.18 |
| 2 | Tc | 2–20 | (20) | 5.2972 | –0.008981 | 0.42758 | 0.9999 | 0.0030 | 19 | 65 747.43 |
| 3 | Pc | 2–20 | (20) | 1.9097 | –0.050147 | –0.24698 | 0.9992 | 0.0191 | 19 | 5084.85 |
| 4 | Cp | 4–16 | (21) | 3.6382 | 0.025666 | 0.66368 | 0.9997 | 0.0099 | 11 | 7888.46 |
| 5 | λ | 5–16 | (22) | –2.7579 | –0.014647 | 0.30762 | 0.9944 | 0.0101 | 7 | 177.44 |
| 6 | ST | 5–16 | (22) | 1.1369 | –0.048077 | 0.86717 | 0.9990 | 0.0108 | 7 | 978.56 |
| 7 | μ | 5–35 | (18) | –5.6286 | 0.043485 | 1.79992 | 0.9996 | 0.0448 | 28 | 14 301.74 |
| 8 | nD | 4–20 | (21) | 0.15997 | –0.003447 | 0.076226 | 0.9987 | 0.0012 | 16 | 2534.24 |
| 9 | Pv | 2–16 | (21) | 7.4528 | –0.461790 | –0.36834 | 1.0000 | 0.0103 | 15 | 347 794.19 |
| 10 | ω | 2–20 | (23) | –3.2314 | –0.005694 | 0.90514 | 0.9997 | 0.0158 | 19 | 13 904.65 |
| 11 | Ip | 2–11 | (19) | 2.5643 | 0.004981 | –0.12156 | 0.9996 | 0.0020 | 10 | 4094.40 |
| 12 | D | 5–16 | (19) | –1.0445 | –0.016174 | 0.30966 | 0.9994 | 0.0025 | 12 | 3850.05 |
| 13 | TF | 3–16 | (24) and (25) | 4.4783 | 0.000031 | 0.45456 | 0.9970 | 0.0213 | 14 | 915.62 |
| 14 | Pw | 2–8 | (26) | 1.1261 | 0.536478 | 0.12495 | 0.9990 | 0.0716 | 6 | 736.35 |
Tb, boiling point (K); Tc, critical temperature (K); Pc, critical pressure (MPa); Cp, heat capacity (J·mol–1·K–1); λ, thermal conductivity (W·m–1 K–1); ST, surface tension (mN·m–1); μ, liquid viscosity [μm Pa·s(300 K)]; nD, refractive Index; Pv, vapor pressure (regression in the form of log Pv); ω, acentric factor; Ip, ionization potential (eV); D, liquid density (g·cm3); TF, flash point (K); and Pw, partition coefficient (regression in the form of log Pw). These properties data are listed in the Supporting Information.
Carbon atom number range.
Number of data points.
The results listed in Table 2 show that the correlation coefficients (R) were all above 0.99 for the correlation equations of 14 properties of n-paraffins, indicating that eq 15 can be used to express the regularity of property changes among homologues using the carbon atom number n. That properties with different change regularities can have the same expression is surprising and has not been reported previously.
We noted that a value of boiling point Tb equation in Table 2 (no. 1) is 4.6973, indicating that ln(P(1)) is 4.6973, and the starting compound boiling point (pseudo-value at n = 1), P(1), is 109.65 K for n-paraffins. The experimental boiling point of methane is known to be 109.15 K, which is very close to 109.65 K.
Interestingly, for the linear change property, PLC(n), of homologues, it was only necessary to directly replace term ln(P(n)) in eq 15 with term PLC(n), as shown in eq 16
| 16 |
For example, for the critical volume21 (Vc) of n-paraffins (n = 2–18), n was used directly to perform regression against Vc, giving eq 17, while eq 16 was used as the model equation to perform regression against Vc to obtain eq 18. Equation 18 showed better correlation compared with eq 17, and the standard error (S) was greatly reduced
| 17 |
| 18 |
As another example, the vaporization enthalpy21 (Hv) of n-paraffins (n = 2–20) was regressed with the methods used in eqs 17 and 18, giving eqs 19 and 20. The correlation of eq 20 was better than that of eq 19, and the standard error (S) of eq 20 was greatly reduced
| 19 |
| 20 |
The above regression analysis results show that eq 16 is more suitable for expressing the linear change properties of homologues compared with directly using n. However, for some linear change properties of homologues, such as molar mass (Mmol), coefficient c in eq 16 was equal to zero, meaning that the term SCNE did not need to be used in such cases.
