Abstract
The 16-kDa protein, an α-crystallin homologue, is one of the most abundant proteins in stationary-phase Mycobacterium tuberculosis. Here, transcription and translation of the hspX gene, which encodes the 16-kDa protein, have been investigated by Northern blotting analysis, primer extension, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis with a microaerophilic stationary-phase model. Two transcripts of about 2.5 and 1.1 kb were demonstrated by Northern blot analysis and hybridized to the hspX gene probe. Primer extension analysis revealed that the transcription start site is located 33 nucleotides upstream of the hspX gene start codon. The cellular level of the hspX mRNA was maximum in log-phase bacilli and was markedly reduced after 20 days in unagitated culture, when the organisms had entered the stationary phase. A third transcript of 0.5 kb was detected 0.6 kb downstream of the hspX gene; this transcript has a transcriptional pattern completely different from that of the 1.1- and 2.5-kb products, suggesting that there may be another gene in this region. In contrast to the high level of hspX mRNA in log-phase bacilli, 16-kDa protein synthesis was low in log-phase bacteria and rose to its maximum after 20 days. In both log-phase and stationary-phase bacteria the mRNA was unstable, with a half-life of 2 min, which indicated that the transcript stability was growth rate independent and not a general means for controlling the gene expression. However, the cellular content of 16-kDa protein, while low in log-phase bacteria, rose to a maximum at 10 days and remained at this high level for up to 50 days, which indicates that this protein is a stable molecule with a low turnover rate. These data suggest that the regulation of hspX expression during entry into and maintenance of stationary phase involves translation initiation efficiency and protein stability as potential mechanisms.
About one-third of the world’s population is infected with Mycobacterium tuberculosis (18). The infection usually occurs in childhood, and the bacteria remain in the body in a nonreplicating or slowly replicating dormant state for the rest of the life of the individual. Most infections pass unnoticed, but about 10% become active, causing tuberculosis, which kills 3 million people each year (18). Dormant M. tuberculosis is important not only because it can survive attack by the immune response but also because it is more resistant to antibacterial agents than actively growing bacteria, leading to the need for prolonged chemotherapy of active disease (10). An in vitro model of nonreplicating or slowly replicating M. tuberculosis has been developed by Wayne (37, 39). The bacilli grow in the top layer of an unagitated culture, where oxygen is available. They settle to the bottom of the container, where there is a low concentration of oxygen, and then slowly adapt to microaerophilic and eventually to anaerobic conditions. After about 20 days, the replication rate becomes lower, and by 30 to 40 days replication cannot be detected, at which point the organisms are in stationary phase (37, 38). The 16-kDa protein, an α-crystallin homologue (34) encoded by the hspX gene (8), is synthesized at a low level in logarithmic-phase cultures, but synthesis increases markedly during the transition from log phase to stationary phase (42). The protein becomes one of the most abundant proteins in stationary-phase bacteria. It has been proposed that the 16-kDa protein is important for the survival of stationary-phase bacteria (42).
The genetic regulation of 16-kDa protein expression has not been described previously. Gene expression in bacteria is usually regulated by the rate of transcription initiation, the stability of the RNA transcript, and the efficiency of translation. Growth rate changes also affect gene expression. In this paper, we describe the transcription and translation of the hspX gene in an extended stationary-phase model (15, 37) as determined by Northern analysis, primer extension, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of [35S]methionine-labeled whole-cell protein extracts. We show that there is an inverse relationship between the steady-state level of the hspX mRNA and synthesis of the 16-kDa protein.
MATERIALS AND METHODS
Bacteria and culture.
M. tuberculosis H37Rv was grown at 37°C in Middlebrook 7H9 medium containing 0.05% Tween 80 supplemented with 10% ADC (Difco Laboratories). Samples of a 10-day mid-log-phase culture were stored at −70°C. They were thawed and subcultivated once for 10 days before being inoculated 1:10 in fresh medium to form the experimental cultures. Microaerophilic growth was achieved by incubating 10-ml cultures in 28-ml screw-capped bottles without shaking for up to 60 days. CFU counts were performed as described previously (15).
DNA manipulations, sequencing, and analysis.
DNA isolation, ethanol precipitation of DNA, and electrophoresis of DNA in agarose were performed by standard procedures (32). Sequencing reactions were carried out on DNA with the T7 Sequenase version 2.0 sequencing kit (U.S. Biochemicals) in accordance with the manufacturer’s instructions, based on the dideoxy chain termination method (33) with α-35S-dATP (specific activity, >1,000 Ci mmol−1; Amersham) as the radioactive label. Computer-aided analysis of the DNA sequence was performed by using the Genetics Computer Group sequence analysis software package (University of Wisconsin Biotechnology Center, Madison).
PCR.
The probes for Northern hybridization were prepared by PCR. The primers used for generating the PCR products are listed in Table 1. The primers were designed to be in the coding regions of the transcripts. PCR was performed in a final volume of 50 μl which contained 5 to 10 ng of M. tuberculosis chromosomal DNA template and 1 μM each primer. Taq DNA polymerase (Promega) was used according to the manufacturer’s instructions. The PCR amplification was carried out for 30 cycles (94°C for 1 min, 58°C for 2 min, and 72°C for 3 min), followed by an extension of 72°C for 7 min. A 20-μl sample of each PCR mixture was subjected to electrophoresis on a 1.5% agarose gel containing ethidium bromide. A DNA ladder (Life Technologies) was used as the molecular size standard. The sequence of the hspX PCR product was determined with a DNA sequencer (ABI 373A) by using the Taq DyeDeoxy terminator chemistry and was identical to the published sequence of the hspX gene (34).
