Abstract
Fluorescence lifetime (FLT) multiplexing and multispectral imaging (MSI) are both frequently employed for in vitro and ex vivo biological studies. In vivo applications of MSI for deep seated fluorophores require consideration of diffusive light propagation in biological tissue. We have previously shown that a well-known redshift of fluorescence spectra in diffusive medium induces a fluorophore cross-talk, which cannot be accounted for even with known optical properties of the medium. In contrast, FLT measurements remain largely unaffected by light propagation in tissue, enabling zero cross-talk and accurate relative quantification. While a fully quantitative estimation of fluorophore concentrations requires depth resolved tomographic imaging, this is often not possible due to the difficulty of estimating tissue optical properties and modelling light propagation in complex tissue geometries. Here, we experimentally investigate the performance of planar (non-tomographic) MSI and FLT multiplexing for the quantitative recovery of multiple near-infrared fluorophores embedded in 4-8 mm thick tissue. We show that FLT multiplexing provides a superior quantification accuracy (error < 10%) compared to MSI (error = 20–107%) in tissue. The error rates for MSI increased with tissue thickness and can be directly attributed to the spectral redshift induced cross-talk between emission spectra. Our data indicate that planar FLT multiplexing can provide high quantification accuracy in thick biological tissue without a need for optical property estimation, thereby offering an important validation tool for rapid quantification of fluorophore concentrations in bulk tissue.
1. Introduction
Optical imaging offers the powerful ability to simultaneously visualize multiple fluorescently labelled tags (“fluorescence multiplexing”) using fluorescence spectral [1–5], and lifetime [6–11], contrast. Fluorescence multiplexing has been widely applied in biological imaging for tracking multiple processes and quantifying fluorescently labeled biomarkers such as cell surface receptors or enzymes in tissue [12–15]. In recent years, in vivo multiplexed imaging has become feasible with the development of several near infrared (NIR) fluorescent probes that can be used for deep tissue molecular imaging in live animals [16–19]. These NIR fluorescent probes exhibit distinct excitation/emission spectra and lifetimes, which can be exploited by both multispectral imaging (MSI) and fluorescence lifetime (FLT) multiplexing to allow in vivo detection of multiple disease biomarkers simultaneously, over time and during therapies.
MSI is typically performed by capturing a series of fluorescence images at different wavelengths and subsequently performing linear unmixing with a known set of spectral ‘basis’ functions [20–22]. This method is particularly useful for analytes with multiple fluorescent species with known spectra that are localized in a spatially overlapping compartment. While fluorescence spectra are readily affected by system-dependent imaging conditions such as the excitation light intensity and collection efficiency, well-standardized imaging conditions can account for these factors to diminish quantification errors during in vitro measurements. However, for in vivo imaging of deep-seated fluorophores (e.g., fluorescence from tumors in internal organs), the quantitative accuracy of MSI is affected by several additional factors, including background tissue autofluorescence, excitation light leakage due to a small Stokes shift of fluorescence, and the influence of spectrally dependent tissue absorption [23,24]. Nonlinear absorption of fluorescence emission spectra in thick biological tissue results in a well-known spectral redshift [25], which introduces a higher degree of spectral overlap between fluorophores. This spectral redshift in thick biological tissue along with the overlapping broad emission spectra of commonly used fluorophores [26], confounds relative quantitation that cannot be accounted for by standardizing the imaging conditions. Further, the degree of spectral redshift is governed by tissue optical properties which are difficult to estimate accurately, and the fluorophore distribution in tissue which is usually not known. We have previously demonstrated, using simulation studies and in vitro tomographic imaging with tissue-mimicking phantoms that MSI provides a significantly higher fluorophore cross-talk compared to FLT multiplexing [27]. This is mathematically described by a reversed order of fluorophore mixing and diffuse light propagation between MSI and FLT multiplexing [27]. For MSI, the mixing of spectral basis functions happens first at the origin voxel, which then propagates through the diffuse medium. The practical implication of this phenomenon is that in MSI, the mixed emission spectra of the fluorophores are further redshifted during the diffuse light transport in tissue, leading to a high cross-talk and significant errors in relative quantitation. Therefore, the application of MSI has generally been limited to fluorophores in solution and in microscopy of ex vivo thin tissue sections.
FLT multiplexing, on the other hand, enables accurate relative quantitation by multiexponential fitting of the asymptotic portion (late photons) of time domain (TD) data with distinct FLTs of the constituent fluorophores [6,28]. In contrast to MSI, TD fluorescence of individual fluorophores are propagated through the diffuse medium first and then mixed with temporal basis functions leading to zero fluorophore cross-talk in FLT multiplexing. We have shown using extensive theoretical and experimental studies [27–31], that FLTs of deep-seated fluorophores are minimally affected by tissue light propagation, provided the FLTs are longer than intrinsic absorption timescales (∼0.2-0.3 ns) [30]. Subsequent multiexponential analysis of the TD data separates the fluorescence decay into multiple FLT components and decay amplitudes corresponding to all fluorophores present in a diffuse medium such as biological tissue [29,32–34]. TD imaging offers an added advantage over MSI by allowing rejection of excitation leakage based on the distinct decay profiles of the excitation and fluorescence light [35]. Therefore, in vivo relative quantitation can be accurately performed using surface measurements (“planar imaging”) in TD imaging, without requiring estimation of tissue optical properties, and modelling light propagation in complex tissue geometries. While our previous reports focused on tomographic FLT multiplexing [36,37], planar imaging may enable rapid and computationally less intensive quantification of fluorophore concentrations for applications where a tomographic recovery of fluorophore depth or location in tissue is not desired. Here we present the first systematic experimental comparison of the relative quantitation performance between MSI and FLT multiplexing for deep-seated (4-8 mm depth) fluorophores.
2. Materials and methods
2.1. Fluorescent dyes and phantoms
NIR fluorescent dye, IRDye 800CW (LI-COR, NE), solutions were prepared in eight concentrations (5, 7, 10, 30, 50, 75, 100 and 200 nM) in a mixture of 1:1 ethanol and water. For the multiplexing experiments, NIR fluorescent dyes IR-806 (Sigma-Aldrich, MO) and Alexa Fluor 750 (Thermo Fisher Scientific, MA) were used. The fluorophore pair was chosen based on their distinct emission spectra and TD decay curves in the same excitation/emission window. 1 µM IR-806 and 100 nM Alexa Fluor 750 (AF750) stock solutions were prepared in a mixture of 1:1 ethanol and water. The stock solutions of IR-806 and AF750 were mixed in five volume ratios, namely VIR-806 / VAF750 = 1:2, 1:3, 1:5, 1:7 and 1:10 to a final volume of 50 µl for each ratio. The final concentration of IR-806 and AF750 in the five volume ratios ranged between 330-90 nM and 90-66 nM, respectively.
2.2. Multispectral imaging (MSI)
A commercial fluorescence imaging device, IVIS SpectrumCT (PerkinElmer, MA), for small animals was used for MSI. Fluorescent dye solutions in transparent tubes were imaged before (in vitro) and after embedding in tissue. A 4 mm or 8 mm thick porcine muscle tissue was placed on top of the fluorescent dye filled tubes between measurements. Care was taken to keep the sample position unchanged while placing the tissue between measurements. The tissues were kept hydrated by immersing into PBS (pH 7.4) between measurements to avoid drying and alterations in tissue optical properties. 710 ± 20 nm excitation and 20 nm bandpass emission filters centered at 760, 780, 800, 820 and 840 nm were used to acquire the multispectral images. Each image consisted of 240 × 240 pixels with a pixel size of 550 µm per pixel. Camera integration time was automatically adjusted between 0.5-15s during image acquisition. Fluorescence emission spectra of pure IRDye 800CW (100 nM), IR-806 (1 µM), AF750 (100 nM) and tissue autofluorescence (AF) were acquired using the same excitation/emission parameters and saved in a spectral library for spectral unmixing studies.
