Summary
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) binds to the angiotensin-converting enzyme 2 (ACE2) receptor, a critical component of the kallikrein-kinin system. Its dysregulation may lead to increased vascular permeability and release of inflammatory chemokines. Interactions between the kallikrein-kinin and the coagulation system might further contribute to thromboembolic complications in COVID-19.
Methods
In this observational study, we measured plasma and tissue kallikrein hydrolytic activity, levels of kinin peptides, and myeloperoxidase (MPO)-DNA complexes as a biomarker for neutrophil extracellular traps (NETs), in bronchoalveolar lavage (BAL) fluid from patients with and without COVID-19.
Findings
In BAL fluid from patients with severe COVID-19 (n = 21, of which 19 were mechanically ventilated), we observed higher tissue kallikrein activity (18·2 pM [1·2-1535·0], median [range], n = 9 vs 3·8 [0·0-22·0], n = 11; p = 0·030), higher levels of the kinin peptide bradykinin-(1-5) (89·6 [0·0-2425·0], n = 21 vs 0·0 [0·0-374·0], n = 19, p = 0·001), and higher levels of MPO-DNA complexes (699·0 ng/mL [66·0-142621·0], n = 21 vs 70·5 [9·9-960·0], n = 19, p < 0·001) compared to patients without COVID-19.
Interpretation
Our observations support the hypothesis that dysregulation of the kallikrein-kinin system might occur in mechanically ventilated patients with severe pulmonary disease, which might help to explain the clinical presentation of patients with severe COVID-19 developing pulmonary oedema and thromboembolic complications. Therefore, targeting the kallikrein-kinin system should be further explored as a potential treatment option for patients with severe COVID-19.
Funding
Research Foundation-Flanders (G0G4720N, 1843418N), KU Leuven COVID research fund.
Keywords: SARS-CoV-2, Kallikreins, Kinins, Extracellular traps, Thromboinflammation
Research in context.
Evidence before this study
Markers of inflammation and coagulation are predictors for clinical outcomes in patients with COVID-19. Binding of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to the angiotensin-converting enzyme 2 receptor, which is involved in kinin breakdown, could interfere with the kallikrein-kinin pathway. SARS-CoV-2 induced dysregulation of this pathway, which links inflammation and coagulation, could be a key trigger for the initial pathophysiological changes that cause severe lung disease. One randomized controlled trial and two small case-control studies indeed observed improvement of length of hospital stay and COVID-19 disease markers, respectively, after kallikrein-kinin pathway inhibition.
Added value of this study
Our data show increased kallikrein activity, higher levels of the most stable kinin peptide bradykinin-(1-5), and higher levels of neutrophil extracellular traps (NETs) in bronchoalveolar lavage fluid samples from patients with severe COVID-19 compared to those in patients without COVID-19. Furthermore, our in vitro data hint towards a FXII-dependent activation of plasma kallikrein by NET components, thus improving understanding of how NETs and the kallikrein-kinin system could be interconnected. These findings may help to explain the clinical presentation and contribute to understanding thromboinflammatory mechanisms in patients with severe pulmonary disease.
Implications of all the available evidence
Our data encourage the investigation of drugs that target the kallikrein-kinin system as a potential treatment option for patients with severe pulmonary disease, especially for those with strong thromboinflammatory responses as observed in COVID-19.
Alt-text: Unlabelled box
Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), a betacoronavirus first associated with outbreaks in Wuhan in December 2019, has caused unprecedented societal and scientific challenges worldwide.1 Thromboinflammation, the combined activation of both procoagulant and inflammatory pathways, provides a crucial clue toward understanding coronavirus disease 2019 (COVID-19) pathophysiology.2 SARS-CoV-2 induced dysregulation of the kallikrein-kinin system, which links inflammation and coagulation, may help to explain the clinical presentation of severe COVID-19 pneumonia potentially evolving in acute respiratory distress syndrome (ARDS).3,4 Moreover, markers of both inflammation and coagulation are linked to clinical outcome in COVID-19,5 thus emphasizing the importance of gaining mechanistic insights into the kallikrein-kinin pathway as a potential key trigger driving thromboinflammation in COVID-19.
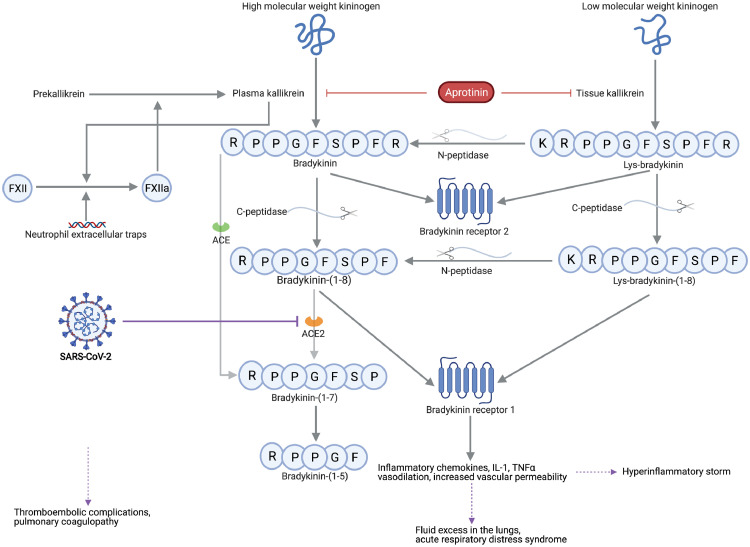
Binding of the viral spike (S) protein of SARS-CoV-2 to its receptor angiotensin-converting enzyme 2 (ACE2), located on alveolar epithelial and vascular endothelial cells, causes receptor internalization.6 Subsequent loss of ACE2 activity may impair degradation of inflammatory kinins that act on both bradykinin receptor 1 and bradykinin receptor 2 (metabolic pathway displayed in Figure 1).7 Bradykinin receptor stimulation results in kinin-driven vasodilation and downstream secretion of interleukin-1, tumour necrosis factor alpha, and inflammatory chemokines, thus mediating local and systemic inflammatory responses.3,7 Inflammatory conditions upregulate the bradykinin receptor 1, further resulting in increased vascular permeability, fluid excess in the lungs, pulmonary oedema, and hypoxic respiratory failure potentially requiring mechanical ventilation.3,8 The interplay between the kallikrein-kinin system and the contact activation pathway of coagulation via factor XII may not only explain the high incidence of thromboembolic complications in COVID-19, but also the pulmonary coagulopathy in severe COVID-19.9 Increased levels of neutrophil extracellular traps (NETs) have also been reported in COVID-1910,11 and constitute another link between inflammation and activation of coagulation (Figure 1).12 Moreover, activated neutrophils could exploit the kallikrein-kinin system to induce vascular endothelial leak during acute inflammation.13 Nonetheless, direct interactions between NETs and the kallikrein-system have not yet been investigated.
Figure 1.
The kallikrein-kinin system links coagulation and inflammation in COVID-19.
The metabolic pathway of bradykinin, Lys-bradykinin and their metabolites. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes downregulation and functional deficiency of angiotensin-converting enzyme 2 (ACE2), which could impair the degradation of kinin peptides that act on bradykinin receptor 1. Excessive activation of bradykinin receptor 1 results in both hyperinflammatory responses and pulmonary edema, whereas factor XII (FXII) activation leads to coagulation activation and provides a feedback loop with activation of the kallikrein-kinin pathway. Aprotinin inhibits plasma and tissue kallikrein, in addition to its in vitro observed antiviral actions. ACE indicates angiotensin-converting enzyme; C-peptidase, carboxypeptidase; IL-1, interleukin-1; N-peptidase, aminopeptidase; TNFα, tumor necrosis factor alpha. This figure was created with BioRender.