TABLE 1.
Probes used for Northern blot analysis in this studya
| Probe | Length (bp) and position | Oligonucleotides used to obtain PCR fragments | EMBL/GenBank accession no. |
|---|---|---|---|
| a | 242 (16996–17237) | 5′-GAAGACGAGATGAAAGAGGGG-3′ | AL021899 |
| 5′-GTAAGAATGCCCTTGTCGTAGG-3′ | |||
| b | 238 (16592–16828) | 5′-ATTACTCGCCGCCTATCG-3′ | AL021899 |
| 5′-AGTTGCTGCGGTGTGATCC-3′ | |||
| c | 289 (16127–16415) | 5′-AGATCGTGATTGCCGTGC-3′ | AL021899 |
| 5′-ACCAGCTCCGCCAACACC-3′ | |||
| d | 246 (15782–16028) | 5′-AGGGCTTTGGTGCGGTAGC-3′ | AL021899 |
| 5′-AGGCTGTAAAGATCCAGACCG-3′ | |||
| 16S | 336 (109–446) | 5′-GCCTGGGAAACTGGGTCTAA-3′ | mtu16srn |
| 5′-TCTCCACCTACCGTCAATCC-3′ |
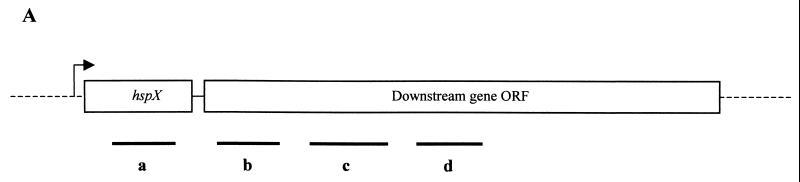
Probe a is hspX gene specific. Probes b, c, and d are representative of three parts of the hspX downstream gene. The positions of probes a, b, c, and d correspond to those in Fig. 1A. 16S indicates the probe specific to 16S rRNA gene.
RNA extraction.
Total RNA extraction from cultures was carried out by using the method of Mangan et al. (24). After isopropanol precipitation, RNA was treated with RNase-free DNase I (Life Technologies) and subjected to phenol extraction and ethanol precipitation. The RNA concentration was determined spectrophotometrically at 260 nm.
Northern (RNA) analysis.
Northern blot analysis was performed by fractionation of RNA samples on a 1.2% agarose-formaldehyde gel, followed by transfer in 20× SSC buffer (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) to a Hybond-N filter (Amersham) (32). To ensure that the same amount of total RNA (20 to 30 μg) was loaded into each well of the gel, a known amount of total RNA was loaded into each gel as an internal RNA standard, and the intensities of 16S and 23S rRNA staining with ethidium bromide was compared to those of the test samples. Sizes were determined with an RNA ladder (Sigma) as the molecular size standard. The probes (Table 1) were labeled with [α-32P]dCTP (specific activity, >3,000 Ci mmol−1; Amersham) by using the random-priming method according to the instructions of the manufacturer (Amersham). Generally, probes were generated with a specific activity of greater than 108 cpm μg−1 and were used in hybridization reactions at 1 × 105 to 5 × 105 cpm ml−1. After prehybridization for 3 h at 42°C in a buffer containing 5× Denhardt’s solution, 5× SSC, 0.2% SDS, 50% formamide, and 100 μg of salmon sperm DNA (Sigma) per ml, the filters were hybridized overnight at 42°C with 32P-labeled probes in the same buffer and washed at high stringency (2× SSC–0.1% SDS, 1× SSC–0.1% SDS, and 0.1× SSC–0.1% SDS at 65°C for 45 min each). Northern hybridization was standardized by using a 16S rRNA gene probe after the same filter was stripped.
The filters were exposed to X-ray films. The films, exposed for various time periods, were scanned with a high-resolution laser Personal Densitometer SI (Molecular Dynamics) linked to ImageQuaNT software (Molecular Dynamics).
Primer extension.
The synthetic oligonucleotides PE1 (5′-CGGGTGGCGCTGAACGGGAA-3′, complementary to nucleotides [nt] 11 to 30 downstream of the ATG start codon in the hspX gene) and PE2 (5′-TCAGAAAACTCGGGGAAGAGG-3′, nt 36 to 56 downstream of the ATG start codon in the hspX gene) were 5′ end labeled with [γ-32P]ATP (specific activity, >3,000 Ci mmol−1; Amersham) and Ready-To-Go T4 polynucleotide kinase (Pharmacia). Total RNAs (40 μg) from different growth phases of microaerophilic cultures were annealed to 5 ng of 5′-end-labeled primer in 5× reverse transcriptase buffer (Life Technologies) at primer melting temperatures for 20 min and then slowly cooled to room temperature for a further 30 min. Primer extension was performed for 1 h at 42°C in the same solution with 500 μM (each) dATP, dCTP, dGTP, and dTTP; 40 U of RNasin (Promega); 5 mM dithiothreitol; and 200 U of SuperScript II transcriptase (Life Technologies). The primer extension products were precipitated with ethanol and sodium acetate at −70°C, washed with 70% ethanol, and dried. The pellets were resuspended in an appropriate amount of formamide dye solution (U.S. Biochemicals) and then separated in a 6% polyacrylamide sequencing gel containing 8 M urea adjacent to a DNA sequence ladder which was generated by using a standard sequencing primer with the single-stranded M13 bacteriophage.
Chemical half-life determination.
