Abstract
Pulmonary artery intimal sarcomas (PAIS) are often misdiagnosed as pulmonary embolisms (PE) as their clinical findings and imaging findings are similar. However, given the clinical outcome of both diseases is different in its prognosis, accurate and rapid diagnosis is mandatory. This is a case report of a histologically-proven PAIS which was initially treated as a PE. The color-coded iodine map using dual-energy computed tomography (dual-energy CT iodine map) well reflected the distribution of the tumor consistent with 18fluoro-2-deoxyglucose-uptake region using positron emission tomography/CT. This case demonstrates the potential of using dual-energy CT iodine map to differentiate PAIS from PE.
Learning objective
Use of a dual-energy computed tomography iodine map to visualize a pulmonary artery intimal sarcoma may provide useful diagnostic information.
Keywords: Pulmonary artery intimal sarcoma, Cardiac tumor, Computed tomography, Positron emission tomography
Introduction
Pulmonary artery intimal sarcomas (PAIS) are rare malignant tumors arising from the intima layer of pulmonary artery. These tumors are often misdiagnosed as a pulmonary embolism (PE) as their clinical findings and imaging findings are similar [1,2]. Therefore, studies have attempted to distinguish the two serious diseases using various imaging modalities [i.e. contrast-enhanced computed tomography (CT), magnetic resonance imaging, and positron emission tomography (PET)-CT] [3]. To date, however, the imaging diagnosis of PAIS is still challenging. We herein report visualization of PAIS by color-coded iodine map using dual-energy CT (dual-energy CT iodine map).
Case report
A 63-year-old woman was referred to our hospital with a 3-month history of cough and progressive dyspnea. At the previous hospital, she was initially administered heparin with suspicion of PE without improvement. Moreover, she had no evidence of deep vein thrombosis. Therefore, PAIS, not PE, was suspected.
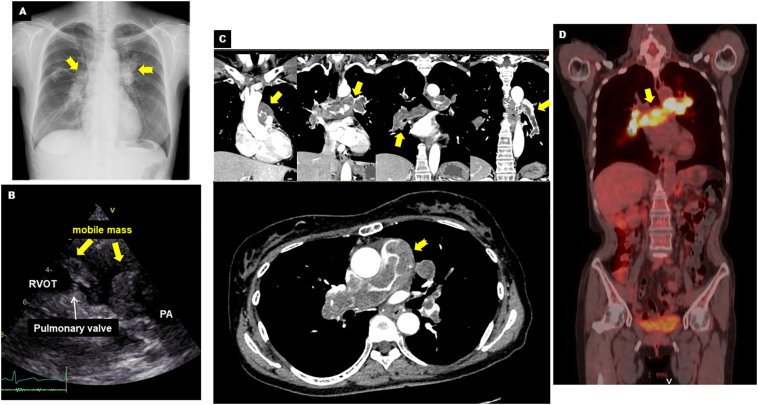
On admission, her blood pressure was 100/60 mmHg and heart rate was 90 bpm with sinus rhythm. D-dimer and brain natriuretic peptide (BNP) levels were slightly elevated (D-dimer = 4.3 μg/ml; reference <1.0 μg/ml and BNP = 62.5 pg/ml; reference ≤18.4 pg/ml). Chest X-ray showed dilated central pulmonary arteries, suggesting pulmonary hypertension (Fig. 1A, yellow arrows). A large mass that moved back and forth across the pulmonary valve was found by echocardiography (Fig. 1B, Online Video 1). A color Doppler ultrasound detected moderate tricuspid regurgitation with a pressure gradient of 88 mmHg, which confirmed the presence of pulmonary hypertension. Contrast-enhanced CT revealed filling defects occupying in the main pulmonary trunk extending to bilateral pulmonary arteries (Fig. 1C, yellow arrows). There was no sign of metastatic tumors originating from other organs. Suspecting a pulmonary artery tumor, 18F-fluorodeoxyglucose (FDG)-PET/CT was performed to rule out PE. Intense FDG-uptake was observed in the pulmonary arteries, particularly at the main pulmonary trunk. This finding strongly suggested pulmonary artery tumor (Fig. 1D, yellow arrow). There was a high maximum standardized uptake (max SUV) of 13.2. The red color-coded region of iodine map (Fig. 2B) using dual-energy CT (Fig. 2A) was a near match for the PET image of the FDG-uptake region (Fig. 2C). The detailed protocol for CT analysis and its validation in other cases are described in the Online materials.
Fig. 1.
Multi-modality imaging of pulmonary artery intimal sarcoma. (A) Chest X-ray showing dilated central pulmonary arteries (yellow arrows). (B) Echocardiography demonstrating mobile mass adjacent to the pulmonary valve between RVOT and PA. (C) Contrast-enhanced CT demonstrating the filling defects in main pulmonary trunk expanding to bilateral pulmonary arteries (yellow arrows). (D) FDG-PET/CT showing the intense FDG uptakes (yellow arrow).
