Abstract
A 58-year-old man suffering from systemic sclerosis was admitted to our hospital because of heart failure. He developed atrioventricular block 4 months previously and had a pacemaker implanted, after which left ventricular wall motion markedly worsened. The global longitudinal strain was already decreased before the onset of atrioventricular block, although the left ventricular ejection fraction was normal. Right ventricular pacing was suspected to have caused overt left ventricular systolic dysfunction. Therefore, right ventricular pacing was upgraded to cardiac resynchronization therapy. After this change, the left ventricular ejection fraction improved to almost normal, but global longitudinal strain remained decreased. The findings in our case suggest that some patients with systemic sclerosis already have subclinical left ventricular systolic dysfunction before the onset of atrioventricular block. Additionally, right ventricular pacing may cause further deterioration of left ventricular systolic function and heart failure.
Learning objective
The possibility of subclinical left ventricular systolic dysfunction associated with systemic sclerosis should be considered when implanting a pacemaker. Speckle-tracking echocardiography may also be useful in the management of patients with systemic sclerosis.
Keywords: Systemic sclerosis, Atrioventricular block, Heart failure, Left ventricular systolic dysfunction, Speckle-tracking echocardiography, Cardiac resynchronization therapy
Introduction
Cardiac involvement associated with systemic sclerosis (SSc) includes myocardial disease, conduction system abnormalities, arrhythmias, and pericardial disease [1], and it contributes to a poor prognosis [2]. Previous histological reports showed that some SSc cases had left ventricular (LV) involvement [3,4]. We experienced a patient with SSc who developed overt LV systolic dysfunction and heart failure after pacemaker implantation. We were able to follow the course of subclinical LV systolic dysfunction by speckle-tracking echocardiography.
Case report
A 58-year-old man was hospitalized for heart failure. He had generalized skin sclerosis and pulmonary fibrosis, and was diagnosed with SSc and SSc-related interstitial pneumonia 12 months before the admission.
Sixteen months before the admission, he had consulted a cardiologist because he had an abnormal electrocardiogram (poor R-wave progression). He underwent coronary angiography, which showed no stenotic lesion. Four months before the admission, he developed advanced atrioventricular block (AVB) (Fig. 1A). He had not taken any cardiovascular medications such as beta blockers that could cause conduction disturbance. He had a pacemaker implanted, and a right ventricular (RV) lead was placed in the right subventricular septum and a right atrial lead was placed in the right atrial appendage. The pacemaker was programmed with the dual chamber pacing mode at 60 beats/min (Fig. 1B). After pacemaker implantation, enalapril 2.5 mg/day and bisoprolol 0.625 mg/day were started. The dose was increased to 5 mg/day and 1.25 mg/day, respectively. He gradually had dyspnea on exertion after pacemaker implantation. Due to worsening heart failure, we decided not to increase the dose of bisoprolol.
Fig. 1.
(A) Electrocardiogram 4 months before admission showing advanced atrioventricular block with escape beats. (B) Electrocardiogram after pacemaker implantation. (C) Electrocardiogram after upgrading to cardiac resynchronization therapy.
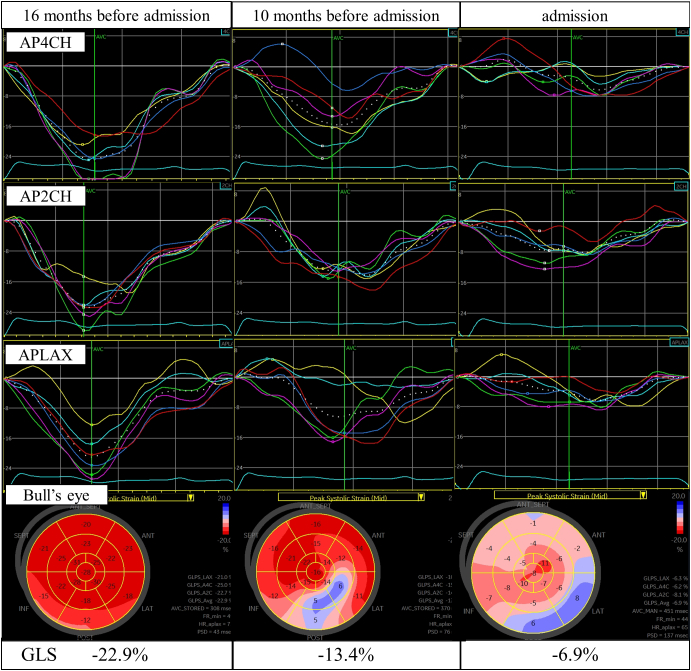
On admission, his blood pressure was 161/99 mm Hg, pulse rate was 86 beats/min, and oxygen saturation was 95% with a reservoir mask of 10 L/min oxygen. On a physical examination, the jugular vein was dilatated, fine crackles were audible in both inferior lung fields, and edema was observed on the face and legs. A laboratory examination showed that the brain natriuretic peptide concentration was 737.9 pg/mL. Chest radiography showed cardiac enlargement and increased pulmonary congestion. An electrocardiogram showed a pacemaker rhythm and multiple premature atrial contractions. Transthoracic echocardiography showed diffusely reduced LV wall motion with a left ventricular ejection fraction (LVEF) of 37.2% and global longitudinal strain (GLS) of −6.9%. Ten months before the admission, GLS was already decreased (−13.4%), although LV wall motion was normal and the LVEF was 61.0% (Fig. 2, Fig. 3). LV diastolic dysfunction was also observed throughout the period (Fig. 3).