Chemical half-lives of the RNA were determined by using log-phase (4-day) and stationary-phase (40-day) microaerophilic cultures. RNA was isolated from the cell samples taken at selected intervals after transcription initiation was inhibited by the addition of 100 μg of rifampin (Sigma) per ml, and the half-lives were determined by Northern blotting analysis. The incorporation of [3H]uridine (activity, 31 to 56 Ci mmol−1; Amersham) into trichloroacetic acid-precipitable RNA was rapidly reduced to 2% of that for the drug-free control (data not shown) after addition of 100 μg of rifampin ml−1, showing that transcription initiation was blocked with this concentration of rifampin.
[35S]methionine labeling of proteins, SDS-PAGE, Western blotting, and protein sequencing.
Microaerophilic cultures which had been incubated for 4 to 60 days were concentrated by centrifugation or by carefully removing part of the supernatant so as to adjust viable counts to 1.25 × 108 to 1.3 × 108 CFU ml−1, and 3 ml of each sample was then placed in a 28-ml sterile plastic universal container. Protein profiles were examined by [35S]methionine labeling of whole-cell proteins and SDS-PAGE by using a method described previously (15). The identification of the 16-kDa protein was performed by N-terminal sequencing and Western blotting. Defined protein antigens of M. tuberculosis were identified by immunoblot analysis of nitrocellulose filters transferred from one-dimensional SDS-PAGE (27). The monoclonal antibody TB68 (7) was used to identify the 16-kDa protein. The 16-kDa protein was separated from the other bacterial proteins by SDS-PAGE and blotted onto a polyvinylidine difluoride (Perkin-Elmer) membrane, the 16-kDa band was cut out, and the N-terminal amino acid sequence was determined by P. Jackson (Applied Biosystems, Perkin-Elmer) by means of Edman degradation on an Applied Biosystems Procise Sequencer.
The functional half-life of the 16-kDa protein was determined by pulse-labeling 5-ml log-phase and stationary-phase cultures with 10 μCi of [35S]methionine ml−1 for selected intervals after addition of rifampin to the culture. After chasing with 10 mM l-methionine, protein was extracted.
Nucleotide sequence accession number.
The nucleotide sequence data shown in Fig. 4 have been assigned EMBL/GenBank accession no. AL021899 (CD sequence 16929 to 17363).
FIG. 4.
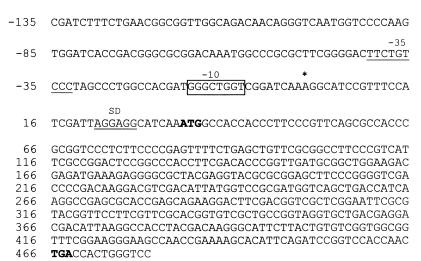
DNA sequence of the hspX gene regulatory region. The regions containing hspX and a 168-bp upstream sequence are shown. The transcription start site is indicated by an asterisk. The putative −10 promoter region is highlighted by a box. The underlined sequence is the −35 sequence. The putative ribosome binding site (SD) is marked with a double line. The start and stop codons are in boldface.
RESULTS
Steady-state level of the hspX mRNA.
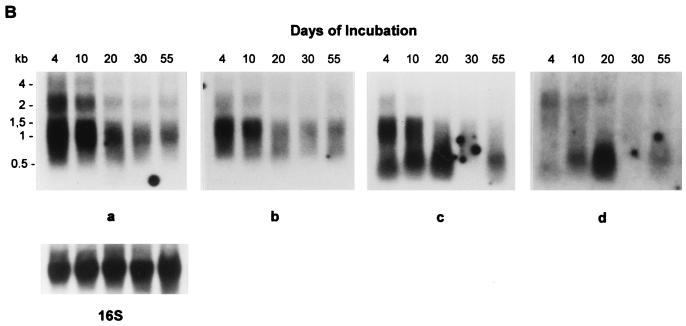
To determine the cellular concentrations of the hspX transcript during log-phase and stationary-phase growth, total RNA was extracted from cultures which were incubated for 4, 10, 20, 30, and 55 days and was analyzed by Northern blotting. When a 0.242-kb DNA fragment from the hspX gene coding region frame was used as a probe (probe a in Fig. 1A), two hybridizing bands were detected (Fig. 1B, panel a). The probe hybridized strongly to two mRNA species of approximately 1,100 and 2,500 nt. The predicted size of the hspX DNA sequence is only about 0.43 kb, considerably shorter than the transcripts detected with the hspX-specific probe. An examination of the DNA sequence of the hspX gene and the downstream genes (from EMBL/GenBank accession no. AL021899, CD sequence 14872 to 16917) shows that the open reading frame of the hspX gene is immediately followed by a gene of 2 kb with unknown function. The intergenic region is 11 nt. No stem-loop structure as a putative transcription terminator was found immediately downstream of the hspX gene. This raised the possibility that the 2.5-kb transcript might be the primary hspX mRNA, which is large enough to include the hspX mRNA and the transcript of the downstream gene. The 1.1-kb transcript which we found was not large enough to include the downstream gene and might be the result of endonucleolytic cleavage of the 2.5 kb transcript or of cotranscription with a part of the downstream gene.
FIG. 1.
