Abstract
Background
Patients often report various symptoms after recovery from acute COVID-19. Previous studies on post-COVID-19 condition have not corrected for the prevalence and severity of these common symptoms before COVID-19 and in populations without SARS-CoV-2 infection. We aimed to analyse the nature, prevalence, and severity of long-term symptoms related to COVID-19, while correcting for symptoms present before SARS-CoV-2 infection and controlling for the symptom dynamics in the population without infection.
Methods
This study is based on data collected within Lifelines, a multidisciplinary, prospective, population-based, observational cohort study examining the health and health-related behaviours of people living in the north of the Netherlands. All Lifelines participants aged 18 years or older received invitations to digital COVID-19 questionnaires. Longitudinal dynamics of 23 somatic symptoms surrounding COVID-19 diagnoses (due to SARS-CoV-2 alpha [B.1.1.7] variant or previous variants) were assessed using 24 repeated measurements between March 31, 2020, and Aug 2, 2021. Participants with COVID-19 (a positive SARS-CoV-2 test or a physician's diagnosis of COVID-19) were matched by age, sex, and time to COVID-19-negative controls. We recorded symptom severity before and after COVID-19 in participants with COVID-19 and compared that with matched controls.
Findings
76 422 participants (mean age 53·7 years [SD 12·9], 46 329 [60·8%] were female) completed a total of 883 973 questionnaires. Of these, 4231 (5·5%) participants had COVID-19 and were matched to 8462 controls. Persistent symptoms in COVID-19-positive participants at 90–150 days after COVID-19 compared with before COVID-19 and compared with matched controls included chest pain, difficulties with breathing, pain when breathing, painful muscles, ageusia or anosmia, tingling extremities, lump in throat, feeling hot and cold alternately, heavy arms or legs, and general tiredness. In 12·7% of patients, these symptoms could be attributed to COVID-19, as 381 (21·4%) of 1782 COVID-19-positive participants versus 361 (8·7%) of 4130 COVID-19-negative controls had at least one of these core symptoms substantially increased to at least moderate severity at 90–150 days after COVID-19 diagnosis or matched timepoint.
Interpretation
To our knowledge, this is the first study to report the nature and prevalence of post-COVID-19 condition, while correcting for individual symptoms present before COVID-19 and the symptom dynamics in the population without SARS-CoV-2 infection during the pandemic. Further research that distinguishes potential mechanisms driving post-COVID-19-related symptomatology is required.
Funding
ZonMw; Dutch Ministry of Health, Welfare, and Sport; Dutch Ministry of Economic Affairs; University Medical Center Groningen, University of Groningen; Provinces of Drenthe, Friesland, and Groningen.
Introduction
After recovery from acute COVID-19, a substantial proportion of patients continue to experience symptoms of a physical, psychological, or cognitive nature.1 These long-term sequelae of COVID-19 have been described as the next public health disaster in the making, and there is an urgent need for empirical data informing on the scale and scope of the problem to support the development of an adequate health-care response.2, 3
Research has been hampered by an absence of a consensus on the prevalence and nature of the post-COVID-19 condition.2 A systematic review examining the frequency and variety of persistent symptoms after COVID-19 reported that the median proportion of patients with at least one persistent symptom was 72·5%.4 However, this estimated prevalence largely depends on the timeframe, population, and symptoms used to define post-COVID-19 condition. The timeframe used varies from 4 weeks to more than 6 months after a COVID-19 diagnosis, with 3 months being the most commonly used.5 Furthermore, most studies have relied on follow-up of hospitalised patients with COVID-19.4 The vast majority of people with COVID-19, however, have mild disease and are not hospitalised,6 and hospitalisation itself is associated with somatic symptoms.7
Another complicating factor is that there is no consensus on the nature of the symptoms that can be attributed to COVID-19. Selection of the symptoms is crucial for charting the scale and scope of post-COVID-19 condition. However, frequently reported post-COVID-19 symptoms are also common in the general population.4, 8, 9 Symptoms such as fatigue and headaches might be worsened during the pandemic also in people without COVID-19, for example, due to anxiety-induced stress or the combination of work and homeschooling.10, 11 An additional complication is that some of the symptoms reported after COVID-19 might already have been present before COVID-19 and might even reflect a pre-existing susceptibility to COVID-19 itself, rather than being a consequence of SARS-CoV-2 infection.
Research in context.
Evidence before this study
We searched PubMed, Google Scholar, and preprint repositories from November, 2019, to February, 2022, for studies published in Dutch or English that investigated the course of post-COVID-19 condition (ie, long COVID) over time, the symptoms associated with post-COVID-19 condition, and the prevalence of post-COVID-19 condition. Furthermore, we searched for studies and policy documents from (global) public health institutes (eg, WHO) that aimed to clinically define post-COVID-19 condition. A formal systematic review was not conducted. Most previous research that assessed the prevalence and symptoms associated with post-COVID-19 condition did not include an adequate control group, and so no adjustments for the prevalence of somatic symptoms in the population without COVID-19 could be made. Additionally, we found no studies that included patients’ symptom prevalence before COVID-19 diagnosis; therefore, the previous studies were unable to assess whether somatic symptoms reported after a COVID-19 diagnosis were already present before SARS-CoV-2 infection. Most research was conducted in a clinical setting, disregarding post-COVID-19 condition in the general population. In the context of these shortcomings, a systematic review estimated that the median proportion of patients with at least one somatic symptom after COVID-19 was 72·5%.
Added value of this study
To our knowledge, this study is the first to include a control group matched for age, sex, and time, enabling us to adjust for symptom presence in the general population and changes herein due to public health measures and seasonal influences. Additionally, the repeated-measures nature of this study enabled us to assess symptom severity in patients with COVID-19 before they had SARS-CoV-2 infection. Therefore, we could assess whether symptom severity was truly increased after a COVID-19 diagnosis, or whether symptoms were a continuation of pre-existing symptoms. Our approach allowed for identification of core symptoms that define post-COVID-19 condition, as these are increased in severity 90–150 days after a COVID-19 diagnosis compared with patient's pre-existing symptom severity.
