Dear Editor,
Almost three years ago, we reported the results of a systematic review on the performance of existing definitions and tests for the diagnosis of invasive aspergillosis (IA) in critically ill, nonneutropenic, adult patients.1 The final qualitative synthesis was aimed at providing the expert panel of the FUNgal infections definitions in intensive care unit (ICU) patients (FUNDICU) initiative with the necessary baseline evidence to guide the discussions over the development of a standard set of definitions for invasive fungal diseases (IFD) in critically ill, adult patients outside classical, immunocompromised populations at risk.2 The main results stemming from our systematic review were as follows: (i) against histology/autopsy as reference, the diagnostic performance for invasive pulmonary aspergillosis (IPA) of the AspICU definition was promising, although with the limitations of small samples and applicability only in the presence of positive respiratory cultures; (ii) there was a consistently better diagnostic performance for IPA of bronchoalveolar lavage fluid (BALF) galactomannan (GM) than serum GM across studies; (iii) the specificity of BALF and serum (1,3)-β-d-glucan (BDG) for the diagnosis of IPA was suboptimal.1 After two years into the coronavirus disease 2019 (COVID-19) pandemic, which temporarily slowed down the FUNDICU project, our initiative is now ready to move forward to the next step of discussing and developing IFD definitions. Before doing that, an update of our previous systematic review was necessary to account for possible novel evidence that became available in the past three years. Using the same methodology (see supplementary methods for details), the literature search was expanded from 2018 up to 31 March 2022.
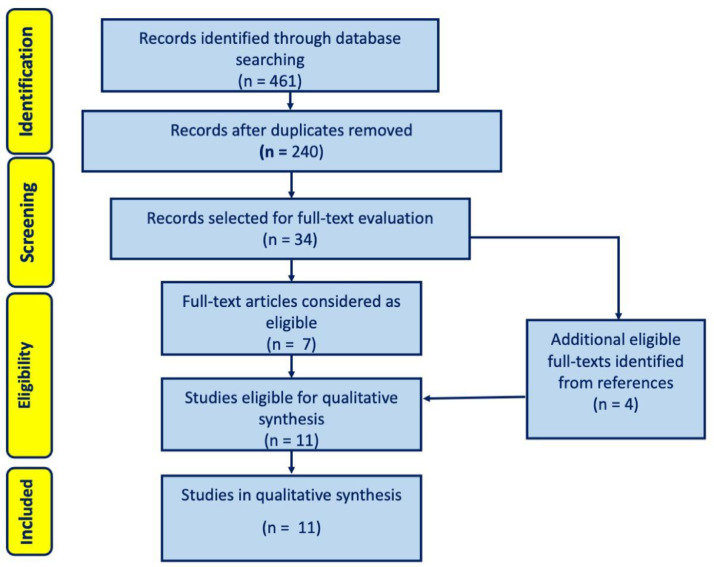
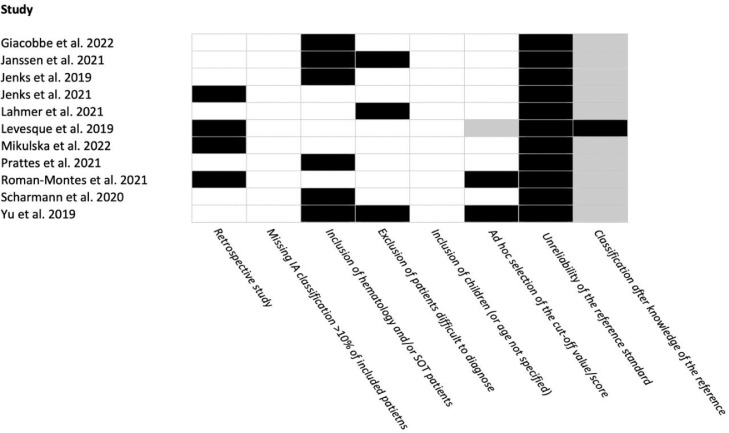
From an initial total of 461 records, we extracted 34 full texts and eventually selected 11 studies for inclusion in the present update (Fig. 1 ). As in the original search, included studies allowed to review the diagnostic performance of tests for the diagnosis of IPA and not of other forms of IA. No studies included in the present update evaluated the diagnostic performance of existing definitions against histology as reference. Overall, the diagnostic performance of serum GM was assessed in 5 studies, of which 4 overlapped with the 6 studies that assessed the diagnostic performance of BALF GM, whereas the diagnostic performance of tracheal aspirate (TA) GM was assessed in 1 study (supplementary Table 1). Regarding tests other than GM, the performance of BALF culture, serum BDG, BALF Aspergillus lateral-flow device (AspLFD), BALF GM-lateral flow assay (GM-LFA), TA GM-LFA, and BALF polymerase chain reaction (PCR) was assessed in 4, 2, 2, 3, 1, and 2 non-mutually exclusive studies, respectively (supplementary Table 2). Overall, GM-LFA was the most investigated test besides GM and culture, showing variable performance based on different cut-offs and reference definitions (supplementary Table 2). The diagnostic performance of combinations of laboratory tests and radiology was assessed in 4 and 1 studies, respectively (supplementary Tables 2 and 3). Finally, 7/11 (64%), 4/11 (36%), and 0/11 (0%) studies had a risk of bias of 〈 3, 3–4, and 〉 4 points according to the scoring system designed for the project (Fig. 2 ).