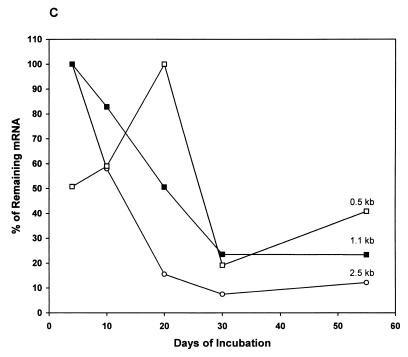
Northern blot analysis of the steady-state level of the hspX mRNA in M. tuberculosis. (A) Graphic representation of the locations of the probes used for Northern blot analysis within the hspX gene and the downstream genes (nt 14872 to 17363; EMBL/GenBank accession no. AL021899). The arrow shows the direction of the transcription. Probes a, b, c, and d are indicated as horizontal bars. ORF, open reading frame. (B) RNA was extracted from cells which had been grown for 4 to 55 days. RNA was then analyzed by formaldehyde-agarose gel electrophoresis and Northern blotting as described in Materials and Methods. The filter was hybridized with the hspX gene-specific probe a (panel a). The blot was stripped and reprobed with probes b, c, and d and a 16S rRNA gene-specific probe (panels b, c, d, and 16S, respectively). (C) Densitometric analysis of the autoradiographs showing the steady-state levels of the 2.5-, 1.1-, and 0.5-kb transcripts. The quantification was based on several exposures for different time periods. The signal obtained from each band was corrected with the corresponding signal from 16S rRNA. The corrected data for the bands were plotted against the days of incubation and were expressed as percentages of the maximum value.
In order to determine the pattern of the hspX gene transcription, the blot was stripped and reprobed with three probes which represent three parts of the downstream gene (probes b, c, and d in Fig. 1A). As seen in Fig. 1B, probes b, c, and d weakly hybridized to a 2.5-kb transcript, and probes b and c, but not probe d, strongly hybridized to a 1.1-kb transcript. In addition, probes c and d hybridized to a small transcript with an approximate length of 0.5 kb.
The cellular concentrations of the hspX transcripts were maximal in 4-day-old log-phase bacilli and then decreased as the cells reached stationary phase. The blot was stripped and reprobed to detect 16S rRNA. As shown in Fig. 1B (16S), the relatively equal intensities of the bands ensured that the same amount of the RNA was loaded into each well of the gel. Densitometric analysis (Fig. 1C) of the autoradiographs shown in Fig. 1B and other autoradiographs from three independent experiments revealed that there were approximately 6.5- ± 0.05- and 10- ± 0.1-fold decreases of the 1.1- and 2.5-kb hspX transcripts, respectively, from log-phase to 30-day-old stationary-phase bacilli. After 30 days, the relative levels of hspX transcripts remained constant for up to 55 days. The steady-state level of the 0.5-kb transcript was low in log-phase bacilli, increased approximately 2.5- ± 0.05-fold when the bacilli reached stationary phase at 20 days, and then decreased in late stationary phase. The level of the hspX mRNA was calculated by using linear regression analysis of the densitometric scan values of the mRNA divided by the values of the 16S rRNA.
Determination of the half-life of the mRNA.
The steady-state level of any single species of mRNA is determined by the rate of transcriptional initiation and the rate of decay. We therefore measured the mRNA half-life in bacilli after inhibition of transcription initiation with 100 μg of rifampin ml−1. Bacteria in both log-phase and stationary-phase growth were used to find out whether the stability of mRNA is growth rate dependent. Figure 2A shows representative Northern blots measuring the decay of the hspX mRNA in 4-day-old and 40-day-old bacilli. The blots were stripped and probed to detect 16S rRNA in order to verify equal loading of the total RNA in each experiment. The autoradiographs (Fig. 2A) and others from two independent experiments, after exposure of the blot to X-ray films for different lengths of time, were scanned.
FIG. 2.
Growth rate-independent decay of the hspX mRNA. (A) Northern blot analysis of decay of hspX mRNA in log-phase and stationary-phase bacilli. Total cellular RNA was extracted from the cells at 0, 3, 5, 10 and 20 min after addition of 100 μg of rifampin ml−1. Analysis of the mRNA decay was performed as described in Materials and Methods. Left panel, RNA was extracted from log-phase bacilli (4 days). Right panel, RNA was extracted from stationary-phase bacilli (40 days). The same blots were stripped and hybridized with the 16S rRNA-specific probe shown under each panel. (B) Densitometric analysis of the autoradiographs in panel A and of two others from independent experiments showing the decay rate of the mRNA. The quantification is based on several exposures of different time periods. The signals of the bands were plotted against the time of the RNA isolation and expressed as percentages of the initial value. The half-lives calculated from the blots were 2.2 ± 0.1 min for log-phase (open circles) and 2.3 ± 0.1 min for stationary-phase (filled circles) bacilli. The values shown are the averages from three independent experiments.
As shown in Fig. 2B, the rate of chemical decay of the mRNA showed no significant change between log-phase and stationary-phase bacilli. The average half-life of both was 2.25 ± 0.1 min, and so the chemical stability of the hspX mRNA appears to be independent of growth rate and it is the rate of transcription initiation which decreases as the rate of the growth falls when the bacteria enter the stationary phase.
The functional stability of the mRNA was measured by analysis of synthesis of the 16-kDa protein by using cell samples taken in parallel with those used for chemical stability measurements. In view of the characteristically low growth rate of M. tuberculosis, [35S]methionine labeling of the protein synthesis requires at least half an hour in log-phase cultures and 1 h in stationary-phase cultures in order to obtain detectable signals in fluorographs (data not shown). Samples were pulse-labeled with [35S]methionine for 1 h after the addition of rifampin. Total protein synthesis rapidly decreased, and no detectable synthesis of the 16-kDa protein was observed over the period of rifampin treatment (data not shown). This indicates that the short functional half-life of the mRNA was consistent with the short chemical half-life.
Primer extension analysis.