2.2.2. Correlation with the Properties of Other Homologues
Regarding whether eq 15 was applicable to other homologues, cycloalkanes, terminal alkenes, terminal alkynes, linear alcohols, 2-methylparaffins, 2,2-dimethylparaffins, 2,3-dimethylparaffins, and 1-cyclopentylparaffins were selected as model compounds for testing. Using the boiling points Tb of cycloalkanes, terminal alkenes, terminal alkynes, linear alcohols, 2-methylparaffins, 2,2-dimethylparaffins, 2,3-dimethylparaffins, and 1-cyclopentylparaffins and the partition coefficient Pw of alcohols as verification examples, quantitative correlation analysis was conducted using model equation15. The results are listed in Table 3, showing that the property correlation was very good for each homologue, and that the correlation coefficients (R) of the nine equations were all greater than 0.999. These results showed that eq 15 is a general equation that can be used to express the change regularity of properties P of various homologues using carbon atom number n.
Table 3. Correlation Equations of Properties for Cycloalkanes, Alkenes, Alkynes, Alcohols, 2-Methylparaffins, 2,2-dimethylparaffins, 2,3-Dimethylparaffins, and 1-Cyclopentylparaffins (Model Equation: ln(P(n)) = a + b(n – 1) + cSCNE).
Pw, partition coefficient (regression in the form of logPw); other symbols are defined in Table 1. These properties data are listed in the Supporting Information.
Carbon atom number range.
Number of data points.
The results obtained in Sections 2.2.1 and 2.2.2 show that, regardless of whether the properties of homologues vary linearly or nonlinearly with carbon atom number n, they can be related using variables (n – 1) and SCNE.
2.2.3. Relationship between Nonlinear Properties of Homologues
Equation 15 not only expresses the change regularity of the properties of homologues with the carbon atom number n but can also link the change regularities of different properties of homologues. If one property of homologue is P(n) and the other property is P′(n), basing on eq 15, we can theoretically deduce eqs 21 and 22
| 21 |
| 22 |
In eq 21, ar = ln(P(1)) – ln(P′(1)), br = ln(α) – ln(α′), and cr = β – β′, while in eq 22, as = ln(P(1)) + ln(P′(1)), bs = ln(α) + ln(α′), and cs = β + β′. For the two specific physicochemical properties, P(n) and P′(n), of the same series of compounds, parameters ar, br, cr, as, bs, and cs can be obtained using the regression method.
When the logarithms of eqs 21 and 22 are removed, they return to eqs 23 and 24
| 23 |
| 24 |
Equations 23 and 24 express the relationship between two properties of a homologues series, where k, l, cr, m, q, and cs are all coefficients. Therefore, one property of homologues can be correlated with another using eqs 23 and 24, providing convenient correlation of the homologue properties.
For example, the relationship between the critical temperature (Tc) and boiling point (Tb) of n-paraffins is shown in eq 25
| 25 |
Equation 25 expresses the quantitative relationship between Tb and Tc for n-paraffins. Using eq 25, the critical temperatures of n-paraffins can be calculated from their boiling points. Table 4 shows the results of critical temperature calculations for n-paraffins with n = 2–32, where the average absolute error between the experimental value (Tc,exp.) and the calculated value (Tc,cal.) was 1.84 K for the 19 compounds (n = 2–20). Figure 2 shows a plot of the calculated and experimental values of critical temperature versus the boiling point of n-paraffins.