Identification of the kallikrein-kinin system as a key trigger driving thromboinflammation in severe COVID-19 would help to identify novel therapeutic strategies. One randomized controlled trial could detect an improvement in length of hospital stay and need of oxygen therapy in patients treated with inhibitors of the kallikrein-kinin system.14 Two other clinical reports also suggest a beneficial effect of inhibitors of the kallikrein-kinin system in severe COVID-19.15,16 Despite the effect of kallikrein-kinin inhibition on clinical outcome, little experimental evidence exists to investigate the underlying pathophysiological mechanisms linking the kallikrein-kinin system with pulmonary disease and activation of coagulation. Therefore, in this study, we measured plasma and tissue kallikrein activity, along with kinins and NETs in bronchoalveolar lavage (BAL) fluid samples from patients with or without COVID-19 pneumonia. In addition to the analyses of clinical samples, we also investigated the role of NET components (DNA and nucleosomes) and free histones in activating plasma kallikrein in vitro.
Methods
Patient cohort, sampling and data collection
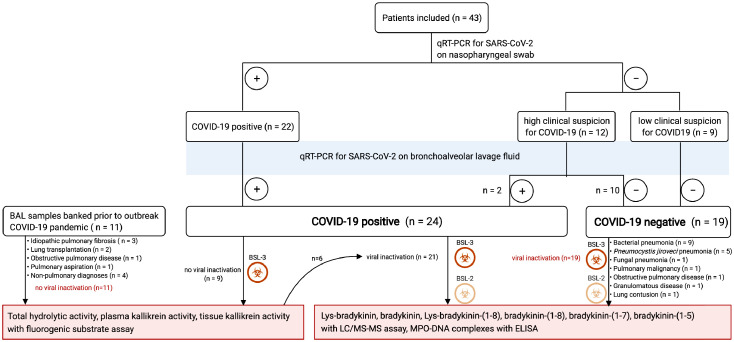
In this prospective single-centre study, adult patients with presumed COVID-19 (based on clinical, laboratory and radiological findings) were recruited at our tertiary care centre in Leuven (Belgium) between March 31st, 2020 and May 28th, 2020. Patients with (i) active haematological malignancy; (ii) active infectious/inflammatory conditions other than presumed COVID-19; (iii) calcineurin-inhibitor treatment, or (iv) patients or legal representatives unable or unwilling to give informed consent were excluded. Definitive diagnosis of COVID-19 was based on clinical symptoms, chest imaging and SARS-CoV-2 RNA-positive testing using quantitative real-time transcription polymerase chain reaction test (qRT-PCR) on a nasopharyngeal swab and/or BAL fluid sample. Non-COVID-19 pneumonia cases all tested negative for SARS-CoV-2 RNA using a qRT-PCR assay on BAL. Patients without COVID-19 comprised (1) patients suspected for COVID-19 with BAL resulting in an alternative diagnosis, (2) patients without COVID-19 who underwent BAL to rule out opportunistic co-infection and/or to remove mucus plugs and who subsequently tested negative for SARS-CoV-2 qRT-PCR on BAL fluid, or (3) patients with pulmonary disease from whom BAL fluid samples were banked prior to the outbreak of the pandemic (Figure 2).
Figure 2.
Characterization of patient groups.
Overview of characterization of patient groups with and without COVID-19 pneumonia to be compared in the different analyses. This figure was created with BioRender.
Bronchoscopy with BAL was performed as part of standard medical care, because of (1) established COVID-19 with clinical deterioration, (2) clinical suspicion of COVID-19 but negative SARS-CoV-2 qRT-PCR on nasopharyngeal swab, or (3) established non-COVID-19 respiratory disease with clinical deterioration (Figure 2). BAL was performed according to routine clinical procedures by instilling approximately 20 mL of sterile saline with a retrieval of approximately 10 mL. 2–3 mL of the retrieved volume was used for clinical purposes and the remaining fraction was used for the experimental analyses. For some patients two sequential volumes could be retrieved, of which the latter volume was used for research purposes.17 BAL fluid was immediately placed on ice, transported to a Biosafety Level 3 (BSL-3) facility (REGA institute, KU Leuven) and centrifuged. The supernatant was frozen at −80°C for batch analyses. Plasma and tissue kallikrein activity were measured in non-virally inactivated BAL fluid samples for a subset of patients because the viral inactivation procedure affects enzyme activity. Before release from the BSL3 laboratory for batch analyses of kinin levels and myeloperoxidase (MPO)-DNA complexes under BSL2 laboratory conditions, the virus in BAL fluid was inactivated by ultraviolet light treatment or by heating at 65°C for 30 min, respectively. Control samples were subjected to the same conditions. Demographic, clinical, laboratory, treatment and outcome data from patient electronic medical records were obtained through a standardized search by four independent researchers (C.P.M., P.V.M., M.M.E., A.O.). This study was conducted according to the principles expressed in the Declaration of Helsinki.
Ethics
Ethical approval was obtained from the Research Ethics Committee of UZ Leuven (S63881; NCT04327570). Informed consent was obtained from all individuals (in presence of witness by patient or by their legal guardians in case of incapacitated patients).
Kinins and their metabolites
Lys-bradykinin and bradykinin were measured in viral inactivated BAL fluid samples using an enzyme-linked immunosorbent assay (ELISA) according to manufacturer instructions (Bradykinin ELISA kit; Enzo Life Sciences, cat. ADI-900-206). In addition, we measured kinins (Lys-bradykinin, bradykinin) and their metabolites (Lys-bradykinin-(1-8), bradykinin-(1-8), bradykinin-(1-7), and bradykinin-(1-5)) using a previously published and more sensitive liquid chromatography with tandem mass spectrometry (LC-MS/MS) assay in collaboration with the University of Düsseldorf, Germany.18,19 For this analysis, 100 µL of BAL fluid was purified by a customized weak cation exchange solid-phase extraction. The resulting eluate was evaporated under a gentle stream of nitrogen at 60°C on a shaker at 350 rpm. The dissolved residue was then analysed using an Agilent 1200 LC-system (Agilent Technologies, Ratingen, Germany) coupled to an API 4000 mass spectrometer (AB Sciex, Darmstadt, Germany). The respective quantification limits for the assessed kinin peptides were: 4·4 pg/mL for Lys-bradykinin, 6·7 pg/mL for bradykinin, 10·6 pg/mL for Lys-bradykinin-(1-8), 7·3 pg/mL for bradykinin-(1-8), 6·5 pg/mL for bradykinin-(1-7), and 22·8 pg/mL for bradykinin-(1-5).