Implications of all the available evidence
Our unique approach allows us to present the core symptoms, namely chest pain, difficulties with breathing, pain when breathing, painful muscles, ageusia or anosmia, tingling extremities, lump in throat, feeling hot and cold alternately, heavy arms or legs, and general tiredness, which could define post-COVID-19 condition. Additionally, we offer an improved working definition of post-COVID-19 condition and provide a reliable prevalence estimate in the general population corrected for pre-existing symptoms, and symptoms in COVID-19-negative controls. Taking into account the symptoms that increased in severity and could be attributed to COVID-19, while correcting for seasonal fluctuations and non-infectious health aspects of the pandemic on symptom dynamics, we estimated that 12·7% of patients with COVID-19 in the general population will experience persistent somatic symptoms after COVID-19. Additionally, these core symptoms have major implications for future research, as these symptoms have the highest discriminative ability to distinguish between post-COVID-19 condition and non-COVID-19-related symptoms.
Therefore, detailed information about symptom dynamics before and after SARS-CoV-2 infection in the general population is needed to provide insight into the scale and scope of post-COVID-19 condition. However, such data—requiring repetitive measurements of symptom scores before and after SARS-CoV-2 infection—have not yet been reported. Furthermore, symptom dynamics need to be compared between people affected by COVID-19 and a matched sample of people without infection to be able to separate the effects of the SARS-CoV-2 infection from the effects of the pandemic, associated social restrictions, and public health measures on symptom dynamics in the general population.12
We aimed to analyse the nature, prevalence, and severity of long-term symptoms related to COVID-19, while correcting for symptoms present before SARS-CoV-2 infection and controlling for the symptom dynamics in the population without infection.
Methods
Study design and participants
This study is based on data collected within the Lifelines COVID-19 cohort study, an add-on study to the multidisciplinary, prospective, population-based, observational Dutch Lifelines cohort study examining the health and health-related behaviours of 167 729 people in the north of the Netherlands (>98% White, 58% female).13, 14 There were no specific inclusion criteria for the Lifelines study. Exclusion criteria for Lifelines were severe mental illness, short life expectancy (<5 years) at time of inclusion, insufficient knowledge of the Dutch language to complete questionnaires, and not being able to visit a general practitioner.14 All Lifelines participants aged 18 years or older with a known email address received invitations to complete digital COVID-19 questionnaires.15 We included data from 24 consecutive measurements collected in the Lifelines COVID-19 cohort study between March 31, 2020, and Aug 2, 2021, for which response rates varied between 28% and 49%. Initially, questionnaires were sent out weekly but from June, 2020, data were collected every 2 weeks, and from August, 2020, data were collected on a monthly basis.15 The Lifelines cohort study and its add-on studies were approved by the Medical Ethical Committee of University Medical Center Groningen (2007/152) and participants provided written informed consent to take part. Further details on the cohort, design considerations, and recruitment procedures, as well as additional information on the COVID-19 pandemic in the Netherlands, have been published previously.13, 14, 15
Procedures
Participants completed digital questionnaires on multiple topics, including sociodemographics and physical and mental health during the COVID-19 pandemic. In January, 2021, the Dutch national immunisation programme for COVID-19 was initiated. On March 1, 2021, only 3·7% of the total Lifelines study population was fully vaccinated, increasing to 9·8% by the end of April, 2021, when the last COVID-19 cases for the current study were included. Until July, 2021, the alpha (B.1.1.7) SARS-CoV-2 variant was dominant in the Netherlands. Participants’ COVID-19 positivity was defined as either a positive SARS-CoV-2 test or a physician's diagnosis of COVID-19, which was based on the evolving clinical case definition issued by the Dutch Institute for Public Health and the Environment. Physician diagnosis of COVID-19 was included as positivity because SARS-CoV-2 testing in the Netherlands was strongly restricted up until August, 2020.6 We only included participants’ first SARS-CoV-2 infections.
We analysed 23 symptoms: headache, dizziness, chest pain, back pain, nausea, painful muscles, difficulties with breathing, feeling hot and cold alternately, tingling extremities, lump in the throat, general tiredness, heavy arms or legs, pain when breathing, runny nose, sore throat, dry cough, wet cough, fever, diarrhoea, stomach pain, ageusia or anosmia, sneezing, and itchy eyes. The first 12 of these symptoms were derived from the validated Symptom CheckList-90 Somatization (SCL-90 SOM) subscale,16 which has been shown to have sufficient measurement invariance, making it suitable to assess symptoms repeatedly over time.17 The remainder of the symptoms were added as these were considered to be related to COVID-19 at the start of the study. All symptoms were assessed using an ordinal 5-point Likert scale that answered to what extent participants were bothered by the respective symptom (1=not at all, 5=extremely) in the past 7 days. The timeframe was changed to the past 14 days when questionnaires were sent out every 2 weeks and monthly. The item assessing sneezing was introduced in the second questionnaire. Stomach pain and diarrhoea were assessed by a combined item in the first two questionnaires, but thereafter these were assessed by separate items. We included these first two measurements for both stomach pain and diarrhoea. Presence of symptoms was defined by a score of at least 3 (ie, moderately bothered by the symptom).
To assess persistence of somatic symptoms after a COVID-19 diagnosis, we first calculated participants’ individual mean pre-COVID-19 score per symptom. We excluded reports on symptom severity collected in the week before COVID-19 diagnosis, as increased symptom severity might have prompted participants to seek SARS-CoV-2 testing or a physician's diagnosis. If no information on symptom severity before the COVID-19 diagnosis was available, participants were excluded from the analyses.
Statistical analysis
Characteristics of the study population, including age, level of education, and presence of chronic disease (appendix p 2), are provided as absolute numbers with concomitant percentages. If appropriate, information on continuous measurements are provided as means with SD. Data were examined for normality using Q-Q plots and histograms. All information, except for age and sex, was self-reported by participants.
