Abstract
The field of “circadian medicine” is a recent addition to chronobiology and sleep research efforts. It represents a logical step arising from the increasing insights into the circadian system and its interactions with life in urbanised societies: apply these insights to the health/disease balance at home and in the medical praxis (outpatient) and clinic (inpatient). Despite its fast expansion and proliferating research efforts, circadian medicine lacks a formal framework to categorise the many observations describing interactions among the circadian system, sleep, and the health/disease balance. A good framework allows us to categorise observations and then assign them to one or more components with hypothesised interactions. Such assignments can lead to experiments that document causal (rather than correlational) relationships and move from describing observations to discovering mechanisms. This paper details such a proposed formal framework for circadian medicine and will hopefully trigger discussion among our colleagues, so that the framework can be improved and expanded.
As the basis of the framework for circadian medicine, we define “circadian health” and how it links to general health. We then define interactions among the circadian system, sleep, and the health/disease balance and put the framework into the context of the literature with examples from six domains of health/disease balance: fertility, cancer, immune system, mental health, cardiovascular, and metabolism.
Keywords: Circadian medicine, circadian health, conditional process model, humans, health
Introduction
The new field of circadian medicine is gaining importance with great momentum (Abbott, Malkani, & Zee, 2018; Allada & Bass, 2021; Cederroth et al., 2019; Fishbein, Knutson, & Zee, 2021). Circadian medicine addresses the interactions among the circadian system, sleep, and the health/disease balance. We define “circadian system” here as a network that includes the pacemaker in the mammalian brain (i.e., the suprachiasmatic nucleus, SCN) and all peripheral tissue and organ clocks. The literature of this new field is growing rapidly, and includes studies on infections (Borrmann, McKeating, & Zhuang, 2021), metabolic syndrome (Roenneberg, Allebrandt, Merrow, & Vetter, 2012; Stenvers, Scheer, Schrauwen, Fleur, & Kalsbeek, 2019), cardiovascular disease (Xu, Jain, & Zhang, 2021), cancer (Patel & Kondratov, 2021; Sahar & Sassone-Corsi, 2009; Tsuchiya, Umemura, & Yagita, 2020) and psychiatry (Kling & Landgraf, 2021).
The conceptual framework for the circadian system was defined by the field’s pioneers Aschoff and Pittendrigh and was instrumental for the elucidation of circadian mechanisms (Daan, 2000). Yet, despite the rapid growth of circadian medicine, a conceptual framework and formal guidelines for circadian medicine are missing. The aims of this review are to provide such a framework by specifically defining these complex interactions among the circadian system, sleep, and the health/disease balance, and to apply this framework in a – by far non-exhaustive – review of the current literature.
Approaching a Conceptual Framework of Circadian Medicine
When trying to understand a complex problem, it is helpful to find its core – an issue, a definition, statement, or hypothesis that is central to the problem and that links most of its components. Once found, this core can be used as a foundation to systematically build a framework: producing a manageable taxonomy in which all components are well defined with hypothesised specific descriptions of their interactions. Only then can frameworks, including their components and interactions, be validated, and extended. Many of the reported relationships between the circadian system, sleep and the health/disease balance are so far only observations and associations. With a good framework, we can categorise observations and then assign them to one or more components with hypothesised interactions. Such assignments can lead to experiments that test causalities where we previously only had correlation.
We propose that the hypothesis “circadian health is associated with general health” can be used as a core to a framework for circadian medicine. The WHO has already defined “general health” as “a state of complete physical, mental and social well-being and not merely the absence of disease and infirmity”; “circadian health” still needs a clear definition. We continue this paper with deriving a definition of circadian health, and with sections on links between circadian and general health, on how sleep and the circadian system interact, and examples from six central domains: fertility, cancer, immune system, mental health, cardiovascular, and metabolism.
Defining circadian health
The definition of “circadian health” can be derived from circadian principles. By evolutionary logic, circadian clocks support fitness in a rhythmic environment, which is only possible if they synchronise (entrain) with an appropriate phase (time) relative to the rhythmic environment. Note that “phase of entrainment” can show variations, which are often referred to as “chronotypes,” within a species. This appropriate phase partially depends on the species-specific temporal niche (Roenneberg, 1992): the most obvious are day-active (diurnal) vs. night-active (nocturnal) species. When resources or other challenges (e.g., food, predators) also vary as part of the rhythmic environment, the tolerance (without negative survival consequences) is lower for deviations from an appropriate entrained phase. Hence, circadian health must be based on appropriate entrainment.
Light (and darkness) is the predominant zeitgeber (“time-giver” or entraining stimulus) for the circadian clocks in practically all organisms on earth, most probably because it is the most reliable predictor for all other rhythmic resources (e.g., temperature, humidity, food, predators). Humans (as other animals and plants) may have circadian clocks in each of their cells, thus these clocks must entrain to both the external and the internal environment and, in some cases, to each other. Because all resources in the pre-industrialised world were tightly coupled to the light-dark cycle (Ekirch, 2001), the central clock in the SCN only needed to follow light. This central clock provides an internal rhythmic environment to which peripheral clocks can entrain. After perturbations (e.g., shift in light exposure by trans-meridian travel or shift-work), recovery is based on resynchronization, both to the external environment and within the internal environment; it may require multiple days until the circadian system resumes a coordinated steady state (e.g., recovering from jetlag). Since such perturbations are associated with health deficits, one can argue that a stable entrainment, i.e., adopting a stable phase relationship to the rhythmic environment, supports health.
The ability of the peripheral clock in the liver to entrain to food (Mistlberger, 2020) may have adaptive value in reflecting a need for flexibility when a food source is only available at specific times within the light-dark cycle in nature. However, for most humans in industrialised societies food is available “24/7”, which may change phase relationships between different components of the circadian system and challenge circadian health. Such internal desynchrony prevents the circadian system from optimally coordinating functions across tissues. To understand circadian health, we need to know more about the ranges and tolerances of phase relationships between components (Roenneberg & Merrow, 2016), which, by oscillator principles, will also affect the amplitudes of organ and tissue clocks.
For industrialised humans, the availability of most resources is barely rhythmic and there appears to be a higher tolerance for a broad variance in entrained phase, especially for timing of both sleep and melatonin: the difference in sleep and in melatonin onset timing between the extreme larks (very “early” people) and the extreme owls (very “late” people) is 12 h, half a 24-h cycle (McHill et al., 2017; Roenneberg, Pilz, Zerbini, & Winnebeck, 2019). This large variance can be explained by profound changes in light environment and self-selected light exposure (Roenneberg et al., 2012; Wright et al., 2013): humans have drastically reduced the strength of the light-dark cycle by hardly being outdoors during the day – even in sunny, warm locations (Cole et al., 1995; Okudaira, Kripke, & Webster, 1983; Scheuermaier, Laffan, & Duffy, 2010) and rarely experiencing full darkness during the night, except when asleep. In addition, industrialised humans eat at day and night (Gill & Panda, 2015) and have little fear of predators.
According to circadian formalisms (the framework established by the pioneers of circadian biology), such weak zeitgebers advance the phase of extreme larks even earlier and delay the phase of all other chronotypes, with owls being delayed more than intermediate chronotypes (Porcheret et al., 2018). Because the physiology underlying entrainment has not changed since industrialisation began (the time span is much too short for genetic adaptation), exposing homo urbanicus to naturally strong light-dark cycles should reverse these predictable consequences of weak zeitgebers and narrow the width of the statistical distribution of circadian phases. This was indeed observed in “camping” studies (Wright et al., 2013). Thus, the “24/7” resource availability indirectly causes the wide chronotype distribution, allowing the consequences of the weak zeitgebers to be expressed in the industrialised population without negative evolutionary outcomes (e.g., starving or being killed).
Even in industrialised societies, not all resources are available “24/7”. Note that ecology regards not only food, but all items and conditions (including places to sleep, avoidance of predation, or access to breeding partners) as resources that can alter fitness. Money has become so dominant as a resource because it can be used as a “resource joker” (or wild card) that can be exchanged into many other resources. Thus, our direct access to obtaining the most important modern resource – money – is limited to work times, even for shift-workers, and our indirect access to money via education is limited to school times. These temporal limitations are defined by local clock time and they may be early for those who are late chronotypes (e.g., some adults and almost all adolescents) (Biller, Molenda, Zerbini, Roenneberg, & Winnebeck, 2022; Vinne et al., 2014; Winnebeck et al., 2019; Zerbini et al., 2017) or may even force people to work during their circadian sleep phases (e.g., night or shift-work). The discrepancy between social (i.e., local clock time set by the society) and biological time is addressed by concepts like “Social Jetlag” (SJL; Roenneberg et al., 2019), Sleep and Circadian Rhythm Disruption (SCRD), Circadian Misalignment, or Circadian Disruption (Zee & Abbott, 2020) and is strongly associated with decreased health.
In summary, we define circadian health as the condition in which circadian clocks are allowed to stably entrain to zeitgebers and thereby establish an appropriate, stable phase relationship to its cyclic environment. This pertains both to organisms entraining to an external cyclic environment and to the organism’s tissues entraining to their respective internal environments, thereby forming appropriate phase relationships to the clocks of other tissue. We note that the distribution of “healthy” phase relationships of this internal entrainment still needs to be determined. While the first part of this definition predominantly applies to people whose clocks are stably entrained to external zeitgebers, the second part still applies if people are not entrained to external zeitgebers, as are many people who suffer from total blindness or who have a circadian rhythm sleep disorder (Bridge et al., 2021; Czeisler et al., 1995; Saeed, Zee, & Abbott, 2019).
Because the circadian system modulates practically all aspects of physiology, from gene expression to sleep, one could argue that circadian health is equal to general health, but the two can be formally separated. In mutant animals without circadian clocks, environmental exposures (e.g., bacteria, diets, toxins) participate in the aetiology of a disease and therapies for recovery can still be still effective. Although these animals may be more vulnerable without a healthy circadian system, their general health can still be protected or restored.
