Summary
Advances in self-organizing cardiac organoids to recapitulate human cardiogenesis have provided a powerful tool for unveiling human cardiac development, studying cardiovascular diseases, testing drugs, and transplantation. Here, we highlight the recent remarkable progress on multicellular cardiac organoids and review the current status for their practical applications. We then introduce key readouts and tools for assessing cardiac organoids for clinical applications, address major challenges and provide suggestions for each assessment method. Lastly, we discuss the current limitations of cardiac organoids as miniature models of the human heart and suggest a direction for moving forward towards building the mini-heart from cardiac organoids.
eTOC
Recent advances and current challenges in multicellular cardiac organoids and tools for assessing cardiac organoids for clinical application are discussed.
Introduction
Cardiac organoids are three-dimensional (3D) self-organized structures that spontaneously assume the shape or function of the heart tissue and contain major cardiac cell types, including cardiomyocytes, cardiac fibroblasts, and endothelial cells. Recently, many studies have revealed how cardiac organoids can model human cardiac development, and have shown substantial progress (Drakhlis et al., 2021, Hofbauer et al., 2021, Rossi et al., 2021).
The term “cardiac organoid” has been widely used in a number of studies using cardiac tissues formed by distinct methods. In most cases, cardiac organoids form spontaneously from self-assembled aggregates of pluripotent stem cells (PSCs) on anti-adhesion surfaces, which then differentiate into cardiac cells (Drakhlis et al., 2021; Hofbauer et al., 2021, Rossi et al., 2021). In some cases, scaffolding materials are added (Kupfer et al., 2020) or differentiated cells are used (Richards et al., 2020) to form organoids. Cardiac spheroids or the spherical microtissues are often formed by mixing pre-differentiated cardiomyocytes, endothelial cells and cardiac fibroblasts in a ratio similar to the cellular composition of the heart (Polonchuk et al., 2017; Sharma and Gentile, 2021). However, the terms “organoid” and “spheroid” are often used interchangeably, and organoids have been broadly referred to as 3D cardiac microtissues. In this perspective, we will focus only on the organoids formed through spontaneous co-differentiation from pluripotent stem cells into various types of cardiac cells.
The term “cardiac organoid” was first coined about 20 years ago (Zimmermann et al., 2002), but its use became popular in 2017 (Mills et al., 2017; Voges et al., 2017). However, using 3D tissues similar to cardiac organoids has a longer history than cardiac organoids, as the early in vitro cardiac differentiation protocols also utilized 3D embryoids (Maltsev et al., 1993). Similar to cardiac organoids, aggregates of pluripotent stem cells, called embryoid bodies, were induced to differentiate into cardiomyocytes using cardiac differentiation media. While the main goal of these early studies was to achieve a high rate of cardiac differentiation (Mummery et al., 2012), recent studies using cardiac organoids aim to recapitulate 3D morphogenesis and pathological processes that are not captured in 2D culture, through cell co-differentiation (Hofbauer et al., 2021). The recent advances were made possible by the development and commercialization of cutting-edge technologies such as human induced pluripotent stem cells (hiPSCs), single-cell analysis (Paik et al., 2020a), and multi-photon microscopy that enable the analysis of complex multicellular organoids. Notably, the adult heart has an extremely limited ability of self-regeneration (Olaf et al., 2009), making it difficult to obtain human cardiomyocytes for research. The advents in hiPSCs and cardiac differentiation protocols have facilitated cardiac research by providing cardiomyocytes and supporting cardiac cells in vitro, typically starting from a small sample of blood (Paik et al., 2020b).
Accordingly, there are a growing number of studies using cardiac organoids. In particular, 2021 saw many remarkable studies being reported (Drakhlis et al., 2021; Hofbauer et al., 2021; Rossi et al., 2021). In this perspective, we will introduce the recent major advances and perspectives in cardiac organoids and their applications, including studies of developmental biology, drug testing, disease modeling, and transplantation. For more successful clinical applications, we will also examine the limitations of cardiac organoids by evaluating their key functions and features. Finally, we will discuss overcoming the current challenges and the future directions, including obtaining mini-hearts starting from organoids.
Modeling early cardiogenesis
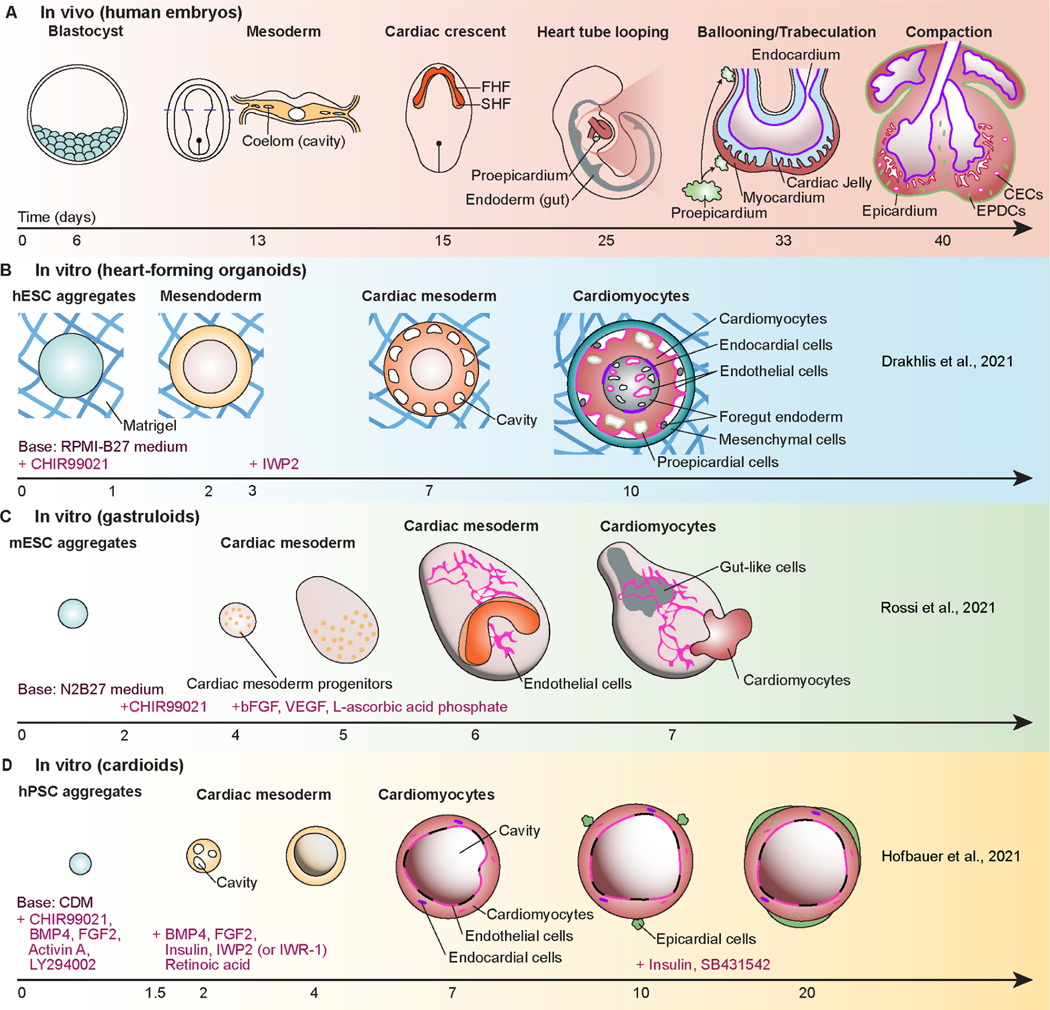
Self-organizing cardiac organoids that mimic cardiogenesis were reported in 2021 (Figure 1). Drakhlis and colleagues induced the formation of mesoderm by CHIR99021 and subsequent cardiac specification by IWP2, after embedding aggregates of human embryonic stem cells (hESCs) or hiPSCs in Matrigel (Drakhlis et al., 2021). They used the MIXL1-GFP hESC reporter line and the NKX2.5-eGFP hESC reporter line to monitor the induction of mesendoderm in the primitive streak during gastrulation and cardiac lineage cells, respectively. During organoid differentiation, MIXL1 showed a transient expression pattern between day 2 and day 4, and one of the earliest markers of the cardiac lineage, NKX2.5 (Behrens et al., 2013) was expressed in a ring shape from day 7. On day 10, the inner layer contained endothelial cells and foregut-like cells with microvilli, and the middle layer consisted of mostly cardiomyocytes with some epicardial cells. The outer layer in these organoids contained mesenchymal cells and liver cells. Notably, a small number of NFATC1+ endocardial cells were seen at the interface of the inner and middle layers. This study also showed formation of cavities in cardiac organoids. In particular, the cavities in the middle layer at day 4 mimicked the formation of coeloms in the mesoderm during human cardiac development (Figures 1A and B), and those in the inner layer at day 10 mimicked foregut cavities and vascular lumina.
Figure 1. Comparison of recently reported cardiac organoids in vitro with cardiac development in vivo.
(A) Early cardiac development of human embryos (first 40 days); FHF: first heart field; SHF: second heart field; EPDCs: epicardial-derived cells; CECs: coronary endothelial cells. (B) Heart-forming organoids mimicking an interplay with foregut endoderm during development; RPMI-B27 medium: Roswell Park Memorial Institute 1640 medium containing B-27 supplement; IWR-2: inhibitor of Wnt production 2. (C) Self-assembled gastruloids with cardiac crescent- and heart tube-like regions; N2B27 medium: a half-half mixture of Dulbecco’s modified essential medium/Ham’s F-12 medium containing N2 supplement and Neurobasal medium containing B-27 supplement; bFGF: basic fibroblast growth factor; VEGF: vascular endothelial growth factor. (D) Chamber-like cardioids with endocardium, myocardium, and epicardium-like layers; BMP4: bone morphogenetic protein 4, FGF2: fibroblast growth factor 2.