We describe COVID-19-positive participants’ moving average symptom report, including the moving average's SE, over time, stratified by symptom and participants’ sex. The moving average was based on an interval of 28 days to avoid fluctuations in symptoms resulting from differences in subsamples of the cohort completing the questionnaire on a specific day. We randomly matched COVID-19-positive participants with COVID-19-negative controls (1:2), by sex (male or female), age (split at the median, ≤52 years or ≥53 years), and time of completing questionnaires that indicated a COVID-19 diagnosis (measurement wave, range 1–24). We matched for time to account for the variation in symptom burden during the pandemic in the population without COVID-19 due to seasonal effects and non-infectious pandemic consequences.
We describe the relative frequency of COVID-19-positive participants and COVID-19-negative matched controls in whom mean symptom severity from 90 to 150 days after COVID-19 was at least moderate (ie, score of ≥3), stratified per symptom. Additionally, we describe the relative frequency of participants in whom mean symptom severity from 90 to 150 days after a COVID-19 diagnosis was increased by at least 1 point compared with before COVID-19, resulting in at least moderate symptom severity (ie, score of ≥3), stratified per symptom. We further assessed whether the presence of symptoms that increased substantially to at least moderate symptom severity from 90 to 150 days after COVID-19 or matched time differed in distribution between COVID-19-positive participants and COVID-19-negative controls via χ2 tests. We maintained a two-sided α level of p<0·001 (0·05 divided by 50; 23 symptoms and two [sub]totals, each tested two times) to correct for the number of performed tests. We assessed mean symptoms at 90–150 days after COVID-19, based on at least one questionnaire, in concordance with the recently proposed WHO clinical case definition for post-COVID-19 condition that states symptoms occur usually 3 months from the onset of COVID-19 and last for at least 2 months. The 1-point difference was assessed as this is the minimal change participants could indicate on the 5-point Likert scale.
Furthermore, in sensitivity analyses, we assessed the prevalence of symptoms at 3 months after COVID-19 restricted to people who had a positive SARS-CoV-2 test.
IBM SPSS (version 25) was used to perform all analyses. In compliance with the SAGER guidelines, we report our findings stratified by participants’ sex if appropriate.18
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
76 422 participants (mean age 53·7 years [SD 12·9], 46 329 [60·8%] were female) completed a total of 883 973 questionnaires. Of these, 4231 (5·5%) participants were COVID-19 positive (mean age 52·4 years [SD 11·7], 2779 [65·7%] were female; table 1 ; appendix p 4); they completed 62 224 questionnaires (appendix p 5). Female COVID-19-positive participants completed a median of 17 questionnaires (IQR 8–23), male COVID-19-positive participants completed a median of 18 (9–23). The maximum follow-up time was 484 days after COVID-19 diagnosis (median 101 days [IQR 43–199]). COVID-19-positive participants were matched to 8462 COVID-19-negative controls who together completed 140 810 questionnaires (appendix p 4). Both male and female control participants completed a median of 20 questionnaires (IQR 12–24) each. The maximum follow-up time of control participants was 481 days after their matched timepoint (median 104 days [IQR 46–201]). The sex-stratified 28-day moving average of control participants’ mean sum score of all 23 assessed symptoms is shown in the appendix (p 3). Men were more frequently hospitalised due to COVID-19 than women (5·0% of male vs 2·5% of female COVID-19-positive participants).
Table 1.
Characteristics of the COVID-19-positive participants
| Male participants (n=1452) | Female participants (n=2779) | ||
|---|---|---|---|
| Age, years | 54·3 (11·5) | 51·4 (11·7) | |
| BMI, kg/m2 | 26·6 (3·7) | 26·3 (4·9) | |
| Level of education | |||
| Low | 193 (13·3%) | 274 (9·9%) | |
| Medium | 694 (47·8%) | 1484 (53·4%) | |
| High | 532 (36·6%) | 896 (32·2%) | |
| Missing | 33 (2·3%) | 125 (4·5%) | |
| Chronic disease* | |||
| Absent | 1225 (84·4%) | 2209 (79·5%) | |
| Present | 110 (7·6%) | 287 (10·3%) | |
| Missing | 117 (8·1%) | 283 (10·2%) | |
| Smoking | |||
| No | 1356 (93·4%) | 2543 (91·5%) | |
| Yes | 63 (4·3%) | 143 (5·1%) | |
| Missing | 33 (2·3%) | 93 (3·3%) | |
| Method of COVID-19 diagnosis | |||
| Physician's diagnosis | 297 (20·5%) | 602 (21·7%) | |
| Positive SARS-CoV-2 test | 1155 (79·5%) | 2177 (78·3%) | |
| Hospitalised with COVID-19 | 72 (5·0%) | 70 (2·5%) | |
Data are mean (SD) or n (%).
See the appendix (p 2) for the full list of included chronic diseases. The characteristics of the COVID-19-negative controls compared with COVID-19-positive participants are provided in the appendix (p 4).
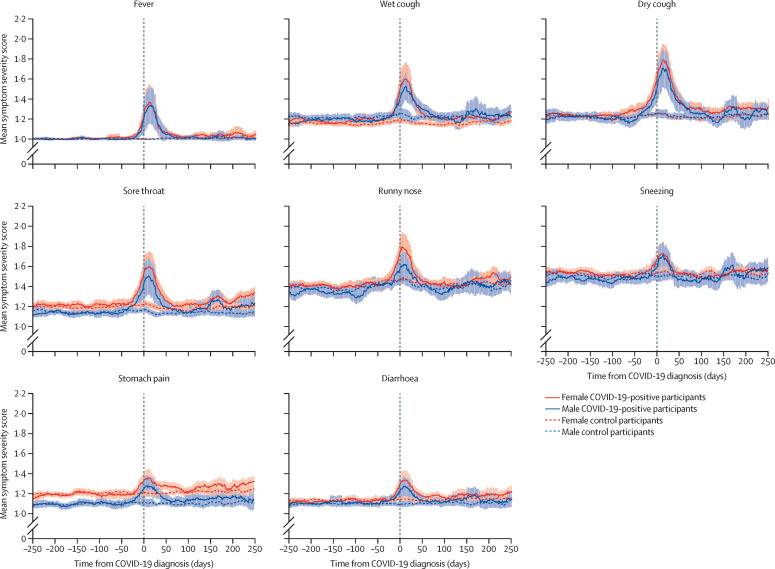
Visual inspection of symptom dynamics over time indicated that almost all assessed symptoms showed an increase in severity in COVID-19-positive participants compared with controls during the acute phase of COVID-19 (Figure 1, Figure 2, Figure 3 ). Diarrhoea and stomach pain, as well as cold-like symptoms including sneezing, wet and dry cough, runny nose, fever, and sore throat on average returned to pre-COVID-19 severity within 50 days of a COVID-19 diagnosis, which suggests that these symptoms were predominantly present during the acute phase of the disease (figure 1).