2.3. MSI data analysis
The Living Image software (PerkinElmer, MA) was used to extract the multispectral fluorescence images and the ImageJ (NIH, Version 1.48u) software was used to scale all fluorescence intensities to 1s integration time before further analysis. An intensity threshold at 80% was first applied to the multispectral images to remove background signal and noise from the raw data. Regions of interest (ROIs) encompassing the full area (>100 pixels) of fluorescent dye filled microcentrifuge tubes were then derived from photographs of tubes before embedding them in tissue. For each fluorophore concentration, a single ROI was used for the quantification of all in vitro and in tissue measurements. Fluorescence spectra for all pixels within the ROIs were extracted using ImageJ and spectral unmixing algorithms were implemented in MATLAB (Mathworks, MA). Fluorescence emission spectra of pure IRDye 800CW ( , 100 nM) in vitro and tissue autofluorescence ( ) were used as basis spectra. A linear unmixing algorithm (Eq. (1)) was used to recover the amplitude of IRDye 800CW for concentration, n:
| (1) |
A concentration scaling factor, , was calculated using the data from 100 nM IRDye 800CW as a reference, where the numerator is dye concentration and is the amplitude of 100 nM IRDye 800CW measured from spectral unmixing. The amplitudes for all other IRDye 800CW solutions were scaled using the concentration scaling factor to recover the measured concentrations.
For spectral multiplexing experiments involving mixtures of two fluorophores in solutions, first, an intensity scaling factor ( ) was calculated as , where and are the in vitro fluorescence intensities of IR-806 ( ) and AF750 (100 nM), respectively, to account for the differences in quantum efficiencies of the two fluorophores. The emission spectra of pure IR-806 ( ) and AF750 ( 100 nM) were used as basis spectra. The emission spectra of each IR-806/AF750 volume ratios were first subtracted by SAF and the amplitudes of IR-806 ( ) and AF750 ( ) were recovered using the following linear unmixing algorithm (Eq. (2)):
| (2) |
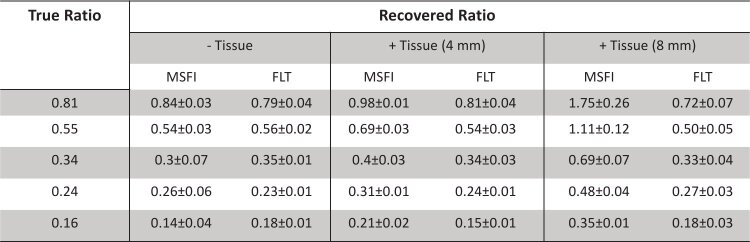
The recovered amplitude ratios ( ) for all solutions in vitro and in tissue (4 mm and 8 mm) were then multiplied with the intensity scaling factor, , to recover the fluorescence yield ratios. Subsequently, the true in vitro fluorescence yield ratios were calculated to be 0.81, 0.55, 0.34, 0.24 and 0.16 for volume ratios 1:2, 1:3, 1:5, 1:7 and 1:10, respectively, by scaling the in vitro fluorescence intensities of 1 µM IR-806 and 100 nM AF750 for their relative volumes in each mixture. The recovered fluorescence yield ratios in vitro and in tissue (4 mm and 8 mm) were statistically compared with the true fluorescence yield ratios.
2.4. FLT multiplexed imaging
Following MSI, FLT multiplexed imaging of the same fluorophore solutions in vitro and in tissue were performed using a previously published custom-built FLT imaging system [38]. Briefly, the imaging system consisted of a supercontinuum laser and tunable filter (EXR-20, SuperK Varia, NKT Photonics, repetition rate: 80 MHz; tuning range: 400-850 nm) providing 770 ± 30-nm excitation, a multimode fiber (Thorlabs) delivering the excitation light into a digital micromirror device (DMD) to project the excitation light on the sample. The average total power across the illumination area (∼5 cm diameter) was 10-20 mW. Fluorescence was collected in reflectance mode using an 835 ± 70 nm emission filter and images were acquired with a gated intensified CCD camera (LaVision, Picostar, 500 V gain, 0.1 to 1 second integration time, 256 × 344 pixels after 4 × 4 hardware binning). Time resolved fluorescence was collected with a gate width of 500 ps and 150 ps delay steps for an acquisition time of 10 ns per duty cycle of the excitation light.
2.5. FLT multiplexed image analysis
Algorithms for time domain (TD) fluorescence data analyses were implemented in MATLAB. An intensity threshold of 80% was first applied to the TD fluorescence images to remove background signal and noise from the raw data. In vitro FLT values of IRDye 800CW (100 nM), IR-806 (1 µM), AF750 (100 nM) and porcine muscle tissue AF were measured by fitting the fluorescence decay curves to a single exponential function as described below. ROIs encompassing the full area (> 100 pixels) of the fluorophore filled microcentrifuge tubes were derived from the photographs of each tube before embedding them in tissue. Subsequently, in vitro and in tissue TD data within the ROIs were analyzed. Fluorescence decay curves for each pixel within the ROIs were plotted as time gate versus log(Intensity) (Fig. 1) and the FLT was obtained by fitting the decay portion of TD fluorescence profiles to a single exponential function, e−t/τ(r), where t denotes time (ns) after excitation and τ(r) denotes FLT of the fluorescent species at pixel location r. IRDye 800CW amplitudes ( ) for all concentrations were recovered using the known FLT values of IRDye 800CW ( ) and tissue AF ( ) in Eq. (3):
| (3) |
is a constant offset to account for background, and is the amplitude of tissue AF. The for each concentration and imaging condition (in vitro or in tissue) were then normalized for camera integration times. A concentration scaling factor, , was calculated using the data from 100 nM IRDye 800CW as a reference, where the numerator is dye concentration and is the amplitude of 100 nM IRDye 800CW measured from FLT multiplexing. The normalized for all other IRDye 800CW solutions were scaled using the concentration scaling factor to recover the measured concentrations.
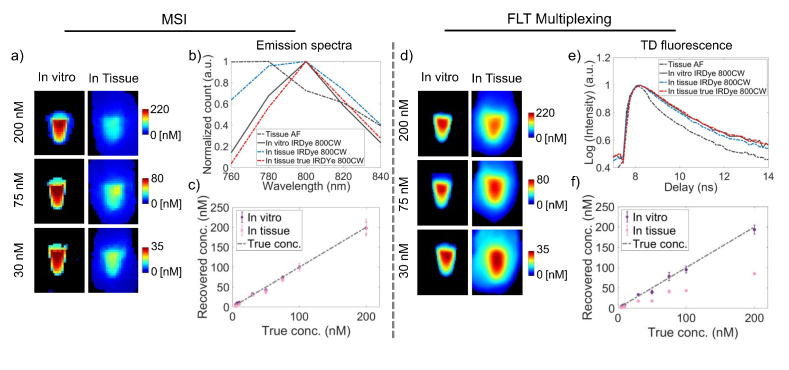
Fig. 1.
Comparison of quantification accuracy of MSI and FLT multiplexing for a single fluorophore in tissue. a) Representative images of recovered IRDye 800CW concentrations (from 200 nM, 75 nM and 30 nM) using MSI in vitro and in tissue are shown. b) Fluorescence spectra of tissue AF (black dashed), in vitro IRDye 800CW (black solid), IRDye 800CW in tissue (blue dashed) and true IRDye 800CW spectra in tissue (red dashed) are shown. The true IRDye 800CW spectra in tissue shows a clear redshift compared to the IRDye 800CW spectra in vitro. (c) Recovered concentrations of IRDye 800CW in vitro and in tissue based on MSI using the tissue AF and in vitro IRDye 800CW spectra as basis functions. d) Representative images of recovered IRDye 800CW concentrations (from 200 nM, 75 nM and 30 nM) using FLT multiplexing in vitro and in tissue are shown. e) TD fluorescence decay curves of tissue AF (black dashed), in vitro IRDye 800CW (black solid), IRDye 800CW in tissue (blue dashed) and true IRDye 800CW decay curve in tissue (red dashed) are shown. The true IRDye 800CW decay curve was nearly identical to the in vitro measured IRDye 800CW decay curve. f) Recovered concentrations of IRDye 800CW in vitro and in tissue based on FLT multiplexing using in vitro TD decay curves of tissue AF and IRDye 800CW. ‘Pink’ and ‘purple’ in (c) and (f) represent data collected in vitro and in tissue, respectively. Gray dotted lines in (c) and (f) represents the true IRDye 800CW concentrations.