Obtaining cardiac cells with foregut and liver cells in a single organoid provided an opportunity to study multi-organ interactions during cardiac development. However, obtaining a large proportion of non-cardiac cells such as foregut endoderm may be due to the embedding of organoids in Matrigel, a tumor matrix preparation that contains numerous growth factors, with batch to batch variability. When cardiac organoids were embedded in growth-factor-reduced Matrigel and collagen I, the layered NKX2.5 pattern did not appear, or the cells died (Drakhlis et al., 2021). In the future, elucidating which particular substance in Matrigel affects cardiogenesis, or the use of alternate cues would greatly improve the robustness and reproducibility of organoid models. As an example, Silva et al. also recently demonstrated multilineage organoids with co-emergence of cardiac and gut cells without Matrigel using epicardial-permissive medium starting from day 7 of cardiac differentiation (Silva et al., 2021).
In a study published in 2021, Rossi and colleagues captured self-organized cardiogenesis in embryonic organoids, which they call gastruloids (Rossi et al., 2021). The mouse embryonic stem cell (mESC) aggregates were first differentiated in the neural stem cell culture medium containing CHIR99021. To also obtain endothelial cells, basic fibroblast growth factor (bFGF), L-ascorbic acid phosphate, and vascular endothelial growth factor (VEGF) were added to the differentiation medium. On day 6, this protocol resulted in the emergence of an in vivo cardiac crescent-like spatial arrangement of first heart field (FHF) and second heart field (SHF) progenitor cells, using a dual FHF/SHF reporter mESC line (HCN4:GFP;TBX1-Cre:RFP) (Figure 1C). The authors claimed that the cardiac crescent-like structure formation and subsequent morphological changes of cardiac lineage cells during days 6–7 could be aligned to the morphogenesis of embryonic cardiac development between E7.5 and E8.5. The emergence of FHF and SHF cells during cardiac lineage specification of hPSC in both 2D culture (Zhang et al., 2019) and 3D cardiac organoids (Lewis-Israeli et al., 2021) has been also reported by other groups.
Besides cardiac lineage cells, the organoids reported by Rossi and colleagues contained networks of endothelial cells and primitive gut tube-like structures (Rossi et al., 2021). In these organoids, a total of 32 cell types were identified, including three germline derivatives and cardiomyocytes, on days 4–7 using single-cell RNA sequencing (scRNA-seq) analysis. In addition, trajectories obtained from RNA velocities showed the gene expression sequence of MESP1 (the earliest marker of cardiac progenitors (Zhang et al., 2017)), NKX2.5 (a marker of cardiac progenitor cells), and HCN4 (a marker of FHF progenitor cells), RYR2, and α-actinin (markers of mature cardiomyocytes) over time in their organoids during differentiation. scRNA-seq results also captured NFATC1+/NPR3+ endocardial cells in the organoids. However, the organoids contained mostly non-cardiac cells and only a few cardiomyocytes, as the differentiation cues did not specifically target cardiac cells, but gastruloids to mimic more general embryonic development. As different cell lines may have different results, this protocol may in fact work better with organoids made from human cells.
In a resource paper by Hofbauer and colleagues, the authors presented remarkable cavity formation in human PSC-derived cardiac organoids (Hofbauer et al., 2021) (Figure 1D), with the presence of major cell types of the heart, including cardiomyocytes, endocardial cells, and epicardial cells. From around day 2.5 of differentiation, when organoids resembled cardiac mesoderm, many small cavities were created that subsequently merged to form large cavities. These organoids showed that the cavity formation requires HAND1 at the beginning of differentiation, possibly because HAND1 expression is needed to become cardiac mesoderm. Accordingly, the cavity formation is most similar to the coelom formation in cardiac mesoderm (Figure 1A), as in the case of Drakhlis et al. (Drakhlis et al., 2021) (Figure 1B). In the inner part of these cavities, the lining of CHD5+ or PECAM1+ endothelial cells was found. In addition, the expression of NFATC1, an endocardial cell marker, was confirmed in organoids from proteomic and scRNA-seq data. Furthermore, to mimic the epicardium from the proepicardial cell clump on the surface of the developing heart, they added the epicardial cells to the surface of their organoids on around day 8 and let them penetrate the tissues. These studies show the potential for cardiac organoids to be used as models of the human fetal heart to recapitulate some aspects of early cardiogenesis, such as cardiac mesoderm and cardiac crescent, and major cell types of human cardiac development.
Disease modelling
Many studies, including the aforementioned cases, have used 3D cardiac organoids to recapitulate the pathology of cardiac diseases in an environment more similar to the native heart than 2D monolayer culture. As CRISPR/Cas9 genome editing is integrated into organoid technology, it is possible to generate disease-carrying mutations in healthy cells or to rescue patients’ cells in organoids (Gopal et al., 2020). Hofbauer et al. (Hofbauer et al., 2021) knocked out HAND1 and NKX2.5 in a hESC line using CRISPR editing to model human cardiac malformations, such as hypoplastic left heart syndrome. Interestingly, knockout (KO) of either HAND1 or NKX2.5 reduced the size of cardia organoids and the cavity formation, but later cardiomyocyte specification (TNNT2+) was still functional. They also generated a developmental injury model by performing localized cryoinjuries in cardiac organoids, which induced the extracellular matrix (ECM) accumulation by endothelial cells or epicardial fibroblast-like cells (Hofbauer et al., 2021).
Drakhlis et al. also constructed cardiac organoids using a NKX2.5 KO hESC line and found that NKX2.5-KO resulted in reduced tissue compaction, reduced adhesion between cardiomyocytes, and larger size of cardiomyocytes, was similar to non-compaction by trabecular overgrowth and hypertrophy captured in Nkx2.5 KO mice (Pashmforoush et al., 2004). Lewis-Israeli et al. cultured cardiac organoids with high glucose and insulin to study the effects of pre-gestational diabetes on cardiac development (Lewis-Israeli et al., 2021), and showed that the diabetic condition resulted in larger organoids, arrhythmia, and lower oxygen consumption. Cardiac diseases caused by developmental abnormalities have been modeled by cardiac organoids. However, current cardiac organoids are similar to fetal hearts, so that modelling of adult heart diseases such as those caused by aging is not yet achieved.
Drug testing
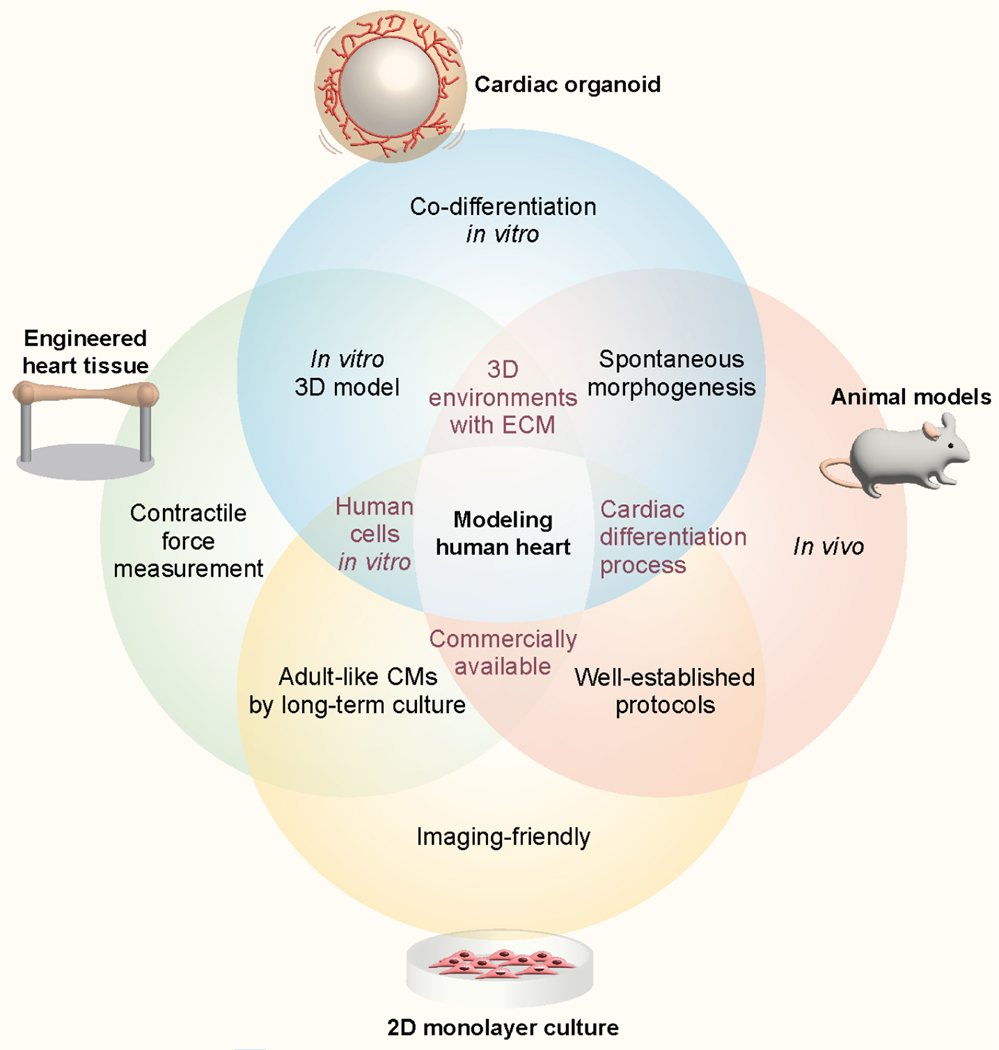
Cardiac organoids have unique advantages that make them suitable for use as drug testing platforms compared with conventional heart models (Figure 2). 2D monolayer culture of human cells and in vivo animal models are widely used and well-established methods in preclinical studies during drug development. 2D monolayer culture is a high throughput system because the preparation and culture are relatively simple and allow cells to be observed at high magnification using a microscope. However, this model may present an oversimplified condition in which many key features in human physiology are not being recapitulated, such as natural structure and cell-ECM interactions (Kapałczyńska et al., 2018; Wnorowski et al., 2019). In addition, animal models provide complex in vivo environments that in vitro models have not yet fully leveraged. Limitations of cardiac animal models are their genetic and functional differences from humans, low throughput, and ethical concerns (Soldatow et al., 2013).