Figure 1.
Acute symptoms
The shaded areas represent the SE of the moving average.
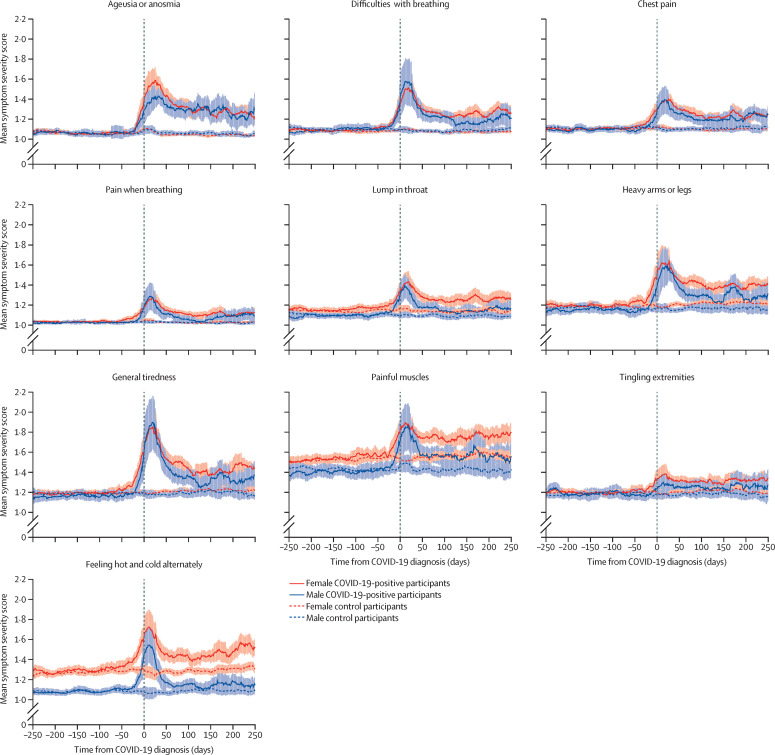
Figure 2.
Core symptoms
The shaded areas represent the SE of the moving average.
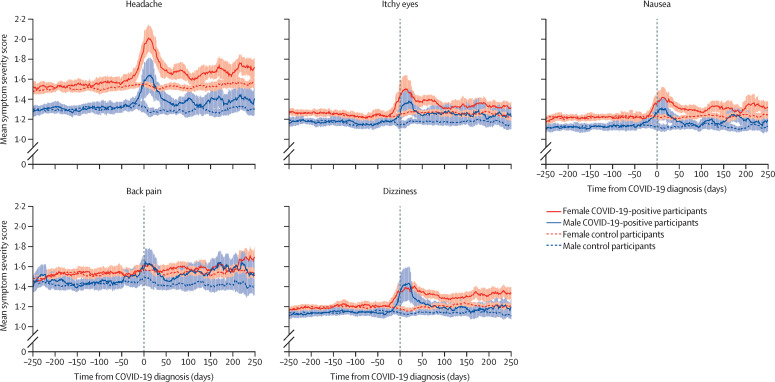
Figure 3.
Other symptoms
The shaded areas represent the SE of the moving average.
Symptoms that were more severe in COVID-19-positive participants 90–150 days after COVID-19 compared with symptom scores before COVID-19 and compared with matched controls (ie, the core symptoms of post-COVID-19 condition) included: cardiopulmonary symptoms (chest pain, difficulties with breathing, and pain when breathing), musculoskeletal symptoms (painful muscles), sensory symptoms (ageusia or anosmia, tingling extremities, lump in throat, and feeling hot and cold alternately), and general symptoms (heavy arms or legs, and general tiredness; figure 2). These symptoms differed based on both visual inspection of symptom dynamics and on the significance of the difference in distribution of symptoms that increased substantially to at least moderate severity in COVID-19-positive participants and control participants (table 2 ). Mean severity for these symptoms appeared to have reached a plateau at 3 months, with no further decline in mean severity thereafter. Symptoms that were not significantly increased in mean severity at 90–150 days after a COVID-19 diagnosis included headache, itchy eyes, dizziness, back pain, and nausea (figure 3).
Table 2.
