We spend most of our time indoors, and the indoor environment is the greatest contributor to human chemical exposures.1,2 Despite its importance, our understanding of indoor chemicals is more limited than that for the outdoor environment. A recent report of the US National Academies3 helps answer a 2-fold question: why does indoor chemistry matter, and what is needed to advance our scientific understanding of indoor chemistry?
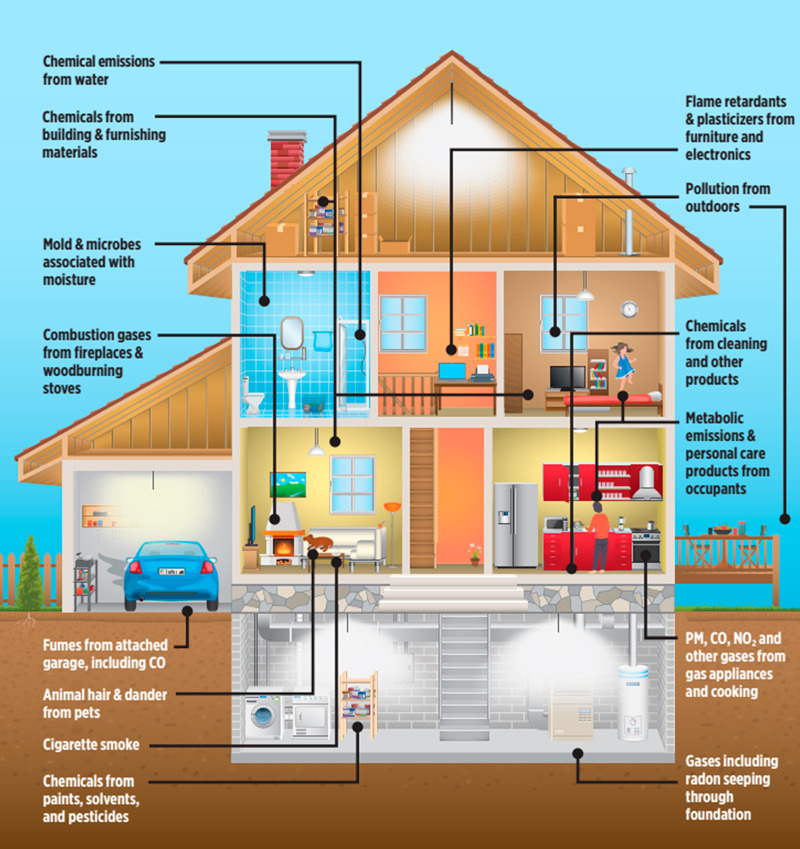
Indoor chemistry is dynamic and complex. Thousands of chemicals are found indoors in air, particles, dust, and surfaces, often at concentrations exceeding those found outdoors. Sources can be primary or secondary, continuous or episodic, and of indoor or outdoor origin (Figure 1). Humans modify indoor chemistry through cooking and the use of personal care products and are a primary source of chemicals in the gas and particle phases.
Figure 1.
This schematic lists examples of the most important primary chemical emission sources and reservoirs found in the indoor environment. CO, carbon monoxide; NO2, nitrogen dioxide; PM, particulate matter. Credit: NASEM, 2022. Reproduced with permission from the National Academy of Sciences, courtesy of the National Academies Press, Washington, DC.
Indoors, chemicals partition between air, airborne particles, dust, water, and surfaces. These surfaces act as primary sources and chemical sinks or reservoirs. Partitioning results in both the removal and release of chemicals from surfaces. Partitioning does not occur instantaneously and may require years to achieve pseudoequilibrium due to the slow rates of molecular transport. Equilibrium conditions change over time and respond to changes in relative humidity, temperature, and other environmental conditions. Indoor chemicals undergo extensive movement within a building and its furnishings. Partitioning can be reversed, resulting in emission of chemicals into the air. Thus, partitioning is a critical determinant of the amount and duration of a chemical’s presence in a particular physical phase and compartment. This directly influences exposure potential for occupants. For example, inhalation exposure increases as chemicals partition to air, while ingestion increases as chemicals partition to dust and surfaces.
Indoor chemicals undergo oxidation, photolysis, hydrolysis, acid–base, and other reactions. Gas-phase oxidation forms highly oxygenated gas-phase species that can lead to the formation of secondary organic aerosols. These reactions not only reduce the concentration of a chemical but also lead to the formation of intermediaries and reaction products with different chemical and physical properties. Products may be more or less toxic than the reactants. Multiphase chemistry occurs on human skin and clothing and surfaces contaminated by cooking or smoking emissions. Ozonolysis of unsaturated organics forms ozonides and volatile oxygenates on surfaces. Chemicals found in air and on surfaces illuminated with sunlight undergo photochemical reactions that produce high oxidant concentrations that drive indoor chemistry. Aqueous surface films facilitate several reactions, including acid–base reactions and nitrogen dioxide disproportionation, that form nitric and nitrous acids. Chemical reactions occur throughout the indoor environment, including attics and other hidden spaces, air ducts, and HVAC systems. This complex environment and the dynamic behavior of chemicals indoors contribute to challenges and uncertainty in measurement, modeling and exposure, and health risk assessments that need to be overcome.