Figure 2. The intersections and unique advantages of cardiac organoids and human heart models for drug testing and disease modelling.
Compared to 2D monolayer cultures, animal models, and engineered heart tissues, cardiac organoids can uniquely provide a platform to investigate spontaneous 3D cardiogenesis through co-differentiation under well-controlled in vitro conditions.
Recently, there have been many meaningful advances in drug testing using engineered heart tissues (EHTs) in which both ends of long rod-shaped 3D cardiac tissues are connected to silicone posts (Cho et al., 2021; Ronaldson-Bouchard et al., 2018). EHTs are mainly prepared by encapsulation of hPSC-derived cardiomyocytes and fibroblasts differentiated in 2D culture into ECM hydrogel, such as fibrin and collagen I (Abilez et al., 2018). This model allows measurements of contractile force (Figure 2), but it is difficult to maintain the tissue without a protease inhibitor in the culture media due to the pre-tension in the tissues generated by the two posts. Furthermore, it is hard to mimic cardiogenesis using EHTs because it is made of already differentiated cells that are at a fetal level of maturity. The throughput of these systems is not high due to the preparation of the molds and a large number of cells for each tissue (~1.5 million cells) (Mannhardt et al., 2017).
Cardiac organoids often exhibit 3D emergent behaviors resulting from cell-cell and cell-ECM interactions in 3D, such as cardiogenesis, not seen in 2D cultures. Organoids can be formed by inducing co-differentiation from hPSCs to multiple cell types in 3D cardiac organoids. These self-organizing cardiac organoids provide much higher throughput than EHTs or animal models in drug screening because the fabrication process is simpler and the number of cells per organoid is much smaller (~5,000 cells) (Drakhlis et al., 2021). In addition, organoids enable personalized medicine approaches using the hiPSCs from patients and to evaluate drug effects on human cardiac development and gene expression pattern. Due to these advantages, cardiac organoids are already used in drug testing for cardiovascular diseases (Lee et al., 2020; Paik et al., 2020b), and may provide new information unavailable from other heart models in traditional preclinical trials. However, there are no standardized organoid protocols or commercially available products yet as cardiac organoid studies are still in the early stage.
Transplantation
In the near future, organoids containing major cell types of target organs may become available for transplantation. In 2018, Mansour and colleagues achieved progressive neuronal maturation of the organoid and growth of axons to multiple regions of the host brain by transplanting hESC-derived brain organoids into adult mouse brain (Mansour et al., 2018). In the same year, Cortez and colleagues transplanted hiPSC-derived intestinal organoids into the mouse mesentery, and 82% of transplanted intestinal organoids were successfully engrafted after 10 weeks (Cortez et al., 2018). Although research on cardiac organoid transplantation has only just begun (Varzideh et al., 2019), we speculate that similar approaches for transplantation of brain and intestinal organoids may be applied to cardiac organoids. Recently, transplantation of cardiac spheroids made from hESC-derived cardiac progenitor cells, endothelial cells, and mesenchymal stem cells into the peritoneal cavity of mice significantly improved the maturity of cardiomyocytes (Varzideh et al., 2019). In addition, it has been shown that hPSC-derived cardiomyocytes, a major component of cardiac organoids, can be used as cell therapy for replenishing damaged myocardium (Oikonomopoulos et al., 2018; Pushp et al., 2021). As an example, transplantation of hiPSC-derived cardiomyocytes into the infarcted hearts of mice showed significant functional improvement by cell therapy (Funakoshi et al., 2016). Also, clinical trials involving the use of hiPSC-derived cardiomyocyte sheets (ClinicalTrials.gov Identifier: NCT04696328) and hESC-derived cardiomyocytes (ClinicalTrials.gov Identifier: NCT05068674) for treating ischemic cardiomyopathy are now underway in Japan and the U.S. As cardiac organoids contain endothelial cells for prevascularization, they are expected to be advantageous for integration into host tissues.
However, the immaturity of the transplanted cells can still cause arrhythmias (Liu et al., 2022; Neofytou et al., 2015; Romagnuolo et al., 2019). Therefore, further studies will be needed to find effective methods for maturation of cardiac organoids, as they currently are at a fetal stages of development. For cardiac organoids to be practical for transplantation into the heart, they need to be fabricated and cultured in chemically defined environments and under good manufacturing practices (GMPs). To achieve this, we should find a GMP-compatible replacement of animal-derived materials, such as Matrigel and basement membrane extract, which have been widely used for both 2D and 3D hPSC culture. Also, using effective mass-production methods for organoids such as stirred suspension culture will substantially increase throughput compared to the conventional static adherent culture (Chen et al., 2015; Marsee et al., 2021).
Assessing cardiac organoids for clinical applications
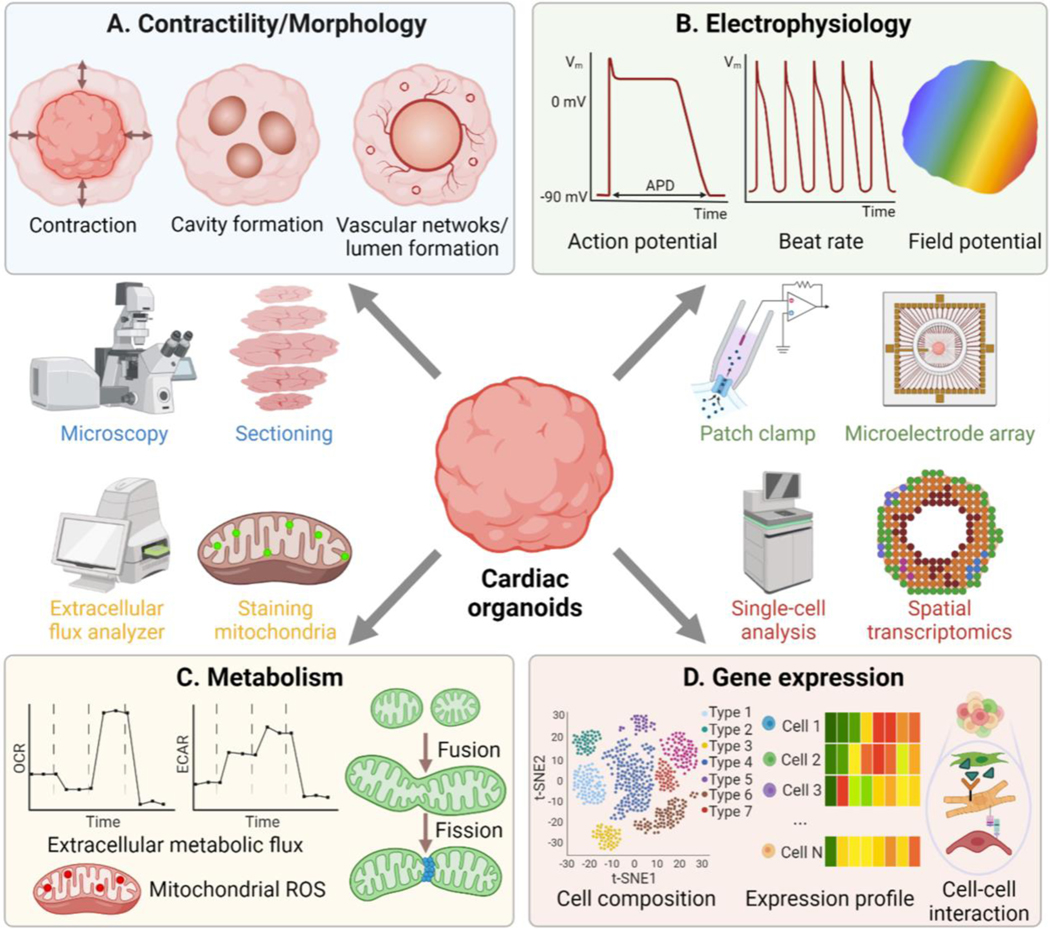
For cardiac organoids to be utilized in various practical applications as aforementioned, it is necessary to accurately evaluate their important functions and monitor their biological status. Previously, factors related to contractility, morphology, electrophysiology, metabolism, and gene expression have been commonly used to evaluate 2D cultured cardiomyocytes (Garg et al., 2018; Zhang et al., 2021a). However, these assays and techniques to assess them mostly require modification or extra preparation steps for application to 3D cardiac organoids. Therefore, we will discuss the challenges and solutions for integrating conventional measurement methods into 3D cardiac organoid research.