Table 4. Experimental and Calculated Critical Temperature, Tc (K), for n-Paraffins H–(CH2)n–H.
| na | Tb,exp.b | Tc,exp.c | Tc,cal.d | na | Tb,exp.b | Tc,exp.c | Tc,cal.d | na | Tb,exp.b | Tc,exp.c | Tc,cal.d |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 184.55 | 305.6 | 307.84 | 13 | 508.29 | 676.1 | 678.36 | 24 | 664.29 | 793.90 | |
| 3 | 231.05 | 369.2 | 368.85 | 14 | 526.69 | 693.1 | 694.86 | 25 | 674.29 | 798.76 | |
| 4 | 272.65 | 425.2 | 421.91 | 15 | 543.59 | 708.1 | 709.23 | 26 | 685.19 | 804.63 | |
| 5 | 309.25 | 469.9 | 466.84 | 16 | 559.99 | 722.1 | 722.82 | 27 | 695.09 | 809.28 | |
| 6 | 342.15 | 507.7 | 505.80 | 17 | 574.79 | 734.1 | 734.23 | 28 | 704.59 | 813.41 | |
| 7 | 371.55 | 540.7 | 539.25 | 18 | 589.09 | 747.1 | 744.90 | 29 | 713.79 | 817.16 | |
| 8 | 398.85 | 568.9 | 569.35 | 19 | 601.99 | 755.4 | 753.71 | 30 | 722.69 | 820.52 | |
| 9 | 423.92 | 594.6 | 596.00 | 20 | 615.99 | 767.6 | 763.81 | 31 | 731.69 | 823.96 | |
| 10 | 447.27 | 617.8 | 620.00 | 21 | 629.49 | 773.20 | 32 | 740.09 | 826.68 | ||
| 11 | 468.79 | 639.1 | 641.24 | 22 | 641.59 | 780.77 | |||||
| 12 | 489.29 | 658.2 | 660.90 | 23 | 653.19 | 787.67 |
Figure 2.
Plot of calculated (solid line) and experimental (open circle) critical temperature (Tc) values versus experimental boiling point (Tb) of n-paraffins (n = 2–32).
As further examples, eqs 23 and 24 were used as model equations to quantitatively correlate the boiling point of n-paraffins with other properties in Table 2. The results are listed in Table 5, showing that any nonlinear change property of n-paraffins could be correlated with the boiling point. As another example, the liquid viscosity (μ) equation in Table 5 (no. 6) was used to calculate the μ values (at 300 K) of n-paraffins using boiling points (Tb), with the results shown in Figure 3. The calculated values were in good agreement with the experimental values.
Table 5. Correlation Equations between the Boiling Point, Tb, and Other Properties of n-Paraffins (Model Equations 23 and 24).
| no. | propertya | range of n | ar(as) | br(bs) | cr(cs) | R | S | N | F |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Tc | 2–20 | 0.593807 | –0.0056465 | –0.07651 | 0.9989 | 0.0042 | 19 | 3715.10 |
| 2 | Pc | 2–20 | –2.79364 | –0.0468120 | –0.75107 | 0.9996 | 0.0235 | 19 | 10 289.86 |
| 3 | Cp | 4–16 | –1.01102 | 0.0314657 | 0.13146 | 0.9988 | 0.0095 | 11 | 1670.97 |
| 4 | λ | 5–16 | –7.39959 | –0.0084513 | –0.22859 | 0.9979 | 0.0104 | 7 | 476.21 |
| 5 | STb | 5–16 | (5.77866) | (−0.0542733) | (1.40338) | 0.9998 | 0.0106 | 7 | 4300.28 |
| 6 | μ | 5–32 | –10.2984 | 0.0487215 | 1.27732 | 0.9992 | 0.0475 | 25 | 6681.44 |
| 7 | nD | 4–20 | –4.48113 | 0.0030487 | –0.46118 | 1.0000 | 0.0019 | 16 | 98 646.53 |
| 8 | Pv | 2–16 | 2.737019 | –0.4605237 | –0.86135 | 1.0000 | 0.0119 | 15 | 339 306.10 |
| 9 | ω | 2–10 | –7.934717 | –0.0023596 | 0.401052 | 0.9991 | 0.0126 | 19 | 4279.41 |
| 10 | Ip | 2–11 | –2.172143 | 0.0013372 | –0.593327 | 0.9999 | 0.0056 | 10 | 19 231.41 |
| 11 | D | 5–16 | –5.686163 | –0.0099647 | –0.226592 | 0.9998 | 0.0026 | 12 | 12752.15 |
| 12 | TFb | 3–16 | (9.143340) | (−0.0048236) | (0.977959) | 0.9993 | 0.0206 | 14 | 4213.98 |
| 13 | Pw | 2–8 | –3.631401 | 0.5256402 | –0.321655 | 0.9985 | 0.0686 | 6 | 487.63 |
Figure 3.
