Survey for prevalence of anti-SARS-CoV-2 antibodies in the blood donor population has been conducted in different countries and provided useful epidemiological information for monitoring the epidemic and estimating the level of herd immunity [1], [2], [3]. In the UK, a study was conducted in March 2021: approximately 3 months after the start of the vaccination campaign, the reported prevalence of around 42 % for anti-spike antibodies reflected both immunity resulting from natural infection and post-vaccination immunity [4]. Recently, a large-scale survey from US blood donations indicated that the combined infection and vaccination-induced seroprevalence of anti-spike SARS-CoV-2 IgG was 83.3 % in May 2021, 5 to 6 months after availability of vaccine to US public [5].
Here, we studied 1,876 samples from consecutive voluntary blood donors collected in the French region of Ile-de-France (departments 75 and 93) in 2021 (875 in January, just before the beginning of the national vaccination campaign and 1001 in early July). During the study period, the Alpha and the Delta strains were predominant in France. All samples were tested for anti-SARS-CoV-2 IgG antibodies by two serological assays: Anti SARS-CoV-2 IgG test, (Euroimmun, Lubeck, Germany) and Access SARS-CoV-2 IgG II (Beckman Coulter, Brea, CA 92821, USA) which target respectively the S1 subunit and the RBD domain of the SARS-CoV-2 spike protein. The anti-S1 assay was a qualitative test while the anti-RBD assay provided quantitative results expressed as IU/mL according to the WHO international standard (NIBSC code 20/136) [6]. Performance for serosurveillance and assays signal half lifes after index donation persistence have been reported for these assays [7].
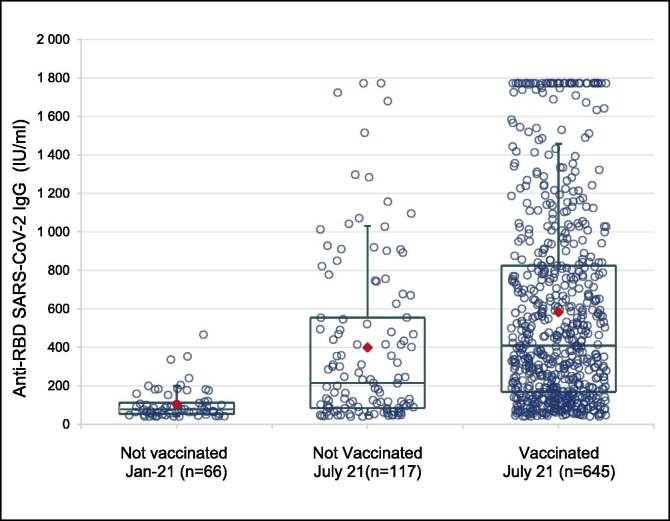
In January 2021, no blood donor reported a history of vaccination and SARS-CoV-2 seroprevalence was 8.57 % and 7.54 % for IgG antibodies to S1 and RBD proteins, respectively. In July 2021, 70.9 % of blood donors reported a full vaccine scheme and seroprevalence was 83 % (anti-S1) and 76.1 % (anti-RBD). Given that the vaccination rate in adults (>18 years old) at that time was close to 50 % [8]; this indicates that the blood donor population adhered to the vaccine recommendations more quickly than the general population. Among seropositive individuals, antibody titres were higher in those who had been vaccinated (Fig. 1 ): the mean titres of anti-RBD antibodies were significantly higher (p < 0.0001 using an ANOVA) in vaccinated donors tested in July (582.7 IU/ml [CI95%: 543.3–622.1]) compared seropositive donors who didn’t reported history of vaccination tested at the same time (398.8 IU/ml [CI95%: 320.4–477.2]), or to seropositive donors sampled in January before the vaccination campaign (103.8 IU/ml [CI95%: 84.2–123.4].
Fig. 1.
Distribution of quantitative results in seropositive individuals when tested using the Access SARS-CoV-2 IgG II, Beckman assay according to the vaccine status. Legend: red diamond = mean; Box = 1st quartile - median – 3rd quartile; Whiskers = 1st decile – 9th decile.
Interestingly, in unvaccinated seropositive donors, mean anti-RBD IgG titres were significantly higher (p < 0.0001 using an ANOVA) in July 2021 than in January 2021. Considering the incidence data of the disease in the French region of Ile de France [9], this difference may be explained in January 2021 by the decrease in antibody titres acquired during the first epidemic peak (seven months before), and in July 2021 by the recent epidemic waves (September to November 2021). Among vaccinated donors, anti-RBD antibody titres did not differ according to ABO blood group.