Defining the Potential Interactions among the Circadian System, Sleep, and the Health/Disease balance
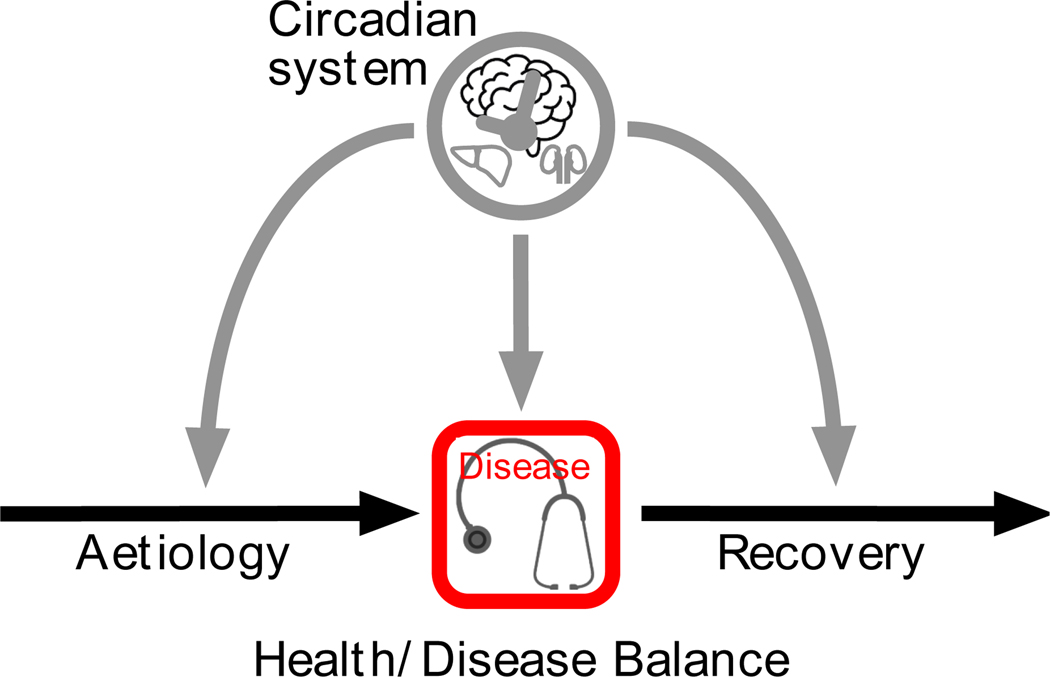
Now that we have defined circadian health, we define the potential interactions between circadian health and the health/disease balance. Health is constantly challenged by exposures (aetiology component in Figure 1), which are either environmental or systemic (e.g., diabetes, cardiovascular diseases, cancer, genetic variations) (Ayres, 2020). The health/disease balance includes resilience to such exposures and thereby reduces the possibility that the exposure leads to disease and becomes clinically relevant (centre of the health/disease pathway in Figure 1). Once a clinically relevant disease process has begun, the balance depends both on the severity of the disease and on the efficacy (both systemic and medical) in fighting the disease (recovery component in Figure 1). Note that the health/disease balance is present throughout the pathway; for example, resilience can be considered a type of recovery (e.g., reducing the effect of the exposure, preventing clinical disease) in the aetiology component. Preventive medicine supports this resilience.
Figure 1:
A graphic of our hypothesis that the circadian system affects the health/disease balance through interactions with all three of its components: aetiology, severity of the actual disease, and recovery (see text for details).
We hypothesise that, in most cases, the circadian system is not directly causal in the aetiology of and the recovery from a disease. It can, instead, significantly modify the health/disease balance by: (i) preventing or reducing its aetiology, e.g., by anticipating exposures; (ii) attenuating its severity, e.g., by ensuring the coordination of disease-defence mechanisms over the course of a 24-hour day; and (iii) facilitating recovery, e.g., by modifying the effectiveness of the therapy and intensity of potential side effects.
We further hypothesise that a healthy circadian system will be more effective in keeping a positive health/disease balance than a challenged circadian system, which we demonstrate with four examples: (i) if the internal biological day is misaligned with the external light phase, the skin is less protected against UV radiation (Plikus & Andersen, 2018); (ii) if we eat at biologically “wrong” times of day and thereby perturb the temporal balance between different metabolic components (e.g., secretion, sensitivity and absorption), we exhibit type-2 diabetes-like metabolic responses (Scheer, Hilton, Mantzoros, & Shea, 2009; Wefers et al., 2018); (iii) obstructive sleep apnoea induces hypoxia which can cause internal misalignment and thereby increase the severity of the damage from obstructive sleep apnoea (Manella et al., 2020); and (iv) chrono-pharmacology in cancer treatments may improve survival (Cederroth et al., 2019). In our review of selected pathologies below, we cover more examples of the circadian system, sleep, and the health/disease balance.
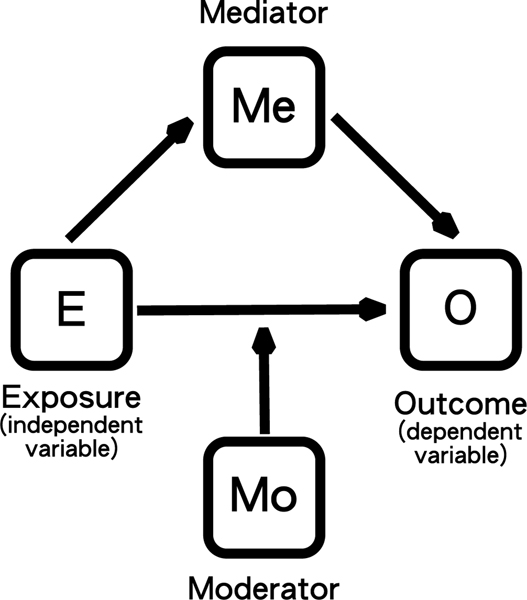
A more functional approach to defining the interactions of circadian rhythms in the health/disease balance is using a conditional process model (Jackson, Redline, & Emmons, 2015; James & Brett, 1984) (Figure 2). These models describe how components in a network act on each other: an independent variable (exposure variable, E) acts on a dependent variable (outcome variable, O) either directly (with or without a moderator variable, Mo) or indirectly via a mediator variable (Me). The advantage this model framework is that there are statistical methods to quantify the strength of relationships between network components (Bolin, 2014).
Figure 2:
Formal aspects of the roles of elements in a network and how they can affect each other.
The circadian effects in Figure 1 have all been drawn as moderators (Mo), but the interactions between the circadian system and the health/disease balance involve many feedback loops and include sleep as an additional active component (see next section and Figure 3). Note that the “independent variable” in the clock-sleep-health network is rarely strictly independent (exposure variable, E) and the “dependent variable” is rarely strictly dependent (outcome variable, O).
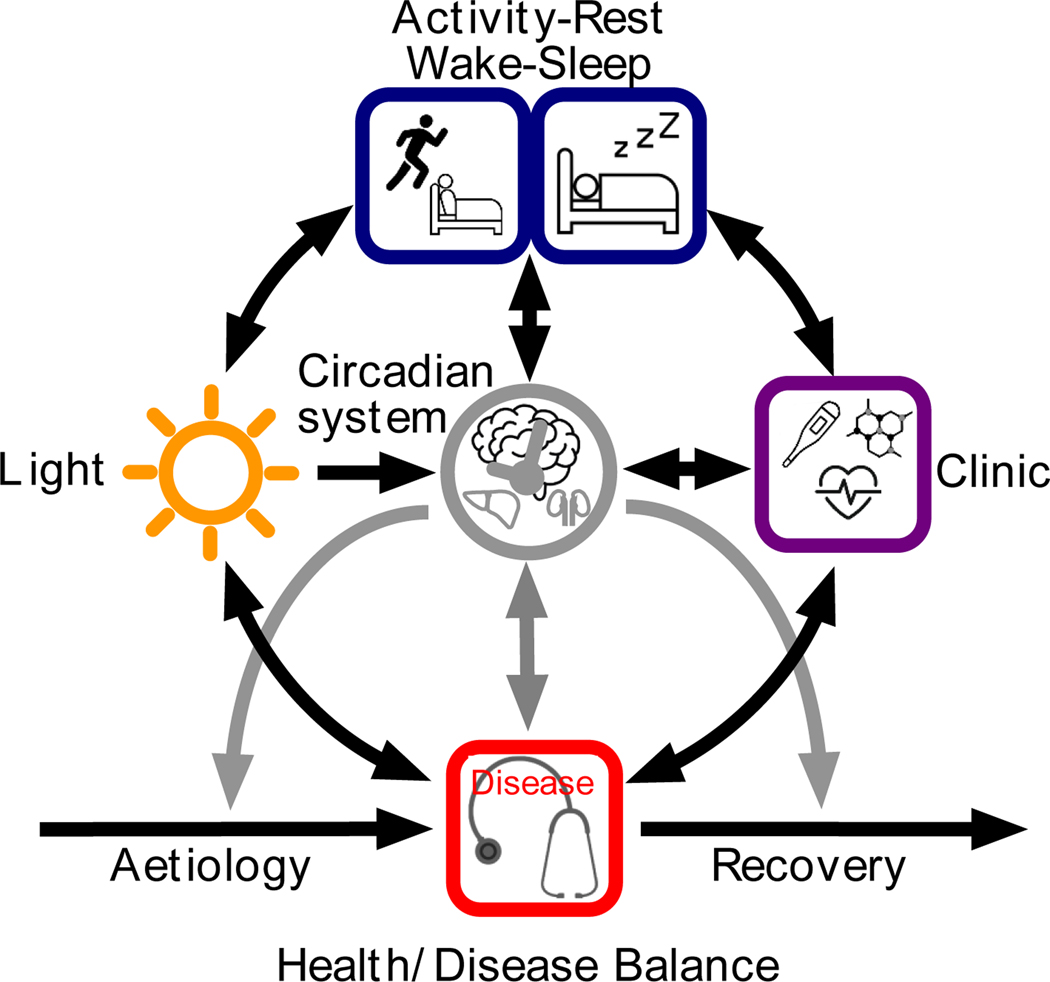
Figure 3:
An example of application of the conceptual framework with the addition of specific components, including Light, Activity-Rest (or Wake-Sleep) and the Clinic (where diseases are diagnosed and treated). Most of the components of the framework interact not only directly but also via feedbacks (see text for details).
The most obvious independent variable (E) in this network is light, which directly affects the clock (O) during the entrainment process (Roenneberg, Daan, & Merrow, 2003). Light (E) can also directly affect sleep (O), e.g., by inducing sleep in nocturnal rodents (Lupi, Oster, Thompson, & Foster, 2008) or inducing wakefulness in humans (Avery et al., 2002). Light (E) can also indirectly affect sleep (O) via phase-shifting the circadian system, in which case the clock adopts the role of mediator (Me).
If functions are moderated by the circadian system or sleep (Mo), they may also proceed at other circadian phases or during wake, albeit with different efficiency; if they are restricted to sleep or specific circadian phases, sleep and the circadian system become mediators (Me). Only if sleep or the circadian system cause an outcome, can they be exposure (E). Thus, some night-specific functions may rely on us actually sleeping (sleep as Me), others may simply be segregated to the biological night as determined by the circadian system (although even then they may be more efficient if we are asleep; sleep as Mo). Note that sleep can be an exposure variable (E) for the circadian system (O), either directly (Deboer, Vansteensel, Détári, & Meijer, 2003) or via light (via darkness by switching off lights, closing eye lids and rolling up the eyeballs), which adopts the mediator role of (Me) in this case.
As indicated in Figure 1, the circadian system acts as moderator (Mo) in all stages of the health/disease balance; one example is moderating responses to vaccines or medications. Sleep can also be a moderator (Mo) for the immune system’s response to vaccines (Lange, Dimitrov, Bollinger, Diekelmann, & Born, 2011) and mediates (Me) many forms of learning, memory sorting and consolidation (Diekelmann & Born, 2010). Circadian rhythms and sleep can also be an outcome of the network (O), either directly or indirectly. For example, light can affect the phase of the circadian system and a person is less likely to sleep after having coffee.