Contractility and morphological features
EHTs allow non-contact, real-time measurements of the contractile force using two elastic posts connected to cardiac tissues. Because cardiac organoids do not have a force sensor, their contractility should be analyzed based on morphological information. To quantify the contractility, the beating of cardiac organoids is recorded as a video through a microscope and then analyzed with a motion vector algorithm program (Huebsch et al., 2014). This provides information that has been commonly used in traditional cardiomyocytes studies such as contraction magnitude, beating rate, and beating velocity. However, unlike 2D monolayer culture, too strong beating of cardiac organoids that are placed on an anti-adhesion surface can change their position and orientation due to the beating. This should be considered in the analysis so that it is not miscalculated as contraction magnitude.
Some 3D morphological parameters of cardiac organoids could not be assessed in 2D cultures, such as cavity formation during cardiogenesis, wall thickness, ejection fraction by contraction, and lumen formation by endothelial cells (Figure 3A). However, because cardiac organoids consist of very dense tissues, the detailed structure and most morphological features deep inside the organoids are not easily visible in real-time. Therefore, we need advanced imaging techniques to capture more detailed morphological features throughout cardiac organoids.
Figure 3. Readouts and methods to assess cardiac functions.
(A) contractility/morphology; (B) electrophysiology; (C) metabolism; (D), gene expression. OCR: oxyzen consumption rate; ECAR: extracellular acidification rate; ROS: reactive oxygen species.
To visualize the beating of the in vivo heart in humans or animals, an echocardiogram using ultrasound waves has been used. The echocardiogram has a high temporal resolution (<5 ms) but poor spatial resolution (0.5–2 mm) for the size of cardiac organoids (0.3–1 mm) (Lin and Alessio, 2009). Confocal microscopy has a high spatial resolution (up to 180 nm) (Schermelleh et al., 2010), but it is difficult to observe cells at a depth of more than 0.3 mm due to the scattering of photons in the dense cardiac organoids. In addition, sample-enhancing methods such as clearing cannot be applied to increase the working depth for live organoid imaging. This severe scattering issue also increases scan time in the deep inner part of cardiac organoids because the averaging of signal over a certain percentage of the pixel time is required due to a low signal-to-noise ratio. The scan time of confocal microscopy (>4 sec/plane, a 512×512 image) is usually much longer than the typical heartbeat rate (<1 beat/sec) (Smith, 2011), thus it is challenging for confocal microscopy to capture internal structural changes during the beating of live cardiac organoids using confocal microscopy.
Light-sheet microscopy, which has a relatively shorter scan time than confocal microscopy by performing point scanning, is also widely used for organoid imaging. However, light-sheet microscopy is limited to scattering within 3 μm depth into cardiac organoids. Two-photon or multi-photon microscopy using long wavelengths could be used to achieve less scattering and less tissue damage deep in the organoids. By combining the fast scan time of light-sheet microscopy with the deep penetration of two-photon microscopy, Richards and colleagues assessed calcium transient profiles of live cardiac organoids using a customized two-photon scanned light-sheet microscope (Richards et al., 2020). This imaging technique allowed monitoring the change in fluorescence of the calcium indicator GCaMP6 in planes deeper than 50 μm (using 4 μm thick optical sections) with high temporal resolution (20 ms). More recently, a preprint on optical coherence tomography describes the internal structures of cardiac organoids such as cavities with a spatial resolution of about 3.5 μm and a temporal resolution of 1 ms without sectioning or staining (Ming et al., 2022). Despite advances in imaging techniques, full scanning of beating cardiac organoids with fluorescence markers at high temporal and spatial resolutions has not been achieved. The use of reporter lines for targeting cell types or software algorithms to supplement the current limitations of 3D imaging can help enhance the quality of real-time organoid imaging.
End-point imaging that allows long scan time and tissue fixation can provide more options for imaging at high spatial resolutions. In particular, high resolution is required when the cell type of interest is only present in very small numbers within the organoids. One of the techniques that can be applied to obtain a full scan of cardiac organoids with a high spatial resolution is tissue clearing, a technique that significantly increases tissue transparency. There are several methods of the clearing. Hydrophobic methods usually shrink tissues, and hydrophilic and hydrogel-based methods result in tissue expansion (Ueda et al., 2020). Due to these structural changes, the selection of tissue clearing method should be carefully considered depending on which morphological parameters in the organoid are of most interest. It also requires optimization of the clearing protocol prior to application to organoids in each research group, which may take a lot of time and cost. Lastly, sectioning causes less deformation than clearing, but can be challenging because of the small size of cardiac organoids (only about 0.3–1 mm in diameter (Hofbauer et al., 2021). In addition, their light color makes them almost invisible in paraffin or frozen optimal cutting temperature (OCT) compounds.
Electrophysiology
The electrophysiology of cardiomyocytes plays an important role in identifying cardiac mechanisms, diseases, and potential therapies (Hayes et al., 2019). The gold standard for assessing electrophysiological characteristics of cardiomyocytes in 2D monolayer culture is the manual patch-clamp and multielectrode array (MEA) assay, as they provide very high temporal resolution (>10 kHz). However, the throughput of the manual patch-clamp that can record at a single-cell resolution is too low for a high-throughput organoid system (Gao et al., 2021). On the other hand, MEA measures an extracellular field potential of multiple organoids or cells at once and also provides information on action potential propagation (Navarrete et al., 2013; Passaro and Stice, 2021) (Figure 3B). In contrast, patch-clamp has a higher spatial resolution (<1 μm) than MEA (>30 μm) (Gu et al., 2002; Müller et al., 2015) and provides quantitative action potentials and the relevant ionic currents (Yamamoto et al., 2021).
Manual patch-clamp and MEA assay, with their respective distinct advantages and limitations, can be used to assess cardiac action potential. However, it is more difficult to apply them to study 3D cardiac organoids because their user interfaces were developed based on 2D culture (Garg et al., 2018). To measure the action potential of ventricular- and atrial-like cardiomyocytes in cardiac organoids, Drakhlis and colleagues first dissociated cardiac organoids into single cells, seeded them on glass coverslips, and recorded them by patch clamping 1–3 days later (Drakhlis et al., 2021). This allowed demonstration of chamber-specific action potential characteristics and identification of cardiac phenotypes. However, the original electrophysiological characteristics in 3D organoids can be altered after dissociating and culturing them on 2D glass coverslips. Moreover, most MEAs use planar electrodes and are limited to recording only the outer edges of cardiac organoids. A flexible thin-film MEA that bends around organoids has also been developed, but it can still record only the outer surfaces (Yang et al., 2020). To solve this problem, several 3D MEAs using mesh nanoelectronics or stretchable arrays have been recently introduced to study brain and cardiac organoids (Passaro and Stice, 2021). In particular, mesh electrodes allow on-line recording across the entire organoids by embedding uniformly spaced nanoelectrodes in the organoids. By integrating these 3D electrodes into cardiac organoids, Li and colleagues were able to determine the presence of synchronized bursting patterns and maturity of cardiomyocytes (Li et al., 2019). Of note, insertion of electrodes into the organoids can affect cardiogenesis, especially in long-term studies.
Besides patch-clamp and MEA, calcium imaging and voltage-sensitive dyes are also widely used in the study of organoid electrophysiology, especially when readouts should be assessed in the intact 3D structure of cardiac organoids at high cellular resolution (Drakhlis et al., 2021; Lee et al., 2020; Passaro and Stice, 2021). Changes in the intercellular calcium concentration by action potential can be monitored through the intensity changes of fluorescent calcium indicators, which enable the study of excitation-contraction coupling and action potential propagation (Eisner et al., 2017; Zhang et al., 2021a). However, calcium imaging alone cannot assess membrane voltage dynamics or individual action potential waveforms that are intrinsic to specific physiological and pathological states (Shroff et al., 2020). Therefore, Lee and colleagues optically mapped the electrophysiological characteristics of cardiac organoids using a voltage-sensitive dye whose intensity changes according to the changes in membrane potential (Lee et al., 2020). They confirmed the presence of ventricular- and atrial-like regions with different action potential durations (APDs) in cardiac organoids. However, due to dye-mediated cytotoxicity and photobleaching, calcium imaging or voltage-sensitive dyes are not suitable methods for long-term culture (Hou et al., 2014). To overcome this limitation, genetically encoded calcium or voltage indicators have been recently utilized in cardiac tissue research (Hou et al., 2014; Shroff et al., 2020). Applying these techniques to cardiac organoids will enable monitoring of electrophysiological properties of cardiac organoids during cardiogenesis over several weeks.
Metabolism
The main role of cardiac metabolism is to generate ATP necessary for the pumping function of the heart from various energy-providing substrates, including glucose, lactate, and fatty acids. There are many different pathways to generate energy, but more than 90% of the energy comes from mitochondrial oxidative phosphorylation (Taegtmeyer et al., 2016). It is known that when hiPSCs are differentiated into cardiomyocytes like cardiac organoids, their metabolic phenotype shifts from anaerobic glycolysis to mitochondrial respiration, as it also occurs during cardiac development (Gaspar et al., 2014). In fact, the shift from glucose to fatty acid metabolic signature is a hallmark of cardiomyocyte maturation to meet high energy demands (Lin et al., 2017; Ronaldson-Bouchard et al., 2018; Taegtmeyer et al., 2016).
Common methods to study the metabolism of cardiac organoids is to measure the concentrations or metabolic fluxes of metabolites that participate in metabolic reactions, including the tricarboxylic acid (TCA) cycle and the mitochondrial electron transport chain occurring in the mitochondria (Lewis-Israeli et al., 2021; Mills et al., 2019) (Figure 3C). The concentrations of metabolites provide valuable information about which metabolic pathways are activated or inhibited. For example, under hypoxia, the concentration of lactate is more than 15 times higher than that of pyruvate (Taegtmeyer et al., 2016). Metabolic fluxes also show responses to changes in the energy demand of cardiac organoids under both physiological and pathological conditions.
Oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) are most widely measured to study metabolic fluxes. OCR is an indicator of mitochondrial respiration, and ECAR is an indicator of proton excretion by glycolysis. The changes in OCR and ECAR reflect the ATP synthesis and consumption rates, which are closely related to ATP turnover (Ferrick et al., 2008). When comparing the metabolism of cardiac organoids under different conditions by OCR and ECAR, it should be taken into account that the cardiac organoids consist of many distinct cell types. In particular, OCR and ECAR become higher when cardiomyocytes mature (Horikoshi et al., 2019), but each cell type has a different metabolism base level (Zhang et al., 2012). In this sense, the exact cell composition of each organoid must be considered for accurate metabolism comparison.
Cardiac metabolism can also be investigated using the staining of mitochondria. Fluorescence dyes enable to analysis of mitochondrial content, mitochondrial fragmentation (balance between fission and fusion), inner mitochondrial membrane potential, and mitochondrial reactive oxygen species (Dorn, 2015; Little et al., 2020).
Gene expression
Gene expression is a key readout to identify the phenotypes of organoids and to show their physiological status. Among multi-omic approaches, proteomics provides information on protein activity such as the abundance of individual protein species as well as post-translational modifications (Matkovich, 2019). Using bulk proteomic analysis, Hofbauer et al. confirmed the proteome expression signatures of cardiomyocytes and endothelial cells in their organoids (Hofbauer et al., 2021). Because cardiac organoids contain many different cell types, most gene expression studies of cardiac organoids were done by sequencing single-cells rather than the bulk population (Figure 3D). Notably, scRNA-seq shows RNA expression of representative cell type markers to precisely reveal cell composition and related properties such as maturation, metabolism, differentiation trajectories, apoptosis, and secretion of ECM (Drakhlis et al., 2021; Paik et al., 2020a; Rossi et al., 2021). scRNA-seq is also more useful than bulk sequencing for analyzing cells present at low numbers and for identifying the underlying mechanisms of biological processes.
The identification of highly expressed ligand-receptor pairs in single-cell transcriptomic information based on known human receptor-ligand pairs (Ramilowski et al., 2015) has enabled the elucidation of intercellular communication and ECM signaling in organoids, which are essential for morphogenesis (Paik et al., 2020a). However, the sequencing depth and coverage of scRNA-seq are not as high as that of bulk sequencing (Chen et al., 2019), so it is important to upgrade the sequencing depth and read length to detect low-abundant transcripts (Rizzetto et al., 2017). Additionally, while droplet-based scRNA-seq recommends loading only up to 10,000 cells per droplet lane (Lafzi et al., 2018), single-cell combinatorial indexing allows for profiling of transcriptomes of more than 2 million cells (Cao et al., 2017, Cao et al., 2019). Recently, there has been a growing use of single-cell multi-omics, which provides not only transcriptomic but also epigenetic and proteomic analyses (Packer and Trapnell, 2018). In the process of sample preparation for single-cell analysis, the organoids are dissociated into single cells, and spatial information of cells within the organoids and cell-cell and cell-ECM communication is lost. In this sense, this technique leaves a gap in organoid research, especially where knowing the location of cells is critical, such as in studies of morphogenesis. To overcome this limitation, spatial transcriptomics, in which location information is retained, was recently applied to heart tissue research (Asp et al., 2019; Mantri et al., 2021). Although the cost of these cutting-edge assays is decreasing as the technology advances, they are still much more expensive than conventional gene expression assays. Cost reduction in the future as the technologies mature would accelerate organoid research.
Current challenges and future perspectives
As we have shown in this perspective, cardiac organoids that retain the emergent behaviors of the developing heart will have a pivotal role not only in basic science but also in clinical applications such as drug testing and transplantation. However, there are challenges that need to be addressed to practically integrate cardiac organoids into preclinical studies of drug development.
Reproducibility of cardiac organoids
Recent studies have shown reproducible modeling of morphogenesis using cardiac organoids. For example, Hofbauer et al. showed that cavities formed in all organoids without foregut endoderm (Hofbauer et al., 2021), and Lewis-Israeli et al. demonstrated that there was no significant difference between organoids derived from three different iPSC lines (Lewis-Israeli et al., 2021). However, as there are still variations in cavity formation (one large cavity vs multiple small cavities) and the overall cardiac organoid shape, both of which are important to model cardiogenesis, we still need to further improve the reproducibility of cardiac organoids, with differentiation being a major issue (Garreta et al., 2021). For this reason, many researchers analyze the results of morphogenesis or differentiation of cardiac organoids using statistical methods (Lee et al., 2020; Silva et al., 2021). Cardiac differentiation has been commonly pursued by activating and inhibiting Wnt/β-catenin signalling at the appropriate times by adding molecules, such as CHIR99021 and IWR-1, to the culture medium. Importantly, there are batch-to-batch variations in cardiac differentiation efficiency in 2D as well as 3D cultures. In 2D monolayers, the efficiency and reproducibility of cardiac differentiation to obtain PSC-derived cardiomyocytes can be substantially improved through glucose starvation, which eliminates non-cardiomyocytes based on the differences in metabolism (Sharma et al., 2015). However, it is not as beneficial for cardiac organoids to apply this very effective method because morphogenesis can be obtained by harmonious cooperation with non-cardiomyocytes, such as endocardial cells and cardiac fibroblasts.
Most studies add soluble factors such as bFGF and VEGF to induce differentiation into endothelial or epicardial cells in addition to the cardiac differentiation medium. This means that only a finite number of cells in tissue can divide into specific proportions for each cell type corresponding to the relative strength of each differentiation cue. For example, cardiac progenitor cells around day 6 of cardiac differentiation can become cardiomyocytes or endothelial cells depending on culture conditions, such as the concentration of VEGF (Paik et al., 2020b). Since this process is not deterministic, many variations occur during the co-differentiation, and the proportion of cardiomyocytes in 3D cardiac organoids is usually lower than in conventional 2D culture (Drakhlis et al., 2021). Moreover, unwanted cell phenotypes can be obtained in cardiac organoids because pluripotency decreases as the passage number of hiPSCs increases.
Wnt activators and inhibitors used for cardiac differentiation are also used for differentiation into other cell lineages. For example, CHIR99021 is used to differentiate into retina, lung, kidney, and blood vessels, and IWR-1 also induces differentiation into the brain, retina, and blood vessel cells. Even if the same molecules are used, the dominant organ type can vary depending on their concentration and timing of application.
If there is a variation in the stimulation regime between cells in an organoid for the same condition, the organoids may contain many different cell types that have similar differentiation conditions. Notably, molecular distributions inside organoids are mostly nonuniform due to diffusion gradients (Richards et al., 2020). For these reasons, cysts similar to pancreatic or liver organoids sometimes appear in cardiac organoids (Lee et al., 2020), and the heart and gut are often seen together in cardiac organoids (Drakhlis et al., 2021; Rossi et al., 2021; Silva et al., 2021). A method to reduce the chance of cells misleading the differentiation cues would increase the robustness of organoid preparation. Recently, transcription factors (TF)-driven differentiation capable of overexpressing specific TF were applied to obtain vascularized cortical organoids (Cakir et al., 2019). The same strategy could be applied to cardiac organoids. However, these engineering interventions should be introduced carefully as they could interfere with self-organization to acquire complex emergent behaviors. Furthermore, biochemical gradients in organoids due to diffusion could be reduced by reducing the size of organoids and using bioreactors.
In addition to these exogenous chemical cues, several important factors can influence organoid reproducibility by activating distinct signaling during differentiation. In multicellular cardiac organoids, each cell type greatly influences the differentiation, function, and behavior of other cell types in numerous ways, including autocrine, paracrine, and juxtacrine signaling within organoids. For example, Lewis-Israeli et al. reported that chemical cues induced differentiation of mesoderm and cardiac lineages through Wnt activation/inhibition/activation (Lewis-Israeli et al., 2021). However, the cavities and non-cardiomyocytes (including endocardial cells, cardiac fibroblasts, and epicardial cells) emerged spontaneously in response to endogenous signaling. However, if the number of cell types that organoids contain increases, the complexity of cell-to-cell interactions will also increase, which could lead to low reproducibility. Moreover, there are also variations in the mechanical environment within an organoid. Most cardiac organoids are formed through spontaneous aggregation on non-adhesion surfaces, and the traction force exerted by cells into the extracellular space results in a spherical tissue shape. During tissue formation, a stress field is generated inside the organoids along with cell-mediated tissue compaction. In particular, the circumferential stress acting on the cells in organoids is much higher at the outer edge than in the core region of a spherical organoid (Lee et al., 2019). This non-uniform mechanical stress field can affect cell differentiation and maturation through mechanotransduction (Abilez et al., 2018; Kaushik and Engler, 2014). Therefore, the effect of the number of desired cell types and mechanical stress field due to a tissue shape should be carefully considered to obtain robust cardiac organoids.