Total RNA was isolated from cell samples taken in parallel with those used for Northern analysis and was subjected to primer extension. The 5′ end of the mRNA was mapped with primer PE1, which corresponds to nt 11 to 30 downstream of the ATG start codon in the hspX gene. As shown in Fig. 3, only one primer extension product was found, with the transcription start site positioned at a nucleotide A, 33 nt upstream from the ATG start codon, suggesting that the transcription of the mRNA is driven by a single promoter upstream of the hspX open reading frame. This result was independently confirmed by using primer PE2 (see Materials and Methods).
FIG. 3.
Mapping of the 5′ end of the hspX transcript by primer extension analysis. The size of the primer extension product was determined by comparison with an unrelated but known DNA sequence ladder generated by the sequencing reactions shown in lanes G, A, T, and C. Lanes 1 to 5, RNA isolated from 4-, 10-, 20-, 30-, and 55-day-old culture, respectively. Lane 6, no-RNA negative control. The 5′ end of the transcript is indicated by an arrow, which corresponds to nucleotide A as shown by the asterisk in Fig. 4.
When sequences centered around −10 and −35 regions upstream from the transcription start point were examined for promoter-like sequences, no significant similarities with the consensus sequence of Mycobacterium promoters which has been published previously (3) were found. However, the sequence 8 nt upstream from the mRNA start site (GGGCTGGT) shows a clear homology to the −10 regions (CGGCAAGT) of the gearbox promoter (1, 2, 4, 35). The sequence has five of eight nucleotide matches to the proposed consensus sequence in the gearbox promoter. No similarities with binding sites of other sigma factors were identified. The transcription initiation site of the transcript together with the putative −10 and −35 regions as well as the putative Shine-Dalgarno (SD) sequence are shown in Fig. 4.
Primer extension analysis was also used to examine the temporal patterns of the hspX gene transcription (Fig. 3). Essentially the same pattern of decreasing mRNA level with respect to the growth rate was observed, which supports the Northern blotting data.
Synthesis of the 16-kDa protein during different growth phases.
We examined both the cellular level and the synthesis of the 16-kDa protein by SDS-PAGE and [35S]methionine labeling with the bacilli from log-phase to stationary-phase cultures. As shown by Coomassie blue staining after SDS-PAGE (Fig. 5A), the cellular level of the 16-kDa protein was very low in early log phase at 1 to 3 days and started to increase at 4 days. The protein level then increased continuously during the incubation, followed by a relatively constant cellular level throughout the stationary phase, and became a dominant band constituting 13% of the total proteins revealed by densitometric analysis. The synthesis of the 16-kDa protein (Fig. 5B) was at a low level over the period of log-phase growth and then increased during the transition to stationary phase, returned to a low level in the late stationary phase, and remained at a low level for up to 50 days. These data contrast with the cellular level of the protein, which was constant throughout stationary phase despite the reduction of the protein synthesis (Fig. 5A).
FIG. 5.
SDS-PAGE analysis of protein profiles of M. tuberculosis H37Rv during different growth phases. (A) Coomassie blue-stained SDS-PAGE of total protein profiles from log phase to stationary phase. Arrow, 16-kDa protein. (B) Fluorograph of [35S]methionine-labeled protein synthesis of the bacilli grown from 4 to 60 days. Each lane represents proteins extracted from 1.25 × 108 CFU of bacilli. Arrow, 16-kDa protein. (C) Western blot analysis of the 16-kDa protein. The results have been confirmed in two independent experiments.
To confirm the identity of the 16-kDa protein band which was observed in SDS-PAGE, Western blotting with the monoclonal antibody TB68 (anti-16-kDa protein) and N-terminal amino acid sequencing were performed. The sequence of the N-terminal 30 residues of the protein, ATTLPVQRHPRSLFPEFSELFQQFPSFAGL, was identical to the sequence of the 16-kDa protein which has been described previously (34). After electrophoresis of the total proteins extracted from a 4-day-old culture and a 40-day-old culture, the gel was blotted onto a nitrocellulose filter (Amersham). A 16-kDa band was observed when the blot was incubated with the monoclonal antibody TB68 (7), confirming the identity of the protein (Fig. 5C). The Western blotting analysis confirmed the result (Fig. 5A) that the 16-kDa protein was accumulated in stationary phase.
DISCUSSION
The molecular mechanisms which contribute to the control of the increased expression of the 16-kDa protein during entry into the stationary phase of growth are transcriptional and posttranscriptional. At the transcriptional level, Northern blotting analysis with the hspX gene-specific probe revealed two transcripts of 1.1 and 2.5 kb, both of which were larger than 0.43 kb predicted from the hspX DNA sequence. One possible explanation for these data is that the 1.1-kb transcript is an endonucleolytic cleavage product of the 2.5-kb transcript, in which case a second 1.4-kb product should be present when a downstream probe is used. However, further analysis (Fig. 1B, panel d) with a probe which spans nucleotides about 1.4 to 1.6 kb from the RNA transcriptional start site does not detect the expected 1.4-kb cleavage product (Fig. 1A). In addition, the 2.5- and 1.1-kb transcripts can be detected by probes b and c, which are close to the 5′ end of the downstream gene, and the 2.5-kb transcript can also be detected by probe d (Fig. 1A and B). This argues against the 1.1-kb product being due to cleavage of the 2.5-kb transcript. Furthermore, an examination of the DNA sequence of the downstream gene reveals that there are no potential sequences such as UAUUUG (29) which can act as RNase target sites in the downstream gene. In addition, probes c and d detect another small transcript of 0.5 kb (Fig. 1B, panels c and d) which has a transcription pattern completely different from that of the 1.1- and 2.5-kb products. The transcription of the 0.5-kb product increases to a maximum at 20 days and then decreases. Two possible explanations for the different transcription pattern of the 0.5 kb transcript are as follows: first, rather than a cleavage product of the 2.5-kb transcript, there is a separate gene whose transcription product is 0.5 kb and is located about 0.6 kb downstream to hspX gene; second, there may be another gene in this region on the opposite strand. Further work on this is under way in our laboratory. Overall, the data do not exclude the possibility of the 1.1-kb transcript being a cleavage product of the 2.5-kb transcript, but an alternative explanation is that the transcription of hspX underwent a readthrough process in the intergenic region between hspX and the downstream genes and ended at different transcriptional termination sites, which resulted in the two products.