For FLT multiplexing with two fluorophores, first an intensity scaling factor ( ) from the in vitro fluorescence intensities of IR-806 ( ) and 100 nM AF750 ( ) was calculated as , to account for the differences in quantum efficiencies of the two fluorophores. Analyses of FLT multiplexing data were performed by first subtracting the tissue AF decay curves from the decay curves for each IR-806/AF750 volume ratios measured in tissue. Amplitudes of IR-806 ( ) and AF750 ( ) were then recovered by fitting the known in vitro FLT values of IR-806 ( ) and AF750 ( ) in the Eq. (4):
| (4) |
The recovered amplitude ratios ( ) for all solutions in vitro and in tissue (4 mm and 8 mm) were then multiplied with an intensity scaling factor, , to recover the fluorescence yield ratios. The resulting recovered fluorescence yield ratios were statistically compared with the true fluorescence yield ratios.
2.6. Statistical analysis
The recovered and true concentrations of IRDye 800CW and the fluorescence yield ratios of IR-806 and AF750 based on MSI, and FLT multiplexing were statistically compared by calculating error rates, correlation coefficient (R2) and slope of a linear curve fit. One-way ANOVA followed by Bonferroni and Holm multiple comparisons were performed to measure differences in errors between MSI and FLT multiplexed imaging. P values less than 0.01 were considered significant: *, p < 0.01.
3. Results and discussion
3.1. Quantifying a single fluorophore in tissue
We first studied the influence of tissue scattering on the quantification accuracy of MSI and FLT multiplexing for a single fluorophore embedded in tissue. We performed spectral unmixing using a spectral basis comprised of tissue AF and the in vitro IRDye 800CW spectra. This is to mimic a realistic scenario when the true spectra of the fluorophore and AF are known and characterized a priori. A performance comparison between MSI and FLT multiplexing for the quantification of IRDye 800CW solutions (5-200nM) is shown in Fig. 1. Figure 1(a) shows images of recovered concentrations based on MSI from three representative IRDye 800CW solutions with true concentrations of 200nM, 75nM and 30nM. We observed that MSI provided accurate quantification of IRDye 800CW under in vitro conditions (Fig. 1(a): left column), while embedding the solutions in a 4 mm thick tissue resulted in significant underestimation of each concentration (Fig. 1(a): right column). To investigate the origin of the underestimation of fluorophore concentration in tissue, we examined the fluorescence spectra of IRDye 800CW obtained in vitro and through tissue. Representative normalized fluorescence spectra (Fig. 1(b)) of tissue AF (black dashed) and 7.0 nM IRDye 800CW (black solid) in vitro showed emission peaks at 760-780 nm and 800 nm, respectively. The fluorescence spectrum of IRDye 800CW embedded in tissue (Fig. 1(b), blue dashed) is blue shifted from the in vitro IRDye 800CW spectrum, whereas one would expect a red-sift due to the spectrally nonlinear light attenuation (tissue absorption and scattering) of the emitted fluorescence traversing tissue [25,27]. The blue shifted spectrum could instead be attributed to the influence of tissue AF, which is blue shifted with respect to IRDye 800CW, and potentially dominates the redshift effect. Indeed, when the pure tissue AF spectra are subtracted from measured IRDye 800CW spectra in tissue at each pixel, a redshifted IRDye 800CW spectrum is realized (Fig. 1(b), red dashed).
Figure 1(c) shows the recovered IRDye 800CW concentrations using MSI in vitro (Fig. 1(c): ‘purple’) and in 4 mm thick tissue (Fig. 1(c): ‘pink’). The recovered IRDye 800CW concentrations were plotted against the true concentrations and a slope of 1 was considered accurate quantification. The recovered IRDye 800CW concentrations in absence of tissue AF closely matched the known concentrations (Fig. 1(b), gray dashed) and presented a slope of 1.1 for a linear fit and an error rate of 1.3% (Table 1) within the experimental concentration range. When IRDye 800CW solutions were embedded in tissue, the recovered IRDye 800CW concentrations were significantly underestimated and we observed a slope of 0.43 with an error rate of 59.8% (Table 1). We observed strong positive correlations (Table 1) between the true and recovered IRDye 800CW concentrations within the experimental concentration range irrespective of the absence (R2 = 0.99) or presence (R2 = 0.99) of tissue AF, which indicated that the spectral redshift during the diffuse light transport in tissue is independent of fluorophore concentration.
Table 1. Statistical parameters (correlation coefficient, slope, and average percent error) comparing MSI and FLT multiplexing for a single fluorophore (IRDye 800CW) in vitro and in tissue.
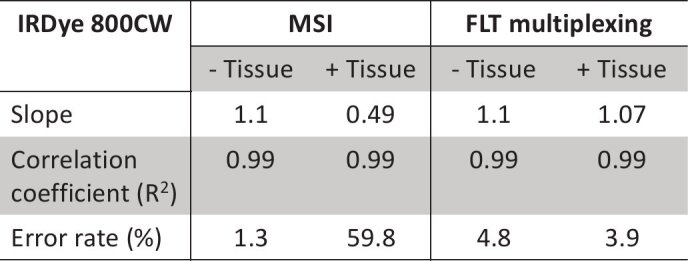
Following MSI, we performed quantification of IRDye 800CW using a FLT basis comprised of FLTs of tissue AF and in vitro IRDye 800CW. Figure 1(d) shows representative images of recovered concentrations in vitro (Fig. 1(d): left column) and in tissue (Fig. 1(d): right column), for three IRDye 800CW solutions with true concentrations of 200nM, 75nM and 30nM. Unlike MSI, the recovered concentrations based on FLT imaging were accurate for both in vitro and in tissue conditions indicating a minimal effect of tissue autofluorescence and diffuse light propagation in FLT-based quantification. Figure 1(e) shows the normalized TD fluorescence decay curves of 7.0 nM IRDye 800CW (Fig. 1(e), black solid) in vitro and tissue AF (Fig. 1(e), black dashed) with FLTs 0.78 ns and 0.55 ns, respectively. In the presence of tissue (thickness: 4 mm), the observed IRDye 800CW decay curve (Fig. 1(e), blue dashed) was a sum of the tissue AF and in vitro IRDye 800CW decay curves. This resulted in a slightly shorter FLT (0.74 ns) in tissue compared to the in vitro FLT (0.78 ns) of IRDye 800CW. The true IRDye 800CW decay curve in tissue was recovered by subtracting the tissue AF from the observed IRDye 800CW decay curve. The resulting AF-subtracted true IRDye 800CW decay curve (Fig. 1(e), red dashed) was nearly identical to the in vitro IRDye 800CW decay curve. This confirms that the measured FLT of IRDye 800CW in tissue is simply a sum of the FLTs of tissue AF and in vitro IRDye 800CW. The identical in vitro and tissue AF subtracted IRDye 800CW decay curves can be considered as the source of the nominal effects of diffuse light propagation on TD measurements. Next, we demonstrated the quantification accuracy of FLT imaging in vitro (Fig. 1(f): ‘purple’) and in a 4 mm thick tissue (Fig. 1(f): ‘pink’). Unlike MSI, the recovered IRDye 800CW concentrations based on FLT imaging closely matched the true concentrations (Fig. 1(f), gray dashed) both in vitro and in tissue with slopes of 1.1 (error rate = 4.8%) and 1.07 (error rate = 3.9%), respectively. The observed error rates for MSI and FLT imaging in vitro and in tissue are summarized inTable 1. It is noted that similar to MSI, the FLT imaging showed a strong positive correlation (R2 = 0.99) between the recovered and true IRDye 800CW concentrations irrespective of the absence or presence of tissue AF (Table 1). The results presented in Fig. 1 demonstrates that in planar imaging, FLT contrast provides higher quantitative accuracy than MSI when a single fluorophore is embedded in thick tissue.