Vascularization
As in any living tissue, cardiac organoids require means to supply nutrients, eliminate waste, and maintain adequate gas exchange, and this is even more critical in highly energetic cells such as cardiac myocytes. In living organisms, vascularization is normally accomplished through the emergence and self-assembly during development of hierarchical vascular networks perfused by blood or blood substitute. Organoids, since they lack vascular perfusion, tend to exhibit some level of necrosis, especially when the tissues attain dimensions in excess of ~1–2 mm in diameter. Recently, methods have been reported for inducing the formation of vascular-like structures that can be identified by expression of endothelial cell markers (e.g., CD31/PECAM-1, CD34). This has been previously been reported for brain (Mansour et al., 2018), kidney (Homan et al., 2019), and liver (Guye et al., 2016) organoids, but only recently have methods been developed - through modulation of the Wnt signaling pathway - to produce similar vascular networks in cardiac organoids (Lewis-Israeli et al., 2021). However, true perfusion via the vasculature has not been achieved, and this has become an area of active investigation with a variety of methods under consideration (Zhang et al., 2021b).
Recapitulating heart features
Recent studies have generated cardiac organoids that successfully captured many important features of the heart, including contractility and the formation of chamber-like cavities that retain the liquid. They were also made of the major cell types present in the developing heart, such as endocardial cells, cardiomyocytes, cardiac fibroblasts, and epicardial cells. However, at this time, cardiac organoids cannot yet recapitulate some pivotal factors of the native heart. One of the missing features in cardiac organoids is the lining of the cavity with endocardial cells. In addition, the chambers in the native heart are formed from a linear heart tube through a sequence of looping, ballooning, trabeculation, and compaction (Kim et al., 2021). These key processes have not yet been seen in cardiac organoids. Unlike some engineered cardiac tissues (Zhao et al., 2019), current cardiac organoids contain chamber-specific (atrial/ventricular) cardiomyocytes without spatial organization and perfusable blood vessels. Notably, the absence of perfusable vasculature limits the size of cardiac organoids, and it is difficult to culture cardiac organoids for a long time as their size increases. Consequently, current cardiac organoids exhibit characteristics similar to those of the fetal rather than adult heart. Currently, adult-like mature cardiac tissues can be obtained as cardiac spheroids via 2D long-term culture before 3D tissue formation (Mattapally et al., 2018; Thomas et al., 2021). However, while these cardiac spheroids beat well, they do not show cardiogenesis.
Cardiac organoid to become mini-hearts
By single-cell analysis, at least 11 different cell types have been identified based on canonical marker genes present in the heart (Litviňuková et al., 2020; Suryawanshi et al., 2020), such as ventricular cardiomyocytes (MYH7, MYL2, FHL2), endothelial cells (VWF, PECAM1, CDH5), and pericytes (RGS5, ABCC9, KCNJ8), with more types that may yet be discovered. It would be difficult to reproduce all of these numerous cell types in cardiac organoids. Instead, essential features and complexity of cardiac organoids will vary depending on their application. For example, a mini-heart to study the pathophysiology of COVID-19 mRNA vaccine-related myocarditis would require immune system, including T helper cells, within cardiac organoids. On the other hand, investigation of arrhythmic cardiomyopathies will require cardiac conduction systems from the sinoatrial node in cardiac organoids. For applications involving the adaptive immune system or those associated with transient ischemic events, vascular perfusion is critical.
There are many difficulties in obtaining multiple cell types in one tissue. High-efficiency differentiation protocols for most cell types are still lacking, and some conditions for different cell types are conflicting. Moreover, the culture medium for multicellular organoids is mostly favorable for the dominant cell type, but may not be suitable for all the cell types in the organoids. Therefore, it will be necessary to develop technologies that only affect cells within the relevant local microenvironment within a single cardiac organoid through advances in bioengineering and biomaterials science. Current cardiac organoids are unlikely to completely replace animal models in preclinical studies, but the gap between in vivo and in vitro applications will be narrowed as we overcome these challenges.
Conclusions
Researchers working on cardiac organoids have made great strides in developmental biology, pathology, culture methods, and analytical assays, enabling capturing some key aspects of human cardiogenesis and cardiovascular diseases. Despite these achievements, current limitations of cardiac organoids such as low reproducibility, limited vascularization, and missing essential features of the heart hamper their widespread use in clinical applications, such as drug discovery by pharmaceutical companies and transplantation in patients with myocardial infarction. With the growing interest and demand for cardiac organoids in recent years, increasing research on cardiac organoids will facilitate improved modeling of the actual human heart. In addition, we believe that cardiac organoid research can transcend current limitations and secure next-generation human heart models through interdisciplinary research in various fields, such as biomaterials, optics, stem cell biology, tissue engineering, and bioengineering, with more active communication among researchers in these fields in the future.
Supplementary Material
Acknowledgments
We would like to acknowledge funding support from the American Heart Association (United States) (grant 20POST35210896 to H.K.), the Wellcome Leap HOPE (United Kingdom) to R.D.K., and National Institutes of Health (United States) (R01 HL113006, R01 HL145676, R01 HL150693, R01 HL163680, R01 HL130020, and UH3 TR002588 to J.C.W.; R01 NS121078 and U54 CA261694 to R.D.K.; and R01 HL076485 and P41 EB027062 to G.V.-N.). Figure 3 was created with BioRender.com.
J.C.W is a cofounder of Greenstone Biosciences and G.V.N is a cofounder of Tara Biosystems. R.D.K is a cofounder of AIM Biotech and receives research support from Biogen and Amgen. However, J.C.W, G.V.N, and R.D.K have no competing interests, as the work presented here is independent. The other authors report no conflicts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abilez OJ, Tzatzalos E, Yang H, Zhao M-T, Jung G, Zöllner AM, Tiburcy M, Riegler J, Matsa E, Shukla P, et al. (2018). Passive Stretch Induces Structural and Functional Maturation of Engineered Heart Muscle as Predicted by Computational Modeling. Stem Cells 36, 265–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asp M, Giacomello S, Larsson L, Wu C, Fürth D, Qian X, Wärdell E, Custodio J, Reimegård J, Salmén F, et al. (2019). A Spatiotemporal Organ-Wide Gene Expression and Cell Atlas of the Developing Human Heart. Cell 179, 1647–1660.e19. [DOI] [PubMed] [Google Scholar]
- Behrens AN, Iacovino M, Lohr JL, Ren Y, Zierold C, Harvey RP, Kyba M, Garry DJ, and Martin CM (2013). Nkx2–5 Mediates Differential Cardiac Differentiation Through Interaction with Hoxa10. Stem Cells Dev. 22, 2211–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang Y-J, Chapeton K, Patterson B, Yuan Y, He C-S, et al. (2019). Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 16, 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Packer JS, Ramani V, Cusanovich DA, Huynh C, Daza R, Qiu X, Lee C, Furlan SN, Steemers FJ, et al. (2017). Comprehensive single-cell transcriptional profiling of a multicellular organism. Science (80-. ). 357, 661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Spielmann M, Qiu X, Huang X, Ibrahim DM, Hill AJ, Zhang F, Mundlos S, Christiansen L, Steemers FJ, et al. (2019). The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Ning B, and Shi T.(2019). Single-Cell RNA-Seq Technologies and Related Computational Data Analysis. Front. Genet 10, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen VC, Ye J, Shukla P, Hua G, Chen D, Lin Z, Liu J, Chai J, Gold J, Wu J, et al. (2015). Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res. 15, 365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Lee C, Skylar-Scott MA, Heilshorn SC, and Wu JC (2021). Reconstructing the heart using iPSCs: Engineering strategies and applications. J. Mol. Cell. Cardiol 157, 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez AR, Poling HM, Brown NE, Singh A, Mahe MM, and Helmrath MA (2018). Transplantation of human intestinal organoids into the mouse mesentery: A more physiologic and anatomic engraftment site. Surgery 164, 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn II GW (2015). Mitochondrial dynamism and heart disease: changing shape and shaping change. EMBO Mol. Med 7, 865–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakhlis L, Biswanath S, Farr C-M, Lupanow V, Teske J, Ritzenhoff K, Franke A, Manstein F, Bolesani E, Kempf H, et al. (2021). Human heart-forming organoids recapitulate early heart and foregut development. Nat. Biotechnol 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner DA, Caldwell JL, Kistamás K, and Trafford AW (2017). Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res 121, 181–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrick DA, Neilson A, and Beeson C.(2008). Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov. Today 13, 268–274. [DOI] [PubMed] [Google Scholar]