Primer extension analysis indicates that the hspX gene is transcribed from a single promoter residing in a region approximately 33 nt upstream of the hspX gene ATG start codon. The start site corresponds to a position near the N terminus of the HspX protein (34) and its predicted SD site (34). Further analysis of the hspX gene promoters revealed a similarity between the −10 consensus sequence, CGGCAAGT, of the Escherichia coli gearbox promoter (1, 2, 4, 35) and the putative hspX gene −10 sequence (GGCTGGT) but no significant homology to the −35 gearbox promoter or to known M. tuberculosis consensus sequences (3). Activation of the gearbox promoter is associated with general stress responses and growth rate changes in E. coli (1, 2, 4, 35). Stationary-phase induction is abolished if the −10 region CGGCTAGT is changed into a sequence including a ς70 consensus sequence, CGTATAAT (2). However, our Northern blotting and primer extension results (Fig. 2 and 3) show a high level of hspX gene transcription in log-phase growth followed by a gradual decrease in transcription initiation rates as the bacilli enter the stationary phase. This suggests that the hspX promoter does not function like most gearbox promoters and is more similar to those promoters which, while containing the gearbox −10 sequence, do not upregulate transcription in response to stress and stationary-phase growth (2, 25). The pattern of the hspX transcription appeared to exhibit a stringent-like control which was similar to that of many genes, such as those for E. coli rRNA and tRNA, whose transcription decreases with decreasing growth rate (9, 13).
It has been reported (42) that synthesis of the 16-kDa protein, while at a low level in log-phase growth, increases when the cells reach stationary phase. Our results (Fig. 5) confirm and extend these data, showing that synthesis of the 16-kDa protein in the Wayne model rises from a low level in log-phase growth to maximum expression at days 20 and 30, followed by a fall back to a low level by 40 days, which is maintained for a further 20 days. This sharply contrasts with the cellular content of the 16-kDa protein, which reached a maximum at 10 days of incubation and then remained at a constant level for up to 50 days. This clearly suggests that the 16-kDa protein is a stable protein with a low turnover rate. It is likely that the constant cellular level of 16-kDa protein in late stationary phase results from the proposed stability of the protein and from the high level of synthesis in early stationary phase, followed by an accumulation of the protein from a low rate of synthesis in late stationary phase.
The increasing rate of synthesis of the 16-kDa protein during entry into stationary phase and the constant cellular level of the protein appeared to be an important strategy for M. tuberculosis to survive in stationary phase and dormancy. The 16-kDa protein is a member of the small heat shock protein (sHSP) family which is homologous to α-crystallin (5). One of the most important features of the proteins in the sHSP family is that they function as molecular chaperones (14, 17). Like other members of the family, the M. tuberculosis 16-kDa protein prevents thermally induced aggregation of other proteins (6, 42), perhaps by reducing undesirable protein-protein interactions and assisting in refolding of denatured proteins.
In our model, entry into stationary phase, defined as slowing of log-phase growth, begins at about 10 to 20 days and ends at 30 to 40 days, when the organisms enter the stationary phase. Thus, the low level of HspX protein synthesis during log phase followed by the high level seen at 20 to 30 days is discordant with the high level of hspX mRNA observed in log phase followed by low levels observed at 20 to 30 days. An increase in protein synthesis was accompanied by a decrease in mRNA accumulation during the transition to stationary-phase growth. When the bacilli were shifted from stationary phase back to exponential phase, a rapid increase in hspX mRNA was observed, without any increase in synthesis of the 16-kDa protein (data not shown). These data show that hspX gene expression during entry into the stationary growth phase is regulated by a posttranscriptional control mechanism. In other bacteria (11, 19, 21, 28), posttranscriptional regulation of gene expression is not uncommon and can involve a change in mRNA stability and a modulation of the efficiency of translation initiation. In E. coli (30) and Bacillus subtilis (31), changes in the stability of certain mRNAs affect the rate of the corresponding protein synthesis in response to growth rate changes. However, in our model, the hspX mRNA half-life was very short in both log phase and stationary phase, which indicates that mRNA stability change is not the mechanism of gene expression regulation in this case. We have no direct evidence for the specific mechanism of posttranscriptional control of hspX gene expression, but changes in the efficiency with which the mRNA is translated are one explanation. It has been reported that upregulation of E. coli cold shock protein A upon a temperature shift from 37 to 15°C is under posttranscriptional control, which is due to a modification of the protein synthesis machinery and an alteration in mRNA stability (12). In E. coli the formation of mRNA secondary and tertiary structures in the ribosome binding site hinders gene expression by shielding important elements such as the initiation codon and the SD nucleotides (16, 22, 23, 26, 41). Translation initiation depends on the unfolding of the initiation region. Presumably, an unknown mechanism operates under inducing conditions such as stationary phase or starvation to release the mRNA secondary structure, allowing translational initiation to take place and therefore enhancing the translational efficiency (19, 21, 26). A detailed analysis of the hspX mRNA secondary and tertiary structures is under way to resolve whether this is an important mechanism of gene control in M. tuberculosis.