Plot of calculated (−) and experimental (△) liquid viscosity, μ, values versus experimental boiling point, Tb, of n-paraffins (n = 5–32).
2.2.4. Relationship between Nonlinear and Linear Properties of Homologues
Equation 16 expresses the change regularity of the linear properties of homologues, while eq 15 expresses the change regularity of the nonlinear properties of homologues. Comparing eq 16 with eq 15 showed that they had the same variables (n – 1) and SCNE. Therefore, a correlation between the nonlinear and linear properties of the homologues can be established by combining eqs 16 and 15, as shown in eq 26
| 26 |
where, for the two specific physicochemical properties, P(n) and PLC(n), of the same series of homologues, parameters a′, b′, and c′ can be obtained by the regression method
Using the boiling point (Tb) and critical volume (Vc) of n-paraffins as an example
| 27 |
Therefore
| 28 |
Here, taking Vc, (n – 1), and SCNE as variables, regression equation 29 can be obtained for ln(Tb)
| 29 |
Equation 29 can be rewritten as eq 30
| 30 |
Using eq 30 and employing parameters Vc, (n – 1), and SCNE of n-paraffins, the Tb values of n-paraffins can be calculated. The average absolute error between the experimental and calculated Tb values was 0.94 K for the 17 n-paraffins with n = 2–18.
2.2.5. Simplification of Equation
Equations 12 and 13 contain the term  , which is inconvenient to use. This section
discusses the simplification of this term. According to mathematical
principles, for the infinite series SS{1, 1/2, 1/3,
..., 1/n}, the sum of the first n terms is shown in eq 31
, which is inconvenient to use. This section
discusses the simplification of this term. According to mathematical
principles, for the infinite series SS{1, 1/2, 1/3,
..., 1/n}, the sum of the first n terms is shown in eq 31
| 31 |
Equation 31 can also be approximately expressed as eq 32
| 32 |
Therefore, the term 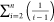 can be expressed as follows
can be expressed as follows
| 33 |
Therefore, eqs 12 and 13 can be simplified to eqs 34 and 35
| 34 |
| 35 |
For example, using eq 35 as a model equation to perform regression analysis of Tb and Tc values of n-paraffins, respectively, afforded eqs 36 and 37
For the boiling point (Tb)
| 36 |
For the critical temperature (Tc)
| 37 |
Equations 36 and 37 still show good correlation, which is only slightly worse than that of the corresponding equations (nos. 1 and 2 of Table 2). However, eqs 36 and 37 are more convenient to use than eqs 12 and 13.
2.2.6. Relationship between SCNE and the Parameter c(G)
Recently, Mukwembi et al.27 proposed a new graph parameter c(G), called the conduction of a graph, and exploited the conduction of a graph to develop a single parameter model for predicting the boiling point of any given alkane. This work is very meaningful, which simplifies the parameters for estimating the boiling point of alkanes. It is interesting to compare and contrast the SCNE parameter with the parameter c(G). Here, we made a plot of exp(SCNE) of this work versus the parameter c(G)27 of n-paraffins (n = 4–14), and obtained Figure 4. From Figure 4, we can see that there is a good linear correlation of exp(SCNE) with the parameter c(G). It means that the parameter exp(SCNE) is intrinsically related to the parameter c(G) for n-paraffins. Whether there is a relationship of exp(SCNE) to other graph theoretical parameters or not is worth studying, and the interested readers can pursue this area.