Frequencies of participants who had presence of, or a substantial increase to, symptoms of at least moderate severity at 90–150 days after COVID-19 diagnosis or matched timepoint
|
Presence of symptom of at least moderate severity |
Substantial increase in symptom severity to at least moderate severity |
|||
|---|---|---|---|---|
| Controls (n=4353) | COVID-19-positive participants (n=1942) | Controls (n=4130) | COVID-19-positive participants (n=1782) | |
| Ageusia or anosmia | 37 (0·8%) | 158 (8·1%)* | 17 (0·4%) | 135 (7·6%)* |
| Difficulties with breathing | 38 (0·9%) | 68 (3·5%)* | 21 (0·5%) | 43 (2·4%)* |
| Chest pain | 44 (1·0%) | 63 (3·2%)* | 24 (0·6%) | 43 (2·4%)* |
| Pain when breathing | 13 (0·3%) | 20 (1·0%)* | <10 (<0·2%) | 16 (0·9%)* |
| Lump in throat | 59 (1·4%) | 61 (3·1%)* | 24 (0·6%) | 42 (2·4%)* |
| Heavy arms or legs | 130 (3·0%) | 126 (6·5%)* | 65 (1·6%) | 75 (4·2%)* |
| General tiredness | 159 (3·7%) | 136 (7·0%)* | 87 (2·1%) | 88 (4·9%)* |
| Painful muscles | 378 (8·7%) | 262 (13·5%)* | 134 (3·2%) | 130 (7·3%)* |
| Tingling extremities | 145 (3·3%) | 98 (5·0%)* | 65 (1·6%) | 52 (2·9%)* |
| Fever | 19 (0·4%) | 16 (0·8%) | 18 (0·4%) | 12 (0·7%) |
| Wet cough | 83 (1·9%) | 58 (3·0%) | 40 (1·0%) | 28 (1·6%) |
| Dry cough | 81 (1·9%) | 50 (2·6%) | 43 (1·0%) | 28 (1·6%) |
| Headache | 239 (5·5%) | 166 (8·5%)* | 111 (2·7%) | 76 (4·3%) |
| Itchy eyes | 143 (3·3%) | 96 (4·9%)* | 78 (1·9%) | 51 (2·9%) |
| Feeling hot and cold alternately | 155 (3·6%) | 112 (5·8%)* | 70 (1·7%) | 63 (2·5%)* |
| Sore throat | 84 (1·9%) | 48 (2·5%) | 51 (1·2%) | 29 (1·6%) |
| Runny nose | 217 (5·0%) | 110 (5·7%) | 94 (2·3%) | 50 (2·8%) |
| Nausea | 128 (2·9%) | 72 (3·7%) | 74 (1·8%) | 37 (2·1%) |
| Sneezing | 210 (4·8%) | 101 (5·2%) | 74 (1·9%)† | 35 (2·1%)‡ |
| Back pain | 413 (9·5%) | 210 (10·8%) | 182 (4·4%) | 88 (4·9%) |
| Stomach pain | 108 (2·5%) | 53 (2·7%) | 58 (1·4%) | 25 (1·4%) |
| Dizziness | 93 (2·1%) | 46 (2·4%) | 56 (1·4%) | 25 (1·4%) |
| Diarrhoea | 80 (1·8%) | 38 (2·0%) | 52 (1·3%) | 19 (1·1%) |
| Total | 1275 (29·3%) | 790 (40·7%)* | 749 (18·1%) | 526 (29·6%)* |
Data are n (%). Symptoms are ordered according to their relative increase in frequency in COVID-19-positive participants compared with controls. A substantial increase in severity was defined as an increase in symptom severity of at least 1 point on the 5-point scale.
p<0·001.
n=3988; sneezing was assessed in 23 surveys instead of 24.
n=1704; sneezing was assessed in 23 surveys instead of 24.
Visual inspection of the core symptoms suggests that in many of these symptoms, including lump in throat, heavy arms or legs, general tiredness, and feeling hot and cold alternately, sex differences were present. Female COVID-19-positive participants showed a longer persistence of increased symptom severity after COVID-19 than male COVID-19-positive participants (figure 2). A similar pattern was observed in acute symptoms, such as dry cough, stomach pain, and diarrhoea (figure 1), and in all symptoms that were not significantly increased in severity at 90–150 days after a COVID-19 diagnosis, except for back pain. Table 2 shows the frequencies of COVID-19-positive participants and controls that had symptoms of at least moderate severity at 90–150 days after COVID-19 or matched timepoint. In total, 790 (40·7%) of 1942 COVID-19-positive participants had at least one symptom of moderate severity at 90–150 days, compared with 1275 (29·3%) of 4353 controls. Painful muscles and back pain were the most frequent symptoms in both COVID-19-positive participants (13·5% and 10·8%, respectively) and controls (8·7% and 9·5%, respectively). This analysis, however, did not consider symptom severity before COVID-19.
A greater proportion of COVID-19-positive participants had a substantial increase in symptom severity resulting in moderate symptom severity of at least one symptom at 90–150 days after COVID-19 diagnosis than control participants during the same period (526 [29·6%] of 1782 participants vs 749 [18·1%] of 4130; table 2). Ageusia or anosmia (135 [7·6%] of 1782 participants), painful muscles (130 [7·3%]) and general tiredness (88 [4·9%]) were most frequently increased to moderate severity in COVID-19-positive participants, while they were increased in 17 (0·4%), 134 (3·2%), and 87 (2·1%) control participants, respectively. The prevalence of ageusia or anosmia of increased severity (7·6%) was 19 times greater in COVID-19-positive participants than in controls (0·4%). Sensitivity analyses in which participants with a physician's diagnosis of COVID-19 were excluded (including only those with a positive SARS-CoV-2 test) showed similar results (appendix pp 6–7).
Restricting the definition of post-COVID-19 condition to core symptoms (figure 2) showed that 381 (21·4%) of 1782 COVID-19-positive participants versus 361 (8·7%) of 4130 controls had at least one symptom substantially increased to at least moderate severity (χ2 [df 1] 181·1; p<0·0001; denominators based on participants with data available for at least 7 days before their SARS-CoV-2 infection or matched timepoint and 90–150 days after their COVID-19 diagnosis or matched timepoint). This finding implies that in 12·7% of patients with COVID-19, the increased core symptoms with moderate severity at 3 months after COVID-19 could be attributed to SARS-CoV-2 infection. Including all assessed symptoms in the definition decreased the prevalence of participants with an increase in symptom severity only slightly (to 11·5%), but resulted in a loss of sensitivity for symptoms that can be attributed to SARS-CoV-2 (ie, the ratio between patients with symptoms due to SARS-CoV-2 infection and those with unrelated symptoms was 2·5 for the core set of symptoms vs 1·6 when including all symptoms).