How the Circadian System and Sleep interact in the Health/Disease balance
The tight coupling between the circadian system and sleep makes it difficult to disentangle their individual contributions in the health/disease balance. As much as adaptation to spatial niches (i.e., sea/land/air) co-evolved with specialisations in many aspects of physiological (from gills and fins to lungs and legs and wings), the adaptation to temporal niches (day/night) is most evident in sensory physiology. As animals adapted to one temporal niche (e.g., day or night), they became less adapted to the other. This specialisation included segregating behaviours, leading to more activity in one niche and more rest in the other; as noted above, the most obvious examples are light-active (diurnal) or dark-active (nocturnal) animals. The more-or-less consolidated sleep of humans is a consequence of this specialisation. Over the course of evolution, more and more functions that we now consider to be sleep-specific were segregated to the circadian/biological “night”. Sleep is commonly regarded as a brain function – mainly because it is associated with decreased responsiveness – but many physiological functions aligned with this temporal segregation, such as brain maintenance (Bushey, Tononi, & Cirelli, 2011; Xie et al., 2013) and metabolic and immune functions (Foster, 2018).
Note that “sleep” and “wake” are placeholders for a wide variety of functions that occur during these states. Before research provided us with insights into sleep’s rich collection of functions, sleep’s depiction was restricted to that of being unconscious and having dreams. Wakefulness can be defined as the opposite of sleep – a state of consciously interacting with reality – without needing the details of its rich collection of functions to define it. These simplistic definitions are used like the modern term “phone”; a mobile phone is used far beyond making phone calls, for sending texts and emails, querying the internet, listening to podcasts or music, looking up a location on a map, or renewing an address book.
Despite their tight coupling, the circadian system and sleep can easily be differentiated on a formal level: the circadian system is a continuous process and sleep is a state within this process. If the circadian system were a Ferris wheel, sleep would be one or more cabins that travel(s) within the Ferris wheel. Within the “sleep” cabin, there may be additional different temporal functions and structures (e.g., ultradian rhythms of NREM sleep and REM sleep). Note that, due to the wide range of chronotypes, the biological night may differ substantially from the actual environmental night. Although we can potentially sleep at any time of the day if we are tired enough, falling asleep is easier at some circadian phases than at others (clock as Mo). The structure (and underlying functions) of sleep also depends on the circadian phases at which we are asleep (Dijk, Duffy, Riel, Shanahan, & Czeisler, 1999; Weitzman, Czeisler, Zimmerman, Moore-Ede, & Ronda, 1980) i.e., the functions within the cabin depend on where the cabin hangs on the Ferris wheel (again, clock as Mo). In addition, sleep/wake state can directly (sleep as Mo) or indirectly (sleep as Me) affect circadian phase responses to light (Jagannath et al., 2021).
Specific experimental conditions are required for distinguishing between circadian and sleep effects on physiology (Czeisler & Klerman, 1999); observational study design confounds the timing of the two since sleep timing in humans is overwhelmingly influenced by work or school times and thus do not occur at all possible circadian times.
Feedback Loops among the Circadian System, Sleep and Health/Disease
How the circadian system and sleep are associated with the health/disease balance is complex due to a network of feedback interactions. The feedbacks schematised in Figure 3 clarify why so many diseases are hypothesised to have reciprocal interactions with the circadian system and sleep (Foster et al., 2013). Many of the examples described above in the context of a conditional process model (Figure 2) are also represented in the network shown in Figure 3. Since light, the circadian system and sleep/wake behaviour form a loop, each of the elements can be regarded as either (E), (Me) or (O). A similar loop can be drawn among the circadian system, clinic (representing both diagnostics and therapeutics) and sleep/wake behaviour. Almost all diseases, from infections to psychiatric pathologies, affect our behaviour. Some enforce bedrest (often accompanied by retreating from bright light) others reduce our outdoor activities - because we lose the motivation to leave our dwellings or we have difficulties in moving around (e.g., due to pain or reduced mobility). Similarly, the moderation of the circadian clock on immune responses is well established (Keller et al., 2009), but growing evidence indicates that the circadian system is also under the influence of the immune system (Coogan & Wyse, 2008)
Examples of Circadian System, Sleep, and Health/Disease Balance in Circadian Medicine
By examining the results from rodent models and studies in humans, the probable impact of a challenged circadian system on human health can be assessed with varying degrees of confidence. Such an examination within our proposed framework will also identify deficits in our knowledge and suggest future observational and experimental approaches. It is difficult to separate sleep loss from time-of-day or circadian factors; this is not surprising in view of the interdependent loops as shown in Figure 3. Therefore, we use the broader term “Sleep and Circadian Rhythm Disruption” (SCRD) to describe such disruptive conditions or agents. For example, circadian misalignment is associated with SRCD and, according to the formal framework introduced above, circadian misalignment challenges circadian health. SCRD conditions are epidemiologically associated with the increased prevalence of practically all diseases (Madeira et al., 2021; Rutters et al., 2014; Wong, Hasler, Kamarck, Muldoon, & Manuck, 2015).
One well known example of SCRD is shift-work, which is associated with circadian misalignment as workers try to sleep during the biological day and work during the biological night. Such behaviours are associated with disrupted sleep, eating at inappropriate circadian phases, light at night, altered sleep timing, and reduced sleep duration. Social jetlag is another example of SCRD. Social jetlag is defined as the difference in temporal behaviour between workdays and work-free days and is almost always associated with sleep loss (Roenneberg et al., 2012).
Note, some “continuous” correlations among the circadian system, sleep and the health/disease balance have to be carefully examined, as for example, the U-shaped association between sleep duration and health (Choi et al., 2008). While the association between short sleep and worse health is supported by proposed biological mechanisms, the risk of long sleep duration for health is still disputed and may be a result of measuring sleep duration with one-question surveys (Lauderdale, Chen, Kurina, Waite, & Thisted, 2016) or the result of a causality reversal, i.e., people with acute or chronic illnesses, including sleep disorders, sleep longer.
We now discuss six areas of medicine in which our framework can be applied.
Infertility
The circadian system is involved at every stage of the menstrual cycle, from the timing of the release of all the key hormones to the responses of the various tissues to those hormones (Kerdelhué et al., 2002; Sellix & Menaker, 2010). The timing of ovulation and release of eggs for fertilisation is a good example of how circadian health (i.e., adequate internal entrainment) ensures general health. The circadian clocks in the SCN, in the hypothalamic GnRH neurons, in the pituitary (LH and FSH release), and in the ovaria (oestrogen and progesterone release) need to be internally entrained for ovulation; disruption or lack of internal entrainment in these tissues contribute to reproductive problems and infertility. One of the key hormones, LH, is affected by sleep/wake state during specific phases of the menstrual cycle (Hall, Sullivan, & Richardson, 2005). Evidence for SCRD being associated with reduced fertility comes from animal experimentation and from human epidemiology. “Clock mutant mice” have disrupted ovulation and reproductive cycles, along with reduced fertility and fewer births (Miller & Takahashi, 2013). Women who experience SCRD due to night shift-work or repeated jetlag have as small but significant increase of irregular and extended menstrual cycles, abnormal levels of reproductive hormones, and a reduction in fertility (Baker & Driver, 2007; Lawson et al., 2011; Nurminen, 1998). Websites and clinical practitioners advise women not to undertake shift-work or multiple trans-meridian flights if they are undergoing in vitro fertilisation (IVF), although no systematic trials have been undertaken to test this advice. Therefore, the circadian system and sleep are involved in the aetiology and probably disease components of this disorder. Restored fertility (recovery component) is expected to occur with reduced circadian misalignment.
Sleep duration is associated with sperm count and semen quality in men (Chen et al., 2016). As noted above, this association is U-shaped and need careful examination.
Cancer
Shift-work studies provide the strongest support for SCRD being involved in the aetiology of cancer. There is increased frequency of breast cancer in women working shift-work (Megdal, Kroenke, Laden, Pukkala, & Schernhammer, 2005). Based on a review of multiple studies, the World Health Organization (WHO) has declared shift-work a carcinogen (Straif et al., 2007). The mechanisms are unknown. One hypothesis concerns “Light at Night” (LAN) in which Light at Night suppresses melatonin, which has been reported to have anti-cancer properties; the evidence is stronger for the circadian effects of shift-work rather than for LAN per se (Kantermann & Roenneberg, 2009; Lunn et al., 2021).
Additional support for the involvement of SCRD in the aetiology of cancer is that risk of cancer varies within a time-zone, with risks increasing from the eastern to the western edge of a time zone. While local (social) clock time remains constant within a time zone, the sun clock (dawn and dusk), which sets the time of the circadian system, gets later by four minutes per degree longitude. Thus, the discrepancy between the circadian time and social time (social jetlag) increases towards the west, equivalent to increasingly earlier “shift” times, even for nine-to-five schedules.
It is not known how SRCD affects cancer once the disease progress has begun. It is very likely that the circadian clock is both involved in cancer aetiology and in the disease itself since the circadian molecular feedback loops are involved in all characteristics features of tumour development and progression: cell cycle, DNA damage/repair, apoptosis, and cancer-specific metabolism (Shafi & Knudsen, 2019).
Time-of-day rhythmicity in response to medications is involved in the recovery from cancer (Mo and Me in Figure 2). For some cancers, administering medication at specific circadian times is associated with increased progression-free and overall survival rates and/or decreased side effects, with a sex-effect in some studies (Innominato et al., 2020; Karaboué et al., 2022; Printezi et al., 2022).
Challenges to the Immune System
Circadian modulation is clearly present in the immune system and its responses to antigens. As the body’s line of defence against pathogens, the immune system is a major player in the aetiology component of the health/disease balance. Multiple studies have shown time-of-day differences in immunity and inflammation related to cellular (e.g., T-cells) and other immune responses, some of which are involved in asthma, rheumatoid arthritis and auto-immune diseases (Gray & Gibbs, 2022; Haspel et al., 2020). The complexity of immune responses, involving the interaction of systemic, SCN-mediated circadian rhythms with the cellular clocks of several different cell types demands a healthy circadian system to ensure coordination (Keller et al., 2009) and avoid inappropriate (e.g., auto-immune) responses. The strong circadian dynamics within the immune system clearly bear on the fact that its defences are dealing with a highly rhythmic world, both externally and internally.