Figure 4.
Plot of exp(SCNE) versus the parameter c(G) of ref (27) for n-paraffins (n = 4–14).
2.2.7. Application of NPOH Equation in Branched Alkanes and Alcohols
For the branched alkanes and organic compounds with functionality located elsewhere along a linear alkyl chain, their physical properties are first affected by the number of carbon atoms in the structure, and second by their molecular structure differences. Therefore, we can establish an estimation model of their properties by adding structural parameters that characterize the molecular carbon atomic skeleton to the NPOH equation. We take the boiling points of alkanes and alcohols for example.
2.2.7.1. Example 1, Boiling Point of Alkanes
Alkane molecules are almost nonpolar, and their boiling points are affected by both the number of carbon atoms and the molecular carbon atomic skeleton in the molecule. The effect of the number of carbon atoms on the boiling point can be expressed with the parameters (n – 1) and SCNE, while that of the molecular carbon atomic skeleton can be expressed using the Odd–Even Index (OEI) difference, ΔOEI.21 That is, for the alkane molecule with n carbon atoms, ΔOEI = OEI (branched alkane) – OEI (normal alkane). Based on Yuan’s method,21 the OEI values of 2-methylpropane and butane are 4.5000 and 5.2222, respectively. Thus, ΔOEI(2-methylpropane) = 4.5000 – 5.2222 = −0.7222; ΔOEI (butane) = 4.5000 – 4.5000 = 0. It means that ΔOEI = 0 for any normal alkane. Then, we employed (n – 1), SCNE, and (ΔOEI/n) as variables to perform the regression analysis of boiling points, Tb(n), of alkanes and obtained eq 38 in which the boiling points for all alkanes were taken from a listing by Cao et al.28 These data are listed in the Supporting Information
| 38 |
In eq 38, the 210 alkanes involve all isomers of C2–C10, and the carbon atoms are in the range of C1–C100. The average absolute error between the experimental value (Tb,exp.) and the calculated value (Tb,cal.) was 3.07 K, and the average percentage error was 0.69%.
2.2.7.2. Example 2, Boiling Point of Alcohols
The molecular structure of alcohol is different from that of alkane in which there is a polar functional group OH. Thus, the boiling points of alcohols are affected by the number of carbon atoms, molecular carbon atomic skeleton, and position of group OH located at the alkyl chain in a molecule. The effect of the latter two factors on the boiling point can be expressed by the polarization effect index PEIOH difference, ΔPEIOH, because the PEIOH of the alcohol is related to the alkyl skeleton and the position of the OH group in the molecule. That is, for the alcohol molecule with n carbon atoms, ΔPEIOH = PEIOH (branched alcohol) – PEIOH (normal alcohol). Taking 2-propanol (CH3)2CHOH (i-PrOH) and 1-propanol CH3CH2CH2OH (PrOH), for example, the PEIOH values of i-Pr and Pr are 1.2811 and 1.1887, respectively. Hence, the difference in PEIOH of i-PrOH is ΔPEIOH(i-PrOH) = 1.2811 – 1.1887 = 0.0924, and the difference in PEIOH of PrOH is ΔPEIOH(PrOH) = 1.1887 – 1.1887 = 0. It means that ΔPEIOH = 0 for any normal alcohol. For the detailed calculation method of PEIOH, one can refer to Cao’s report.28 Thus, we employed (n – 1), SCNE, and (ΔPEIOH/n) as variables to perform regression analysis of boiling points Tb(n) of alcohols and obtained eq 39 in which the boiling points for all alcohols were taken from a listing by Cao et al.28 These data are listed in the Supporting Information
| 39 |
In eq 39, the 105 alcohols involving the carbon atoms are in the range of C2–C11. The average absolute error between the experimental value (Tb,exp.) and the calculated value (Tb,cal.) was 4.40 K, and the average percentage error was 1.01%.