CT, computed tomography; FDG, 18fluoro-2-deoxyglucose; PA, pulmonary artery; PET, positron emission tomography; RVOT, right ventricular outflow tract.
Fig. 2.
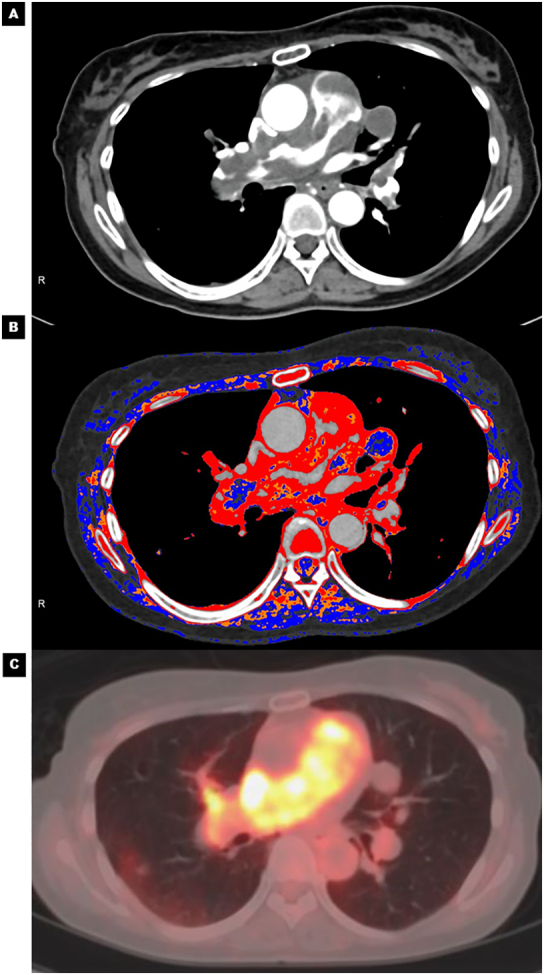
Visualization of pulmonary artery intimal sarcoma by color-coded iodine map: comparison between contrast-enhanced CT, iodine map, and PET/CT. (A) Contrast-enhanced CT showing the filling defects showing the disease distribution. (B) Dual-energy CT shows good correspondence between the red color-coded region of the iodine map and (C) the intense FDG-uptake region using PET/CT.
CT, computed tomography; FDG, 18fluoro-2-deoxyglucose; PET, positron emission tomography.
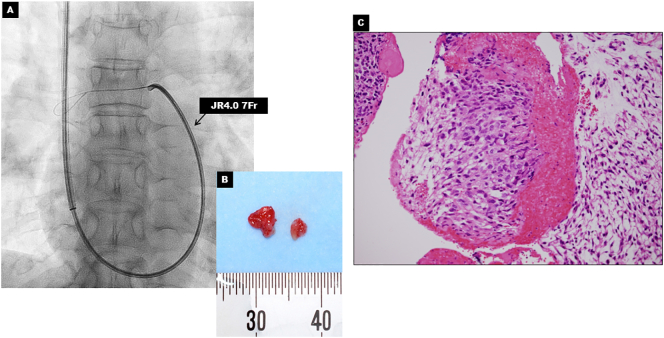
Pathological examination by biopsy was required to obtain definitive diagnosis. We decided to perform a safe and minimally invasive transcatheter aspiration biopsy [4]. Assisted by a pulmonary arteriograph, aspiration biopsy was performed using a 7Fr JR4.0 (Fig. 3A). Tissue samples were successfully obtained without complication (Fig. 3B) and PAIS was proved by pathological examination (Fig. 3C).
Fig. 3.
Transcatheter aspiration biopsy and pathological findings. (A) Fluoroscopy image showing transcatheter aspiration biopsy using the 7F JR4.0. (B) Gross appearance of tissue sample. (C) Biopsy specimen shows spindle cell sarcoma with prominent nuclear atypia that are consistent with pulmonary artery intimal sarcoma (hematoxylin-eosin staining x400).
The time from onset of symptoms to diagnosis was 93 days. Given her advanced disease, the patient was placed into hospice care where she died 6 months later.
Discussion
PAIS carries an extremely poor prognosis. Life expectancy is as short as 6 weeks without treatment [1]. Therefore, early and accurate diagnosis leading to proper treatment have great clinical impact. Currently, the median time from symptom onset to diagnosis is reported to be 100 days (interquartile range 30–210) [2]. Consistent with a previous report, the diagnosis was also delayed in the present case. Kim et al. reported that the most common misdiagnoses leading to the delay were PE followed by Takayasu arteritis [3].