The importance of dealing with the circadian rhythms and sleep of the internal (i.e., host) world are especially evident in viral-host interactions (i.e., the disease component of the framework) since the “aggressor” reproduces by using the hosts replication machinery that itself is highly rhythmic (Borrmann et al., 2021; Ehlers et al., 2017; Zhuang et al., 2021). If the circadian system is compromised, as in arrhythmic mice mutants, influenza A produces a higher viral burden and increased inflammatory responses (Edgar et al., 2016; Sengupta et al., 2019). A time-of-day variation in SARS-CoV2 virus load is also observed (McNaughton, Adams, Johnson, Ward, & Lasko, 2021). The hosts circadian system regulates virus progression and thereby symptoms (Edgar et al., 2016; Scheiermann, Gibbs, Ince, & Loudon, 2018), and Herpes virus has been shown to differentially target molecular clock components (Baxter & Ray, 2020). Inflammation related diseases also demonstrate these rhythms: asthma has a circadian variation in symptoms (Scheer et al., 2021), independent of sleep/wake behaviour; and rheumatoid arthritis also has a circadian variation in symptoms (Gray & Gibbs, 2022). Mice with mutations in circadian clock components have altered timing and severity of a disease related to multiple sclerosis (Gray & Gibbs, 2022). Sleep is a “container” (as E, Mo, Me) for many immune functions and decreased sleep is also associated with reduced immune response (Besedovsky, Lange, & Haack, 2019; Dimitrov, Lange, Tieken, Fehm, & Born, 2004; Everson, 1993; Haspel et al., 2020; Irwin et al., 1996; Lange et al., 2011; Lange, Perras, Fehm, & Born, 2003; Langlois, Smolensky, Glezen, & Keitel, 1995; Long et al., 2016)
Circadian rhythms of the external (i.e., pathogen) world affect disease processes for some parasite-host interactions. For example, the malaria parasite has an intrinsic circadian clock gating the daily bursting of red blood in mice (Rijo-Ferreira et al., 2020), which may account for the daily variation in malaria symptoms.
In view of the strong rhythmicity within the immune system, it is not surprising that responses to vaccinations (i.e., the recovery component of the framework) also show time of day effects (Kirby, 2016; Kurupati et al., 2017; Phillips, Gallagher, Carroll, & Drayson, 2008; Wang et al., 2021). The benefit of specific timing of medications for asthma, arthritis, and general allergy (e.g., skin, rhinitis) has also been documented in multiple studies (Scheer et al., 2021).
Psychiatric Disorders
For issues related to mental health, it is especially difficult to separate sleep loss from time-of-day or circadian factors. Many psychiatric disorders have associated sleep complaints (Freeman, Sheaves, Waite, Harvey, & Harrison, 2020) and there is major overlap of symptoms between mood disorders and sleep problems: both have symptoms of fatigue, sleep disturbance/loss, loss of interest, decreased motivation, problems with attention/concentration, decreased libido, and or mood variability.
For aetiology and disease components, sleep loss is a known trigger for mood changes in bipolar disorder (Lewis et al., 2017). Insomnia and/or being awake at night is a known risk factor relapse for substance use disorders and for new or recurrent major depressive disorder (Buysse et al., 2008; Dew et al., 1997; Perlis, Giles, Buysse, Tu, & Kupfer, 1997; Pigeon et al., 2008). Suicides are more common at night after adjustment for percentage of people awake (Perlis et al., 2016). Multiple components of behaviours associated with mental health, including mood and addiction-type disorders are worse at night and or after sleep loss (Tubbs, Fernandez, Grandner, Perlis, & Klerman, 2022).
Multiple time-of-day factors are involved in the aetiology and disease components of psychiatric disorders. There are different time-of-day rhythms in positive vs. negative affect (Emens et al., 2020). For Seasonal Affective Disorder, a circadian-based hypothesis for its aetiology is supported by the fact that the efficacy of lighting and melatonin interventions depend on the time of day of intervention (Lewy et al., 2007). Variation in some circadian clock genes is also associated with increased risk of winter depression (Partonen et al., 2007) and with other psychiatric disorders (Charrier, Olliac, Roubertoux, & Tordjman, 2017; Sato et al., 2021; Schuch, Genro, Bastos, Ghisleni, & Tovo-Rodrigues, 2017). Late chronotypes and individuals with social jet lag also are at higher risk of psychiatric disorders (Levandovski et al., 2011).
For recovery, reduction in sleep complaints, including reducing sleep timing variability – which would be expected to affect the amplitude and phase of circadian rhythms – is associated with improvement. For example, treatment of insomnia reduces the risk of relapse of substance use disorder and major depressive disorder (Gebara et al., 2018; Manber et al., 2016; McCall et al., 2019).
Cardiovascular disease (CVD)
The blockage or rupture of a blood vessel in the brain, e.g., ischemic or haemorrhagic stroke or transient ischemic attack (TIA) or in the heart (e.g., myocardial infarction or “heart attack”) deprives these highly metabolically active organs of glucose, oxygen, and other key nutrients. All these events vary in their likelihood by time of day. All subtypes (ischemic, haemorrhagic, transient ischemic attacks) of strokes have a significantly higher chance to occur between 6 am and noon compared to the rest of the day (all types: 49%; ischemic strokes: 55%; haemorrhagic strokes: 34%; transient ischemic attacks 50%; Elliott, 1998). Similar findings have been documented repeatedly for myocardial infarction, ventricular arrhythmias, and sudden cardiac arrest (Butt, Zakaria, & Hussain, 2009; Manfredini et al., 2005; Thosar, Butler, & Shea, 2018).
SRCD is implied (aetiology component) in the increased likelihood of strokes, heart attacks and coronary events that are more likely in rotating night shift-workers (Brown et al., 2009; Vetter et al., 2016). (Vyas et al., 2012). Some risk factors for cardiovascular disease are increased in experiments in which circadian misalignment is induced (Morris, Purvis, Hu, & Scheer, 2016).
In this scenario, the circadian system acts as moderator (Mo). While it is not the cause of strokes and heart attacks, normal morning physiology makes them more likely if the cardiovascular system is weakened. Between 6 am and noon, heart rate and blood pressure rise in anticipation of being awake, switching from sleep to consciousness, from rest to activity, thereby increasing the supply of oxygen and nutrients. The circadian system regulates blood pressure and heart rate via the sympathetic branch of the autonomic nervous system. Major changes in activity and posture following wake contribute to this cardiovascular activation (Stubblefield & Lechleiter, 2019). Increased physical activity is accompanied by additional circadian changes including increased cortisol, testosterone, insulin, and glucose release, all of which help drive a higher metabolic rate. Concurrently to this morning-specific cardiovascular activation, there is a circadian increase in pro-clotting factors in the blood, including the activation of platelets (Scheer et al., 2011) and in blood clotting (McLoughlin, Haines, & FitzGerald, 2014). Importantly, individuals who have had a myocardial infarction during the morning experience more heart damage and a poorer chance of recovery compared with heart attacks at other times of the day (Suárez-Barrientos et al., 2011), implying circadian effects in the disease and recovery components of Figure 1 also.
The circadian system could act as a moderator (Mo) on the aetiology of CVD in two ways, which are described by the following analogies.
Interaction 1: if one would pump up a tire regularly every morning between 6 am and noon (keeping the pressure in the recommended range), the chances of the tire to burst would be increased during that temporal window. The medical outcome of this interaction would be short-term, in form of a stroke or heart attack.
Interaction 2: the chance for the tire to burst depends on weaknesses in its wall. The outcome of interaction 2 would long-term, concerning all the factors that lead to this, including higher cholesterol, stiffer vessel walls, higher blood pressure. While interaction 1 has mechanic reasons, interaction 2 has metabolic and inflammatory reasons (see respective sections).
In Figure 1, we argue that preventive medicine supports resilience in the aetiology component, which is under circadian moderation and therefore medication should take circadian phase into account. For example, when antihypertensives are taken before bedtime, rather than in the morning, they significantly improve overall blood pressure levels (Bowles, Thosar, Herzig, & Shea, 2018) and reduce the risk of cardiovascular death, including heart failure and stroke (Peirson & Foster, 2011). Similarly, low-dose aspirin reduces platelet activation much more efficiently when taken before bedtime rather than in the morning (Bonten et al., 2015; Buurma, Diemen, Thijs, Numans, & Bonten, 2019). In the most extensive trial to-date antihypertensive medicine at bedtime was associated with both improved blood pressure control (i.e., recovery component for blood pressure) and a near halving of cardiovascular deaths and cardiovascular problems when compared with morning medication (Peirson & Foster, 2011).
As shown in Figure 1, circadian moderation also extends to the recovery component. Reducing SCRD after a stroke or heart attack aids recovery (Fleming et al., 2020; Hodor, Palchykova, Baracchi, Noain, & Bassetti, 2014; Zunzunegui, Gao, Cam, Hodor, & Bassetti, 2011). Of note, many treatments for stroke have been developed in nocturnal rodents. However, nocturnal rodents have different sleep and activity rhythms relative to circadian phase than humans (Peirson & Foster, 2011), which may explain why many treatments that were found to be successful in mice, subsequently failed in humans (Esposito et al., 2020).
Metabolic Syndrome
The circadian system influences every aspect of metabolism, from hunger and digestion to the regulation of the metabolic hormones (Thie, Kato, Bader, Montplaisir, & Lavigne, 2002). The human stomach empties faster after identical meals in the morning than in the evening (Duboc, Coffin, & Siproudhis, 2020). Gastric acid secretion varies depending on when we eat, and there is an underlying daily rhythm with increased production towards the evening (Vaughn, Rotolo, & Roth, 2014). Daily rhythms in glucose, insulin and feeding behaviour are abolished when circadian coordination by the pacemaker is absent in SCN-lesioned animals (Pol & Powley, 1979; Yamamoto, Nagai, & Nakagawa, 1987).
The liver is an excellent example for entrainment (in this case, intra-organismal) being essential for circadian health and thereby for general health. When the SCN provides an internal cyclic environment, the liver produces a daily rhythm in blood glucose, which in humans, peaks in the middle/late part of the day. However, circadian rhythms in liver cell clocks – although still rhythmic – lose synchrony within the tissue when the SCN stops providing a systemic circadian environment thereby losing daily rhythms in blood glucose (Pol & Powley, 1979). Abnormalities in clock genes have also been linked to altered glucose metabolism, type 2 diabetes and obesity - in both humans and mice (Kalsbeek, Fleur, & Fliers, 2014). Mice with defective circadian rhythms due to mutations in key clock genes fail to show a clear night/day feeding rhythm, eat excessively, are obese and develop metabolic abnormalities including fatty liver disease and insulin resistance (Turek et al., 2005). Metabolic abnormalities in conjunction with eating during the biological night (Dashti et al., 2020) may work via similar desynchrony mechanisms, i.e., misalignment between peripheral circadian clocks and the SCN (Hara et al., 2001; Stokkan, Yamazaki, Tei, Sakaki, & Menaker, 2001).
In summary, while individual liver cell clocks may contribute to a rhythmic glucose output, this output can only be coordinated when the internal environment is rhythmic, and a lack of liver clock synchrony leads to metabolic problems on the aetiology component of the health/disease balance, including obesity and insulin resistance (Chaix, Lin, Le, Chang, & Panda, 2019). Note that internal desynchrony formally always leads to a loss of amplitude in the circadian system. It is therefore not surprising that night shift work is also associated with a flattened circadian rhythm in leptin, along with an overall increase in appetite (Drongelen, Boot, Merkus, Smid, & Beek, 2011). Regular and longer duration of food restriction, especially if eating only occurs in the morning and afternoon, positively affects metabolism and increases weight loss (recovery component) (Allison et al., 2021; Garaulet et al., 2013), possibly via a stronger synchrony of circadian rhythms across the tissues involved in food processing.