The results indicate that eqs 38 and 39 provide very satisfactory results. We noted that it is difficult to compare the results of this work with those of other works because different data sets and different number of variables were employed in various works. In particular, many of the other works only include alkanes with below 11 carbon atoms. Nevertheless, it is instructive that Allison et al.,29 in 2022, used a graph convolutional neural network to predict the normal boiling point and obtained an average absolute error of 6 K (corresponding to an average percentage error of 1.32%). Cordes and Rarey30 compared some group contribution methods for predicting the boiling points of acyclic alkanes and found that these methods produced average absolute errors ranging from 6.5 to 26.7 K. Palatinus et al.31 proposed eq 40 to calculate the YR parameter and then used the calculated YR values and eq 41 to predict the boiling point for linear and branched alkanes in which the average absolute deviation between experimental and predicted boiling points was 1.45 K, and the average percentage error was 0.32%. Liu et al.32 used the QSPR method to predict the boiling points of alcohols and obtained the standard error of 5.69 K
| 40 |
| 41 |
The above two examples show that the NPOH equation can also be used in property correlation for structures with functionality located elsewhere along a linear alkyl chain as well as for branched organic compounds.
3. Conclusions
This research shows that various nonlinear change properties of homologues can be expressed using a general equation, named the NPOH equation (eqs 14 or 15), while various linear change properties of homologues can be expressed by general equation16. Therefore, both linear and nonlinear change properties of homologues can be correlated with the number of carbon atoms (n) and the “sum of carbon number effects” (SCNE). Furthermore, using the two parameters n and SCNE, a quantitative correlation equation can be established between any two properties of each homologue, providing convenient mutual estimation of properties of a series of homologues. Finally, for nonstraight-chain isomers, using the NPOH equation, we can add structural parameters that characterize the molecular carbon atomic skeleton to establish an estimation model for their properties. The study on the properties of branched chain organic compounds will be reported in a subsequent paper.
This work can provide a new perspective for studying quantitative structure–property relationships (QSPRs).
Acknowledgments
The project was supported by the Hunan Natural Science Foundation (2020JJ5155), the Research Foundation of Education Bureau of Hunan Province, China (Grant Nos. 20B224 and 18C0327), and the National Natural Science Foundation of China (21672058). The authors thank Simon Partridge, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn/) for editing the English text of a draft of this manuscript.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c02828.
Physicochemical properties of n-alkanes (Table S1); physicochemical properties of cycloalkanes, alkenes, alkynes, alcohols, 2-methylparaffins, 2,2-dimethylparaffins, 2,3-dimethylparaffins, and 1-cyclopentylparaffins (Table S2); boiling points of 210 alkanes (Table S3); and boiling points of 105 alcohols (Table S4) (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Kontogeorgis G. M.; Dohrn R.; Economou I. G.; Hemptinne J.-C.; Kate A.; Kuitunen S.; Mooijer M.; Žilnik L. F.; Vesovic V. Industrial Requirements for Thermodynamic and Transport Properties: 2020. Ind. Eng. Chem. Res. 2021, 60, 4987–5013. 10.1021/acs.iecr.0c05356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto-Draghi C.; Fayet G.; Creton B.; Rozanska X.; Rotureau P.; Hemptinne J.-C.; Ungerer P.; Rousseau B.; Adamo C. A General Guidebook for the Theoretical Prediction of Physicochemical Properties of Chemicals for Regulatory Purposes. Chem. Rev. 2015, 115, 13093–13164. 10.1021/acs.chemrev.5b00215. [DOI] [PubMed] [Google Scholar]