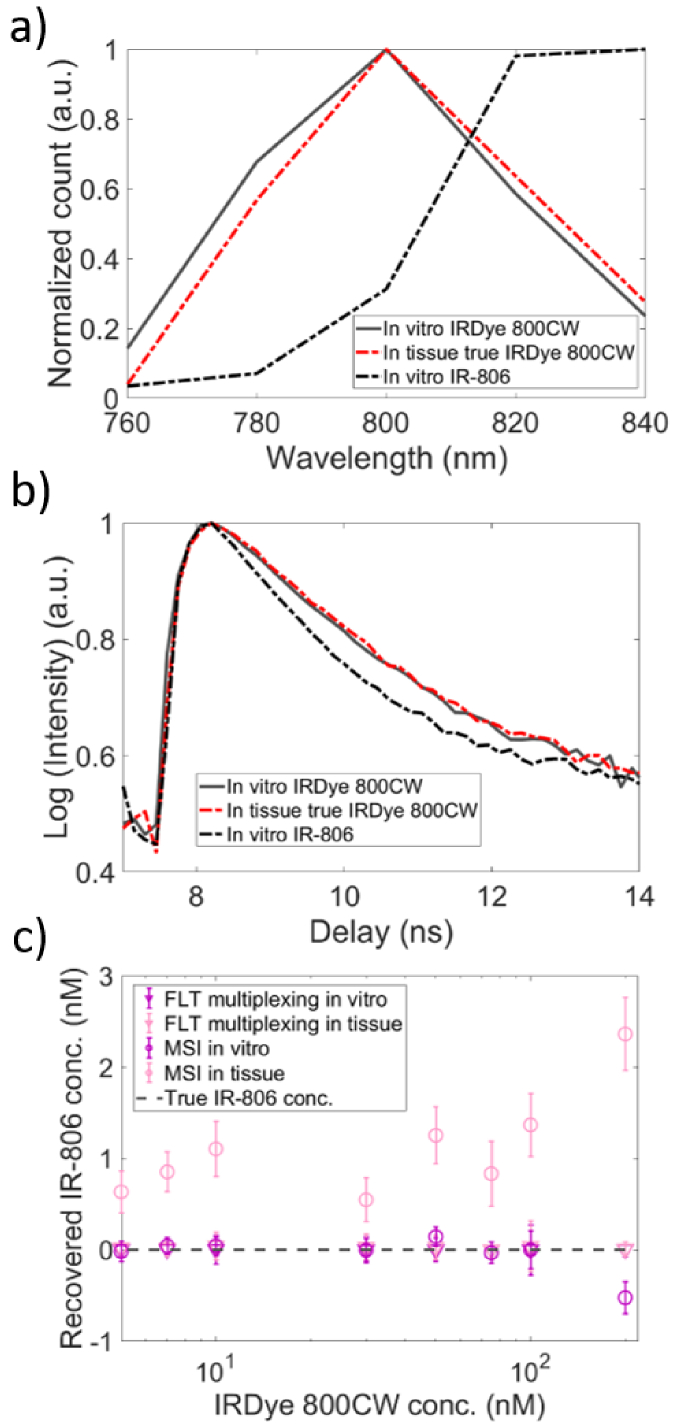
We next examined fluorophore cross-talk in MSI and FLT multiplexing. As we have shown before [27], an effective approach to evaluate crosstalk in multiplexing is to introduce an additional numerical “phantom” dye in the basis function, even though this is not present in the actual measurement. Here we consider the spectral and lifetime characteristics of NIR dye, IR-806 as the phantom dye and perform unmixing of the spectral and TD fluorescence data collected with IRDye 800CW alone in vitro or embedded in tissue. Figure 2(a) shows the emission spectra of in vitro IRDye 800CW (black solid) and IR-806 (black dashed) with emission peaks at 800 nm and 820-840 nm, respectively. As before, the true IRDye 800CW spectrum in tissue (red dashed) was recovered by subtracting the tissue AF spectra from the measured IRDye 800CW spectra in tissue. A clear redshift of the true IRDye 800CW spectra was observed in tissue. Figure 2(b) shows the TD fluorescence data for in vitro IRDye 800CW (black solid) and IR-806 (black dashed) with FLTs 0.78 ns and 0.6 ns, respectively. The AF-corrected IRDye 800CW decay curve (red dashed) in tissue (obtained by subtracting tissue AF decay curve from the measured IRDye 800CW decay curve in tissue) was once again nearly identical to the true in vitro IR-806 decay curve. When multiplexing analyses were performed using the in vitro basis functions of IRDye 800CW and IR-806, the recovered IR-806 concentrations by FLT multiplexing were negligible (< 0.2 nM) in both in vitro (Fig. 2(c), ‘▾’, ‘purple’) and in tissue (Fig. 2(c), ‘▾’, ‘pink’). While MSI also recovered negligible IR-806 concentrations (< 0.2 nM) under in vitro conditions (Fig. 2(c), ‘O’, ‘purple’), it consistently recovered a spurious IR-806 component in tissue (Fig. 2(c), ‘O’, ‘pink’). These results confirm the presence of high fluorescence cross-talk and poor quantification accuracy with MSI even for one fluorophore in the presence of tissue. It is noted that the AF-subtracted IRDye 800CW spectrum in tissue (Fig. 2(a), red dashed) displays a higher overlap with the IR-806 spectra (black dashed), compared to the in vitro IRDye 800CW spectrum (Fig. 2(a), black solid). This increased spectral overlap plays a key role in introducing cross-talks between the two fluorophores during MSI-based quantification of fluorophore concentrations.
Fig. 2.
Comparison of MSI and FLT multiplexing for fluorophore cross-talks between IRDye 800CW and IR-806 in tissue. (a) Fluorescence emission spectra of in vitro IRDye 800CW (black solid), in vitro IR-806 (black dashed) and true IRDye 800CW in tissue (red dashed) are shown. The redshifted true IRDye 800CW spectra in tissue displays a greater overlap with in vitro IR-806 spectra. (b) TD fluorescence decay curves of in vitro IRDye 800CW (black solid), in vitro IR-806 (black dashed) and true IRDye 800CW in tissue (red dashed) are shown. (c) Recovered IR-806 concentrations (indicating pure cross talk) in IRDye 800CW solutions in vitro (‘purple’) and in tissue (‘pink’) using MSI (‘O’) and FLT multiplexing (‘’). Gray dashed line in (c) represents the true concentration of IR-806 (0 nM) in all IRDye 800CW concentrations.