- Funakoshi S, Miki K, Takaki T, Okubo C, Hatani T, Chonabayashi K, Nishikawa M, Takei I, Oishi A, Narita M, et al. (2016). Enhanced engraftment, proliferation and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci. Rep 6, 19111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, Liao C, Liu S, Xia T, and Jiang G.(2021). Nanotechnology: new opportunities for the development of patch‐clamps. J. Nanobiotechnology 19, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg P, Garg V, Shrestha R, Sanguinetti MC, Kamp TJ, and Wu JC (2018). Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes as Models for Cardiac Channelopathies. Circ. Res 123, 224–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garreta E, Kamm RD, Chuva de Sousa Lopes SM, Lancaster MA, Weiss R, Trepat X, Hyun I, and Montserrat N.(2021). Rethinking organoid technology through bioengineering. Nat. Mater 20, 145–155. [DOI] [PubMed] [Google Scholar]
- Gaspar JA, Doss MX, Hengstler JG, Cadenas C, Hescheler J, and Sachinidis A.(2014). Unique Metabolic Features of Stem Cells, Cardiomyocytes, and Their Progenitors. Circ. Res 114, 1346–1360. [DOI] [PubMed] [Google Scholar]
- Gopal S, Rodrigues AL, and Dordick JS (2020). Exploiting CRISPR Cas9 in Three-Dimensional Stem Cell Cultures to Model Disease. Front. Bioeng. Biotechnol 8, 692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Gorelik J, Spohr HA, Shevchuk A, Lab MJ, Harding SE, Vodyanoy I, Klenerman D, and Korchev YE (2002). High-resolution scanning patch-clamp: new insights into cell function. FASEB J. 16, 748–750. [DOI] [PubMed] [Google Scholar]
- Guye P, Ebrahimkhani MR, Kipniss N, Velazquez JJ, Schoenfeld E, Kiani S, Griffith LG, and Weiss R.(2016). Genetically engineering self-organization of human pluripotent stem cells into a liver bud-like tissue using Gata6. Nat. Commun 7, 10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes HB, Nicolini AM, Arrowood CA, Chvatal SA, Wolfson DW, Cho HC, Sullivan DD, Chal J, Fermini B, Clements M, et al. (2019). Novel method for action potential measurements from intact cardiac monolayers with multiwell microelectrode array technology. Sci. Rep 9, 11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer P, Jahnel SM, Papai N, Giesshammer M, Deyett A, Schmidt C, Penc M, Tavernini K, Grdseloff N, Meledeth C, et al. (2021). Cardioids reveal self-organizing principles of human cardiogenesis. Cell. [DOI] [PubMed] [Google Scholar]
- Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, Mau D, Valerius MT, Ferrante T, Bonventre JV, et al. (2019). Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat. Methods 16, 255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi Y, Yan Y, Terashvili M, Wells C, Horikoshi H, Fujita S, Bosnjak ZJ, and Bai X.(2019). Fatty Acid-Treated Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes Exhibit Adult Cardiomyocyte-Like Energy Metabolism Phenotypes. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou JH, Kralj JM, Douglass AD, Engert F, and Cohen AE (2014). Simultaneous mapping of membrane voltage and calcium in zebrafish heart in vivo reveals chamber-specific developmental transitions in ionic currents. Front. Physiol 5, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebsch N, Loskill P, Mandegar MA, Marks NC, Sheehan AS, Ma Z, Mathur A, Nguyen TN, Yoo JC, Judge LM, et al. (2014). Automated Video-Based Analysis of Contractility and Calcium Flux in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes Cultured over Different Spatial Scales. Tissue Eng. Part C Methods 21, 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapałczyńska M, Kolenda T, Przybyła W, Zajączkowska M, Teresiak A, Filas V, Ibbs M, Bliźniak R, Łuczewski Ł, and Lamperska K.(2018). 2D and 3D cell cultures - a comparison of different types of cancer cell cultures. Arch. Med. Sci 14, 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik G, and Engler AJ (2014). Chapter Nine - From Stem Cells to Cardiomyocytes: The Role of Forces in Cardiac Maturation, Aging, and Disease. In Mechanotransduction, Engler AJ, and S.B.T.-P. in M.B. and Kumar TS, eds. (Academic Press; ), pp. 219–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Wang M, and Paik DT (2021). Endothelial-Myocardial Angiocrine Signaling in Heart Development. Front. Cell Dev. Biol 9, 1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer ME, Lin W-H, Ravikumar V, Qiu K, Wang L, Gao L, Bhuiyan DB, Lenz M, Ai J, Mahutga RR, et al. (2020). In Situ Expansion, Differentiation, and Electromechanical Coupling of Human Cardiac Muscle in a 3D Bioprinted, Chambered Organoid. Circ. Res 127, 207–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafzi A, Moutinho C, Picelli S, and Heyn H.(2018). Tutorial: guidelines for the experimental design of single-cell RNA sequencing studies. Nat. Protoc 13, 2742–2757. [DOI] [PubMed] [Google Scholar]
- Lee J, Sutani A, Kaneko R, Takeuchi J, Sasano T, Kohda T, Ihara K, Takahashi K, Yamazoe M, Morio T, et al. (2020). In vitro generation of functional murine heart organoids via FGF4 and extracellular matrix. Nat. Commun 11, 4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Kalashnikov N, Mok S, Halaoui R, Kuzmin E, Putnam AJ, Takayama S, Park M, McCaffrey L, Zhao R, et al. (2019). Dispersible hydrogel force sensors reveal patterns of solid mechanical stress in multicellular spheroid cultures. Nat. Commun 10, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Israeli YR, Wasserman AH, Gabalski MA, Volmert BD, Ming Y, Ball KA, Yang W, Zou J, Ni G, Pajares N, et al. (2021). Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat. Commun 12, 5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Nan K, Le Floch P, Lin Z, Sheng H, Blum TS, and Liu J.(2019). Cyborg Organoids: Implantation of Nanoelectronics via Organogenesis for Tissue-Wide Electrophysiology. Nano Lett. 19, 5781–5789. [DOI] [PubMed] [Google Scholar]
- Lin E, and Alessio A.(2009). What are the basic concepts of temporal, contrast, and spatial resolution in cardiac CT? J. Cardiovasc. Comput. Tomogr 3, 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B, Lin X, Stachel M, Wang E, Luo Y, Lader J, Sun X, Delmar M, and Bu L.(2017). Culture in Glucose-Depleted Medium Supplemented with Fatty Acid and 3,3′,5Triiodo-l-Thyronine Facilitates Purification and Maturation of Human Pluripotent Stem Cell-Derived Cardiomyocytes. Front. Endocrinol 8, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little AC, Kovalenko I, Goo LE, Hong HS, Kerk SA, Yates JA, Purohit V, Lombard DB, Merajver SD, and Lyssiotis CA (2020). High-content fluorescence imaging with the metabolic flux assay reveals insights into mitochondrial properties and functions. Commun. Biol 3, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litviňuková M, Talavera-López C, Maatz H, Reichart D, Worth CL, Lindberg EL, Kanda M, Polanski K, Heinig M, Lee M, et al. (2020). Cells of the adult human heart. Nature 588, 466–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Feng X, Li G, Gokulnath P, and Xiao J.(2022). Generating 3D human cardiac constructs from pluripotent stem cells. EBioMedicine 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltsev VA, Rohwedel J, Hescheler J, and Wobus AM (1993). Embryonic stem cells differentiate in vitro into cardiomyocytes representing sinusnodal, atrial and ventricular cell types. Mech. Dev 44, 41–50. [DOI] [PubMed] [Google Scholar]
- Mannhardt I, Saleem U, Benzin A, Schulze T, Klampe B, Eschenhagen T, and Hansen A.(2017). Automated Contraction Analysis of Human Engineered Heart Tissue for Cardiac Drug Safety Screening. JoVE e55461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, Johnston S, Parylak SL, Jin X, and Gage FH (2018). An in vivo model of functional and vascularized human brain organoids. Nat. Biotechnol 36, 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantri M, Scuderi GJ, Abedini-Nassab R, Wang MFZ, McKellar D, Shi H, Grodner B, Butcher JT, and De Vlaminck I.(2021). Spatiotemporal single-cell RNA sequencing of developing chicken hearts identifies interplay between cellular differentiation and morphogenesis. Nat. Commun 12, 1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsee A, Roos FJM, Verstegen MMA, Marsee A, Roos F, Verstegen M, Clevers H, Vallier L, Takebe T, Huch M, et al. (2021). Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell 28, 816–832. [DOI] [PubMed] [Google Scholar]
- Matkovich SJ (2019). Multiomic approaches to delineate the pathogenesis of cardiac disease. Curr. Opin. Cardiol 34. [DOI] [PubMed] [Google Scholar]
- Mattapally S, Zhu W, Fast VG, Gao L, Worley C, Kannappan R, Borovjagin AV, and Zhang J.(2018). Spheroids of cardiomyocytes derived from human-induced pluripotent stem cells improve recovery from myocardial injury in mice. Am. J. Physiol. Circ. Physiol 315, H327–H339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RJ, Titmarsh DM, Koenig X, Parker BL, Ryall JG, Quaife-Ryan GA, Voges HK, Hodson MP, Ferguson C, Drowley L, et al. (2017). Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc. Natl. Acad. Sci 114, E8372–LP-E8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills RJ, Parker BL, Quaife-Ryan GA, Voges HK, Needham EJ, Bornot A, Ding M, Andersson H, Polla M, Elliott DA, et al. (2019). Drug Screening in Human PSCCardiac Organoids Identifies Pro-proliferative Compounds Acting via the Mevalonate Pathway. Cell Stem Cell 24, 895–907.e6. [DOI] [PubMed] [Google Scholar]