Plasma and tissue kallikrein
Plasma and tissue kallikrein activity were measured in BAL fluid samples without prior viral inactivation using the synthetic fluorogenic substrate H-Pro-Phe-Arg-AMC (Bachem, cat. I-1295.0050). BAL fluid samples were diluted (typically 3/10 or 1/10 (v/v)) in a reaction mixture composed of 20 mM Tris-HCl, 150 mM NaCl, 1 mM EDTA, 0.1 % (v/v) PEG-6000-8000, 0.1 % (v/v) Triton X-100 (pH 7.5) with or without aprotinin (Sigma-Aldrich, cat. A6279, 5 µM final), or THR-149 (100 nM final, provided by Oxurion N.V., Leuven, Belgium). The use of aprotinin, which inhibits both plasma and tissue kallikrein, and THR-149, a bicyclic peptide which selectively inhibits plasma kallikrein,20 allows for identification of the fraction of total hydrolytic activity attributed to either plasma or tissue kallikrein. Substrate hydrolysis was monitored by recording the increase in fluorescence at 480 nm with excitation at 360 nm in 96-well plate format using either a Spectramax M2e plate reader (Molecular Devices) or a Spark multimode microplate reader (Tecan) in such a way that no more than 10% of the substrate was hydrolysed. All hydrolytic data were normalized with a well containing a fixed concentration of human plasma kallikrein (Molecular Innovations, cat. HPKA-3900, typically 0.4 nM final) and expressed as human plasma kallikrein equivalent concentration.
MPO-DNA complexes
MPO-DNA complexes, a biomarker for NETs, were measured in heat-treated virus inactivated BAL fluid samples using an in-house ELISA modified from the Cell Death Detection ELISA (Roche).21,22 A 96-well Nunc immunoassay plate (MediSORP, ThermoFisher) was coated overnight with polyclonal anti-MPO antibody (1:1000 dilution, ThermoFisher PA5-16672) in 0.05 M sodium carbonate/sodium bicarbonate buffer (pH 9.6). After four washes with 300 μL PBS containing 0.05% Tween-20, wells were blocked with incubation buffer from the Cell Death Detection ELISA kit. Samples were diluted in incubation buffer (ranging from 1:20 to 1:2000) and incubated for 90 min. Following washing with four times 300 μL PBS containing 0,05% Tween-20, wells were incubated for 90 min with mouse anti-DNA monoclonal antibody conjugated with peroxidase from the Roche Cell Death Detection ELISA, washed, and detected with ready-to-use TMB substrate (Life Technologies, 2023). The reaction was stopped with 1 N hydrochloric acid and the plate read at 450 nm with 630 nm background subtraction using a Biotek Gen5 microplate reader. A standard curve was prepared by incubating a known quantity of an in-house-generated MPO-DNA mixture complex consisting of enzymatically inactive MPO standard (from the LEGEND MAX Human MPO ELISA kit, Biolegend), lambda-DNA (Invitrogen) and human native nucleosomes (Sigma). Reagent information and validation can be found in the supplemental data.
Assessment of plasma kallikrein activity induction by NET components
Plasma kallikrein activity was determined using a commercial colorimetric assay kit (BioVision incorporated, CA) according to the manufacturer's instructions. To investigate whether the NET components DNA, nucleosomes and/or free histones can activate plasma kallikrein in a FXII-dependent manner, we incubated 3 µL of normal human plasma pool and human plasma deficient in factors XII or IX, with either DNA (final concentration 7.14 µg/mL, Invitrogen), nucleosomes (final concentration 14.3 µg/mL, Merck Millipore), histone H3/H4 (14.3 µg/mL) or plasma kallikrein activator provided in the kit, in the presence of a synthetic pNA-based peptide substrate. Nucleosomes were co-incubated with DNase to investigate the effect on plasma kallikrein activation of components of nucleosomes other than DNA. An inhibitor control was included for each condition by adding plasma kallikrein specific inhibitor (PKSI, BioVision incorporated, CA). The absorbance of released pNA was measured at 405 nm in kinetic mode for 1 h at 37°C. The linear region of kinetic progress curves was selected and used to calculate a slope. Slopes obtained from activated samples were subtracted by slopes of their corresponding inhibitor controls. Plasma kallikrein activity was then calculated using a standard curve according to the manufacturer's instructions. Plasma kallikrein activity is expressed in mU/mL.
Quantification and statistical analysis
Descriptive statistics are presented as mean (standard deviation), median [range] and frequencies (n) with proportions (%) for normally distributed continuous, non-normally distributed continuous and categorical variables, respectively. Comparisons between patients with and without COVID-19 were performed by using Mann-Whitney U tests for continuous variables and chi-squared or Fisher exact tests for categorical variables. For comparison between dependent groups, Wilcoxon signed test for paired data and McNemar exact test are used for continuous and categorical variables, respectively. Correlation analyses were made using Spearman correlation coefficient. Linear mixed effects model was used to assess the relationship between individual kinin peptide levels. Multiple comparisons for levels of kallikrein activity, kinin peptides, and MPO-DNA complexes between patient groups were corrected for multiple testing using Benjamini-Hochberg correction for multiple testing.23 Statistical analyses for levels of kallikrein activity, kinin peptides and NETs, were performed with the two-sided alternative hypothesis at the 5% significance level using R (version 4.0.3, R Foundation for Statistical Computing, R Core Team, Vienna, Austria). For graphical representation, data have been transformed using the logarithmic transformation except as indicated otherwise. All data figures and statistical analyses for the assessment of plasma kallikrein activity induction by NET components and free histones, were made with GraphPad Prism version 9.0.0
Role of the funding source
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Results
Patients
We included 24 hospitalized patients with COVID-19 and 19 hospitalized patients without COVID-19 (Figure 2) in April 2020. Demographic and clinical data including cardiovascular co-morbidities (body-mass index, arterial hypertension, diabetes mellitus, chronic use of statins, ACE-inhibitors or angiotensin receptor blockers, and smoking behaviour) were collected for COVID-19 patients of whom both kallikrein activity, kinin peptides and MPO-DNA levels were measured, and for hospitalized control patients without COVID-19 (Table 1). Fifteen out of 21 (71%) patients with COVID-19 were male, mean age was 64·9 years (standard deviation 9·6 years). Patients with COVID-19 had a higher rate of intensive care unit admission, a higher need for mechanical ventilation and more often received heparin treatment as compared to hospitalized patients without COVID-19 (Table 1). There was no difference in in-hospital mortality rate between both patient groups. Bronchoscopy with BAL fluid collection was performed later in the disease course for patients with COVID-19 (21·7 days after hospital admission on average) than for those without COVID-19 (11·5 days after hospital admission on average). Demographic and clinical data of control patients who provided biobanked material (n = 11) for measurement of total hydrolytic activity, tissue and plasma kallikrein levels are provided in Supplementary Table 1.
Table 1.
Demographics and characteristics of enrolled patients (kinin peptides, MPO-DNA analyses).