We know little about human exposures to chemicals across phases, exposure pathways, and time scales. Our understanding of whether indoor chemicals have synergistic or antagonistic effects is also lacking. Advanced targeted and nontargeted analytical methods are needed to fully characterize and quantify chemical mixtures and model their emissions, fate, and transport. There is a critical need to develop chemical process models that account for indoor chemistry across phases and compartments. These models can be linked with human exposure and uptake models and toxicological data. Linked models can estimate internal dose and subsequent health effects of mixtures across exposure pathways. Improved knowledge of molecular reaction rates, kinetics, emission rates, building and environmental factors, and human behaviors and time activity patterns along with their uncertainties are needed to refine exposure and health risk models. This scientific foundation will further our understanding of indoor chemistry and its impacts on health and the environment and support regulatory action and guidelines.
Regulatory actions can include banning or phasing out chemicals; however, recycling of materials can inadvertently reintroduce flame retardants and other phased-out chemicals back into the indoor environment. Similarly, replacement chemicals often have less data available on their emission rates, exposure potential, and health hazards. A lack of transparency on chemicals in consumer products and incomplete chemical inventories complicate regulation of indoor chemicals.
The COVID-19 pandemic has raised global awareness of the importance of the indoor environment on viral transmission and human health. The pandemic increased use of bleach, quaternary ammonium compounds, and other indoor air cleaning devices with unknown impacts on indoor chemistry. While filtration-based devices can be beneficial, unsubstantiated claims around removal efficacy, safety, and personal health risks and benefits are rampant. Air cleaners that rely on ozonolysis, photolysis, ionization, or other chemical transformations to “remove” chemicals indoors can potentially generate harmful secondary chemicals with no clear evidence of health benefits. Research is needed to understand the unintended consequences of these devices, and standardized consensus test methods could help regulators determine whether oversight is warranted.
Commercial grade or “low-cost” sensors have gained widespread use. These sensors increase spatiotemporal coverage of air quality monitoring indoors and outdoors, especially in areas with poor coverage or environmental justice concerns. There is a critical need to develop effective public health messaging regarding these sensors. For example, current messaging is often based on ambient air quality risk thresholds that may not translate directly to indoor environments.
In conclusion, important data gaps remain, and research is needed to increase our understanding of indoor chemistry, exposure pathways and routes, and health risks and to provide scientific evidence to support efforts to protect human health and the environment. Studies of indoor chemistry in realistic and diverse settings are needed, especially in environmental justice communities. There is a need for strategic investment in collaborative and interdisciplinary research to accelerate knowledge of indoor chemistry, translate the science into practice that benefits public health and the environment, and promote active stakeholder engagement and dissemination. Researchers, planners, and engineers should integrate indoor chemistry considerations into building materials, operations, and design. Finally, given the importance of indoor chemistry and remaining knowledge gaps, federal and state agencies and research funders should prioritize studies of indoor chemistry and its impacts on human exposures and health.
Acknowledgments
The authors gratefully acknowledge the consensus report’s peer reviewers, the subject-matter experts who provided input at the committee’s information-gathering workshop, and the staff of the National Academies Research Center.
Glossary
ABBREVIATIONS
- HVAC
heating, ventilation, and air conditioning.
Biography

Megan E. Harries is a Program Officer with the Board on Chemical Sciences and Technology at the National Academies of Sciences, Engineering, and Medicine. She is the director of the Committee on Emerging Science on Indoor Chemistry, which authored the report described in this Viewpoint. Trained as an analytical chemist, Dr. Harries received a BA from Fordham University and a Ph.D. from the University of Colorado Boulder. Prior to joining the National Academies, she was the recipient of a National Research Council Research Associateship, which she spent at the National Institute of Standards and Technology developing methods for more sensitive and repeatable chemical characterization of trace forensic evidence.
This work was sponsored in part by the Alfred P. Sloan Foundation, the Centers for Disease Control and Prevention, the Environmental Protection Agency, and the National Institute of Environmental Health Sciences.
These opinions, recommendations, findings, and conclusions do not necessarily reflect the views or policies of the National Institute of Standards and Technology (NIST) or the United States Government.
The authors declare no competing financial interest.
References
- Spengler J. D.; Sexton K. Indoor air pollution: a public health perspective. Science 1983, 221 (4605), 9–17. 10.1126/science.6857273. [DOI] [PubMed] [Google Scholar]
- Samet J. M.; Spengler J. D. Indoor environments and health: moving into the 21st century. American Journal of Public Health 2003, 93 (9), 1489–1493. 10.2105/AJPH.93.9.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine (NASEM) . Why Indoor Chemistry Matters; The National Academies Press: Washington, DC, 2022; 10.17226/26228. [DOI] [PubMed]