Discussion
This study shows post-COVID-19 condition might occur in about one out of eight people with COVID-19 in the general population. Core symptoms of post-COVID-19 condition include chest pain, difficulties with breathing, lump in throat, pain when breathing, painful muscles, heavy arms or legs, ageusia or anosmia, feeling hot and cold alternately, tingling extremities, and general tiredness. To our knowledge, this is the first study to provide a reliable assessment of the prevalence of post-COVID-19 condition, while correcting for individual symptoms present before SARS-CoV-2 infection and for the dynamics of symptoms reported by sex-matched and age-matched controls without infection in the same period during the pandemic. This corrected prevalence remained nearly unaltered irrespective of the use of the core symptoms versus a broader range of symptoms as a definition of post-COVID-19 condition. However, when including a broader range of symptoms, the ratio between patients with symptoms due to SARS-CoV-2 infection and those with unrelated symptoms decreased. Increased knowledge on both the nature of the core symptoms and the prevalence of post-COVID-19 condition in the general population represents a major step forward in our ability to design studies that ultimately inform an adequate health-care response to the long-term sequelae of COVID-19.
The major strengths of this study are the large sample size of COVID-19-positive participants identified in a general population cohort, as well as the multiple repeated measurements of symptom severity in the participants. This allowed for the calculation of pre-COVID-19 symptom severity in each participant. In addition, we were able to compare COVID-19-positive participants’ symptom severity with controls matched by sex and age who provided measurements at the same time period as the cases. Finally, the SCL-90 SOM subscale is a validated instrument, suitable for assessing symptoms in large-scale cohort studies. The addition of other COVID-19-related symptoms allowed for detailed insights into participants’ symptom dynamics.
Before interpreting the results, some limitations of this study should be acknowledged. First, COVID-19 cases can be asymptomatic and remain undetected.8 Therefore, the prevalence of COVID-19 in this study might have been underestimated. Second, the assessed symptoms were included in the Lifelines COVID-19 cohort study at the beginning of the pandemic. Although at that time these symptoms were considered to be related to COVID-19, other symptoms such as cognitive symptoms (eg, brain fog) and post-exertional malaise were identified later during the pandemic as potentially relevant for a working definition of post-COVID-19 condition.7 Third, as all participants in the Lifelines COVID-19 cohort study were aged 18 years or older, we could not assess paediatric post-COVID-19 condition. Fourth, the exact date of COVID-19 diagnosis was unknown; we therefore used the date of the first questionnaire in which COVID-19 positivity was indicated as date of diagnosis. This might have led to an underestimation of post-COVID-19 time. Lastly, as this study was conducted in the northern region of the Netherlands, these results might not be generalisable to other areas.
Multiple studies have assessed the persistence of somatic symptoms after COVID-19, with timeframes of follow-up varying from 21 days to 6 months.4, 19 Some studies included participants from post-COVID-19 support groups or predominantly patients who were hospitalised, leading to biased results.20, 21 A systematic review analysed 11 studies that assessed the persistence of symptoms 90–180 days after COVID-19 in outpatients.19 The sample sizes ranged from 59 to 2915 patients with COVID-19 and the number of assessed symptoms ranged from six to 21. The most prevalent symptom was fatigue (11–42% of patients), followed by dyspnoea (8–37%), painful muscles (7–24%), and ageusia or anosmia (3–24%). Thoracic pain was reported in 3–14% of patients at 90–180 days after COVID-19. Although we found similar prevalence rates for some of these symptoms, we also showed that these rates were lower when patients’ symptom severity before COVID-19 was taken into account. Additionally, we showed that the most prevalent symptoms are not the most distinctive symptoms for post-COVID-19 condition. Furthermore, many studies with clinical cohorts did not include a matched control group and were therefore unable to distinguish between effects of SARS-CoV-2 infection and those of the pandemic on symptoms.12 Studies that included a control group could not distinguish between symptoms resulting from a SARS-CoV-2 infection and pre-existing symptoms. A large study that included 106 578 patients with COVID-19 and matched controls with influenza, which assessed the persistence of seven somatic symptoms at 90–180 days after diagnosis, found that somatic symptoms, such as headache, chest pain, and fatigue, were more frequently present in patients with COVID-19 than in the controls.22 The study found higher prevalence rates for most assessed somatic symptoms than our study—for example, breathing difficulties occurred in 7·9% of patients with COVID-19 and chest pain occurred in 5·7%. Painful muscles was the only symptom that was less frequently reported (1·5% of patients). The difference in observed prevalence rates might be explained by the previous study only including patients with COVID-19 who sought help for their persistent symptoms from a health-care provider, and not adjusting for patients’ symptoms before COVID-19.
Additionally, a study in France that included 1091 SARS-CoV-2-positive participants and 25 732 controls suggested that the belief of being infected with SARS-CoV-2 was more strongly associated with the severity of symptoms 8 weeks after SARS-CoV-2 infection than laboratory confirmed COVID-19 diagnosis.23 This conclusion is potentially stigmatising,24 and the study has some limitations. First, serological assays were used to detect SARS-CoV-2 infection, but patients affected by post-COVID-19 condition might have lower antibody responses.25 Second, the cross-sectional nature of the study with retrospective assessments is problematic, as persistent physical symptoms might have confounded recall of past illness and thus the belief in having been infected. Third, confounding by other viruses might have occurred, which might have caused both the belief of having been infected with SARS-CoV-2 and the persistent symptoms. Our study overcame these limitations by performing sensitivity analyses restricted to participants with a COVID-19 diagnosis based on a positive SARS-CoV-2 test and by the study's prospective design. Nevertheless, our study cannot provide definitive information on the underlying mechanisms driving post-COVID-19-related symptoms. Therefore, additional research assessing the causes of post-COVID-19-related symptoms is required.
To our knowledge, this is the first study that is able to identify which persistent symptoms are particularly related to SARS-CoV-2 infection, and we used these core symptoms of post-COVID-19 condition for an empirically based working definition of the condition. Notably, in the absence of adequate control data, case definitions might be biased towards highly prevalent symptoms. Experts in a WHO Delphi procedure constructed a case definition that identified fatigue and dyspnoea as the most important symptoms of post-COVID-19 condition (78% of the panel agreed on their importance for the case definition).26 Our empirical analyses showed that these were among the core symptoms, but the most distinctive symptoms also included chest pain and ageusia or anosmia (considered important for the case definition by 55% and 57% of the Delphi panel, respectively). Additionally, tingling extremities were considered important by merely 39% of the experts, while 56% considered headache to be important for the case definition. Our results, however, suggest that tingling extremities is a core symptom whereas headache is not related to SARS-CoV-2 infection. These differences clearly show the importance of longitudinal cohort studies in the general population with pre-infection data and controls without infection to study the scale and scope of post-COVID-19 condition.