| Control (n = 19) | COVID-19 (n = 21) | |
|---|---|---|
| Age – mean (SD), year | 69·2 (7·6) | 64·9 (9·6) |
| Male sex – no.(%) | 14 (73·7%) | 15 (71·4%) |
| Caucasians – no.(%) | 19 (100·0%) | 20 (95·2%) |
| Body-mass Indexa, mean (SD), kg/m2 | 25·3 (3·8) | 27·2 (3·6) |
| Arterial hypertension – no.(%) | 11 (57·9%) | 16 (76·2%) |
| Diabetes mellitus – no.(%) | 8 (42·1%) | 10 (47·6%) |
| Chronic use of | ||
| Statins – no.(%) | 5 (26·3%) | 14 (66·7%) |
| Angiotensin-converting enzyme inhibitors – no.(%) | 5 (26·3%) | 10 (47·6%) |
| Angiotensin receptor blockers – no.(%) | 3 (15·8%) | 2 (9·5%) |
| Active smoking – no(%) | 3 (15·8%) | 2 (9·5%) |
| Onset symptoms till BAL, mean (SD), days | 11·5 (10·3) | 21·7 (8·4) |
| Intensive care unit admission – no.(%) | 6 (31·6%) | 19 (90·5%) |
| Ventilatory support at time of sampling | ||
| No respiratory support – no.(%) | 6 (31·6%) | 0 (0·0%) |
| Non-invasive ventilation no.(%) | 11 (57·9%) | 2 (9·5%) |
| Mechanical ventilation – no.(%) | 2 (10·5%) | 17 (81·0%) |
| Extracorporeal membrane oxygenation – no.(%) | 0 (0·0%) | 2 (9·5%) |
| Biochemical signs of hyperinflammationb - no.(%) | 3 (15·8%) | 10 (47·6%) |
| Death in hospital – no.(%) | 2 (10·5%) | 2 (9·5%) |
Data are means +/- standard deviation or n (%). Characteristics that differ between patients with and without COVID-19 are shown in bold. Percentages may not add to 100 because of rounding.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
’Biochemical signs of hyperinflammation’ is defined as: (a) absolute lymphocyte count < 1000 cells/mL and (b) two of the following: i. ferritin > 800 ng/mL ii. LDH > 400 U/L iii. D-Dimers > 1000 ng/mL iiii. CRP > 100 mg/L.
Kinins
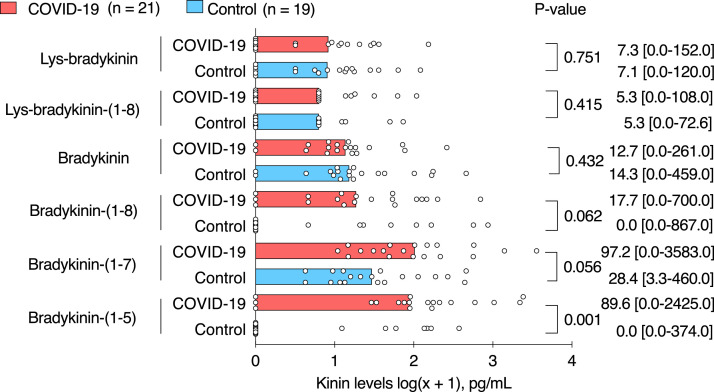
Levels of kinin peptides (bradykinin and Lys-bradykinin) and their metabolites (Lys-bradykinin-(1-8), bradykinin-(1-8), bradykinin-(1-7), and bradykinin-(1-5)) measured in BAL fluid by LC-MS/MS are summarized in Table 2. Levels of Lys-bradykinin and bradykinin measured with an ELISA fell below the lower detection limit (data not shown). Levels of Lys-bradykinin, bradykinin, and Lys-bradykinin-(1-8) were generally low with no detected difference in BAL fluid samples from patients with COVID-19 compared to those from patients without COVID-19. In BAL fluid samples from patients with COVID-19 vs in those from patients without COVID-19, the levels of bradykinin-(1-8) were 17·7 pg/mL [0·0-700·0] (median [range]) vs 0·0 pg/mL [0·0-867·0], p = 0·062 (Mann-Whitney U test); the levels of bradykinin-(1-7) were 97·2 pg/mL [0·0-3583·0] vs 28·4 pg/mL [3·3 - 460·0], p = 0·056 (Mann-Whitney U test); and the levels of bradykinin-(1-5) were 89·6 pg/mL [0·0 - 2425·0] vs 0·0 pg/mL [0·0 - 374·0], p = 0·001 (Mann-Whitney U test), p = 0·005 after correcting for multiple testing (Benjamini-Hochberg method) (Figure 3). Per-patient individual profile of lys-bradykinin, bradykinin, bradykinin-(1-8) and bradykinin-(1-5) levels, showed an overall increase in the levels of downstream metabolites per patient in bronchoalveolar lavage fluid from patients with COVID-19 (p = 0·0211, linear mixed effects model) (Supplementary Figure 1).
Table 2.
Kallikrein activity and kinin peptides measured in bronchoalveolar lavage fluid.
| Control | COVID-19 | p-value | |
|---|---|---|---|
| Lys-bradykinin (pg/mL) | 7·1 [0·0-120·0] | 7·3 [0·0-152·0] | 0·751 |
| Lys-bradykinin-(1-8) (pg/mL) | 5·3 [0·0-72·6] | 5·3 [0·0-108·0] | 0·415 |
| Bradykinin (pg/mL) | 14·3 [0·0-459·0] | 12·7 [0·0-261·0] | 0·432 |
| Bradykinin-(1-8) (pg/mL) | 0·0 [0·0-867·0] | 17·7 [0·0-700·0] | 0·062 |
| Bradykinin-(1-7) (pg/mL) | 28·4 [3·3-460·0] | 97·2 [0·0-3583·0] | 0·056 |
| Bradykinin-(1-5) (pg/mL) | 0·0 [0·0-374·0] | 89·6 [0·0-2425·0] | 0·001 |
| Total hydrolytic activity (eq. PKal, pM) | 14·4 [2·4-52·0] | 41·9 [7·8-1574·0] | 0·037 |
| Plasma kallikrein activity (pM) | 0·3 [0·0-2·4] | 2·1 [0·0-41·7] | 0·173 |
| Tissue kallikrein activity (pM) | 3·8 [0·0-22·0] | 18·2 [1·2-1535·0] | 0·030 |
Results are reported as medians with [min – max range]. Kinin peptides measurements are available for 21 patients with COVID-19 and 19 control patients (characteristics shown in Table 1). Kallikrein activity measurements are available for 9 patients with COVID-19 and 11 control patients.
Figure 3.
Levels of kinin peptides in bronchoalveolar lavage fluid from patients with and without COVID-19.
Graphs show individual dots representing patient data and bar representing median of each patient group. For graphical representation, data were transformed using the logarithmic transformation. Pairwise comparisons were made between patients with COVID-19 (n = 21) and patients without COVID-19 (n = 19). Median [range] and p-value (Mann-Whitney U test, not corrected for multiple testing) are shown.
Kallikrein activity
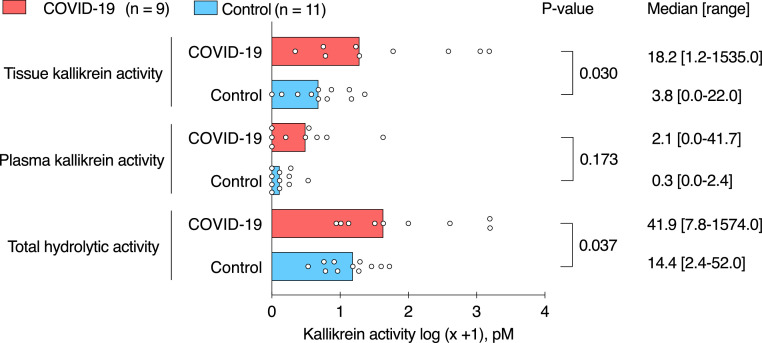
Total hydrolytic activity of the kallikrein substrate was higher in BAL fluid from COVID-19 patients than in banked BAL fluid samples from patients without COVID-19 (41·9 pM [7·8-1574·0] (median [range]) vs 14·4 pM [2·4-52·0], respectively, p = 0·037 (Mann-Whitney U test), p = 0·055 after correcting for multiple testing (Benjamini-Hochberg method)) (Table 2, Figure 4). The overall hydrolytic activity was mainly due to tissue kallikrein activity, which was up to fourfold higher in BAL fluid samples from patients with COVID-19 than in banked BAL fluid from patients without COVID-19 (18·2 pM [1·2-1535·0] vs 3·8 pM [0·0-22·0], respectively, p = 0·030 (Mann-Whitney U test), p = 0·055 after correcting for multiple testing (Benjamini-Hochberg method)). Tissue kallikrein activity levels of more than 50 pM were observed in four out of nine patients with COVID-19, in contrast to no tissue kallikrein activity of more than 50 pM in patients without COVID-19 (p = 0·026, Fisher exact test). There was no statistically significant difference for plasma kallikrein activity between BAL fluid of COVID-19 and control patients. Plasma kallikrein activity in BAL fluid of COVID-19 patients was lower than tissue kallikrein activity (p = 0·004, Wilcoxon signed test for paired data).