Fig. 2.
Left ventricular longitudinal strain and Bull's eye mapping by two-dimensional speckle-tracking echocardiography at 16 months before admission, 10 months before admission, and on admission. The white dotted line shows GLS in each view.
AP4CH, apical four-chamber view; APLAX, apical long-axis view; AP2CH, apical two-chamber view; GLS, global longitudinal strain.
Fig. 3.
Clinical course of the patient. The solid and dotted lines show the trends in the LVEF and GLS, respectively. GLS declined before the decline in the LVEF 10 months before admission. In addition, the trend of each left ventricular diastolic function index is shown below the graph. Four months before admission, we could not measure them due to AVB.
A, late transmitral flow velocity; AVB, atrioventricular block; Ave, averaged; CAG, coronary angiography; CRT, cardiac resynchronization therapy; E, early transmitral flow velocity; e′, early diastolic mitral annular velocity; GLS, global longitudinal strain; LVEF, left ventricular ejection fraction; NA, not available; TRV, tricuspid regurgitant velocity.
Cardiac contrast-enhanced magnetic resonance imaging and 18F-fluorodeoxyglucose-positron emission tomography were performed to investigate cardiomyopathy, but no specific findings were found. Despite treatment for heart failure, dyspnea on exertion remained and the brain natriuretic peptide concentration was still high (507.6 pg/mL). Because RV pacing may have caused overt LV systolic dysfunction and heart failure, we upgraded to cardiac resynchronization therapy (CRT) (Fig. 1C). After upgrading to CRT, we titrated bisoprolol up to 2.5 mg/day and then to 5 mg/day. Shortly after upgrading to CRT, dyspnea markedly improved, and the brain natriuretic peptide concentration decreased to 94.9 pg/mL. One month later, the LVEF improved to 52.5%, while GLS remained decreased (−9.0%). We have continued to carefully follow-up the patient.
Discussion
The findings in this case showed that some patients with SSc may already have subclinical LV systolic dysfunction before the onset of AVB. Additionally, RV pacing may cause further deterioration of LV systolic function in such patients.
Previous histological studies showed myocardial fibrosis in up to 80% of patients with SSc [3,4]. Therefore, a certain number of these patients are estimated to have subclinical LV dysfunction. However, many patients with SSc do not show clinical signs, and their LV dysfunction is likely to be underdiagnosed [1]. In recent years, GLS has been the focus as a method of detecting subclinical LV systolic dysfunction. An example of this use of GLS is that, in the field of onco-cardiology, GLS has become widely used to detect chemotherapy-related cardiac dysfunction. Spethmann et al. conducted conventional echocardiography in 22 patients with SSc and age- and sex-matched healthy subjects, and also conducted speckle-tracking echocardiography to assess global and regional LV systolic function [5]. They found that GLS of the LV was significantly lower in the SSc group compared with controls. They also compared the echocardiographic results of 19 patients with SSc at baseline and 2 years later. They found that GLS was significantly decreased, but the LVEF was unchanged [6]. Based on these previous studies and our case, GLS appears to be a more sensitive marker than the LVEF in detecting the presence and progression of subclinical LV systolic dysfunction in patients with SSc.
A previous study reported that second- and third-degree AVB is uncommon and is estimated to occur in less than 2% of patients with SSC [7]. There have been a few case reports of patients with SSc who had AVB and underwent pacemaker implantation. In 2007, Ciurzyński et al. reported the clinical course of a 59-year-old man with SSc complicated by complete AVB and pacemaker implantation [8]. Six months after pacemaker implantation, the patient had increased dyspnea, an elevated N-terminal pro-B-type natriuretic peptide concentration, and progressive pulmonary hypertension. The clinical course of the patient was similar to that of our case, but LV function by echocardiography, including GLS, was not specified. In other reports, there have been no cases in which LV systolic dysfunction progressed after pacemaker implantation, as in our case.