3.2. Relative quantification of two fluorophores in mixture
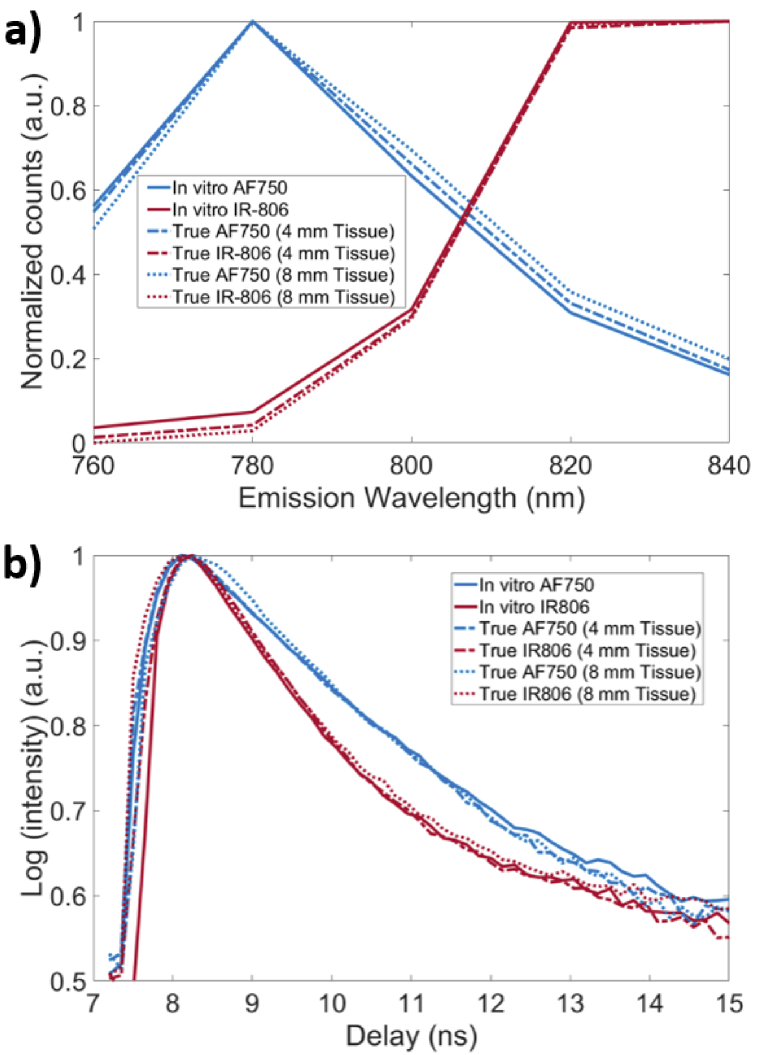
We next compared the relative quantitation accuracies of MSI and FLT multiplexing using mixtures of IR-806 and Alexa Fluor 750 (AF750) solutions. Using in vitro fluorescence spectra and FLTs of IR-806 and AF750 as the basis functions, we performed MSI and FLT multiplexing of five separate IR-806/AF750 mixtures with distinct fluorescence yield ratios. Accounting for the differences in quantum efficiencies of IR-806 and AF750 under the experimental conditions, the true fluorescence yield ratios of IR-806/AF750 in mixed solutions were measured to be 0.81, 0.55, 0.34, 0.24 and 0.16. Figure 3(a) shows the in vitro fluorescence spectra of AF750 (blue solid) and IR-806 (red solid). When the same fluorophore solutions were imaged through a 4 mm thick tissue, the recovered true fluorescence spectra of both AF750 (Fig. 3(a), blue dashed) and IR-806 (Fig. 3(a), red dashed) were redshifted with respect to the in vitro spectrum. Correspondingly, imaging through an 8 mm thick tissue resulted in a greater redshift of the true fluorescence spectra (Fig. 3(a), AF750: blue dotted and IR-806: red dotted). In contrast, the recovered TD fluorescence decay curves of AF750 and IR-806 (Fig. 3(b)), were minimally affected in the presence of 4 mm or 8 mm thick tissue and resulted in FLTs that were nearly identical (<5% experimental error) to the in vitro FLTs of IR-806 and AF750 of 0.6 ns and 0.9 ns, respectively.
Fig. 3.
Fluorescence emission spectra and TD fluorescence decay curves of IR-806 and AF750 in thick biological tissue. Emission spectra and TD fluorescence of IR-806 and AF750 are represented in ‘red’ and ‘blue’ colors, respectively. (a) In vitro emission spectra of IR-806 (red solid) and AF750 (blue solid) are shown along with the true emission spectra of IR-806 (red dashed) and AF750 (blue dashed) in 4 mm thick tissue. Redshifted emission spectra were observed for both IR-806 and AF750 in tissue. A further redshift of IR-806 (red dotted) and AF 750 (blue dotted) emission spectra was observed in 8 mm thick tissue. (b) In vitro TD fluorescence decay curves of IR-806 (red solid) and AF750 (blue solid) with distinct FLTs (0.6 ns and 0.9 ns, respectively) are shown. True fluorescence decay curves of IR-806 and AF750 in 4 mm (dashed) or 8 mm (dotted) thick tissue were nearly identical to the in vitro decay curves.
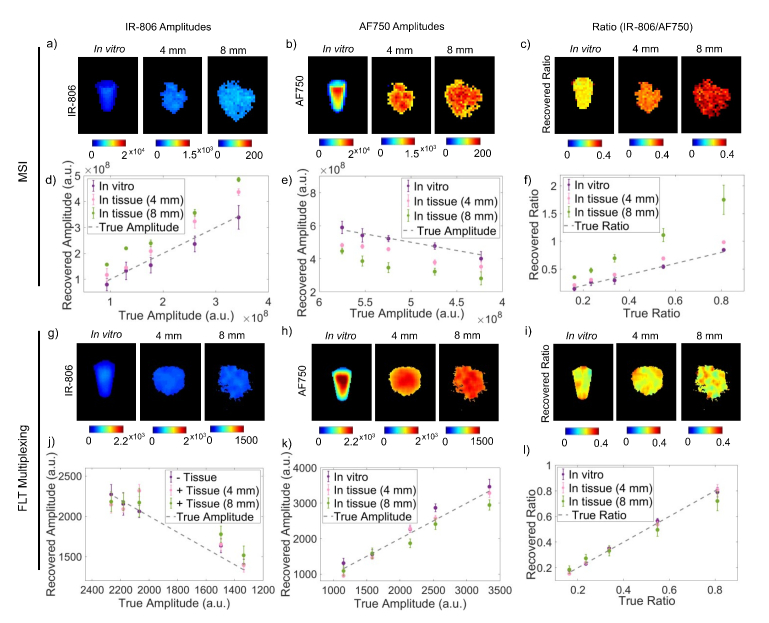
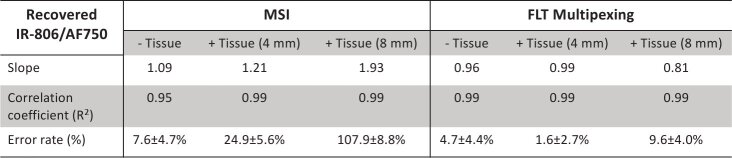
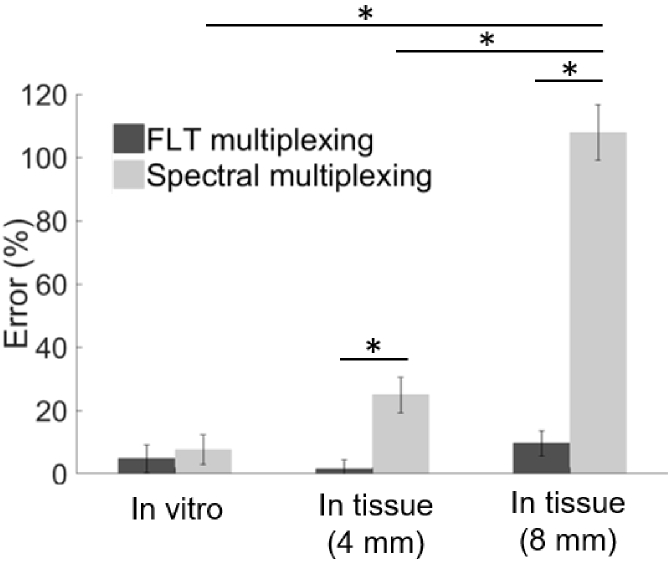
Figure 4 describes a comprehensive summary of the imaging results from MSI and FLT multiplexing. Figures 4(a) and 4(b) shows images of recovered IR-806 and AF750 yields, respectively, based on MSI from a representative IR-806/AF750 yield ratio (0.24) under the in vitro condition and in tissues of 4 mm and 8 mm thickness. The corresponding IR-806/AF750 yield ratio for the three imaging conditions are presented in Fig. 4(c). The images in Fig. 4(c) clearly indicated that the recovered yield ratio for the in vitro condition (0.26 ± 0.06) closely matched the true yield ratio (0.24), however in presence of tissue, the ratios were greatly overestimated (0.31 ± 0.01 for 4 mm thick tissue and 0.48 ± 0.04 for 8 mm thick tissue) with a margin of error up to 100%. Figures 4(d) and 4(e) shows the MSI-based recovered IR-806 and AF750 yields (scaled for camera integration time), respectively, plotted against the true yields for all five mixtures under the three experimental conditions (in vitro, in 4 mm thick tissue and in 8 mm thick tissue). The gray dashed lines in Fig. 4(d) and 4(e) represent the true yields of IR-806 and AF750, respectively. Under in vitro conditions (Fig. 4(d) and 4(e), ‘purple’), the yields of IR-806 and AF750 for all five mixtures were accurately recovered using MSI. However, the quantification accuracy of MSI was greatly affected in the presence of tissue. Specifically, we observed an overestimation of IR-806 amplitudes and an underestimation of AF750 yields when the fluorophore solutions were embedded in tissue. The degrees of overestimation (for IR-806) or underestimation (for AF750) were higher for increasing tissue thickness (Fig. 4(a) and 4(b), 4 mm: ‘pink’; 8 mm: ‘green’). This can be explained by the redshifted fluorescence spectra of AF750 (Fig. 3(a)) in tissue, which causes a greater crosstalk between AF750 and IR-806, compared to the in vitro measurements. As a result, the recovered fluorescence yield ratios of IR-806 and AF750 (Fig. 4(f) andTable 2) in presence of 4 mm (Fig. 4(f), ‘pink’) and 8 mm (Fig. 4(f), ‘green’) thick tissue were significantly higher than the true yield ratios (Fig. 4(f), gray dashed line).Table 3 shows the slopes and correlation coefficients of a linear fit for the data presented in Fig. 4(f). We observed an increased slope while the correlation coefficients remained unaffected (R2 > 0.95) with increasing tissue thickness. This indicated that the overestimation or underestimation of the recovered amplitudes rely on the tissue thickness and not on the individual fluorophore concentrations. Figures 4 g and 4 h shows images of recovered IR-806 and AF750 yields, respectively, based on FLT multiplexing from a representative IR-806/AF750 mixture with a yield ratio of 0.24. The corresponding IR-806/AF750 yield ratio for the three imaging conditions (i.e., in vitro and in tissues of 4 mm and 8 mm thickness) are presented in Fig. 4(i). Unlike MSI (Fig. 4(c)), the recovered yield ratios from FLT multiplexing for in vitro (0.23 ± 0.01), in 4 mm thick tissue (0.24 ± 0.01) and in 8 mm thick tissue (0.27 ± 0.03) closely matched with the true yield ratio of 0.24. Furthermore, the recovered yields (scaled for camera integration time) for IR-806 (Fig. 4(j)) and AF750 (Fig. 4(k)) across all five mixtures were also comparable to the true yields (gray dashed lines in Fig. 4(j) and 4(k)). Therefore, the recovered fluorescence yield ratios closely matched the true yield ratios (Fig. 4l and Table 2) and the slope and correlation coefficient approached 1 (Table 3) irrespective of the imaging conditions (i.e., in vitro and in tissues of 4 mm and 8 mm thickness). We next compared the average error rates (Fig. 5 andTable 3) in recovered fluorescence yield ratios for MSI and FLT multiplexing. Under in vitro conditions, we observed low error rates for both MSI (error = 7.6 ± 4.7%) and FLT multiplexing (error = 4.7 ± 4.4%), while in presence of 4 mm thick tissue, MSI showed a significantly higher (p < 0.01) error rate (24.9 ± 5.63%) compared to FLT multiplexing (1.6 ± 2.7%). As conceivable from Fig. 4, imaging through an 8 mm thick tissue resulted in a larger error rate for MSI (error = 107.9 ± 8.78%), while FLT multiplexing showed an error rate of 9.6 ± 4.0%.