Figure 4.
Kallikrein hydrolytic activity in bronchoalveolar lavage fluid from patients with and without COVID-19.
Graphs show individual dots representing patient data and bar representing median of each patient group. For graphical representation, data were transformed using the logarithmic transformation. Pairwise comparisons were made between patients with COVID-19 (n = 9) and patients without COVID-19 (n = 11). Median [range] and p-value (Mann-Whitney U test, not corrected for multiple testing) are shown.
MPO-DNA complexes
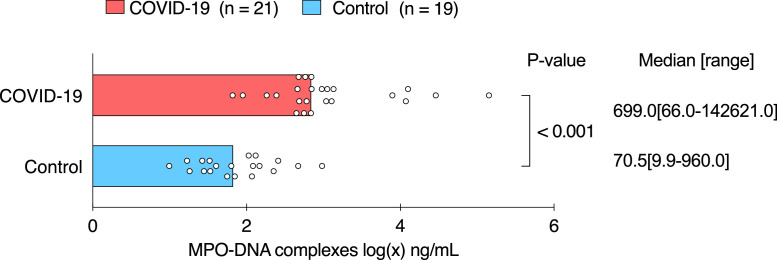
Levels of MPO-DNA complexes, a biomarker for NETs, were up to tenfold higher in BAL fluid from COVID-19 patients than in BAL fluid from hospitalized patients without COVID-19 (699·0 ng/mL [66·0-142621·0] median [range] vs 70·5 ng/mL [9·9-960·0], respectively, p < 0·001 (Mann-Whitney U test)) (Figure 5). Moreover, levels of MPO-DNA complexes were correlated with levels of the bradykinin-(1-5) peptide in BAL fluid samples from patients with COVID-19 (r = 0·64, p = 0·002, Spearman correlation).
Figure 5.
MPO-DNA complexes in bronchoalveolar lavage fluid from patients with and without COVID-19.
Graphs show individual dots representing patient data and bar representing median of each patient group. For graphical representation, data were transformed using the logarithmic transformation. Pairwise comparisons were made between patients with COVID-19 (n = 21) and patients without COVID-19 (n = 19). Median [range] and p-value are shown (Mann-Whitney U test).
Plasma kallikrein activity induced by DNA or nucleosomes, but not free histones
Levels of plasma kallikrein activity in normal plasma from healthy volunteers were increased when incubated with either DNA ((383·3 (371·3 - 446·4) mU/ml; median (interquartile range)) or nucleosomes (41·3 (13·5 – 65·5) mU/ml) as compared to plasma without activator (0·7 (−12·8 - 27·3) mU/ml; p < 0·0001 and p = 0·0100, respectively; Mann-Whitney test). Free histones were not able to induce plasma kallikrein activity (4·1 (0·7 - 11·8) mU/ml) as compared to plasma without activator in normal human plasma (0·7 (−12·8 - 27·3) mU/ml; p = 0·6820, Mann-Whitney test). Of note, the increase in plasma kallikrein activity induced by DNA was significantly higher in normal human plasma (383·3 (371·3 - 446·4) mU/ml) as compared to plasma deficient in FXII (14·3 (5·1 - 35·9) mU/ml; p = 0·0014), but not as compared to plasma deficient in FIX (399·3 (319·3 - 419·0) mU/ml; p > 0·9999) (Kruskal-Wallis test with post hoc Dunn's multiple comparisons test) (Supplementary Figure 2).
Discussion
In this observational prospective study, we report elevated levels of kinin peptides and high kallikrein activity in bronchoalveolar lavage fluid from patients with severe COVID-19 pulmonary disease. Notably, we measured an increase in bradykinin-(1-5) levels, the most downstream kinin metabolite with the longest half-life, pointing towards a general increase in kinin generation in patients with severe COVID-19 pneumonia. Kallikrein activity in BAL fluid was mainly driven by tissue kallikrein, whereas plasma kallikrein contributed less to the overall kallikrein activity in bronchoalveolar lavage fluid.
In line with our findings, Garvin et al. reported increased kininogen and kallikrein gene expressions in BAL fluid from patients with severe COVID-19.24 Our findings are also consistent with those of Lipcsey et al.,25 who reported decreased levels of prekallikrein and high molecular weight kininogen in plasma samples from patients with severe COVID-19.
Notwithstanding SARS-CoV-2 enters the cell via the ACE2 receptor resulting in receptor internalization and potential deficiency of its role in degradation of kinins and peptides from the renin-angiotensin-aldosterone system, existing evidence does not allow us to claim any COVID-19 specific mechanism of action. ACE2 has indeed also shown a lung-protective effect in inflammatory conditions induced by other viruses26,27 and prothrombotic disease phenotypes have not merely been observed in patients with COVID-19. Therefore, further research is needed to investigate the pathophysiological mechanisms linking SARS-CoV-2 cell entry via ACE2 and subsequent dysregulation of the kallikrein-kinin system.