- Ming Y, Hao S, Xu Z, Goestenkors A, Lewis-Israeli YR, Volmert BD, Aguirre A, and Zhou C.(2022). Longitudinal Morphological and Functional Characterization of Human Heart Organoids Using Optical Coherence Tomography. BioRxiv 2022.01.19.476972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller J, Ballini M, Livi P, Chen Y, Radivojevic M, Shadmani A, Viswam V, Jones IL, Fiscella M, Diggelmann R, et al. (2015). High-resolution CMOS MEA platform to study neurons at subcellular, cellular, and network levels. Lab Chip 15, 2767–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, and Kamp TJ (2012). Differentiation of Human Embryonic Stem Cells and Induced Pluripotent Stem Cells to Cardiomyocytes. Circ. Res 111, 344–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete EG, Liang P, Lan F, Sanchez-Freire V, Simmons C, Gong T, Sharma A, Burridge PW, Patlolla B, Lee AS, et al. (2013). Screening Drug-Induced Arrhythmia Using Human Induced Pluripotent Stem Cell–Derived Cardiomyocytes and Low-Impedance Microelectrode Arrays. Circulation 128, S3–S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neofytou E, O’Brien CG, Couture LA, and Wu JC (2015). Hurdles to clinical translation of human induced pluripotent stem cells. J. Clin. Invest 125, 2551–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonomopoulos A, Kitani T, and Wu JC (2018). Pluripotent Stem Cell-Derived Cardiomyocytes as a Platform for Cell Therapy Applications: Progress and Hurdles for Clinical Translation. Mol. Ther 26, 1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaf B, D. BR, Samuel B, Sofia Z, Fanie B-H, Stuart W, Joel Z, Kanar A, A. BB, Henrik D, et al. (2009). Evidence for Cardiomyocyte Renewal in Humans. Science (80-. ). 324, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer J, and Trapnell C.(2018). Single-Cell Multi-omics: An Engine for New Quantitative Models of Gene Regulation. Trends Genet. 34, 653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik DT, Cho S, Tian L, Chang HY, and Wu JC (2020a). Single-cell RNA sequencing in cardiovascular development, disease and medicine. Nat. Rev. Cardiol 17, 457–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik DT, Chandy M, and Wu JC (2020b). Patient and Disease-Specific Induced Pluripotent Stem Cells for Discovery of Personalized Cardiovascular Drugs and Therapeutics. Pharmacol. Rev 72, 320–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashmforoush M, Lu JT, Chen H, Amand TS, Kondo R, Pradervand S, Evans SM, Clark B, Feramisco JR, Giles W, et al. (2004). Nkx2–5 Pathways and Congenital Heart Disease: Loss of Ventricular Myocyte Lineage Specification Leads to Progressive Cardiomyopathy and Complete Heart Block. Cell 117, 373–386. [DOI] [PubMed] [Google Scholar]
- Passaro AP, and Stice SL (2021). Electrophysiological Analysis of Brain Organoids: Current Approaches and Advancements. Front. Neurosci 14, 1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonchuk L, Chabria M, Badi L, Hoflack J-C, Figtree G, Davies MJ, and Gentile C.(2017). Cardiac spheroids as promising in vitro models to study the human heart microenvironment. Sci. Rep 7, 7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushp P, Nogueira DES, Rodrigues CAV, Ferreira FC, Cabral JMS, and Gupta MK (2021). A Concise Review on Induced Pluripotent Stem Cell-Derived Cardiomyocytes for Personalized Regenerative Medicine. Stem Cell Rev. Reports 17, 748–776. [DOI] [PubMed] [Google Scholar]
- Ramilowski JA, Goldberg T, Harshbarger J, Kloppmann E, Lizio M, Satagopam VP, Itoh M, Kawaji H, Carninci P, Rost B, et al. (2015). A draft network of ligand–receptor-mediated multicellular signalling in human. Nat. Commun 6, 7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards DJ, Li Y, Kerr CM, Yao J, Beeson GC, Coyle RC, Chen X, Jia J, Damon B, Wilson R, et al. (2020). Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat. Biomed. Eng 4, 446–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzetto S, Eltahla AA, Lin P, Bull R, Lloyd AR, Ho JWK, Venturi V, and Luciani F.(2017). Impact of sequencing depth and read length on single cell RNA sequencing data of T cells. Sci. Rep 7, 12781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnuolo R, Masoudpour H, Porta-Sánchez A, Qiang B, Barry J, Laskary A, Qi X, Massé S, Magtibay K, Kawajiri H, et al. (2019). Human Embryonic Stem Cell-Derived Cardiomyocytes Regenerate the Infarcted Pig Heart but Induce Ventricular Tachyarrhythmias. Stem Cell Reports 12, 967–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, and Vunjak-Novakovic G.(2018). Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556, 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G, Broguiere N, Miyamoto M, Boni A, Guiet R, Girgin M, Kelly RG, Kwon C, and Lutolf MP (2021). Capturing Cardiogenesis in Gastruloids. Cell Stem Cell 28, 230–240.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermelleh L, Heintzmann R, and Leonhardt H.(2010). A guide to super-resolution fluorescence microscopy. J. Cell Biol 190, 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, and Gentile C.(2021). Cardiac Spheroids as in vitro Bioengineered Heart Tissues to Study Human Heart Pathophysiology. JoVE e61962. [DOI] [PubMed] [Google Scholar]
- Sharma A, Li G, Rajarajan K, Hamaguchi R, Burridge PW, and Wu SM (2015). Derivation of Highly Purified Cardiomyocytes from Human Induced Pluripotent Stem Cells Using Small Molecule-modulated Differentiation and Subsequent Glucose Starvation. JoVE e52628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff SN, Das SL, Tseng H, Noueihed J, Fernandez F, White JA, Chen CS, and Han X.(2020). Voltage Imaging of Cardiac Cells and Tissue Using the Genetically Encoded Voltage Sensor Archon1. IScience 23, 100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Matthys OB, Joy DA, Kauss MA, Natarajan V, Lai MH, Turaga D, Blair AP, Alexanian M, Bruneau BG, et al. (2021). Co-emergence of cardiac and gut tissues promotes cardiomyocyte maturation within human iPSC-derived organoids. Cell Stem Cell 28, 2137–2152.e6. [DOI] [PubMed] [Google Scholar]
- Smith CL (2011). Basic Confocal Microscopy. Curr. Protoc. Neurosci 56, 2.2.1–2.2.18. [DOI] [PubMed] [Google Scholar]
- Soldatow VY, LeCluyse EL, Griffith LG, and Rusyn I.(2013). In vitro models for liver toxicity testing. Toxicol. Res. (Camb) 2, 23–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryawanshi H, Clancy R, Morozov P, Halushka MK, Buyon JP, and Tuschl T.(2020). Cell atlas of the foetal human heart and implications for autoimmune-mediated congenital heart block. Cardiovasc. Res 116, 1446–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taegtmeyer H, Young ME, Lopaschuk GD, Abel ED, Brunengraber H, Darley-Usmar V, Des Rosiers C, Gerszten R, Glatz JF, Griffin JL, et al. (2016). Assessing Cardiac Metabolism. Circ. Res 118, 1659–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Kim H, Lopez N, and Wu JC (2021). Fabrication of 3D Cardiac Microtissue Arrays using Human iPSC-Derived Cardiomyocytes, Cardiac Fibroblasts, and Endothelial Cells. JoVE e61879. [DOI] [PubMed] [Google Scholar]
- Ueda HR, Ertürk A, Chung K, Gradinaru V, Chédotal A, Tomancak P, and Keller PJ (2020). Tissue clearing and its applications in neuroscience. Nat. Rev. Neurosci 21, 61–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varzideh F, Pahlavan S, Ansari H, Halvaei M, Kostin S, Feiz M-S, Latifi H, Aghdami N, Braun T, and Baharvand H.(2019). Human cardiomyocytes undergo enhanced maturation in embryonic stem cell-derived organoid transplants. Biomaterials 192, 537–550. [DOI] [PubMed] [Google Scholar]
- Voges HK, Mills RJ, Elliott DA, Parton RG, Porrello ER, and Hudson JE (2017). Development of a human cardiac organoid injury model reveals innate regenerative potential. Development 144, 1118–1127. [DOI] [PubMed] [Google Scholar]
- Wnorowski A, Yang H, and Wu JC (2019). Progress, obstacles, and limitations in the use of stem cells in organ-on-a-chip models. Adv. Drug Deliv. Rev 140, 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Hirose S, Wuriyanghai Y, Yoshinaga D, and Makiyama T.(2021). Electrophysiological Analysis of hiPSC-Derived CardiomyocytesHiPSC-derived cardiomyocytes (hiPSC-CMs) Using a Patch-ClampPatch clampTechnique BT - Pluripotent Stem-Cell Derived Cardiomyocytes. Yoshida Y, ed. (New York, NY: Springer US; ), pp. 121–133. [Google Scholar]
- Yang W, Fan QH, and Li W.(2020). A Fully Transparent, Flexible μECoG Array Based on Highly Conductive and Anti-reflective PEDOT:PSS-ITO-Ag-ITO Thin Films. In 2020 IEEE 15th International Conference on Nano/Micro Engineered and Molecular System (NEMS), pp. 124–129. [Google Scholar]
- Zhang F, Wang L, Li Y, Liu W, Duan F, Huang R, Chen X, Chang SC-N, Du Y, and Na J.(2017). Optimizing mesoderm progenitor selection and three-dimensional microniche culture allows highly efficient endothelial differentiation and ischemic tissue repair from human pluripotent stem cells. Stem Cell Res. Ther 8, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Nuebel E, Wisidagama DRR, Setoguchi K, Hong JS, Van Horn CM, Imam SS, Vergnes L, Malone CS, Koehler CM, et al. (2012). Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat. Protoc 7, 1068–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ, Termglinchan V, Shao N-Y, Itzhaki I, Liu C, Ma N, Tian L, Wang VY, Chang ACY, Guo H, et al. (2019). A Human iPSC Double-Reporter System Enables Purification of Cardiac Lineage Subpopulations with Distinct Function and Drug Response Profiles. Cell Stem Cell 24, 802–811.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JZ, Zhao SR, Tu C, Pang P, Zhang M, and Wu JC (2021a). Protocol to measure contraction, calcium, and action potential in human-induced pluripotent stem cell-derived cardiomyocytes. STAR Protoc. 2, 100859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wan Z, and Kamm RD (2021b). Vascularized organoids on a chip: strategies for engineering organoids with functional vasculature. Lab Chip 21, 473–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, Aggarwal P, Zhang B, Conant G, Ronaldson-Bouchard K, et al. (2019). A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 176, 913–927.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W-H, Schneiderbanger K, Schubert P, Didié M, Münzel F, Heubach JF, Kostin S, Neuhuber WL, and Eschenhagen T.(2002). Tissue Engineering of a Differentiated Cardiac Muscle Construct. Circ. Res 90, 223–230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.