Furthermore, although sex differences are known to be present in persistent somatic symptoms of COVID-19, this is the first study of our knowledge to stratify symptom dynamics by sex both before and after COVID-19. Multiple somatic symptoms—for example, feeling hot and cold alternately, lump in throat, and general tiredness—were shown to be more severe after COVID-19 in women than in men, compared with controls. Research has shown that women report more severe common somatic symptoms than men and that these symptoms are more frequently persistent.27, 28, 29 Multiple explanations have been proposed for this phenomenon. First, women are thought to have a heightened sensitivity to pain compared with men, due to biological differences rooted in, among others, sex hormones and genotype.30 Second, women might be more aware of bodily sensations than men, allowing for an easier and earlier perception of somatic symptoms in women than in men.29 However, the female preponderance in symptom experience is not only due to differences in biology (ie, sex), but also in societal expectations of women and men (ie, gender roles).27, 28 Feminine gender roles, for example, are thought to be associated with poorer access to health care, which might also explain health-related gender differences.31
A list of empirically validated core symptoms of post-COVID-19 condition, used for a working definition of the condition, is essential to adequately study pathophysiological mechanisms,2 which is especially important given the risk of simple psychogenic explanations and the resulting consequences for patients.24 Our results support a working definition at least based on the core symptoms, given the improved sensitivity ratio between cases and controls compared with a broader definition. These core symptoms were increased at 3–5 months after COVID-19, and are likely to limit functioning, prompt help-seeking, and have plausible underlying pathophysiological mechanisms. Nevertheless, research shows that COVID-19 might also affect brain functioning and mental health.32, 33 Therefore, future research should not overlook mental health symptoms (eg, depression and anxiety symptoms), nor additional post-infectious symptoms that were not assessed in this study (eg, brain fog, insomnia, and post-exertional malaise). Additionally, future intersectional research should assess how ethnicity, gender, age, socioeconomic status, other social identities, and the presence of underlying chronic diseases are associated with symptom dynamics surrounding COVID-19 and risk of post-COVID-19 condition. Further research will focus on the clustering of COVID-19 symptoms in participants, and whether symptom clusters are associated with subtypes and distinct pathophysiological mechanisms underlying post-COVID-19 condition. We will also study genetic and environmental risk factors, and how post-COVID-19 condition affects (work) functioning and wellbeing. Additionally, as research suggests that vaccination before SARS-CoV-2 infection only partly mitigates the risk of long-term symptom sequelae 6 months after COVID-19,34 further studies should assess the effect of SARS-CoV-2 vaccination and the timing thereof, and the effect of SARS-CoV-2 variants, on symptom dynamics in both adults and children.
In conclusion, we present a starting point for core symptoms that could define post-COVID-19 condition, offer an improved working definition of post-COVID-19 condition, and provide a reliable prevalence estimate in the general population of the northern region of the Netherlands corrected for pre-existing symptoms and symptoms in participants without infection. Taking into account those symptoms that increased in severity and could be attributed to COVID-19, while correcting for seasonal fluctuations and non-infectious health aspects of the pandemic on symptom dynamics,2, 5, 12 we found that about one in every eight patients are affected by persistent symptoms after COVID-19. This finding shows that post-COVID-19 condition is an urgent problem with a mounting human toll.
Data sharing
Lifelines data will not be shared publicly. Access to the Lifelines data is organised according to a strict data access procedure. For all types of access, a research proposal must be submitted for evaluation by the Lifelines Research Office. The evaluation is performed to align the goals of the researchers with the goals of Lifelines (which are in turn aligned with the informed consent form signed by Lifelines participants). Further information on Lifelines data can be obtained by contacting the Lifelines Research Office (https://www.lifelines.nl).
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We thank Nord van den Bos (University of Groningen, University Medical Center Groningen, Groningen, Netherlands) for his contributions and critical thinking during the initial stages of this study, and Martijn Nawijn (University of Groningen, University Medical Center Groningen, Groningen, Netherlands) for commenting on the draft manuscript. This work was supported by the ZonMw Gender and Health Program (849200013) and the ZonMw COVID-19 Program (10430302110002). The Lifelines initiative has been made possible by subsidy from the Dutch Ministry of Health, Welfare, and Sport, the Dutch Ministry of Economic Affairs, the University Medical Center Groningen, University of Groningen, and the Provinces in the north of the Netherlands (Drenthe, Friesland, and Groningen).
Contributors
AVB analysed the data, conceptualised the analyses, and wrote the first version of the manuscript. SKRvZ and TCoH helped with conceptualising the analyses, interpreting the results, and critically revised the manuscript. AVB and SKRvZ accessed and verified the reported underlying data. JGMR conceived the study's design, helped conceptualise the analyses, interpreted the results, and critically revised the manuscript. The Lifelines Corona Research Initiative collected the data.