Nevertheless, our finding of dysregulation of the kallikrein-kinin system in patients with severe pulmonary disease may help to understand part of the initial pathophysiological changes triggering local pulmonary inflammation and subsequent propagation from local inflammation in the pulmonary alveolar space to a systemic inflammatory and prothrombotic reaction. The kallikrein-kinin system is indeed linked with the contact pathway of coagulation,28 but its true contribution can only be interpretated as being one component of a complex network of multiple downstream pathways within the coagulation system, the fibrinolytic system, the renin-angiotensin system, and the complement system.2,26,29,30 Here, the role of thrombin-thrombomodulin as a central ‘switch’ between a pro- and anti-thromboinflammatory reaction, and the importance of the activated protein C pathway in regulating inflammation and thrombosis in severe inflammatory response syndrome, have been well-studied.31
In this regard, neutrophils emerged as potential promotors of thromboinflammation in COVID-19, as their numbers are increased in bronchoalveolar lavage fluid of COVID-19 patients,17 and their interconnection with the kallikrein-kinin system has been postulated before.13 However, the link between NETs and the kallikrein-kinin system had not yet been experimentally addressed. Here we report a quantitative comparison of NETs in BAL fluid samples from patients with severe COVID-19 compared to those from hospitalized patients without COVID-19. Our data corroborate the previously published evidence of NET formation both in plasma and in the airways of patients with COVID-19.10,11 Furthermore, we show that, in vitro, DNA and nucleosomes, the major structural components of NETs, are able to induce plasma kallikrein activity in human plasma if FXII is present, thus providing in vitro evidence hinting towards a potential FXII-dependent link between NETs and the kallikrein-kinin system. As expected, positively charged free histones not complexed with DNA did not activate plasma kallikrein. Interestingly, moderately negatively charged nucleosomes are able to activate plasma kallikrein to a lesser extent than negatively charged DNA. NETs have previously been shown to initiate the contact activation pathway via activation of factor XII,12 with defective NET clearance indeed contributing to elevated factor XII activation in COVID-19,32 but activation of factor XII can also result in plasma kallikrein activation, thus creating a powerful feedback loop (Figure 1).33 Although NET components have previously been linked to contact activation,33, 34, 35, 36 the consequence of this on activation of prekallikrein to kallikrein had not directly been shown other than by purified DNA.37 Furthermore, activated neutrophils are not only able to activate the kallikrein-kinin pathway,13 but neutrophils could also be recruited by cytokine signals following stimulation of the bradykinin receptor 1 and 2.7,38
Our work encourages further investigation of drugs that target the kallikrein-kinin pathway as a treatment option for patients with severe infectious or inflammatory pulmonary disease. In the context of the COVID-19 pandemic, one randomised controlled trial showed an improvement in length of hospital stay and need for oxygen therapy following aprotinin inhalation in hospitalized patients with COVID-19.14 Aprotinin is a broad-spectrum protease inhibitor that inhibits both tissue and plasma kallikrein, as well as plasmin, and has been used to modulate thromboinflammation in high-risk cardiothoracic surgery.39 Furthermore, aprotinin was shown to inhibit replication of SARS-CoV-2 in vitro by inhibiting the transmembrane serine protease-2 (TMPRSS-protease) responsible for proteolytic modification of the spike (S) protein.40 Our finding that the increase in total hydrolytic activity in BAL fluid samples from patients with severe COVID-19 was mainly driven by an increase in tissue kallikrein activity could potentially explain the beneficial effect of inhibiting both tissue and plasma kallikrein as compared to inhibition of plasma kallikrein alone. Nonetheless, the role of plasma kallikrein triggering thromboinflammation in plasma, which could be mediated through interactions with NETs, remains to be explored. Whether or not systemic administration of aprotinin could reinforce its beneficial effect, has been investigated in a randomized controlled clinical trial coordinated at our hospital.41
In addition to aprotinin, inhibition of other targets in the kallikrein-kinin system in small case-control studies could show a beneficial effect on surrogate endpoints for severe COVID-19. Mansour et al. reported that the bradykinin receptor 2 antagonist icatibant and inhibitor of C1 esterase/kallikrein improve blood eosinophil count and chest CT severity scores,16 whereas van de Veerdonk et al. observed a reduction in oxygen need for icatibant treated patients.15 Several larger clinical trials investigating KKS inhibitors are currently ongoing, including the plasma kallikrein inhibitor lanadelumab (NCT 04422509), icatibant15 (NCT 04488081) and the recombinant soluble ACE2 (APN01; NCT 04335136). Targeting NETs using aerosolized DNase, speculated to act upstream of the KKS cascade, is also under investigation (reviewed in;42 NCT04541979).
Limitations
In this study we performed in-depth analysis of bradykinin and Lys-bradykinin peptides and their metabolites with a validated LC-MS/MS assay,18,19 while being challenged by logistical difficulties to collect highly contagious BAL fluid samples from patients with COVID-19, the complex set-up of a BSL-3 facility and the period of extreme pressure on the healthcare system. The challenging circumstances of this study led to a number of inherent limitations. First and foremost, given the invasive nature of bronchoscopy with BAL and the associated risks to patients and staff, recruitment and sampling was guided by clinical indication. This observational study is thus not case-controlled, introducing bias in the composition of our study cohorts. Specifically, a large majority of COVID-19 patients were severely ill, as evidenced by the need for mechanical ventilation or ECMO, whereas this was the case for only a few control patients (Table 1). Consequently, our results are applicable and clinically relevant for severely ill COVID-19 patients, but should not be generalised to mild COVID-19 cases. Although being triggered by the hypothesis of SARS-CoV-2 induced dysregulation of the kallikrein-kinin system upon its cell entry via ACE2, the clinical heterogeneity in our cohort implies we cannot claim a COVID-19 specific disease mechanism. Direct viral activation of contact activation and indirect activation of coagulation via necrotic cell death signals in the context of severe inflammation or via mechanical ventilation-induced stress,43 might also contribute to dysregulation of the kallikrein-kinin system in infectious or inflammatory diseases other than COVID-19.
Furthermore, COVID-19 patients more often received heparin treatment than those patients without COVID-19, whose final diagnoses comprised both non-COVID-19 related infectious and non-infectious diseases, and many patients were treated with different classes of antibiotics and antifungals. Pharmacokinetic or -dynamic interactions between these drugs and the kallikrein-kinin system are poorly studied, but might have influenced our results.
Secondly, with regards to sampling procedure and timing, a single sampling time point per patient was obtained as clinically indicated (generally more delayed in the COVID-19 group compared to the control group). This precluded an assessment of the impact of the disease phase on kallikrein-kinin activity, yet serial and/or fixed timepoint bronchoscopies with BAL would not have been feasible clinically. Furthermore, we cannot exclude partial ex vivo activation of the KKS during sampling (e.g. catheter induced contact activation) contributing to the measured levels of kallikrein activity and kinin peptides.44 However, all samples from COVID-19 or non-COVID-19 control patients have been collected and treated in the same way, making it unlikely that the large differences found are only a consequence of contact activation due to sample collection.
Thirdly, from an analytical point of view, the small sample size limits statistical power and does not allow to correct for or investigate the influence of the above mentioned patient and disease characteristics on the kallikrein-kinin system.45 In this study we were able to assess kallikrein activity in a limited number of non-virally inactivated BAL fluid samples. When correcting for multiple testing of these three variables (total, tissue and plasma kallikrein activity) in this small cohort, the difference in total hydrolytic activity and tissue kallikrein activity between patients with and without COVID-19 is no longer significant (p = 0·055). However, the effect sizes (Supplementary Table 2) do support a large effect size for tissue kallikrein activity, indeed suggesting that the borderline non-significance reflects the small power of the study. Moreover, the proposed downstream effect, an increase of bradykinin-(1-5), remained statistically significant after correcting for multiple testing. Besides, because the analyses with non-virally inactivated BAL fluid samples had to be performed in a BSL-3 facility, banked samples were used as a control. Finally, we cannot comment on which step of the kallikrein-kinin system is most affected in severe COVID-19, nor on the exact underlying aetiology, probably because only the most downstream stable kinin metabolite bradykinin-(1-5) could be quantified reliably. Per-patient profiles of kinin peptides indeed show a general increase in bradykinin-(1-5) levels as compared to the upstream metabolites, which is in line with the different half-lives of these kinin peptides (Supplementary Table 3).
Higher levels of kinin peptides and increased kallikrein activity in BAL fluid samples from patients with severe COVID-19 suggest that dysregulation of the kallikrein-kinin system contributes to pulmonary thromboinflammation in severe COVID-19. Due to inherent limitations, we cannot identify the exact pathophysiological mechanism which leads to dysregulation of the kallikrein-kinin system, nor to which extent this is unique for COVID-19 pneumonia. Nevertheless, our data help us to gain mechanistic insights and emphasize the need to further investigate the kallikrein-kinin system as part of a complex network triggering thromboinflammation in other infectious and inflammatory respiratory illnesses. Moreover, our findings encourage the initiation of larger studies that characterize and investigate drugs that target the kallikrein-kinin system as a potential treatment option for patients with severe pulmonary disease, especially for those with strong thromboinflammatory responses as observed in COVID-19.