Main limitations of our study are (i) date of infection(s) was not recorded and chronology of infection(s) and vaccination could not be used for a better understanding of increase or decrease of IgG anti-SARS-Co-2 levels; (ii) the proportion of individuals dually vaccinated and infected cannot be formally estimated; (iii) the proportion of seroreversion resulting from the waning of antibody levels with time cannot be assessed both in those with COVID-19 and those who have only been vaccinated.
In conclusion, the SARS-CoV-2 seroprevalence (around 80 %) observed in July 2021 in French Ile-de France blood donors convincingly reflects the respective contributions of natural (around 10 % according to January data) and vaccine (around 70 % according to donors' self-reporting) immunity. Our data together with previous observations [10], suggest that anti-SARS-coV-2 antibody prevalence and titres represent a compromise between seroconversions (new infections and vaccinations) and waning of titres overtime that may ultimately lead to seroreversion. Follow-up in blood donor’s population of seroprevalence rates and quantitative levels of SARS-CoV-2 antibodies may provide useful information to health authorities concerning dynamics and level of immunity in general population.
Acknowledgments
Acknowledgements
We are indebted to blood donors who participated in the study; to director (S. Noel) and medical staff of the Ile de France regional blood service for their implication and support: and to C. Isnard for invaluable technical contribution.
Conflicts of Interest
PG, AS, LM and PM are employed by the French transfusion public service (Etablissement Français du Sang) in charge of blood products manufacturing and issuing in France. “The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results”.
Funding
This study was performed with the financial support of the Agence Nationale de la Recherche (ANR-20-COVI-0073-01, France) and of REACTing, a French multi-disciplinary collaborative network working on emerging infectious diseases.
References
- 1.Gallian P., Pastorino B., Morel P., Chiaroni J., Ninove L., de Lamballerie X. Lower prevalence of antibodies neutralizing SARS-CoV-2 in group O French blood donors. Antiviral Res. 2020;181 doi: 10.1016/j.antiviral.2020.104880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone M., Di Germanio C., Wright D.J., Sulaeman H., Dave H., et al. Use of U.S. Blood Donors for National Serosurveillance of SARS-CoV-2 Antibodies: Basis for an Expanded National Donor Serosurveillance Program. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slot E.d., Hogema B.M., Reusken C.B.E.M., Reimerink J.H., Molier M., Karregat J.H.M., et al. Low SARS-CoV-2 seroprevalence in blood donors in the early COVID-19 epidemic in the Netherlands. Nat Commun. 2020;11(1) doi: 10.1038/s41467-020-19481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitaker H.J., Elgohari S., Rowe C., Otter A.D., Brooks T., et al. Impact of COVID-19 vaccination program on seroprevalence in blood donors in England, 2021. J Infect. 2021;83:237–279. doi: 10.1016/j.jinf.2021.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones J.M., Stone M., Sulaeman H., Fink R.V., Dave H., Levy M.E., et al. Estimated US Infection- and Vaccine-Induced SARS-CoV-2 Seroprevalence Based on Blood Donations, July 2020-May 2021. JAMA. 2021;326(14):1400. doi: 10.1001/jama.2021.15161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kristiansen P.A., Page M., Bernasconi V., Mattiuzzo G., Dull P., Makar K., et al. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397(10282):1347–1348. doi: 10.1016/S0140-6736(21)00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone M., Grebe E., Sulaeman H., Di Germanio C., Dave H., Kelly K., et al. Evaluation of Commercially Available High-Throughput SARS-CoV-2 Serologic Assays for Serosurveillance and Related Applications. Emerg Infect Dis. 2022;28(3):672–683. doi: 10.3201/eid2803.211885. PMID: 35202525; PMCID: PMC8888213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Centre for Disease Prevention and Control. COVID-19 Vaccine Tracker. Avaiable at : https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html#uptake-tab (accessed 2022, July 20).
- 9.Information COVID 19. Avaiable at : https://www.gouvernement.fr/info-coronavirus/carte-et-donnees#vue_d_ensemble-nombre_moyen_de_nouvelles_hospitalisations_quotidiennes (accessed 2022, July 20).
- 10.Perreault J., Tremblay T., Fournier M.-J., Drouin M., Beaudoin-Bussières G., Prévost J., et al. Waning of SARS-CoV-2 RBD antibodies in longitudinal convalescent plasma samples within 4 months after symptom onset. Blood. 2020;136(22):2588–2591. doi: 10.1182/blood.2020008367. [DOI] [PMC free article] [PubMed] [Google Scholar]