Supplementary Material
References
- 1.Callard F, Perego E. How and why patients made long COVID. Soc Sci Med. 2021;268 doi: 10.1016/j.socscimed.2020.113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips S, Williams MA. Confronting our next national health disaster—long-haul COVID. N Engl J Med. 2021;385:577–579. doi: 10.1056/NEJMp2109285. [DOI] [PubMed] [Google Scholar]
- 3.Crook H, Raza S, Nowell J, Young M, Edison P. Long COVID—mechanisms, risk factors, and management. BMJ. 2021;374 doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 4.Nasserie T, Hittle M, Goodman SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond) 2021;53:737–754. doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballering AV, Oertelt-Prigione S, olde Hartman TC, et al. Sex and gender-related differences in COVID-19 diagnoses and SARS-CoV-2 testing practices during the first wave of the pandemic: the Dutch Lifelines COVID-19 cohort study. J Womens Health (Larchmt) 2021;30:1686–1692. doi: 10.1089/jwh.2021.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- 8.Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Cuadrado ML, Florencio LL. Defining post-COVID symptoms (post-acute COVID, long COVID, persistent post-COVID): an integrative classification. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acevedo-Mesa A, Tendeiro JN, Roest A, Rosmalen JGM, Monden R. Improving the measurement of functional somatic symptoms with item response theory. Assessment. 2021;28:1960–1970. doi: 10.1177/1073191120947153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bekhuis E, Schoevers RA, van Borkulo CD, Rosmalen JG, Boschloo L. The network structure of major depressive disorder, generalized anxiety disorder and somatic symptomatology. Psychol Med. 2016;46:2989–2998. doi: 10.1017/S0033291716001550. [DOI] [PubMed] [Google Scholar]
- 11.Janssens KAM, Rosmalen JGM, Ormel J, van Oort FV, Oldehinkel AJ. Anxiety and depression are risk factors rather than consequences of functional somatic symptoms in a general population of adolescents: the TRAILS study. J Child Psychol Psychiatry. 2010;51:304–312. doi: 10.1111/j.1469-7610.2009.02174.x. [DOI] [PubMed] [Google Scholar]
- 12.Amin-Chowdhury Z, Ladhani SN. Causation or confounding: why controls are critical for characterizing long COVID. Nat Med. 2021;27:1129–1130. doi: 10.1038/s41591-021-01402-w. [DOI] [PubMed] [Google Scholar]
- 13.Klijs B, Scholtens S, Mandemakers JJ, Snieder H, Stolk RP, Smidt N. Representativeness of the Lifelines cohort study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scholtens S, Smidt N, Swertz MA, et al. Cohort profile: Lifelines, a three-generation cohort study and biobank. Int J Epidemiol. 2015;44:1172–1180. doi: 10.1093/ije/dyu229. [DOI] [PubMed] [Google Scholar]
- 15.McIntyre K, Lanting P, Deelen P, et al. Lifelines COVID-19 cohort: investigating COVID-19 infection and its health and societal impacts in a Dutch population-based cohort. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-044474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zijlema WL, Stolk RP, Löwe B, Rief W, White PD, Rosmalen JGM. How to assess common somatic symptoms in large-scale studies: a systematic review of questionnaires. J Psychosom Res. 2013;74:459–468. doi: 10.1016/j.jpsychores.2013.03.093. [DOI] [PubMed] [Google Scholar]
- 17.Rytilä-Manninen M, Fröjd S, Haravuori H, et al. Psychometric properties of the Symptom Checklist-90 in adolescent psychiatric inpatients and age- and gender-matched community youth. Child Adolesc Psychiatry Ment Health. 2016;10:23. doi: 10.1186/s13034-016-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heidari S, Babor TF, De Castro P, Tort S, Curno M. Sex and Gender Equity in Research: rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev. 2016;1:2. doi: 10.1186/s41073-016-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen NN, Hoang VT, Dao TL, Dudouet P, Eldin C, Gautret P. Clinical patterns of somatic symptoms in patients suffering from post-acute long COVID: a systematic review. Eur J Clin Microbiol Infect Dis. 2022;41:515–545. doi: 10.1007/s10096-022-04417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goërtz YMJ, Van Herck M, Delbressine JM, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res. 2020;6:542. doi: 10.1183/23120541.00542-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matta J, Wiernik E, Robineau O, et al. Association of self-reported COVID-19 infection and SARS-CoV-2 serology test results with persistent physical symptoms among French adults during the COVID-19 pandemic. JAMA Intern Med. 2022;182:19–25. doi: 10.1001/jamainternmed.2021.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ballering A, olde Hartman T, Rosmalen J. Long COVID-19, persistent somatic symptoms and social stigmatisation. J Epidemiol Community Health. 2021;75:603–604. doi: 10.1136/jech-2021-216643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García-Abellán J, Padilla S, Fernández-González M, et al. Antibody response to SARS-CoV-2 is associated with long-term clinical outcome in patients with COVID-19: a longitudinal study. J Clin Immunol. 2021;41:1490–1501. doi: 10.1007/s10875-021-01083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballering AV, Wardenaar KJ, olde Hartman TC, Rosmalen JGM. Female sex and femininity independently associate with common somatic symptom trajectories. Psychol Med. 2020 doi: 10.1017/S0033291720004043. published online Nov 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ballering AV, Bonvanie IJ, olde Hartman TC, Monden R, Rosmalen JGM. Gender and sex independently associate with common somatic symptoms and lifetime prevalence of chronic disease. Soc Sci Med. 2020;253 doi: 10.1016/j.socscimed.2020.112968. [DOI] [PubMed] [Google Scholar]
- 29.Barsky AJ, Peekna HM, Borus JF. Somatic symptom reporting in women and men. J Gen Intern Med. 2001;16:266–275. doi: 10.1046/j.1525-1497.2001.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111:52–58. doi: 10.1093/bja/aet127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelletier R, Humphries KH, Shimony A, et al. Sex-related differences in access to care among patients with premature acute coronary syndrome. CMAJ. 2014;186:497–504. doi: 10.1503/cmaj.131450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santomauro DF, Mantilla Herrera AM, Shadid J, et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398:1700–1712. doi: 10.1016/S0140-6736(21)02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Douaud G, Lee S, Alfaro-Almagro F, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Aly Z, Bowe B, Xie Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. 2022 doi: 10.1038/s41591-022-01840-0. published online May 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Lifelines data will not be shared publicly. Access to the Lifelines data is organised according to a strict data access procedure. For all types of access, a research proposal must be submitted for evaluation by the Lifelines Research Office. The evaluation is performed to align the goals of the researchers with the goals of Lifelines (which are in turn aligned with the informed consent form signed by Lifelines participants). Further information on Lifelines data can be obtained by contacting the Lifelines Research Office (https://www.lifelines.nl).