Contributors
P.V. and T.V. designed the experiments and acquired financial support for the project leading to this publication. P.V.M., J.W., E.W., R.V., A.V.H., M.M.E., A.O., C.V., P.M., G.H., and A.W. were responsible for sample collection. P.V.M., L.L., E.H., S.J., J.N., and P.V. were responsible for sample processing and shipment. Following authors performed experiments, analysed and verified data: M.V., B.N., H.C., and J.H.M.F. (kallikrein activity and bradykinin and Lys-bradykinin ELISA); T.G. and B.B.B. (kinin peptides using liquid chromatography with mass spectrometry assay); L.C.V.P. and K.M. (MPO-DNA complexes); S.K., M.J. and K.M. (plasma kallikrein activity induced by nucleosomes and DNA). C.P.M., P.V.M., M.M.E., and A.O. extracted data from the medical files. C.P.M. and M.M.E. verified these data. I.G. did the statistical analyses. P.V. and T.V. verified all the data and take responsibility for the integrity of the data and the accuracy of the data analysis. C.P.M. wrote the initial draft with input from P.V. and T.V.; M.V., T.G., B.B.B., L.C.V.P., S.K., K.M., and I.G. wrote methodology sections for the manuscript. C.P.M. and P.V.M. wrote the revised manuscript with important input from K.M., P.V. and T.V. C.P.M. made tables and figures with input from T.G., B.B.B., P.V., and T.V. P.V. and T.V. coordinated and directed the entire project. All authors had full access to all the data in the study, reviewed the manuscript and approved the final version for submission.
Data sharing statement
The original data (deidentified patient data) are available upon request to the corresponding author.
Declaration of interests
M.V., B.N., H.C., and J.H.M.F. are employees of Oxurion NV. Oxurion NV and KU Leuven LRD submitted a patent on kallikrein inhibitors. K.M. is an inventor on the granted patent US9642822 awarded to Children's Medical Center Corporation covering the targeting of NETs in thrombosis and lung injury and the pending patent WO20180271953A1. She reports issued patent US9642822B2 and pending patents US2019167680A1. K.M. also reports consulting fees from PEEL Therapeutics. She holds a grant from Flanders Research Foundation (FWO) and a Horizon 2020 grant, as well as a grant from the International Society on Thrombosis and Haemostasis. T.V. is a board member and vice-president of the Belgian Society for Thrombosis and Haemostasis. P.V. and T.V. are co-holders of a research chair for clinical research with Trasylol (DAWN-AntiCo). P.V. holds an institutional grant funded by Bayer AG and by BMS/Pfizer. He reports consulting fees from Anthos Therapeutics, Bayer AG, Boehringer,BMS, Pfizer, Daiichi Sankyo, and Portola/Actelion, and honoraria as a speaker from Bayer, BMS, Pfizer, Daiichi Sankyo, and Leo Pharma. P.V. has participated in a DSMB for clinical trials sponsored by Bayer and Boehringer Ingelheim. R.V. and A.V. report participation in advisory board for Takeda and involvement in ERS, ESOT/ECTTA boards. J.W. is a holder of research grants funded by MSD, Pfizer, and Gilead. He reports speakers fees and support for attending meetings from Gilead, MSD, and Pfizer, and participation on an advisory board for Gilead. He has received study medication for clinical trials from MSD. The other authors declare no conflict of interest.
Acknowledgements
The authors acknowledge Tina Van Buyten (Laboratory of Virology and Chemotherapy, Department of Microbiology, Immunology and Transplantation, Rega Institute, KU Leuven, Leuven, Belgium) for technical assistance in sample processing and performing of experiments, Cato Jacobs and Sofie Coenen (Laboratory for Clinical Infectious and Inflammatory Diseases, Department of Microbiology and Immunity, KU Leuven, Leuven, Belgium) for technical assistance in sample collection of hospitalized patients, Bart M. Vanaudenaerde (Department CHROMETA, Laboratory of respiratory Diseases and Thoracic Surgery (BREATHE), KU Leuven, Leuven, Belgium) for collection of banked bronchoalveolar fluid samples, and Isabel Spriet en Pieter Vermeersch for their valuable input. This study was supported by research funding from Research Foundation-Flanders (G0G4720N to P.V., T.V., and J.W. (FWO, COVID-related research) and 1843418N (“fundamenteel klinisch mandaat”, FWO, T.V. = promotor)), and the COVID research fund of the KU Leuven (COVID1-O2010, to K.M.). A.V., C.P.M. and P.V.M. are fellows of the Research-Foundation Flanders (FWO; 11B0621N and 1S66020N, resp.). T.V. (1843418N), P.V., R.V., J.W. (1833317N), and G.H. (1805116N), are Senior Clinical research Fellows of the FWO. P.V., B.B. and T.G. are funded by Life Sciences Research Foundation.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2022.104195.
Appendix. Supplementary materials
References
- 1.Tran BX, Ha GH, Nguyen LH, et al. Studies of novel coronavirus disease 19 (COVID-19) pandemic: a global analysis of literature. Int J Environ Res Public Health. 2020;17(11):1–16. doi: 10.3390/ijerph17114095. 4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henry BM, Vikse J, Benoit S, Favaloro EJ, Lippi G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta. 2020;507:167–173. doi: 10.1016/j.cca.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van de Veerdonk FL, Netea MG, van Deuren M, et al. Kallikrein-kinin blockade in patients with COVID-19 to prevent acute respiratory distress syndrome. Elife. 2020;9:1–9. doi: 10.7554/eLife.57555. 57555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Maat S, de Mast Q, Danser AHJ, van de Veerdonk FL, Maas C. Impaired breakdown of bradykinin and its metabolites as a possible cause for pulmonary edema in COVID-19 infection. Semin Thromb Hemost. 2020;46(7):835–837. doi: 10.1055/s-0040-1712960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorlinger K, Dirkmann D, Gandhi A, Simioni P. COVID-19-associated coagulopathy and inflammatory response: what do we know already and what are the knowledge gaps? Anesth Analg. 2020;131(5):1324–1333. doi: 10.1213/ANE.0000000000005147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sodhi CP, Wohlford-Lenane C, Yamaguchi Y, et al. Attenuation of pulmonary ACE2 activity impairs inactivation of des-Arg(9) bradykinin/BKB1R axis and facilitates LPS-induced neutrophil infiltration. Am J Physiol Lung Cell Mol Physiol. 2018;314(1):L17–L31. doi: 10.1152/ajplung.00498.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Passos GF, Fernandes ES, Campos MM, et al. Kinin B1 receptor up-regulation after lipopolysaccharide administration: role of proinflammatory cytokines and neutrophil influx. J Immunol. 2004;172(3):1839–1847. doi: 10.4049/jimmunol.172.3.1839. [DOI] [PubMed] [Google Scholar]
- 9.Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Middleton EA, He XY, Denorme F, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136(10):1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouwendijk WJD, Raadsen MP, van Kampen JJA, et al. Neutrophil extracellular traps persist at high levels in the lower respiratory tract of critically ill COVID-19 patients. J Infect Dis. 2021;223(9):1512–1521. doi: 10.1093/infdis/jiab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sorvillo N, Cherpokova D, Martinod K, Wagner DD. Extracellular DNA NET-works with dire consequences for health. Circ Res. 2019;125(4):470–488. doi: 10.1161/CIRCRESAHA.119.314581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenne E, Rasmuson J, Renne T, et al. Neutrophils engage the kallikrein-kinin system to open up the endothelial barrier in acute inflammation. FASEB J. 2019;33(2):2599–2609. doi: 10.1096/fj.201801329R. [DOI] [PubMed] [Google Scholar]