- Katritzky A. R.; Kuanar M.; Slavov S.; Hall C. D.; Karelson M.; Kahn I.; Dobchev D. A. Quantitative Correlation of Physical and Chemical Properties with Chemical Structure: Utility for Prediction. Chem. Rev. 2010, 110, 5714–5789. 10.1021/cr900238d. [DOI] [PubMed] [Google Scholar]
- Leidner F.; Yilmaz N. K.; Schiffer C. A. Deciphering Antifungal Drug Resistance in Pneumocystis Jirovecii DHFR with Molecular Dynamics and Machine Learning. J. Chem. Inf. Model. 2021, 61, 2537–2541. 10.1021/acs.jcim.1c00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Wei S. C.; Dong C. F.; Wang B.; Liang Y.; Wang Y. J.; Chen Q. Progress in QSPR Modelling Methods. Chin. Sci. Bull. 2021, 66, 2832–2844. 10.1360/TB-2020-1362. [DOI] [Google Scholar]
- Needbam D. E.; Wei I.-C.; Seybold P. G. Molecular Modeling of the Physical Properties of the Alkanes. J. Am. Chem. Soc. 1988, 110, 4186–4194. 10.1021/ja00221a015. [DOI] [Google Scholar]
- Cao C.-T.; Chen M.; Fang Z.; Au C.; Cao C. Relationship investigation between C(sp2)-X and C(sp3)-X bond energies based on substituted benzene and methane. ACS Omega 2020, 5, 19304–19311. 10.1021/acsomega.0c02964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C.-T.; Yan L.; Cao C.; Qu J. Influence of substituent and push-pull effect on the chemical shifts of the carbon in bridging bond of 1-furyl/thienyl-2-arylethylene. J. Phys. Org. Chem. 2021, 34, e4160 10.1002/poc.4160. [DOI] [Google Scholar]
- Cao C. T.; Cao C.; Qu J. Quantifying and Fine Adjusting the Solid-state Fluorescence Wavelength of 1-Thienyl-2-arylethylene. J. Lumin. 2021, 233, 117895 10.1016/j.jlumin.2021.117895. [DOI] [Google Scholar]
- Redlich O.; Kwong J. On the Thermodynamics of Solutions. V. An Equation of State. Fugacities of Gaseous Solutions. Chem. Rev. 1949, 44, 233–244. 10.1021/cr60137a013. [DOI] [PubMed] [Google Scholar]
- Peng D.-Y.; Robinson D. A New Two-Constant Equation of State. Ind. Eng. Chem. Fundam. 1976, 15, 59–64. 10.1021/i160057a011. [DOI] [Google Scholar]
- Kreglewsky A.; Zwolinsky B. J. A New Relation for Physical Properties of n-Alkanes and n-Alkyl Compounds. J. Phys. Chem. A 1961, 65, 1050–1052. 10.1021/j100824a505. [DOI] [Google Scholar]
- Gasem K. A. M.; Robinson R. L. Jr. In Prediction of Phase Behavior for CO2 + Heavy Normal Paraffins Using Generalized-Parameter Soave and Peng–Robinson Equations of State, AIChE Annual Meeting, Houston, TX, March 26, 1985.
- Gasem K. A. M.; Ross C. H.; Robinson R. L. Jr. Prediction of Ethane and CO2 Solubilities in Heavy Normal Paraffins Using Generalized-Parameter Soave and Peng-Robinson Equations of State. Can. J. Chem. Eng. 1993, 77, 805–816. 10.1002/cjce.5450710520. [DOI] [Google Scholar]
- Marano J. J.; Holder G. D. General Equation for Correlating the Thermophysical Properties of n-Paraffins, n-Olefins, and Other Homologous Series. 1. Formalism for Developing Asymptotic Behavior Correlations. Ind. Eng. Chem. Res. 1997, 36, 1887–1894. 10.1021/ie960511n. [DOI] [Google Scholar]
- Zenkevich I. G. Approximation of any Physicochemical Constants of Homologues with the use of Recurrent Functions. J. Math. Chem. 2009, 46, 913–933. 10.1007/s10910-009-9559-8. [DOI] [Google Scholar]