Fig. 1.
Flow diagram of the study selection process, Modified from Moher et al.3.
Fig. 2.
Risk of bias in included studies.
Three major considerations stem from the present literature search update: (i) the updated evidence is in line with the conclusions of the original study on the better performance of BALF GM than serum GM and the suboptimal specificity of serum BDG for the diagnosis of IPA; (ii) four studies assessing the diagnostic performance for IPA of GM-LFA met our inclusion criteria, providing a structured baseline evidence for guiding panel discussion on this test in the next phases of the FUNDICU project; (iii) six of the included studies (55%) assessed the diagnostic performance of laboratory markers for the diagnosis of COVID-19-associated pulmonary aspergillosis (CAPA), an entity which was obviously unknown three years ago. Regarding CAPA, incorporation bias (i.e., presence of the evaluated laboratory test in the reference mycological criteria for CAPA) was predominant in studies conducted in critically ill patients with COVID-19 and may have been relevant in biasing the diagnostic performance of mycological tests in the respiratory tract, with overestimation of their diagnostic accuracy for CAPA in some of the main analyses. Considering that in many studies different mycological tests were performed on the specimens from the respiratory tract (where their positivity may also reflect either colonization or growth without invasive disease), the mostly appreciable consequence due to incorporation bias was likely that of overestimating specificity (frequently very close to 100% for both GM and culture, see supplementary tables), although concomitant, more subtle biases on sensitivity cannot be excluded.
Since recent data suggest a possible unfavorable prognostic effect of positive mycological markers (serum GM and/or the combination of positive BALF GM and BALF culture) in critically ill patients with COVID-19,4 , 5 overdiagnosis of CAPA in colonized patients (or in patients with early localized disease unlikely to progress to manifest invasive disease) might be considered, at least in part, a somewhat acceptable compromise from a clinical perspective (pending more precise diagnostic algorithms), aiming not to delay or miss the treatment of true CAPA cases.6 However, for the goal of improving comparability and standardization of research findings, an overestimation of diagnostic accuracy remains an important limitation. The concept of improving diagnostic accuracy by combining classical mycological markers with PCR or other innovative tests is certainly promising, but the related evidence is still preliminary, as also testified by the heterogeneity of evaluated combinations across the few studies that met the inclusion criteria for the present review. In addition, the lack of included studies on the diagnostic performance of mycological tests against histology further precludes a firm assessment of their true accuracy for the diagnosis of CAPA. For all these reasons, the evidence resulting from the present review may ultimately not add to expert considerations and opinions that already allowed to develop shared definitions of CAPA during the pandemic.7 Consequently, the expert panel of the FUNDICU project will evaluate whether to develop a novel definition of CAPA or to support already existing definitions pending further evidence. For similar reasons, the possibility of supporting already existing definitions will also be considered for influenza-associated pulmonary aspergillosis (IAPA).8
In conclusion, the present update mostly confirms the statements from our original review and will serve as the necessary baseline evidence for the development of standard definitions of IPA in critically ill, nonneutropenic patients in ICU.
Funding
The present project did not require additional funding from routine research activities. Costs for open access publications will be covered by research funds of the main authors.
Ethical statement
No ethical approval was required as the research in this systematic review is based on previously published data.
Declaration of Competing Interest
Outside the submitted work, DRG reports investigator-initiated grants from Pfizer, Shionogi, and Gilead Italia, and speaker and/or advisory board fees from Pfizer and Tillotts Pharma. Outside the submitted work, MB reports research grants and/or advisor/consultant and/or speaker/chairman fees from Bayer, BioMérieux, Cidara, Cipla, Gilead, Menarini, MSD, Pfizer, and Shionogi. The other authors have no conflict of interests to disclose.