Fig. 4.
Comparison of relative quantitation of two fluorophores in mixture using MSI and FLT multiplexing. a) and b) shows images (in vitro and in 4 mm or 8 mm thick tissue) of the MSI-based recovered amplitudes of IR-806 and AF750, respectively. Representative images are shown from a mixture with IR-806/AF750 volume ratio of 1:7 (true yield ratio = 0.24). (c) MSI-based recovered yield ratios calculated from the images presented in (a) and (b). Recovered amplitudes for all ratios of IR-806 and AF750 based on MSI are shown in (d) and (e), respectively. Purple, pink and green data points represent experiments performed in vitro, in 4 mm thick tissue and in 8 mm thick tissue, respectively. (f) MSI based recovered fluorescence yield ratios of five IR-806 and AF750 mixtures (true yield ratios: 0.16, 0.24, 0.34, 0.55 and 0.81) are shown for imaging under in vitro conditions and in 4 mm or 8 mm thick tissue along with the true yield ratios (gray dashed). g) and h) shows images (in vitro and in 4 mm or 8 mm thick tissue) of the recovered amplitudes of IR-806 and AF750, respectively, based on FLT multiplexing. Representative FLT multiplexed images are shown from a mixture with IR-806/AF750 volume ratio of 1:7 (true yield ratio = 0.24). (i) Recovered yield ratios calculated from the FLT multiplexed images presented in (g) and (h). (j) IR-806 and (k) AF750 amplitudes recovered based on FLT multiplexing are shown for in vitro conditions and in tissue when the fluorophore solutions were embedded in 4 mm or 8 mm thick tissue. (l) The recovered yield ratios based on FLT multiplexing are shown for imaging in vitro, in 4 mm and 8 mm thick tissue along with the true yield ratios (gray dashed). The true amplitudes of IR-806 in (d) and (e), and AF750 in (j) and (k) are represented by gray dashed lines. The true ratios in (f) and (l) (gray dotted) are adjusted for camera integration time and the differences in quantum efficiency of IR-806 and AF750 under the experimental conditions. Error bars represent standard deviations across three separate measurements.
Table 2. Recovered and true IR-806/AF750 fluorescence yield ratios for MSI and FLT multiplexing.
Table 3. Statistical parameters (correlation coefficient, slope, and average percent error) comparing relative quantitation for MSI and FLT multiplexing in vitro and in tissue.
Fig. 5.
Percent error of relative quantitation by MSI and FLT multiplexing. Bar graphs represents the mean of percent errors calculated for five mixed solutions of IR-806 and AF750 under in vitro conditions and in tissue of varying thickness (4 mm and 8 mm). Standard deviations represent variance of recovered fluorescence yield ratios. Black: FLT multiplexing. Gray: MSI. p * < 0.01
4. Conclusions
In summary, we have presented a systematic experimental comparison of the quantification accuracies between MSI and FLT multiplexing, with implications for whole body planar imaging of NIR fluorescent probes. We showed that in the absence of a scattering medium (e.g., biological tissue), both MSI and FLT multiplexing provide accurate quantification of one or more fluorophores in solution. However, when fluorophores are embedded in a light scattering and absorbing medium, FLT multiplexing provides significantly higher quantification accuracy (error rate < 10%) than MSI (error rate 20–107%). The high error rates in MSI are attributed to an increased cross-talk between fluorescence spectra of the constituent fluorescent species in tissue. However, the cross-talk is negligible in the TD measurements, which leads to the observed high accuracy of relative quantification for FLT multiplexing even for fluorophores at 8 mm depth in tissue. These results confirmed that in planar imaging of fluorophores embedded in thick biological tissue, FLT multiplexing can provide more accurate and reliable relative quantitation compared to MSI. We note that while the average quantification error for FLT multiplexing for imaging at a depth of 8 mm remained below 10%, the error increased from 4 mm to 8 mm depth. Therefore, the actual quantification error for in vivo imaging will depend on the fluorophore depth and specific imaging conditions. However, the general conclusions of this study, namely the superior quantitative accuracy of FLT multiplexing compared to MSI will hold true, irrespective of the fluorophore depth. While the benefits of tomographic FLT multiplexing have been previously reported by our group [27,29], the findings in this paper highlights the important implications of planar FLT multiplexing in biological imaging, specifically to facilitate studies where estimation of fluorophore concentration in bulk tissue is desired without the knowledge of fluorophore depth and tissue optical properties. Fluorescence tomography is ideally required to identify the precise locations of fluorophores and the relative quantification of multiple spatially overlapping fluorophore species in tissue. However, a complex instrumentation and data collection protocols, and computationally intensive analysis methods has limited a widespread adoption of fluorescence tomography in preclinical imaging applications. Planar FLT multiplexed imaging on the other hand can provide a necessary means for straightforward and rapid estimation of fluorophore concentration in tissue with relatively low error rates.