In addition to control of translation initiation efficiency as a potential mechanism at work in this system, a mechanism involving regulation of protein stability is also possible. In E. coli, the cellular concentration of ςS is controlled by the regulation of protein stability (19). Also it has been suggested that α-crystallins and sHSPs, including the 16-kDa protein, share a C-terminal structural domain that is very stable (40). α-Crystallin is exceptionally thermostable (36), with an extremely long half-life in vivo. Some of the sHSPs are also very stable in vivo (20). Our data suggest that the 16-kDa protein is a stable molecule with a low turnover rate, and so this could be an important factor contributing to control of the cellular level of the 16-kDa protein during stationary phase. Our results suggest that the regulation of the hspX gene expression in response to stationary phase is unique and is controlled not only by translation initiation efficiency but also by protein stability.
ACKNOWLEDGMENTS
We thank the British Medical Research Council for funding to A.R.M.C.
We thank P. Butcher for helpful advice. We are grateful to P. Jackson (Applied Biosystems, Perkin-Elmer) for the peptide sequencing and to J. Mangan for technical advice.
REFERENCES
- 1.Aldea M, Garrido T, Hernández-Chico C, Vicente M, Kushner S R. Induction of a growth-phase-dependent promoter triggers transcription of bolA, an Escherichia coli morphogene. EMBO J. 1989;8:3923–3931. doi: 10.1002/j.1460-2075.1989.tb08573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldea M, Garrido T, Pla J, Vicente M. Division genes in Escherichia coli are expressed coordinately to cell septum requirements by gearbox promoters. EMBO J. 1990;9:3787–3794. doi: 10.1002/j.1460-2075.1990.tb07592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashyam M D, Kaushal D, Dasgupta S K, Tyagi A K. A study of the mycobacterial transcriptional apparatus: identification of novel features in promoter elements. J Bacteriol. 1996;178:4847–4853. doi: 10.1128/jb.178.16.4847-4853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohannon D E, Connell N, Keener J, Tormo A, Espinosa-Urgel A, Zambrano M M, Kolter R. Stationary-phase-inducible “gearbox” promoters: differential effects of katF mutations and role of ς70. J Bacteriol. 1991;173:4482–4492. doi: 10.1128/jb.173.14.4482-4492.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caspers G-J, Leunissen J A M, de Jong W W. The expanding small heat shock family, and structure predictions of the conserved “α-crystallin domain.”. J Mol Evol. 1995;40:238–248. doi: 10.1007/BF00163229. [DOI] [PubMed] [Google Scholar]
- 6.Chang Z, Primm T P, Jakana J, Lee I H, Serysheva I, Chiu W, Gilbart H F, Quiocho F A. Mycobacterium tuberculosis 16kDa antigen (Hsp16.3) functions as an oligomeric structure in vitro to suppress thermal aggregation. J Biol Chem. 1996;271:7218–7223. [PubMed] [Google Scholar]
- 7.Coates A R M, Hewitt J, Allen B W, Ivanyi J, Mitchison D A. Antigenic diversity of Mycobacterium tuberculosis and Mycobacterium bovis detected by means of monoclonal antibodies. Lancet. 1981;ii:167–169. doi: 10.1016/s0140-6736(81)90355-x. [DOI] [PubMed] [Google Scholar]
- 8.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry III C E, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Rajandream M-A, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston J E, Taylor K, Whitehead S, Barrell B G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 9.Dennis P P. Regulation of ribosomal and transfer RNA synthesis in Escherichia coli B/r. J Biol Chem. 1972;247:2842–2845. [PubMed] [Google Scholar]
- 10.Dickinson J M, Mitchison D A. Experimental models to explain the high sterilizing activity of rifampin in the chemotherapy of tuberculosis. Am Rev Respir Dis. 1981;123:367–371. doi: 10.1164/arrd.1981.123.4.367. [DOI] [PubMed] [Google Scholar]
- 11.Gold L. Posttranscriptional regulation mechanisms in Escherichia coli. Ann Rev Biochem. 1988;57:199–233. doi: 10.1146/annurev.bi.57.070188.001215. [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg D, Azar I, Oppenheim A B, Brandi A, Pon C L, Gualerzi C O. Role of Escherichia coli cspA promoter sequences and adaptation of translational apparatus in the cold shock response. Mol Gen Genet. 1997;256:282–290. doi: 10.1007/s004380050571. [DOI] [PubMed] [Google Scholar]
- 13.Gourse R L, de Boer H A, Normura M. DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, anti-termination. Cell. 1986;44:197–205. doi: 10.1016/0092-8674(86)90498-8. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz J. α-Crystallin can act as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu Y M, Butcher P D, Sole K, Mitchison D A, Coates A R M. Protein synthesis is shutdown in dormant Mycobacterium tuberculosis and is reversed by oxygen or heat shock. FEMS Microbiol Lett. 1998;158:139–145. doi: 10.1111/j.1574-6968.1998.tb12813.x. [DOI] [PubMed] [Google Scholar]
- 16.Iserentant D, Fiers W. Secondary structure of mRNA and efficiency of translation initiation. Gene. 1980;9:1–12. doi: 10.1016/0378-1119(80)90163-8. [DOI] [PubMed] [Google Scholar]
- 17.Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1992;268:1517–1520. [PubMed] [Google Scholar]