Sleep restriction is also associated with decreased metabolic health. i.e., reduced levels of leptin (satiation hormone) and increased levels ghrelin (hunger hormone), and people with self-selected short sleep duration (and therefore sleep restriction) show increased hunger and the consumption of food (Cauter, Spiegel, Tasali, & Leproult, 2008; Froy, 2009; Schmid, Hallschmid, Jauch-Chara, Born, & Schultes, 2008; Spiegel, Tasali, Penev, & Cauter, 2004). Sleep loss and sleep disruption in in shift workers is also associated with a higher risk of metabolic syndrome (Beihl, Liese, & Haffner, 2009; Gangwisch et al., 2007; Study, Meisinger, Heier, & Loewel, 2005).
The circadian system may be also involved in metabolic diseases themselves due to feedbacks between the circadian system and metabolism; while the former moderates key metabolic processes, the latter can alter the circadian molecular machinery (Lamia et al., 2009) and these feedbacks could contribute to the severity of the disease. We know more about the negative effects of sleep restriction/debt on metabolic health; we still know too little about how recovery sleep can recover metabolic health (Killick, Banks, & Liu, 2012).
Summary
Here we propose a conceptual framework for circadian medicine that can be used to understand and clearly document the roles of the circadian system and sleep in the three components of the health/disease balance (aetiology, disease, recovery). The conceptual process model of relationships among variables allows more specific questions about these associations and interactions, which in turn should guide experiments, analyses, and their interpretation. Our examples and literature review have been limited on purpose; we encourage others to (re)analyse in consideration of this framework – and to use the framework when planning new work. Most studies in our literature review document relationships between the circadian system, sleep, and the aetiology component of the health/disease balance, a few in the recovery component, and fewest in the disease component. We therefore encourage more studies on disease and recovery. This attempt of a framework will only be useful if considered broadly in circadian medicine and will flourish with a dynamic process of revisions, expansions, and reductions.
Acknowledgements
We would like to acknowledge Luisa Klaus Pilz and Nicoli Bertoul Xavier, whose exciting work on the relationship between circadian parameters and health parameters in the Brazilian Quilombo population has inspired the core of the framework “circadian health is associated with general health”.
Support:
RGF: Wellcome Trust: 106174/Z/14/Z, 106174/Z/14/A, 214571/Z/18/Z, 206500/Z/17/Z; Leducq Foundation: 21CVD04; BBSRC: BB/S015817/1
EBK: Leducq Foundation, US NIH R01NS114526-02S1, R21DA052861, U54AG062322, U01NS114001, R01NS099055; US Department of Defense W81XWH2010776
Disclosures:
EBK: Consulting for Circadian Therapeutics, The National Sleep Foundation, Yale University Press
TR: Founder and CSO of Chronsulting
RGF: Co-founder of Circadian Therapeutics. Consultant for The National Sleep Foundation.
References
- Abbott SM, Malkani R, & Zee PC (2018). Circadian disruption and human health: a bidirectional relationship. The European Journal of Neuroscience, 51(1), 567–583. 10.1111/ejn.14298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allada R, & Bass J.(2021). Circadian Mechanisms in Medicine. The New England Journal of Medicine, 384(6), 550–561. 10.1056/nejmra1802337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison KC, Hopkins CM, Ruggieri M, Spaeth AM, Ahima RS, Zhang Z, … Goel N.(2021). Prolonged, Controlled Daytime versus Delayed Eating Impacts Weight and Metabolism. Current Biology, 31(3), 650–657.e3. 10.1016/j.cub.2020.10.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery DH, Kouri ME, Monaghan K, Bolte MA, Hellekson C, & Eder D.(2002). Is dawn simulation effective in ameliorating the difficulty awakening in seasonal affective disorder associated with hypersomnia? Journal of Affective Disorders, 69(1–3), 231–236. 10.1016/s0165-0327(00)00360-8 [DOI] [PubMed] [Google Scholar]
- Ayres JS (2020). The Biology of Physiological Health. Cell, 181(2), 250–269. 10.1016/j.cell.2020.03.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter M, & Ray DW (2020). Circadian rhythms in innate immunity and stress responses. Immunology, 161(4), 261–267. 10.1111/imm.13166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beihl DA, Liese AD, & Haffner SM (2009). Sleep Duration as a Risk Factor for Incident Type 2 Diabetes in a Multiethnic Cohort. Annals of Epidemiology, 19(5), 351–357. 10.1016/j.annepidem.2008.12.001 [DOI] [PubMed] [Google Scholar]
- Besedovsky L, Lange T, & Haack M.(2019). The Sleep-Immune Crosstalk in Health and Disease. Physiological Reviews, 99(3), 1325–1380. 10.1152/physrev.00010.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller AM, Molenda C, Zerbini G, Roenneberg T, & Winnebeck EC (2022). Sleep improvements on days with later school starts persist after 1 year in a flexible start system. Scientific Reports, 12(1), 2787. 10.1038/s41598-022-06209-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin JH (2014). Hayes Andrew F. (2013). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression‐Based Approach. New York, NY: The Guilford Press. Journal of Educational Measurement, 51(3), 335–337. 10.1111/jedm.12050 [DOI] [Google Scholar]
- Bonten TN, Snoep JD, Assendelft WJJ, Zwaginga JJ, Eikenboom J, Huisman MV, … Bom JG van der. (2015). Time-dependent effects of aspirin on blood pressure and morning platelet reactivity: a randomized cross-over trial. Hypertension (Dallas, Tex. : 1979), 65(4), 743–750. 10.1161/hypertensionaha.114.04980 [DOI] [PubMed] [Google Scholar]
- Borrmann H, McKeating JA, & Zhuang X.(2021). The Circadian Clock and Viral Infections. Journal of Biological Rhythms, 36(1), 9–22. 10.1177/0748730420967768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles NP, Thosar SS, Herzig MX, & Shea SA (2018). Chronotherapy for Hypertension. Current Hypertension Reports, 20(11), 97. 10.1007/s11906-018-0897-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge H, Morjaria R, Peirson SN, Coullon GSL, Warnaby CE, Pothecary CA, … Downes SM (2021). Functional Brain Imaging During Extra-Ocular Light Stimulation in Anophthalmic and Sighted Participants: No Evidence for Extra-Ocular Photosensitive Receptors. Frontiers in Neuroscience, 15, 744543. 10.3389/fnins.2021.744543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DL, Feskanich D, Sánchez BN, Rexrode KM, Schernhammer ES, & Lisabeth LD (2009). Rotating night shift work and the risk of ischemic stroke. American Journal of Epidemiology, 169(11), 1370–1377. 10.1093/aje/kwp056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushey D, Tononi G, & Cirelli C.(2011). Sleep and Synaptic Homeostasis: Structural Evidence in Drosophila. SCIENCE, 332(6037), 1576–1581. 10.1126/science.1202839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt M-U-RA, Zakaria M, & Hussain HM (2009). Circadian pattern of onset of ischaemic and haemorrhagic strokes, and their relation to sleep/wake cycle. JPMA. The Journal of the Pakistan Medical Association, 59(3), 129–132. [PubMed] [Google Scholar]
- Buurma M, Diemen JJK van Thijs A, Numans ME, & Bonten TN (2019). Circadian Rhythm of Cardiovascular Disease: The Potential of Chronotherapy With Aspirin. Frontiers in Cardiovascular Medicine, 6, 84. 10.3389/fcvm.2019.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, & Rössler W.(2008). Prevalence, Course, and Comorbidity of Insomnia and Depression in Young Adults. Sleep, 31(4), 473–480. 10.1093/sleep/31.4.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauter EV, Spiegel K, Tasali E, & Leproult R.(2008). Metabolic consequences of sleep and sleep loss. Sleep Medicine, 9 Suppl 1, S23–8. 10.1016/s1389-9457(08)70013-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederroth CR, Albrecht U, Bass J, Brown SA, Dyhrfjeld-Johnsen J, Gachon F, … Canlon B.(2019). Medicine in the Fourth Dimension. Cell Metabolism, 30(2), 238–250. 10.1016/j.cmet.2019.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Lin T, Le HD, Chang MW, & Panda S.(2019). Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metabolism, 29(2), 303–319.e4. 10.1016/j.cmet.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier A, Olliac B, Roubertoux P, & Tordjman S.(2017). Clock Genes and Altered Sleep–Wake Rhythms: Their Role in the Development of Psychiatric Disorders. International Journal of Molecular Sciences, 18(5), 938. 10.3390/ijms18050938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Yang H, Zhou N, Sun L, Bao H, Tan L, … Cao J.(2016). Inverse U-shaped Association between Sleep Duration and Semen Quality: Longitudinal Observational Study (MARHCS) in Chongqing, China. Sleep, 39(01), 79–86. 10.5665/sleep.5322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KM, Lee JS, Park HS, Baik SH, Choi DS, & Kim SM (2008). Relationship between sleep duration and the metabolic syndrome: Korean National Health and Nutrition Survey 2001. International Journal of Obesity, 32(7), 1091–1097. 10.1038/ijo.2008.62 [DOI] [PubMed] [Google Scholar]
- Cole RJ, Kripke DF, Wisbey J, Mason WJ, Gruen W, Hauri PJ, & Juarez S.(1995). Seasonal Variation in Human Illumination Exposure at Two Different Latitudes. Journal of Biological Rhythms, 10(4), 324–334. 10.1177/074873049501000406 [DOI] [PubMed] [Google Scholar]
- Coogan AN, & Wyse CA (2008). Neuroimmunology of the circadian clock. Brain Research, 1232, 104–112. 10.1016/j.brainres.2008.07.087 [DOI] [PubMed] [Google Scholar]
- Czeisler CA, & Klerman EB (1999). Circadian and sleep-dependent regulation of hormone release in humans. Recent Progress in Hormone Research, 54, 97–130; discussion 130–2. [PubMed] [Google Scholar]
- Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, … Rizzo JF (1995). Suppression of Melatonin Secretion in Some Blind Patients by Exposure to Bright Light. New England Journal of Medicine, 332(1), 6–11. 10.1056/nejm199501053320102 [DOI] [PubMed] [Google Scholar]
- Daan S.(2000). The Colin S. Pittendrigh Lecture. Colin Pittendrigh, Jürgen Aschoff, and the natural entrainment of circadian systems. [DOI] [PubMed] [Google Scholar]