Although clinical manifestations of PAIS and PE are similar (i.e. cough, chest pain, or hemoptysis), the symptoms of PE are often dramatic. In contrast to PE, the symptoms of PAIS are often gradual onset and worsen [2,5]. Thus, the clinical course can help the diagnosis of PAIS. However, pulmonary artery occlusion due to rapidly progressing PAIS may present with dramatic and acute symptoms similar to PE. Therefore, diagnosis based on clinical symptoms alone may lead to misdiagnosis and should be combined with blood sampling results and imaging. Laboratory results for D-dimer and BNP level may be helpful in obtaining a differential diagnosis [2,3]. D-dimer and BNP levels may be elevated in both diseases, but these values are typically lower in PAIS than PE, as was found in our case. It has been reported that a unilateral, central, lobulated filling defect on contrast-enhanced CT are more likely to be observed in PAIS rather than PE [6,7]. Moreover, Kim et al. previously reported that tumor extension patterns (i.e. tumoral impaction, diffuse/focal wall thickening, or cauliflower-like polypoid lesion) or heterogeneous attenuation may help diagnosis of PAIS [3]. However, these findings vary depending on the stage of the PAIS and may also be seen in PE. The visualization by color-coded iodine map could provide incremental benefit for differentiating a tumor from a thrombus. Likewise, imaging with PET-CT to evaluate FDG-uptake is also useful in differentiating PAIS from PE [8]. Ito et al. reported that the mean max SUV in PAIS was significantly higher than that in PE (7.6 vs. 2.3) [8]. Our case also had a high max SUV value. Disadvantages of PET-CT include high cost and low availability. Our case demonstrated that iodine map using dual-energy CT well reflected the FDG-uptake region. Similarly, Chang et al. previously reported dual-energy CT-based iodine quantification could be used to differentiate PAIS and PE [6]. Use of a dual-energy CT iodine map is more cost efficient compared with PET-CT and appears to be a good option for diagnosing PAIS; however, validation and feasibility studies are still needed.
The following are the supplementary data related to this article.
Echocardiography showing mobile mass adjacent to the pulmonary valve.
Protocol for CT analysis and its validation
Sources of funding
None.
Declaration of competing interest
The authors declare that there is no conflict of interest.
References
- 1.Krüger I., Borowski A., Horst M., de Vivie E.R., Theissen P., Gross-Fengels W. Symptoms, diagnosis, and therapy of primary sarcomas of the pulmonary artery. Thorac Cardiovasc Surg. 1990;38:91–95. doi: 10.1055/s-2007-1014001. [DOI] [PubMed] [Google Scholar]
- 2.Bandyopadhyay D., Panchabhai T.S., Bajaj N.S., Patil P.D., Bunte M.C. Primary pulmonary artery sarcoma: a close associate of pulmonary embolism-20-year observational analysis. J Thorac Dis. 2016;8:2592–2601. doi: 10.21037/jtd.2016.08.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim C., Kim M.Y., Kang J.W., Song J.S., Lee K.Y., Kim S.S. Pulmonary artery intimal sarcoma versus pulmonary artery thromboembolism: CT and clinical findings. Korean J Radiol. 2018;19:792–802. doi: 10.3348/kjr.2018.19.4.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujii Y., Koizumi J., Hara T., Sekiguchi T., Itou C., Asano K., et al. Endovascular catheter biopsy for the diagnosis of pulmonary artery sarcoma. Ann Vasc Dis. 2019;12:256–259. doi: 10.3400/avd.hdi.19-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Mehisen R., Al-Halees Z., Alnemri K., Al-Hemayed W., Al-Mohaissen M. Primary pulmonary artery sarcoma: a rare and overlooked differential diagnosis of pulmonary embolism. Clues to diagnosis. Int J Surg Case Rep. 2019;65:15–19. doi: 10.1016/j.ijscr.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang S., Hur J., Im D.J., Suh Y.J., Hong Y.J., Lee H.J., et al. Dual-energy CT-based iodine quantification for differentiating pulmonary artery sarcoma from pulmonary thromboembolism: a pilot study. Eur Radiol. 2016;26:3162–3170. doi: 10.1007/s00330-015-4140-2. [DOI] [PubMed] [Google Scholar]
- 7.Wittram C., Maher M.M., Yoo A.J., Kalra M.K., Shepard J.A., McLoud T.C. CT angiography of pulmonary embolism: diagnostic criteria and causes of misdiagnosis. Radiographics. 2004;24:1219–1238. doi: 10.1148/rg.245045008. [DOI] [PubMed] [Google Scholar]
- 8.Ito K., Kubota K., Morooka M., Shida Y., Hasuo K., Endo H., et al. Diagnostic usefulness of 18F-FDG PET/CT in the differentiation of pulmonary artery sarcoma and pulmonary embolism. Ann Nucl Med. 2009;23:671–676. doi: 10.1007/s12149-009-0292-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Echocardiography showing mobile mass adjacent to the pulmonary valve.
Protocol for CT analysis and its validation