- 18.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 19.Lange R, Hengge-Aronis R. The cellular concentration of the ςS subunit of RNA-polymerase in Escherichia coli is controlled at the levels of transcription, translation and protein stability. Genes Dev. 1994;8:1600–1612. doi: 10.1101/gad.8.13.1600. [DOI] [PubMed] [Google Scholar]
- 20.Langry J, Chrètien P, Laszlo A, Lambert H. Phosphorylation of HSP27 during development and decay of thermotolerance in Chinese hamster cells. J Cell Physiol. 1991;147:93–101. doi: 10.1002/jcp.1041470113. [DOI] [PubMed] [Google Scholar]
- 21.Loewen P C, von Ossowski I, Switala J, Mulvey M R. KatF (ςS) synthesis in Escherichia coli is subject to posttranscriptional regulation. J Bacteriol. 1993;175:2150–2153. doi: 10.1128/jb.175.7.2150-2153.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Looman A C, Bodlaender J, de Gruyter M, Vogelaar A, van Knippenberg P H. Secondary structure as primary determination of the efficiency of ribosomal binding sites in Escherichia coli. Nucleic Acids Res. 1986;14:5481–5497. doi: 10.1093/nar/14.13.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maarten, de Smit H, van Duin J. Secondary structure of the ribosome binding site determines translational efficiency: a quantitative analysis. Proc Natl Acad Sci USA. 1990;87:7668–7672. doi: 10.1073/pnas.87.19.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangan J A, Sole K M, Mitchison D A, Butcher P D. An effective method of RNA extraction from bacteria refractory to disruption, including mycobacteria. Nucleic Acids Res. 1997;25:675–676. doi: 10.1093/nar/25.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matin A. The molecular basis of carbon-starvation-induced general resistance in Escherichia coli. Mol Microbiol. 1991;5:3–10. doi: 10.1111/j.1365-2958.1991.tb01819.x. [DOI] [PubMed] [Google Scholar]
- 26.McCann M P, Fraley C D, Matin A. The putative ς factor KatF is regulated posttranscriptionally during carbon starvation. J Bacteriol. 1993;175:2143–2149. doi: 10.1128/jb.175.7.2143-2149.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehlert A, Young D B. Biochemical and antigenic characterization of the Mycobacterium tuberculosis 71 kD antigen, a member of the 70 kD heat-shock protein family. Mol Microbiol. 1989;3:125–130. doi: 10.1111/j.1365-2958.1989.tb01801.x. [DOI] [PubMed] [Google Scholar]
- 28.Melin L, Rutberg L, von Gabain A. Transcriptional and posttranscriptional control of the Bacillus subtilis succinate dehydrogenase operon. J Bacteriol. 1989;171:2110–2115. doi: 10.1128/jb.171.4.2110-2115.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mudd E A, Prentki P, Belin D, Krisch H M. Processing of unstable bacteriophage T4 gene 32 mRNAs into a stable species requires Escherichia coli ribonuclease E. EMBO J. 1988;7:3601–3607. doi: 10.1002/j.1460-2075.1988.tb03238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsson G, Belasco J G, Cohen S N, Von Gabain A. Growth-rate dependent regulation of mRNA stability in Escherichia coli. Nature (London) 1984;312:75–77. doi: 10.1038/312075a0. [DOI] [PubMed] [Google Scholar]
- 31.Resnekov O, Butberg L, von Gabain A. Changes in the stability of specific mRNA species in response to growth stage in Bacillus subtilis. Proc Natl Acad Sci USA. 1990;87:8355–8359. doi: 10.1073/pnas.87.21.8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 33.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verbon A, Hartskeerl R A, Schuitema A, Kolk A H, Young D B, Lathigra R. The 14,000-molecular-weight antigen of Mycobacterium tuberculosis is related to the alpha crystallin family of low-molecular-weight heat shock proteins. J Bacteriol. 1992;174:1352–1359. doi: 10.1128/jb.174.4.1352-1359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vicente M, Kushner S R, Garrido T, Aldea M. The role of the ‘gearbox’ in the transcription of essential genes. Mol Microbiol. 1991;5:2085–2091. doi: 10.1111/j.1365-2958.1991.tb02137.x. [DOI] [PubMed] [Google Scholar]
- 36.Walsh M T, Sen A C, Chakrabarti B. Micellar subunit assembly in a three-layer model of oliomeric α-crystallin. J Biol Chem. 1991;266:20079–20084. [PubMed] [Google Scholar]
- 37.Wayne L G. Dynamics of submerged growth of Mycobacterium tuberculosis under aerobic and microaerophilic conditions. Am Rev Respir Dis. 1976;114:807–811. doi: 10.1164/arrd.1976.114.4.807. [DOI] [PubMed] [Google Scholar]
- 38.Wayne L G, Lin K Y. Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect Immun. 1982;37:1042–1049. doi: 10.1128/iai.37.3.1042-1049.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wayne L G. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur J Clin Microbiol Infect Dis. 1994;13:908–914. doi: 10.1007/BF02111491. [DOI] [PubMed] [Google Scholar]
- 40.Wistow G. Domain structure and evolution in α-crystallins and small heat-shock proteins. FEBS Lett. 1985;181:1–6. doi: 10.1016/0014-5793(85)81102-9. [DOI] [PubMed] [Google Scholar]
- 41.Wood C R, Boss M A, Patel T P, Emtage J S. The influence of messenger RNA secondary structure on expression of an immunoglobulin heavy chain in Escherichia coli. Nucleic Acids Res. 1984;12:3937–3950. doi: 10.1093/nar/12.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan Y, Crane D D, Barry C E., III Stationary phase-associated protein expression in Mycobacterium tuberculosis: function of the mycobacterial α-crystallin homolog. J Bacteriol. 1996;178:4484–4492. doi: 10.1128/jb.178.15.4484-4492.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]