- Dashti HS, Gómez-Abellán P, Qian J, Esteban A, Morales E, Scheer FAJL, & Garaulet M.(2020). Late eating is associated with cardiometabolic risk traits, obesogenic behaviors, and impaired weight loss. The American Journal of Clinical Nutrition, 113(1), 154–161. 10.1093/ajcn/nqaa264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deboer T, Vansteensel MJ, Détári L, & Meijer JH (2003). Sleep states alter activity of suprachiasmatic nucleus neurons. Nature Neuroscience, 6(10), 1086–1090. 10.1038/nn1122 [DOI] [PubMed] [Google Scholar]
- Dew MA, Reynolds CF, Houck PR, Hall M, Buysse DJ, Frank E, & Kupfer DJ (1997). Temporal Profiles of the Course of Depression During Treatment: Predictors of Pathways Toward Recovery in the Elderly. Archives of General Psychiatry, 54(11), 1016. 10.1001/archpsyc.1997.01830230050007 [DOI] [PubMed] [Google Scholar]
- Diekelmann S, & Born J.(2010). The memory function of sleep. Nature Reviews Neuroscience, 11(2), 114–126. 10.1038/nrn2762 [DOI] [PubMed] [Google Scholar]
- Dijk D, Duffy JF, Riel E, Shanahan TL, & Czeisler CA (1999). Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. The Journal of Physiology, 516(2), 611–627. 10.1111/j.1469-7793.1999.0611v.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, Tieken S, Fehm HL, & Born J.(2004). Sleep associated regulation of T helper 1/T helper 2 cytokine balance in humans. Brain, Behavior, and Immunity, 18(4), 341–348. 10.1016/j.bbi.2003.08.004 [DOI] [PubMed] [Google Scholar]
- Drongelen A.van Boot C, Merkus S, Smid T, & Beek AJ (2011). The effects of shift work on body weight change − a systematic review of longitudinal studies. Scandinavian Journal of Work, Environment & Health, 37(4), 263–275. 10.5271/sjweh.3143 [DOI] [PubMed] [Google Scholar]
- Duboc H, Coffin B, & Siproudhis L.(2020). Disruption of Circadian Rhythms and Gut Motility: An Overview of Underlying Mechanisms and Associated Pathologies. Journal of Clinical Gastroenterology, 54(5), 405–414. 10.1097/mcg.0000000000001333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RS, Stangherlin A, Nagy AD, Nicoll MP, Efstathiou S, O’Neill JS, & Reddy AB (2016). Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proceedings of the National Academy of Sciences of the United States of America, 113(36), 10085–10090. 10.1073/pnas.1601895113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers A, Xie W, Agapov E, Brown S, Steinberg D, Tidwell R, … Haspel JA (2017). BMAL1 links the circadian clock to viral airway pathology and asthma phenotypes. Mucosal Immunology, 11(1), 97–111. 10.1038/mi.2017.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekirch AR (2001). Sleep We Have Lost: Pre-industrial Slumber in the British Isles. The American Historical Review, 106(2), 343–386. 10.1086/ahr/106.2.343 [DOI] [PubMed] [Google Scholar]
- Elliott WJ (1998). Circadian Variation in the Timing of Stroke Onset: A Meta-analysis. Stroke, 29(5), 992–996. 10.1161/01.str.29.5.992 [DOI] [PubMed] [Google Scholar]
- Emens JS, Berman AM, Thosar SS, Butler MP, Roberts SA, Clemons NA, … Shea SA (2020). Circadian rhythm in negative affect: Implications for mood disorders. Psychiatry Research, 293, 113337. 10.1016/j.psychres.2020.113337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito E, Li W, Mandeville ET, Park J-H, Şencan I, Guo S, … Lo EH (2020). Potential circadian effects on translational failure for neuroprotection. Nature, 582(7812), 395–398. 10.1038/s41586-020-2348-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson CA (1993). Sustained sleep deprivation impairs host defense. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 265(5), R1148–R1154. 10.1152/ajpregu.1993.265.5.r1148 [DOI] [PubMed] [Google Scholar]
- Fishbein AB, Knutson KL, & Zee PC (2021). Circadian disruption and human health. The Journal of Clinical Investigation, 131(19). 10.1172/jci148286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MK, Smejka T, Slater DH, Gils V. van, Garratt E., Kara EY, & Johansen-Berg H. (2020). Sleep Disruption After Brain Injury Is Associated With Worse Motor Outcomes and Slower Functional Recovery. Neurorehabilitation and Neural Repair, 34(7), 661–671. 10.1177/1545968320929669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster RG (2018). There is no mystery to sleep: Why do we sleep? PsyCh Journal, 7(4), 206–208. 10.1002/pchj.247 [DOI] [PubMed] [Google Scholar]
- Foster RG, Peirson SN, Wulff K, Winnebeck E, Vetter C, & Roenneberg T.(2013). Sleep and Circadian Rhythm Disruption in Social Jetlag and Mental Illness. In Progress in Molecular Biology and Translational Science: Vol. 119 (pp. 325–346). 10.1016/b978-0-12-396971-2.00011-7 [DOI] [PubMed] [Google Scholar]
- Freeman D, Sheaves B, Waite F, Harvey AG, & Harrison PJ (2020). Sleep disturbance and psychiatric disorders. The Lancet Psychiatry, 7(7), 628–637. 10.1016/s2215-0366(20)30136-x [DOI] [PubMed] [Google Scholar]
- Froy O.(2009). Metabolism and Circadian Rhythms—Implications for Obesity. Endocrine Reviews, 31(1), 1–24. 10.1210/er.2009-0014 [DOI] [PubMed] [Google Scholar]
- Gangwisch JE, Heymsfield SB, Boden-Albala B, Buijs RM, Kreier F, Pickering TG, … Malaspina D.(2007). Sleep Duration as a Risk Factor for Diabetes Incidence in a Large US Sample. Sleep, 30(12), 1667–1673. 10.1093/sleep/30.12.1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee Y-C, Ordovás JM, & Scheer FAJL (2013). Timing of food intake predicts weight loss effectiveness. International Journal of Obesity, 37(4), 604–611. 10.1038/ijo.2012.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebara MA, Siripong N, DiNapoli EA, Maree RD, Germain A, Reynolds CF, … Karp JF (2018). Effect of insomnia treatments on depression: A systematic review and meta-analysis. Depression and Anxiety, 35(8), 717–731. 10.1002/da.22776 [DOI] [PubMed] [Google Scholar]
- Gill S, & Panda S.(2015). A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metabolism, 1–20. 10.1016/j.cmet.2015.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KJ, & Gibbs JE (2022). Adaptive immunity, chronic inflammation and the clock. Seminars in Immunopathology, 44(2), 209–224. 10.1007/s00281-022-00919-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JE, Sullivan JP, & Richardson GS (2005). Brief Wake Episodes Modulate Sleep-Inhibited Luteinizing Hormone Secretion in the Early Follicular Phase. The Journal of Clinical Endocrinology & Metabolism, 90(4), 2050–2055. 10.1210/jc.2004-2033 [DOI] [PubMed] [Google Scholar]
- Hara R, Wan K, Wakamatsu H, Aida R, Moriya T, Akiyama M, & Shibata S.(2001). Restricted feeding entrains liver clock without participation of the suprachiasmatic nucleus: Restricted feeding-induced Per genes in the liver. Genes to Cells, 6(3), 269–278. 10.1046/j.1365-2443.2001.00419.x [DOI] [PubMed] [Google Scholar]
- Haspel JA, Anafi R, Brown MK, Cermakian N, Depner C, Desplats P, … Solt LA (2020). Perfect timing: circadian rhythms, sleep, and immunity — an NIH workshop summary. JCI Insight, 5(1), e131487. 10.1172/jci.insight.131487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodor A, Palchykova S, Baracchi F, Noain D, & Bassetti CL (2014). Baclofen facilitates sleep, neuroplasticity, and recovery after stroke in rats. Annals of Clinical and Translational Neurology, 1(10), 765–777. 10.1002/acn3.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innominato PF, Ballesta A, Huang Q, Focan C, Chollet P, Karaboué A, … Lévi FA (2020). Sex-dependent least toxic timing of irinotecan combined with chronomodulated chemotherapy for metastatic colorectal cancer: Randomized multicenter EORTC 05011 trial. Cancer Medicine, 9(12), 4148–4159. 10.1002/cam4.3056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin M, McClintick J, Costlow C, Fortner M, White J, & Gillin JC (1996). Partial night sleep deprivation reduces natural killer and celhdar immune responses in humans. The FASEB Journal, 10(5), 643–653. 10.1096/fasebj.10.5.8621064 [DOI] [PubMed] [Google Scholar]
- Jackson CL, Redline S, & Emmons KM (2015). Sleep as a Potential Fundamental Contributor to Disparities in Cardiovascular Health. Annual Review of Public Health, 36(1), 417–440. 10.1146/annurev-publhealth-031914-122838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannath A, Varga N, Dallmann R, Rando G, Gosselin P, Ebrahimjee F, … Vasudevan SR (2021). Adenosine integrates light and sleep signalling for the regulation of circadian timing in mice. Nature Communications, 12(1), 2113. 10.1038/s41467-021-22179-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- James LR, & Brett JM (1984). Mediators, moderators, and tests for mediation. Journal of Applied Psychology, 69(2), 307–321. 10.1037/0021-9010.69.2.307 [DOI] [Google Scholar]
- Kalsbeek A, Fleur S.la, & Fliers, E. (2014). Circadian control of glucose metabolism. Molecular Metabolism, 3(4), 372–383. 10.1016/j.molmet.2014.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantermann T, & Roenneberg T.(2009). Is light-at-night a health risk factor or a health risk predictor? Chronobiology International, 26(6), 1069–1074. 10.3109/07420520903223984 [DOI] [PubMed] [Google Scholar]
- Karaboué A, Collon T, Pavese I, Bodiguel V, Cucherousset J, Zakine E, … Lévi F.(2022). Time-Dependent Efficacy of Checkpoint Inhibitor Nivolumab: Results from a Pilot Study in Patients with Metastatic Non-Small-Cell Lung Cancer. Cancers, 14(4), 896. 10.3390/cancers14040896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk H-D, … Maier B.(2009). A circadian clock in macrophages controls inflammatory immune responses. Proceedings of the National Academy of Sciences, 106(50), 21407–21412. 10.1073/pnas.0906361106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdelhué B. d, Brown S, Lenoir V Jr, JT Q, Jones GS, Scholler R, & HW J Jr (2002). Timing of Initiation of the Preovulatory Luteinizing Hormone Surge and Its Relationship with the Circadian Cortisol Rhythm in the Human. Neuroendocrinology, 75(3), 158–163. 10.1159/000048233 [DOI] [PubMed] [Google Scholar]
- Killick R, Banks S, & Liu PY (2012). Implications of Sleep Restriction and Recovery on Metabolic Outcomes. The Journal of Clinical Endocrinology & Metabolism, 97(11), 3876–3890. 10.1210/jc.2012-1845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby T.(2016). Influenza vaccination in the morning improves response. The Lancet. Respiratory Medicine, 4(6), 435. 10.1016/s2213-2600(16)30100-x [DOI] [PubMed] [Google Scholar]