Some cases with LV systolic dysfunction progress after pacemaker implantation in the right ventricle, which is known as pacing-induced cardiomyopathy. The abnormal electrical and mechanical activation of the ventricles due to RV pacing is thought to result in interventricular dyssynchrony and contribute to LV systolic dysfunction. According to a previous report, pacemaker-induced cardiomyopathy is more likely to occur when baseline GLS is decreased [9]. In our case, the LV systolic dysfunction had subclinically progressed before the onset of AVB, and the RV pacing may have caused further deterioration of LV systolic function. The LV wall motion improved globally one month after upgrading to CRT. Khurshid et al. reported that in patients with pacemaker-induced cardiomyopathy, 86% of patients showed an improvement in LVEF of 5% or more, with the majority of improvement occurring within 3 months after upgrading to CRT [10]. From the individual patient data listed in the supplemental data of that study, there was a case in which the LVEF improved from 35% to 50% in 42 days after upgrading to CRT, which is similar to the course of our case. Early improvement in LVEF after upgrading to CRT may not be common, but it is a possible process. On the other hand, GLS did not recover to normal and LV diastolic function remained decreased after upgrading to CRT. Therefore, the LV systolic and diastolic dysfunction associated with SSc appeared to be ongoing.
How to manage after early detection of subclinical systolic dysfunction in patients with SSc is not yet known. If a decrease in GLS is observed, we may have to shorten the follow-up period and start cardioprotective drugs such as renin-angiotensin inhibitors and beta blockers, although the usefulness of GLS as a predictor of mortality and cardiovascular events in patients with SSc has not yet been established.
In conclusion, the findings in our case suggest that some patients with SSc may already have subclinical LV systolic dysfunction before the onset of AVB, and RV pacing may cause further deterioration of LV systolic function in such patients. In patients with SSc, speckle-tracking echocardiography may be useful for detecting subclinical LV systolic dysfunction.
Declaration of competing interest
The authors declare that there is no conflict of interest.
Acknowledgments
Acknowledgments
None.
Funding
None.
References
- 1.Champion H.C. The heart in scleroderma. Rheum. Dis. Clin. N. Am. 2008;34:181–190. doi: 10.1016/j.rdc.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steen V.D., Medsger T.A., Jr. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43:2437–2444. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 3.D’Angelo W.A., Fries J.F., Masi A.T., Shulman L.E. Pathologic observations in systemic sclerosis (scleroderma). A study of fifty-eight autopsy cases and fifty-eight matched controls. Am. J. Med. 1969;46:428–440. doi: 10.1016/0002-9343(69)90044-8. [DOI] [PubMed] [Google Scholar]
- 4.Follansbee W.P., Miller T.R., Curtiss E.I., Orie J.E., Bernstein R.L., Kiernan J.M., et al. A controlled clinicopathologic study of myocardial fibrosis in systemic sclerosis (scleroderma) J. Rheumatol. 1990;17:656–662. [PubMed] [Google Scholar]
- 5.Spethmann S., Dreger H., Schattke S., Riemekasten G., Borges A.C., Baumann G., et al. Two-dimensional speckle tracking of the left ventricle in patients with systemic sclerosis for an early detection of myocardial involvement. Eur. Heart J. Cardiovasc. Imaging. 2012;13:863–870. doi: 10.1093/ehjci/jes047. [DOI] [PubMed] [Google Scholar]
- 6.Spethmann S., Rieper K., Riemekasten G., Borges A.C., Schattke S., Burmester G.R., et al. Echocardiographic follow-up of patients with systemic sclerosis by 2D speckle tracking echocardiography of the left ventricle. Cardiovasc. Ultrasound. 2014;12:13. doi: 10.1186/1476-7120-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janosik D.L., Osborn T.G., Moore T.L., Shah D.G., Kenney R.G., Zuckner J. Heart disease in systemic sclerosis. Semin. Arthritis Rheum. 1989;19:191–200. doi: 10.1016/0049-0172(89)90032-2. [DOI] [PubMed] [Google Scholar]
- 8.Ciurzyński M., Bienias P., Szewczyk A., Lichodziejewska B., Błaszczyk M., Liszewska-Pfejfer D., et al. Advanced systemic sclerosis complicated by pulmonary hypertension and complete atrioventricular block: a case report. Med. Sci. Monit. 2007;13:CS124–7. [PubMed] [Google Scholar]
- 9.Chin J.Y., Kang K.W., Park S.H., Choi Y.J., Jung K.T., Lee S., et al. Pre-implant global longitudinal strain as an early sign of pacing-induced cardiomyopathy in patients with complete atrioventricular block. Echocardiography. 2021;38:175–182. doi: 10.1111/echo.14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khurshid S., Obeng-Gyimah E., Supple G.E., Schaller R., Lin D., Owens A.T., et al. Reversal of pacing-induced cardiomyopathy following cardiac resynchronization therapy. JACC Clin Electrophysiol. 2018;4:168–177. doi: 10.1016/j.jacep.2017.10.002. [DOI] [PubMed] [Google Scholar]