Footnotes
FUNDICU investigators (collaborators): M. Akova, A. Alastruey-Izquierdo, S. Arikan-Akdagli, E. Azoulay, S. Blot, A. Cortegiani, O. A. Cornely, C. Grecchi, C. Lass-Flörl, P. Koehler, M. Cuenca-Estrella, D.W. de Lange, F.G. De Rosa, J.J. De Waele, G. Dimopoulos, J. Garnacho-Montero, M. Hoenigl, S.S. Kanj, F. Lamoth, J. Maertens, I. Martin-Loeches, P. Muñoz, B.J. Kullberg, C. Agvald-Ohman, G. Poulakou, C. Rebuffi, J. Rello, M. Sanguinetti, F.S. Taccone, J-F. Timsit, A. Torres, J.A. Vazquez, J. Wauters, T. Calandra, S. Tejada, I. Karaiskos, M. Peghin, A. Vena, K.L. Mortensen, C. Lebihan, T. Mercier.
from the Study Group for Infections in Critically Ill Patients (ESGCIP) and the Fungal Infection Study Group (EFISG) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), the European Society of Intensive Care Medicine (ESICM), the European Confederation of Medical Mycology (ECMM), and the Mycoses Study Group Education and Research Consortium (MSGERC).
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2022.08.003.
Contributor Information
FUNDICU investigators (collaborators):
M. Akova, A. Alastruey-Izquierdo, S. Arikan-Akdagli, E. Azoulay, S. Blot, A. Cortegiani, O.A. Cornely, C. Grecchi, C. Lass-Flörl, P. Koehler, M. Cuenca-Estrella, D.W. de Lange, F.G. De Rosa, J.J. De Waele, G. Dimopoulos, J. Garnacho-Montero, M. Hoenigl, S.S. Kanj, F. Lamoth, J. Maertens, I. Martin-Loeches, P. Muñoz, B.J. Kullberg, C. Agvald-Ohman, G. Poulakou, C. Rebuffi, J. Rello, M. Sanguinetti, F.S. Taccone, J-F. Timsit, A. Torres, J.A. Vazquez, J. Wauters, T. Calandra, S. Tejada, I. Karaiskos, M. Peghin, A. Vena, K.L. Mortensen, C. Lebihan, and T. Mercier
Appendix. Supplementary materials
References
- 1.Bassetti M., Giacobbe D.R., Grecchi C., Rebuffi C., Zuccaro V., Scudeller L., et al. Performance of existing definitions and tests for the diagnosis of invasive aspergillosis in critically ill, adult patients: a systematic review with qualitative evidence synthesis. J Infect. 2020;81:131–146. doi: 10.1016/j.jinf.2020.03.065. [DOI] [PubMed] [Google Scholar]
- 2.Bassetti M., Scudeller L., Giacobbe D.R., Lamoth F., Righi E., Zuccaro V., et al. Developing definitions for invasive fungal diseases in critically ill adult patients in intensive care units. Protocol of the fungal infections definitions in ICU patients (FUNDICU) project. Mycoses. 2019;62:310–319. doi: 10.1111/myc.12869. [DOI] [PubMed] [Google Scholar]
- 3.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 4.Ergun M., Bruggemann R.J.M., Alanio A., Delliere S., van Arkel A., Bentvelsen R.G., et al. Aspergillus test profiles and mortality in critically ill COVID-19 patients. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.01229-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giacobbe D.R., Prattes J., Wauters J., Dettori S., Signori A., Salmanton-Garcia J., et al. Prognostic impact of bronchoalveolar lavage fluid galactomannan and aspergillus culture results on survival in COVID-19 intensive care unit patients: a post hoc analysis from the european confederation of medical mycology (ECMM) COVID-19-associated pulmonary aspergillosis study. J Clin Microbiol. 2022;60 doi: 10.1128/jcm.02298-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verweij P.E., Bruggemann R.J.M., Azoulay E., Bassetti M., Blot S., Buil J.B., et al. Taskforce report on the diagnosis and clinical management of COVID-19 associated pulmonary aspergillosis. Intensive Care Med. 2021;47:819–834. doi: 10.1007/s00134-021-06449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koehler P., Bassetti M., Chakrabarti A., Chen S.C.A., Colombo A.L., Hoenigl M., et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21:e149–e162. doi: 10.1016/S1473-3099(20)30847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verweij P.E., Rijnders B.J.A., Bruggemann R.J.M., Azoulay E., Bassetti M., Blot S., et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020;46:1524–1535. doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.