- Kling C, & Landgraf D.(2021). Stress: Genetics, Epigenetics and Genomics. 95–108. 10.1016/b978-0-12-813156-5.00008-x [DOI] [Google Scholar]
- Kurupati RK, Kossenkoff A, Kannan S, Haut LH, Doyle S, Yin X, … Ertl HCJ (2017). The effect of timing of influenza vaccination and sample collection on antibody titers and responses in the aged. Vaccine, 35(30), 3700–3708. 10.1016/j.vaccine.2017.05.074 [DOI] [PubMed] [Google Scholar]
- Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, … Evans RM (2009). AMPK Regulates the Circadian Clock by Cryptochrome Phosphorylation and Degradation. Science, 326(5951), 437–440. 10.1126/science.1172156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange T, Dimitrov S, Bollinger T, Diekelmann S, & Born J.(2011). Sleep after Vaccination Boosts Immunological Memory. The Journal of Immunology, 187(1), 283–290. 10.4049/jimmunol.1100015 [DOI] [PubMed] [Google Scholar]
- Lange T, Perras B, Fehm HL, & Born J.(2003). Sleep Enhances the Human Antibody Response to Hepatitis A Vaccination. Psychosomatic Medicine, 65(5), 831–835. 10.1097/01.psy.0000091382.61178.f1 [DOI] [PubMed] [Google Scholar]
- Langlois PH, Smolensky MH, Glezen WP, & Keitel WA (1995). Diurnal Variation in Responses to Influenza Vaccine. Chronobiology International, 12(1), 28–36. 10.3109/07420529509064497 [DOI] [PubMed] [Google Scholar]
- Lauderdale DS, Chen J-H, Kurina LM, Waite LJ, & Thisted RA (2016). Sleep duration and health among older adults: associations vary by how sleep is measured. Journal of Epidemiology and Community Health, 70(4), 361. 10.1136/jech-2015-206109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levandovski R, Dantas G, Fernandes LC, Caumo W, Torres I, Roenneberg T, … Allebrandt KV (2011). Depression scores associate with chronotype and social jetlag in a rural population. Chronobiology International, 28(9), 771–778. 10.3109/07420528.2011.602445 [DOI] [PubMed] [Google Scholar]
- Lewis KS, Gordon-Smith K, Forty L, Florio AD, Craddock N, Jones L, & Jones I.(2017). Sleep loss as a trigger of mood episodes in bipolar disorder: individual differences based on diagnostic subtype and gender. The British Journal of Psychiatry : The Journal of Mental Science, 211(3), 169–174. 10.1192/bjp.bp.117.202259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewy A, Rough JN, Songer JB, Mishra N, Yuhas K, & Emens JS (2007). The phase shift hypothesis for the circadian component of winter depression. Dialogues in Clinical Neuroscience, 9(3), 291–300. 10.31887/dcns.2007.9.3/alewy [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JE, Drayson MT, Taylor AE, Toellner KM, Lord JM, & Phillips AC (2016). Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial. Vaccine, 34(24), 2679–2685. 10.1016/j.vaccine.2016.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn RM, Schwingl PJ, Atwood ST, Mehta SS, Jahnke GD, & Garner SC (2021). National Toxicology Program Cancer Hazard Assessment Report on Night Shift Work and Light at Night. National Toxicology Program Public Health Service U.S. Department of Health and Human Services. [PubMed] [Google Scholar]
- Lupi D, Oster H, Thompson S, & Foster RG (2008). The acute light-induction of sleep is mediated by OPN4-based photoreception. Nature Neuroscience, 11(9), 1068–1073. 10.1038/nn.2179 [DOI] [PubMed] [Google Scholar]
- Madeira SG, Reis C, Paiva T, Moreira CS, Nogueira P, & Roenneberg T.(2021). Social jetlag, a novel predictor for high cardiovascular risk in blue‐collar workers following permanent atypical work schedules. Journal of Sleep Research, 30(6), e13380. 10.1111/jsr.13380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manber R, Buysse DJ, Edinger J, Krystal A, Luther JF, Wisniewski SR, … Thase ME (2016). Efficacy of Cognitive-Behavioral Therapy for Insomnia Combined With Antidepressant Pharmacotherapy in Patients With Comorbid Depression and Insomnia: A Randomized Controlled Trial. The Journal of Clinical Psychiatry, 77(10), e1316–e1323. 10.4088/jcp.15m10244 [DOI] [PubMed] [Google Scholar]
- Manfredini R, Boari B, Smolensky MH, Salmi R, Cecilia O. la Malagoni AM, … Manfredini F (2005). Circadian Variation in Stroke Onset: Identical Temporal Pattern in Ischemic and Hemorrhagic Events. Chronobiology International, 22(3), 417–453. 10.1081/cbi-200062927 [DOI] [PubMed] [Google Scholar]
- Marshall NS, Glozier N, & Grunstein RR (2008). Is sleep duration related to obesity? A critical review of the epidemiological evidence. Sleep Medicine Reviews, 12(4), 289–298. 10.1016/j.smrv.2008.03.001 [DOI] [PubMed] [Google Scholar]
- McCall WV, Benca RM, Rosenquist PB, Youssef NA, McCloud L, Newman JC, … Krystal AD (2019). Reducing Suicidal Ideation Through Insomnia Treatment (REST-IT): A Randomized Clinical Trial. The American Journal of Psychiatry, 176(11), 957–965. 10.1176/appi.ajp.2019.19030267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHill AW, Phillips AJ, Czeisler CA, Keating L, Yee K, Barger LK, … Klerman EB (2017). Later circadian timing of food intake is associated with increased body fat. The American Journal of Clinical Nutrition, 106(5), 1213–1219. 10.3945/ajcn.117.161588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin SC, Haines P, & FitzGerald GA (2014). Clocks and cardiovascular function. Methods in Enzymology, 552, 211–228. 10.1016/bs.mie.2014.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton CD, Adams NM, Johnson CH, Ward MJ, & Lasko TA (2021). Diurnal variation in SARS-CoV-2 PCR test results: Test accuracy may vary by time of day. MedRxiv, 2021.03.12.21253015. 10.1101/2021.03.12.21253015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megdal SP, Kroenke CH, Laden F, Pukkala E, & Schernhammer ES (2005). Night work and breast cancer risk: A systematic review and meta-analysis. European Journal of Cancer, 41(13), 2023–2032. 10.1016/j.ejca.2005.05.010 [DOI] [PubMed] [Google Scholar]
- Mistlberger RE (2020). Food as circadian time cue for appetitive behavior. F1000Research, 9, 61. 10.12688/f1000research.20829.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Purvis TE, Hu K, & Scheer FAJL (2016). Circadian misalignment increases cardiovascular disease risk factors in humans. Proceedings of the National Academy of Sciences of the United States of America, 113(10), E1402–11. 10.1073/pnas.1516953113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okudaira N, Kripke DF, & Webster JB (1983). Naturalistic studies of human light exposure. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 245(4), R613–R615. 10.1152/ajpregu.1983.245.4.r613 [DOI] [PubMed] [Google Scholar]
- Partonen T, Treutlein J, Alpman A, Frank J, Johansson C, Depner M, … Schumann G.(2007). Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Annals of Medicine, 39(3), 229–238. 10.1080/07853890701278795 [DOI] [PubMed] [Google Scholar]
- Patel SA, & Kondratov RV (2021). Clock at the Core of Cancer Development. Biology, 10(2), 150. 10.3390/biology10020150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirson SN, & Foster RG (2011). Bad light stops play. EMBO Reports, 12(5), 380–380. 10.1038/embor.2011.70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, Giles DE, Buysse DJ, Tu X, & Kupfer DJ (1997). Self-reported sleep disturbance as a prodromal symptom in recurrent depression. Journal of Affective Disorders, 42(2–3), 209–212. 10.1016/s0165-0327(96)01411-5 [DOI] [PubMed] [Google Scholar]
- Perlis ML, Grandner MA, Chakravorty S, Bernert RA, Brown GK, & Thase ME (2016). Suicide and sleep: Is it a bad thing to be awake when reason sleeps? Sleep Medicine Reviews, 29, 101–107. 10.1016/j.smrv.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AC, Gallagher S, Carroll D, & Drayson M.(2008). Preliminary evidence that morning vaccination is associated with an enhanced antibody response in men. Psychophysiology, 45(4), 663–666. 10.1111/j.1469-8986.2008.00662.x [DOI] [PubMed] [Google Scholar]
- Pigeon WR, Hegel M, Unützer J, Fan M-Y, Sateia MJ, Lyness JM, … Perlis ML (2008). Is Insomnia a Perpetuating Factor for Late-Life Depression in the IMPACT Cohort? Sleep, 31(4), 481–488. 10.1093/sleep/31.4.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plikus MV, & Andersen B.(2018). Skin as a window to body-clock time. Proceedings of the National Academy of Sciences, 115(48), 201817419. 10.1073/pnas.1817419115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol ANVD, & Powley T.(1979). A fine-grained anatomical analysis of the role of the rat suprachiasmatic nucleus in circadian rhythms of feeding and drinking. Brain Research, 160(2), 307–326. 10.1016/0006-8993(79)90427-x [DOI] [PubMed] [Google Scholar]
- Porcheret K, Wald L, Fritschi L, Gerkema M, Gordijn M, Merrrow M, … Foster RG (2018). Chronotype and environmental light exposure in a student population. Chronobiology International, 35(10), 1–10. 10.1080/07420528.2018.1482556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Printezi MI, Kilgallen AB, Bond MJG, Štibler U, Putker M, Teske AJ, … Laake LW van. (2022). Toxicity and efficacy of chronomodulated chemotherapy: a systematic review. The Lancet. Oncology, 23(3), e129–e143. 10.1016/s1470-2045(21)00639-2 [DOI] [PubMed] [Google Scholar]
- Rijo-Ferreira F, Acosta-Rodriguez VA, Abel JH, Kornblum I, Bento I, Kilaru G, … Takahashi JS (2020). The malaria parasite has an intrinsic clock. Science, 368(6492), 746–753. 10.1126/science.aba2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg Pilz, Zerbini & Winnebeck (2019). Chronotype and Social Jetlag: A (Self-) Critical Review. Biology, 8(3), 54–19. 10.3390/biology8030054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T.(1992). Spatial and Temporal Environment. Universitas, 3, 202–210. [Google Scholar]
- Roenneberg T, Allebrandt KV, Merrow M, & Vetter C.(2012). Social Jetlag and Obesity. Current Biology, 22(10), 939–943. 10.1016/j.cub.2012.03.038 [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Daan S, & Merrow M.(2003). The art of entrainment. Journal of Biological Rhythms, 18(3), 183–194. 10.1177/0748730403018003001 [DOI] [PubMed] [Google Scholar]
- Roenneberg T, & Merrow M.(2016). The Circadian Clock and Human Health. Current Biology, 26(10), R432–R443. 10.1016/j.cub.2016.04.011 [DOI] [PubMed] [Google Scholar]