- 14.Redondo-Calvo FJ, Padin JF, Munoz-Rodriguez JR, et al. Aprotinin treatment against SARS-CoV-2: a randomized phase III study to evaluate the safety and efficacy of a pan-protease inhibitor for moderate COVID-19. Eur J Clin Invest. 2022;52(6) doi: 10.1111/eci.13776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Veerdonk FL, Kouijzer IJE, de Nooijer AH, et al. Outcomes associated with use of a kinin B2 receptor antagonist among patients with COVID-19. JAMA Netw Open. 2020;3(8) doi: 10.1001/jamanetworkopen.2020.17708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansour E, Palma AC, Ulaf RG, et al. Safety and outcomes associated with the pharmacological inhibition of the kinin-kallikrein system in severe COVID-19. Viruses. 2021;13(2):1–16. doi: 10.3390/v13020309. 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wauters E, Van Mol P, Garg AD, et al. Discriminating mild from critical COVID-19 by innate and adaptive immune single-cell profiling of bronchoalveolar lavages. Cell Res. 2021;31:272–290. doi: 10.1038/s41422-020-00455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gangnus T, Burckhardt BB. Improving sensitivity for the targeted LC-MS/MS analysis of the peptide bradykinin using a design of experiments approach. Talanta. 2020;218 doi: 10.1016/j.talanta.2020.121134. [DOI] [PubMed] [Google Scholar]
- 19.Gangnus T, Burckhardt BB. Sensitive mass spectrometric determination of kinin-kallikrein system peptides in light of COVID-19. Sci Rep. 2021;11(1):3061. doi: 10.1038/s41598-021-82191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teufel DP, Bennett G, Harrison H, et al. Stable and long-lasting, novel bicyclic peptide plasma kallikrein inhibitors for the treatment of diabetic macular edema. J Med Chem. 2018;61(7):2823–2836. doi: 10.1021/acs.jmedchem.7b01625. [DOI] [PubMed] [Google Scholar]
- 21.Kessenbrock K, Krumbholz M, Schonermarck U, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15(6):623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanderbeke L, Van Mol P, Van Herck Y, et al. Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity. Nat Commun. 2021;12(1):4117. doi: 10.1038/s41467-021-24360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamini Y HY. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 24.Garvin MR, Alvarez C, Miller JI, et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife. 2020;9:1–9. doi: 10.7554/eLife.59177. 59177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipcsey M, Persson B, Eriksson O, et al. The outcome of critically Ill COVID-19 patients is linked to thromboinflammation dominated by the kallikrein/kinin system. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.627579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Curran CS, Rivera DR, Kopp JB. COVID-19 usurps host regulatory networks. Front Pharmacol. 2020;11:1278. doi: 10.3389/fphar.2020.01278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmaier AH. The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities. J Thromb Haemost. 2016;14(1):28–39. doi: 10.1111/jth.13194. [DOI] [PubMed] [Google Scholar]
- 29.Carvalho PR, Sirois P, Fernandes PD. The role of kallikrein-kinin and renin-angiotensin systems in COVID-19 infection. Peptides. 2020;135 doi: 10.1016/j.peptides.2020.170428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020;133 doi: 10.1016/j.cyto.2020.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van de Wouwer M, Collen D, Conway EM. Thrombomodulin-protein C-EPCR system: integrated to regulate coagulation and inflammation. Arterioscler Thromb Vasc Biol. 2004;24(8):1374–1383. doi: 10.1161/01.ATV.0000134298.25489.92. [DOI] [PubMed] [Google Scholar]
- 32.Englert H, Rangaswamy C, Deppermann C, et al. Defective NET clearance contributes to sustained FXII activation in COVID-19-associated pulmonary thrombo-inflammation. EBioMedicine. 2021;67 doi: 10.1016/j.ebiom.2021.103382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renne T, Stavrou EX. Roles of factor XII in innate immunity. Front Immunol. 2019;10:2011. doi: 10.3389/fimmu.2019.02011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noubouossie DF, Whelihan MF, Yu YB, et al. In vitro activation of coagulation by human neutrophil DNA and histone proteins but not neutrophil extracellular traps. Blood. 2017;129(8):1021–1029. doi: 10.1182/blood-2016-06-722298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song DY, Gu JY, Yoo HJ, et al. Activation of factor XII and kallikrein-kinin system combined with neutrophil extracellular trap formation in diabetic retinopathy. Exp Clin Endocrinol Diabetes. 2021;129(8):560–565. doi: 10.1055/a-0981-6023. [DOI] [PubMed] [Google Scholar]
- 36.Busch MH, Timmermans S, Nagy M, et al. Neutrophils and contact activation of coagulation as potential drivers of COVID-19. Circulation. 2020;142(18):1787–1790. doi: 10.1161/CIRCULATIONAHA.120.050656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oehmcke S, Morgelin M, Herwald H. Activation of the human contact system on neutrophil extracellular traps. J Innate Immun. 2009;1(3):225–230. doi: 10.1159/000203700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sodhi CP, Nguyen J, Yamaguchi Y, et al. A dynamic variation of pulmonary ACE2 is required to modulate neutrophilic inflammation in response to Pseudomonas aeruginosa lung infection in mice. J Immunol. 2019;203(11):3000–3012. doi: 10.4049/jimmunol.1900579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Later AF, Maas JJ, Engbers FH, et al. Tranexamic acid and aprotinin in low- and intermediate-risk cardiac surgery: a non-sponsored, double-blind, randomised, placebo-controlled trial. Eur J Cardiothorac Surg. 2009;36(2):322–329. doi: 10.1016/j.ejcts.2008.11.038. [DOI] [PubMed] [Google Scholar]
- 40.Bojkova D, Bechtel M, McLaughlin K-M, et al. Aprotinin inhibits SARS-CoV-2 replication. Cells. 2020;9(11):1–13. doi: 10.3390/cells9112377. 2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vanassche T, Engelen MM, Van Thillo Q, et al. Correction to: a randomized, open-label, adaptive, proof-of-concept clinical trial of modulation of host thromboinflammatory response in patients with COVID-19: the DAWn-Antico study. Trials. 2020;21(1):1033. doi: 10.1186/s13063-020-04991-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonaventura A, Vecchie A, Dagna L, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21(5):319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Riordan TG, Weinstein MD, Abraham WM, Forteza R. Elevated tissue kallikrein activity in airway secretions from patients with tracheobronchitis associated with prolonged mechanical ventilation. Lung. 2003;181(5):237–244. doi: 10.1007/s00408-003-1019-9. [DOI] [PubMed] [Google Scholar]
- 44.Yau JW, Stafford AR, Liao P, Fredenburgh JC, Roberts R, Weitz JI. Mechanism of catheter thrombosis: comparison of the antithrombotic activities of fondaparinux, enoxaparin, and heparin in vitro and in vivo. Blood. 2011;118(25):6667–6674. doi: 10.1182/blood-2011-07-364141. [DOI] [PubMed] [Google Scholar]
- 45.Hamid S, Rhaleb IA, Kassem KM, Rhaleb NE. Role of kinins in hypertension and heart failure. Pharmaceuticals. 2020;13(11):1–25. doi: 10.3390/ph13110347. 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.