Molecular imaging with fluorescent probes that target unique disease markers is an active area of investigation. Antibodies or small molecules to diseases biomarkers are often conjugated with fluorescent dyes that act as molecular beacons. These beacons are widely applied in preclinical studies to track the expression of target molecules [39], estimate drug uptake in specific compartments in the whole body [40–42], and monitor responses to therapeutic interventions. We have recently shown in clinical and preclinical studies that FLT imaging using a therapeutic antibody tagged to a fluorescent molecule enables accurate detection and quantification of a well-documented cancer biomarker, epidermal growth factor receptor (EGFR) [39,43]. Imaging technologies that allow accurate in vivo quantification of these molecular beacons are of paramount importance in preclinical studies as a first step towards the clinical translation of molecular imaging. In the current era of personalized medicine and combination therapies, imaging, and monitoring of multiple physiological processes in vivo and over time are crucial. With the advancements in the field of biocompatible NIR fluorescent probes [44,45], we anticipate a significant role of planar FLT multiplexed imaging for in vivo preclinical and bulk tissue imaging.
Acknowledgements
This work was supported by NIH grants R01-CA211084 and R01-CA260857.
Funding
National Institutes of Health10.13039/100000002 (R01-CA211084, R01-CA260857).
Disclosures
The authors declare no conflict of interest.
Data availability
Data underlying the results presented in this paper may be obtained from the authors upon reasonable request.
References
- 1.Dickinson ME, Bearman G, Tille S, Lansford R, Fraser SE., “Multi-spectral imaging and linear unmixing add a whole new dimension to laser scanning fluorescence microscopy,” BioTechniques 31(6), 1272–1278 (2001) 4-6, 8. 10.2144/01316bt01 [DOI] [PubMed] [Google Scholar]
- 2.Hiraoka Y., Shimi T., Haraguchi T., “Multispectral imaging fluorescence microscopy for living cells,” Cell Struct. Funct. 27(5), 367–374 (2002). 10.1247/csf.27.367 [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi H., Hama Y., Koyama Y., Barrett T., Regino C. A., Urano Y., Choyke P. L., “Simultaneous multicolor imaging of five different lymphatic basins using quantum dots,” Nano Lett. 7(6), 1711–1716 (2007). 10.1021/nl0707003 [DOI] [PubMed] [Google Scholar]
- 4.Levenson R. M., Lynch D. T., Kobayashi H., Backer J. M., Backer M. V., “Multiplexing with multispectral imaging: from mice to microscopy,” ILAR J. 49(1), 78–88 (2008). 10.1093/ilar.49.1.78 [DOI] [PubMed] [Google Scholar]
- 5.Holzapfel H. Y., Stern A. D., Bouhaddou M., Anglin C. M., Putur D., Comer S., Birtwistle M. R., “Fluorescence Multiplexing with Spectral Imaging and Combinatorics,” ACS Comb. Sci. 20(11), 653–659 (2018). 10.1021/acscombsci.8b00101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rice W. L., Kumar A. T., “Preclinical whole body time domain fluorescence lifetime multiplexing of fluorescent proteins,” J. Biomed. Opt. 19(4), 046005 (2014). 10.1117/1.JBO.19.4.046005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao R., Pankayatselvan V., Houston J. P., “Cytometric sorting based on the fluorescence lifetime of spectrally overlapping signals,” Opt. Express 21(12), 14816 (2013). 10.1364/OE.21.014816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen K. J., Peterson K. C., Muretta J. M., Higgins S. E., Gillispie G. D., Thomas D. D., “Fluorescence lifetime plate reader: resolution and precision meet high-throughput,” Rev. Sci. Instrum. 85(11), 113101 (2014). 10.1063/1.4900727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W., Houston K. D., Houston J. P., “Shifts in the fluorescence lifetime of EGFP during bacterial phagocytosis measured by phase-sensitive flow cytometry,” Sci. Rep. 7(1), 40341 (2017). 10.1038/srep40341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodwolf R., Volz-Rakebrand P., Stellmacher J., Wolff C., Unbehauen M., Haag R., Schafer-Korting M., Zoschke C., Alexiev U., “Faster, sharper, more precise: Automated Cluster-FLIM in preclinical testing directly identifies the intracellular fate of theranostics in live cells and tissue,” Theranostics 10(14), 6322–6336 (2020). 10.7150/thno.42581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bitton A., Sambrano J., Valentino S., Houston J. P., “A Review of New High-Throughput Methods Designed for Fluorescence Lifetime Sensing From Cells and Tissues,” Front Phys. 9, 648553 (2021). 10.3389/fphy.2021.648553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montet X., Ntziachristos V., Grimm J., Weissleder R., “Tomographic fluorescence mapping of tumor targets,” Cancer Res. 65(14), 6330–6336 (2005). 10.1158/0008-5472.CAN-05-0382 [DOI] [PubMed] [Google Scholar]
- 13.Smith M. Q., Staley C. A., Kooby D. A., Styblo T., Wood W. C., Yang L., “Multiplexed fluorescence imaging of tumor biomarkers in gene expression and protein levels for personalized and predictive medicine,” Curr. Mol. Med. 9(8), 1017–1023 (2009). 10.2174/156652409789712765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haugwitz M., Nourzaie O., Garachtchenko T., Hu L., Gandlur S., Olsen C., Farmer A., Chaga G., Sagawa H., “Multiplexing bioluminescent and fluorescent reporters to monitor live cells,” Curr. Chem. Genomics 1, 11–19 (2008). 10.2174/1875397300801010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhalla R. M., Hulsemann M., Verkhusha P. V., Walker M. G., Shcherbakova D. M., Hodgson L., “Multiplex Imaging of Rho GTPase Activities in Living Cells,” Methods Mol Biol. 2350, 43–68 (2021). 10.1007/978-1-0716-1593-5_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filonov G. S., Piatkevich K. D., Ting L. M., Zhang J., Kim K., Verkhusha V. V., “Bright and stable near-infrared fluorescent protein for in vivo imaging,” Nat. Biotechnol. 29(8), 757–761 (2011). 10.1038/nbt.1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shcherbakova D. M., Verkhusha V. V., “Near-infrared fluorescent proteins for multicolor in vivo imaging,” Nat. Methods 10(8), 751–754 (2013). 10.1038/nmeth.2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Busch C., Schroter T., Grabolle M., Wenzel M., Kempe H., Kaiser W. A., Resch-Genger U., Hilger I., “An in vivo spectral multiplexing approach for the cooperative imaging of different disease-related biomarkers with near-infrared fluorescent forster resonance energy transfer probes,” J Nucl Med 53(4), 638–646 (2012). 10.2967/jnumed.111.094391 [DOI] [PubMed] [Google Scholar]
- 19.Day K. E., Sweeny L., Kulbersh B., Zinn K. R., Rosenthal E. L., “Preclinical comparison of near-infrared-labeled cetuximab and panitumumab for optical imaging of head and neck squamous cell carcinoma,” Mol Imaging Biol 15(6), 722–729 (2013). 10.1007/s11307-013-0652-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neher R., Neher E., “Optimizing imaging parameters for the separation of multiple labels in a fluorescence image,” J. Microsc. (Oxford, U. K.) 213(1), 46–62 (2004). 10.1111/j.1365-2818.2004.01262.x [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann T., Rietdorf J., Girod A., Georget V., Pepperkok R., “Spectral imaging and linear un-mixing enables improved FRET efficiency with a novel GFP2-YFP FRET pair,” FEBS Lett. 531(2), 245–249 (2002). 10.1016/S0014-5793(02)03508-1 [DOI] [PubMed] [Google Scholar]