- Rutters F, Lemmens SG, Adam TC, Bremmer MA, Elders PJ, Nijpels G, & Dekker JM (2014). Is Social Jetlag Associated with an Adverse Endocrine, Behavioral, and Cardiovascular Risk Profile? Journal of Biological Rhythms, 29(5), 377–383. 10.1177/0748730414550199 [DOI] [PubMed] [Google Scholar]
- Saeed Y, Zee PC, & Abbott SM (2019). Clinical Neurophysiology: Diseases and Disorders. Handbook of Clinical Neurology, 161(Sleep Med Clin 6 2011), 369–380. 10.1016/b978-0-444-64142-7.00061-8 [DOI] [PubMed] [Google Scholar]
- Sahar S, & Sassone-Corsi P.(2009). Metabolism and cancer: the circadian clock connection. Nature Reviews Cancer, 9(12), 886–896. 10.1038/nrc2747 [DOI] [PubMed] [Google Scholar]
- Sato S, Bunney B, Mendoza-Viveros L, Bunney W, Borrelli E, Sassone-Corsi P, & Orozco-Solis R.(2021). Rapid-acting antidepressants and the circadian clock. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 47(4), 805–816. 10.1038/s41386-021-01241-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FAJL, Hilton MF, Evoniuk HL, Shiels SA, Malhotra A, Sugarbaker R, … Shea SA (2021). The endogenous circadian system worsens asthma at night independent of sleep and other daily behavioral or environmental cycles. Proceedings of the National Academy of Sciences of the United States of America, 118(37), e2018486118. 10.1073/pnas.2018486118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FAJL, Hilton MF, Mantzoros CS, & Shea SA (2009). Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences of the United States of America, 106(11), 4453–4458. 10.1073/pnas.0808180106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer FAJL, Michelson AD, Frelinger AL, Evoniuk H, Kelly EE, McCarthy M, … Shea SA (2011). The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PloS One, 6(9), e24549. 10.1371/journal.pone.0024549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C, Gibbs J, Ince L, & Loudon A.(2018). Clocking in to immunity. Nature Reviews Immunology, 18(7), 423–437. 10.1038/s41577-018-0008-4 [DOI] [PubMed] [Google Scholar]
- Scheuermaier K, Laffan AM, & Duffy JF (2010). Light Exposure Patterns in Healthy Older and Young Adults. Journal of Biological Rhythms, 25(2), 113–122. 10.1177/0748730410361916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SM, Hallschmid M, Jauch-Chara K, Born J, & Schultes B.(2008). A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. Journal of Sleep Research, 17(3), 331–334. 10.1111/j.1365-2869.2008.00662.x [DOI] [PubMed] [Google Scholar]
- Schuch JB, Genro JP, Bastos CR, Ghisleni G, & Tovo-Rodrigues L.(2017). The role of CLOCK gene in psychiatric disorders: Evidence from human and animal research. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 177(2), 181–198. 10.1002/ajmg.b.32599 [DOI] [PubMed] [Google Scholar]
- Sellix MT, & Menaker M.(2010). Circadian clocks in the ovary. Trends in Endocrinology and Metabolism: TEM, 21(10), 628–636. 10.1016/j.tem.2010.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Tang SY, Devine JC, Anderson ST, Nayak S, Zhang SL, … FitzGerald GA (2019). Circadian control of lung inflammation in influenza infection. Nature Communications, 10(1), 4107. 10.1038/s41467-019-11400-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafi AA, & Knudsen KE (2019). Cancer and the Circadian Clock. Cancer Research, 79(15), 3806–3814. 10.1158/0008-5472.can-19-0566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel K, Tasali E, Penev P, & Cauter EV (2004). Brief Communication: Sleep Curtailment in Healthy Young Men Is Associated with Decreased Leptin Levels, Elevated Ghrelin Levels, and Increased Hunger and Appetite. Annals of Internal Medicine, 141(11), 846. 10.7326/0003-4819-141-11-200412070-00008 [DOI] [PubMed] [Google Scholar]
- Stenvers DJ, Scheer FAJL, Schrauwen P, Fleur SE la, & Kalsbeek, A. (2019). Circadian clocks and insulin resistance. Nature Reviews Endocrinology, 15(2), 75–89. 10.1038/s41574-018-0122-1 [DOI] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, & Menaker M.(2001). Entrainment of the circadian clock in the liver by feeding. SCIENCE, 291(5503), 490–493. 10.1126/science.291.5503.490 [DOI] [PubMed] [Google Scholar]
- Stubblefield JJ, & Lechleiter JD (2019). Time to Target Stroke: Examining the Circadian System in Stroke. The Yale Journal of Biology and Medicine, 92(2), 349–357. [PMC free article] [PubMed] [Google Scholar]
- Study TMAC, Meisinger C, Heier M, & Loewel H.(2005). Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia, 48(2), 235–241. 10.1007/s00125-004-1634-x [DOI] [PubMed] [Google Scholar]
- Suárez-Barrientos A, López-Romero P, Vivas D, Castro-Ferreira F, Núñez-Gil I, Franco E, … Ibanez B.(2011). Circadian variations of infarct size in acute myocardial infarction. Heart (British Cardiac Society), 97(12), 970–976. 10.1136/hrt.2010.212621 [DOI] [PubMed] [Google Scholar]
- Thie NMR, Kato T, Bader G, Montplaisir JY, & Lavigne GJ (2002). The significance of saliva during sleep and the relevance of oromotor movements. Sleep Medicine Reviews, 6(3), 213–227. 10.1053/smrv.2001.0183 [DOI] [PubMed] [Google Scholar]
- Thosar SS, Butler MP, & Shea SA (2018). Role of the circadian system in cardiovascular disease. Journal of Clinical Investigation, 128(6), 2157–2167. 10.1172/jci80590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya Y, Umemura Y, & Yagita K.(2020). Circadian clock and cancer: From a viewpoint of cellular differentiation. International Journal of Urology, 27(6), 518–524. 10.1111/iju.14231 [DOI] [PubMed] [Google Scholar]
- Tubbs AS, Fernandez F-X, Grandner MA, Perlis ML, & Klerman EB (2022). The Mind After Midnight: Nocturnal Wakefulness, Behavioral Dysregulation, and Psychopathology. Frontiers in Network Physiology, 1. 10.3389/fnetp.2021.830338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, … Bass J.(2005). Obesity and Metabolic Syndrome in Circadian Clock Mutant Mice. Science, 308(5724), 1043–1045. 10.1126/science.1108750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn B, Rotolo S, & Roth H.(2014). Circadian rhythm and sleep influences on digestive physiology and disorders. ChronoPhysiology and Therapy, 67. 10.2147/cpt.s44806 [DOI] [Google Scholar]
- Vetter C, Devore EE, Wegrzyn LR, Massa J, Speizer FE, Kawachi I, … Schernhammer ES (2016). Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. JAMA: The Journal of the American Medical Association, 315(16), 1726. 10.1001/jama.2016.4454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinne V.van der Zerbini G, Siersema A, Pieper A, Merrow M, Hut RA, … Kantermann T.(2014). Timing of Examinations Affects School Performance Differently in Early and Late Chronotypes. Journal of Biological Rhythms. [DOI] [PubMed] [Google Scholar]
- Vyas MV, Garg AX, Iansavichus AV, Costella J, Donner A, Laugsand LE, … Hackam DG (2012). Shift work and vascular events: systematic review and meta-analysis. BMJ (Clinical Research Ed.), 345(jul26 1), e4800. 10.1136/bmj.e4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Balfe P, Eyre DW, Lumley SF, O’Donnell D, Warren F, … McKeating JA (2021). Time of Day of Vaccination Affects SARS-CoV-2 Antibody Responses in an Observational Study of Health Care Workers. Journal of Biological Rhythms, 37(1), 124–129. 10.1177/07487304211059315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefers J, Moorsel D.van Hansen J, Connell NJ, Havekes B, Hoeks J, … Schrauwen P.(2018). Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America, 115(30), 7789–7794. 10.1073/pnas.1722295115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman ED, Czeisler CA, Zimmerman JC, Moore-Ede MC, & Ronda JM (1980). Sleep duration, sleep stages and waking time are related to circadian phase in young and older men during nonentrained conditions. Transactions of the American Neurological Association, 105, 371–374. [PubMed] [Google Scholar]
- Winnebeck EC, Vuori-Brodowski MT, Biller AM, Molenda C, Fischer D, Zerbini G, & Roenneberg T.(2019). Later school start times in a flexible system improve teenage sleep. Sleep, 1–45. 10.1093/sleep/zsz307/5678526 [DOI] [PubMed] [Google Scholar]
- Wong PM, Hasler BP, Kamarck TW, Muldoon MF, & Manuck SB (2015). Social Jetlag, Chronotype, and Cardiometabolic Risk. The Journal of Clinical Endocrinology & Metabolism, 100(12), jc.2015–2923. 10.1210/jc.2015-2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KP, McHill AW, Birks BR, Griffin BR, Rusterholz T, & Chinoy ED (2013). Entrainment of the Human Circadian Clock to the Natural Light-Dark Cycle. Current Biology, 23(16), 1554–1558. 10.1016/j.cub.2013.06.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, … Nedergaard M.(2013). Sleep drives metabolite clearance from the adult brain. SCIENCE, 342(6156), 373–377. 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Jain MK, & Zhang L.(2021). Molecular link between circadian clocks and cardiac function: a network of core clock, slave clock, and effectors. Current Opinion in Pharmacology, 57, 28–40. 10.1016/j.coph.2020.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Nagai K, & Nakagawa H.(1987). Role of SCN in Daily Rhythms of Plasma Glucose, FFA, Insulin and Glucagon. Chronobiology International, 4(4), 483–491. 10.3109/07420528709078539 [DOI] [PubMed] [Google Scholar]
- Zee PC, & Abbott SM (2020). Circadian Rhythm Sleep-Wake Disorders. CONTINUUM: Lifelong Learning in Neurology, 26(4), 988–1002. 10.1212/con.0000000000000884 [DOI] [PubMed] [Google Scholar]
- Zerbini G, Vinne V.van der, Otto LKM., Kantermann T., Krijnen WP, Roenneberg T., & Merrow M. (2017). Lower school performance in late chronotypes: underlying factors and mechanisms. Scientific Reports, (7), 4385. 10.1038/s41598-017-04076-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Tsukuda S, Wrensch F, Wing PAC, Schilling M, Harris JM, … McKeating JA (2021). The circadian clock component BMAL1 regulates SARS-CoV-2 entry and replication in lung epithelial cells. IScience, 24(10), 103144. 10.1016/j.isci.2021.103144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunzunegui C, Gao B, Cam E, Hodor A, & Bassetti CL (2011). Sleep Disturbance Impairs Stroke Recovery in the Rat. Sleep, 34(9), 1261–1269. 10.5665/sleep.1252 [DOI] [PMC free article] [PubMed] [Google Scholar]