- Cao C.; Liu S.; Li Z. On Molecular Polarizability: 2. Relationship to the Boiling Point of Alkanes and Alcohols. J. Chem. Inf. Comput. Sci. 1999, 39, 1105–1111. 10.1021/ci990055o. [DOI] [Google Scholar]
- Cai G.; Liu Z.; Zhang L.; Zhao S.; Xu C. Quantitative Structure–Property Relationship Model for Hydrocarbon Liquid Viscosity Prediction. Energy Fuels 2018, 32, 3290–3298. 10.1021/acs.energyfuels.7b04075. [DOI] [Google Scholar]
- CRC Handbook of Chemistry and Physics, 91st ed.; Haynes W. M., Ed.; CRC Press, 2011; pp 199–216. [Google Scholar]
- Wu K.; Chen Z.; Li J.; Lei Z.; Xu J.; Wang K.; Li R.; Dong X.; Peng Y.; Yang S.; Zhang F.; Chen Z.; Gao Y. Nanoconfinement Effect on n-Alkane Flow. J. Phys. Chem. C 2019, 123, 16456–16461. 10.1021/acs.jpcc.9b03903. [DOI] [Google Scholar]
- Yuan H.; Cao C. Topological Indices Based on Vertex, Edge, Ring, and Distance: Application to Various Physicochemical Properties of Diverse Hydrocarbons. J. Chem. Inf. Comput. Sci. 2003, 43, 501–512. 10.1021/ci0202988. [DOI] [PubMed] [Google Scholar]
- Kauffman G. W.; Jurs P. C. Prediction of Surface Tension, Viscosity, and Thermal Conductivity for Common Organic Solvents Using Quantitative Structure-Property Relationships. J. Chem. Inf. Comput. Sci. 2001, 41, 408–418. 10.1021/ci000139t. [DOI] [PubMed] [Google Scholar]
- Yaws C. L.Chemical Properties Handbook; Beijing World Publishing Corporation/McGraw-Hill: Beijing, 1999; pp 1–29. [Google Scholar]
- Atabati M.; Emamalizadeh R. A Quantitative Structure Property Relationship for Prediction of Flash Point of Alkanes Using Molecular Connectivity Indices. Chin. J. Chem. Eng. 2013, 21, 420–426. 10.1016/S1004-9541(13)60483-8. [DOI] [Google Scholar]
- Mathieu D. Inductive Modeling of Physico-chemical Properties: Flash Point of Alkanes. J. Hazard. Mater. 2010, 179, 1161–1164. 10.1016/j.jhazmat.2010.03.081. [DOI] [PubMed] [Google Scholar]
- Klopman G.; Li J.-Y.; Wang S.; Dimayuga M. Computer Automated logP Calculations Based on an Extended Group Contribution Approach. J. Chem. Inf. Comput. Sci. 1994, 34, 752–781. 10.1021/ci00020a009. [DOI] [Google Scholar]
- Mukwembi S.; Nyabadza F. A New Model for Predicting Boiling Points of Alkanes. Sci. Rep. 2021, 11, 24261 10.1038/s41598-021-03541-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C.; Liu S.; Li Z. On Molecular Polarizability: 2. Relationship to the Boiling Point of Alkanes and Alcohols. J. Chem. Inf. Comput. Sci. 1999, 39, 1105–1111. 10.1021/ci990055o. [DOI] [Google Scholar]
- Qu C.; Kearsley A. J.; Schneider B. I.; Keyrouz W.; Allison T. C. Graph Convolutional Neural Network Applied to the Prediction of Normal Boiling Point. J. Mol. Graphics Model. 2022, 112, 108149 10.1016/j.jmgm.2022.108149. [DOI] [PubMed] [Google Scholar]
- Cordes W.; Rarey J. A New Method for the Estimation of the Normal Boiling Point of Nonelectrolyte Organic Compounds. Fluid Phase Equilib. 2002, 201, 409–433. 10.1016/S0378-3812(02)00050-X. [DOI] [Google Scholar]
- Palatinus J. A.; Sams C. M.; Beeston C. M.; Carroll F. A.; Argenton A. B.; Quina F. H. Kinney Revisited: An Improved Group Contribution Method for the Prediction of Boiling Points of Acyclic Alkanes. Ind. Eng. Chem. Res. 2006, 45, 6860–6863. 10.1021/ie0604425. [DOI] [Google Scholar]
- Liu W.; Cao C. Quantitative Structure-property Relationship of Normal Boiling Point of Aliphatic Oxygen-containing Organic Compounds. CIESC J. 2012, 63, 3739–3746. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