- 22.Nadrigny F., Rivals I., Hirrlinger P. G., Koulakoff A., Personnaz L., Vernet M., Allioux M., Chaumeil M., Ropert N., Giaume C., Kirchhoff F., Oheim M., “Detecting fluorescent protein expression and co-localisation on single secretory vesicles with linear spectral unmixing,” Eur. Biophys. J. 35(6), 533–547 (2006). 10.1007/s00249-005-0040-8 [DOI] [PubMed] [Google Scholar]
- 23.Garofalakis A., Zacharakis G., Meyer H., Economou E. N., Mamalaki C., Papamatheakis J., Kioussis D., Ntziachristos V., Ripoll J., “Three-dimensional in vivo imaging of green fluorescent protein-expressing T cells in mice with noncontact fluorescence molecular tomography,” Mol. Imaging 6(2), 7290.2007.00007 (2007). 10.2310/7290.2007.00007 [DOI] [PubMed] [Google Scholar]
- 24.Zhu B., Rasmussen J. C., Lu Y., Sevick-Muraca E. M., “Reduction of excitation light leakage to improve near-infrared fluorescence imaging for tissue surface and deep tissue imaging,” Med. Phys. 37(11), 5961–5970 (2010). 10.1118/1.3497153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pera V., Brooks D. H., Niedre M., “Multiplexed fluorescence tomography with spectral and temporal data: demixing with intrinsic regularization,” Biomed. Opt. Express 7(1), 111 (2016). 10.1364/BOE.7.000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zimmermann T., Rietdorf J., Pepperkok R., “Spectral imaging and its applications in live cell microscopy,” FEBS Lett. 546(1), 87–92 (2003). 10.1016/S0014-5793(03)00521-0 [DOI] [PubMed] [Google Scholar]
- 27.Hou S. S., Bacskai B. J., Kumar A. T., “Comparison of tomographic fluorescence spectral and lifetime multiplexing,” Opt. Lett. 41(22), 5337 (2016). 10.1364/OL.41.005337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar A. T., Carp S. A., Yang J., Ross A., Medarova Z., Ran C., “Fluorescence lifetime-based contrast enhancement of indocyanine green-labeled tumors,” J. Biomed. Opt. 22(4), 040501 (2017). 10.1117/1.JBO.22.4.040501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raymond S. B., Boas D. A., Bacskai B. J., Kumar A. T., “Lifetime-based tomographic multiplexing,” J. Biomed. Opt. 15(4), 046011 (2010). 10.1117/1.3469797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar A. T., Raymond S. B., Boverman G., Boas D. A., Bacskai B. J., “Time resolved fluorescence tomography of turbid media based on lifetime contrast,” Opt. Express 14(25), 12255 (2006). 10.1364/OE.14.012255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar A. T., Raymond S. B., Bacskai B. J., Boas D. A., “Comparison of frequency-domain and time-domain fluorescence lifetime tomography,” Opt. Lett. 33(5), 470 (2008). 10.1364/OL.31.000470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rice W. L., Hou S., Kumar A. T., “Resolution below the point spread function for diffuse optical imaging using fluorescence lifetime multiplexing,” Opt. Lett. 38(12), 2038 (2013). 10.1364/OL.38.002038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice W. L., Shcherbakova D., Verkhusha V., Kumar A. T., “In vivo tomographic imaging of deep seated cancer using fluorescence lifetime contrast,” Cancer Res. 75(7), 1236–1243 (2015). 10.1158/0008-5472.CAN-14-3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar A. T., Chung E., Raymond S. B., van de Water J. A. J. M., Shah K., Fukumura D., Jain R. K., Bacskai B. J., Boas D. A., “Feasibility of in vivo imaging of fluorescent proteins using lifetime contrast,” Opt. Lett. 34(13), 2066 (2009). 10.1364/OL.34.002066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diamandis E. P., “Immunoassays with time-resolved fluorescence spectroscopy: principles and applications,” Clin. Biochem. 21(3), 139–150 (1988). 10.1016/0009-9120(88)90001-X [DOI] [PubMed] [Google Scholar]
- 36.Hou S. S., Bacskai B. J., Kumar A. T. N., “The Resolution Matrix in Tomographic Multiplexing: Optimization of Inter-Parameter Cross-Talk, Relative Quantitation, and Localization,” IEEE Trans. Biomed. Eng. 66(8), 2341–2351 (2019). 10.1109/TBME.2018.2889043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou S. S., Rice W. L., Bacskai B. J., Kumar A. T., “Tomographic lifetime imaging using combined early- and late-arriving photons,” Opt. Lett. 39(5), 1165 (2014). 10.1364/OL.39.001165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han H. H., Kang H., Kim S. J., Pal R., Kumar A. T. N., Choi H. S., Hahn S. K., “Fluorescent nanodiamond - hyaluronate conjugates for target-specific molecular imaging,” RSC Adv. 11(37), 23073–23081 (2021). 10.1039/D1RA03936A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pal R., Kang H., Choi H. S., Kumar A. T. N., “Fluorescence Lifetime-Based Tumor Contrast Enhancement Using an EGFR Antibody-Labeled Near-Infrared Fluorophore,” Clin. Cancer Res. 25(22), 6653–6661 (2019). 10.1158/1078-0432.CCR-19-1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasquez K. O., Casavant C., Peterson J. D., “Quantitative whole body biodistribution of fluorescent-labeled agents by non-invasive tomographic imaging,” PLoS One 6(6), e20594 (2011). 10.1371/journal.pone.0020594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Z., Leon J., Martin M., Harder J. W., Zhang R., Liang D., Lu W., Tian M., Gelovani J. G., Qiao A., Li C., “Pharmacokinetics and biodistribution of near-infrared fluorescence polymeric nanoparticles,” Nanotechnology 20(16), 165101 (2009). 10.1088/0957-4484/20/16/165101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Korideck H., Peterson J. D., “Noninvasive quantitative tomography of the therapeutic response to dexamethasone in ovalbumin-induced murine asthma,” J. Pharmacol. Exp. Ther. 329(3), 882–889 (2009). 10.1124/jpet.108.147579 [DOI] [PubMed] [Google Scholar]
- 43.Pal R, Hom ME, Berg NS van den, Lwin TM, Lee YJ, Prilutskiy A, Faquin W, Yang E, Saladi SV, Varvares MA, Rosenthal EL, Kumar ATN, “First Clinical Results of Fluorescence Lifetime-Enhanced Tumor Imaging Using Receptor-targeted Fluorescent Probes,” Clin. Cancer Res. 28(11), 2373–2384 (2022). 10.1158/1078-0432.CCR-21-3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chu L., Wang S., Li K., Xi W., Zhao X., Qian J., “Biocompatible near-infrared fluorescent nanoparticles for macro and microscopic in vivo functional bioimaging,” Biomed. Opt. Express 5(11), 4076 (2014). 10.1364/BOE.5.004076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin C. C., Chang W. H., Cheng T. M., Chiu L. H., Wang Y. H., Lin C. J., Ho Y. S., Zuo C. S., Wang Y. M., Lai W. T., “Two new, near-infrared, fluorescent probes as potential tools for imaging bone repair,” Sci. Rep. 10(1), 2580 (2020). 10.1038/s41598-020-59522-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data underlying the results presented in this paper may be obtained from the authors upon reasonable request.