Graphical abstract

Keywords: Pea protein, High-intensity ultrasound, pH-shifting, Solubility, Emulsifying
Highlights
-
•
High-intensity ultrasound and pH-shifting improve pea protein solubility.
-
•
The combined treatment reduced the particle size of pea protein.
-
•
The combined treatment exposed hydrophobic residues of pea protein.
-
•
High-intensity ultrasound and pH-shifting promote the emulsion physical stability.
Abstract
The most important factors restricting research and application in the food industry are the poor solubility and emulsification of pea protein isolate (PPI). This study investigates the effect of high-intensity ultrasound (HIU, 0–600 W) and pH-shifting treatment, alone or combined, on the structure, solubility, and emulsification of PPI, as well as its potential mechanism. The results revealed that the PPI solubility significantly increases when treated with the combination, corresponding to a decrease in the protein particle size, especially at 500 W of HIU power (p < 0.05). Correspondingly, the emulsion prepared from it was less prone to phase separation during storage. According to the structural analysis, the structural changes caused by protein unfolding (i.e., the exposure of hydrophobic and polar sites and the loss of the α-helix) seemed to be the primary reasons for increased PPI solubility. In addition, confocal laser scanning microscopy indicated that the combination treatment accelerated the adsorption of PPI at the oil/water interface and strengthened the compactness of the interface film. Improved interfacial properties and intermolecular forces played a critical role in the resistance to droplet coalescence in PPI emulsion. In conclusion, ultrasound and pH-shifting treatments have a synergistic effect on improving the solubility and emulsification of PPI.
1. Introduction
The increasing global population will lead to rapid growth in protein demand in the coming years. Plant-derived proteins are emerging as the main alternative to animal proteins (dairy, meat, and eggs) and functional ingredients in product formulation due to the growing concerns regarding food health, environmental damage, and social factors. This emergence is primarily due to (1) the potential health benefits of plant-based foods for consumers, (2) the increasing emergence of vegetarianism, (3) agro-industrial waste and by-products as potential sustainable plant protein sources, and (4) environmentally friendly and lower production costs [1].
Pea protein isolate (PPI), as a high-quality protein with high content, high availability, high antioxidant potential, hypoallergenicity, nontransgenic status, and potential economic benefits, has attracted widespread attention [2]. In addition, PPI is rich in essential amino acids (e.g., phenylalanine, arginine, leucine, and isoleucine) and especially lysine, which is a limited amino acid in cereals [1]. However, limitations related to the low solubility of PPI in aqueous media in the raw state directly affect emulsifying properties, limiting its application in the food industry.
In addition, PPI comprises 10 % to 20 % albumin and 70 % to 80 % globulin. Albumins (approximately 5–80 kDa, 2 S) are water-soluble metabolic proteins, whereas globulins are salt-soluble storage proteins, which is the main reason for the poor water solubility of PPI. Furthermore, globulins are divided into legumin (approximately 300–400 kDa, 11 S), vicilin (approximately 150–170 kDa, 7 S), and convicilin (approximately 70 kDa, 7 S) according to the sedimentation coefficient [3]. Legumin has a relatively rigid structure due to the highly ordered quaternary conformation, intermolecular hydrophobic interactions, and disulfide bridges, which is detrimental to the water dissolution of PPI and the development of oil–water interfacial protein layers in emulsion [4].
Due to these characteristics, protocols have been devised to treat PPI to enhance its solubility and emulsification in aqueous media. According to Zha et al. [5], poorly soluble legumin and vicilin subunits form conjugates with hydrophilic gum arabic via a controlled Maillard reaction, increasing the PPI solubility and improving the physical and oxidative stability of their emulsions. Likewise, tryptic hydrolysis increases the water solubility of PPI, and Klost et al. [6] indicated that this is associated with the release of hydrophilic amino acids and small peptides carrying high-density charges during hydrolysis.
Despite being valid approaches to improving the solubility and emulsifying properties of PPI, these strategies present some defects. For instance, the loss of lysine during the Maillard reaction reduces nutritional value and digestibility [2]. In addition, the conditions and degree of enzymatic hydrolysis are difficult to control, which may produce unpleasant flavors, such as bitterness and astringency [7]. Therefore, better strategies that achieve the purpose of improving the solubility and emulsification of PPI are needed.
Moreover, as a simple, mild, and effective chemical modification method, pH-shifting can enhance functional properties. The exposure of protein solutions to extremely alkaline or acidic conditions causes molecular repulsion between the charged side chain groups of amino acids resulting in the partial unfolding of the protein structure. Then, the pH is repositioned to neutral, and the unfolded structure cannot be fully recovered, resulting in a more flexible protein structure called a molten globule [8]. Several studies have demonstrated that the pH-shifting method improves protein solubility and emulsification, such as in soy protein and faba bean protein [9]. However, a complementary technique to improve the modification efficiency must be found for PPI with a rigid structure.
High-intensity ultrasound (HIU) is a nonthermal physical technique and has become widely used for food protein modification. The acoustic cavitation effect induced by ultrasound, a periodic process in which bubbles grow rapidly and collapse violently, can generate strong mechanical forces (e.g., shear force, shock waves, and microscopic turbulence) [10]. These forces disperse protein particles or aggregates and expose the hydrophobic and sulfhydryl groups in the protein, further improving the functional properties of proteins [11].
A fascinating hypothesis is that the cavitation caused by HIU due to physical modification, combined with the unfolding effect of pH-shifting on the PPI structure, could more effectively improve the functional properties of PPI. Once successful, the emergence of modified PPI can meet the development needs of a variety of food products, such as beverage, ice cream, fat substitute, plant meat, and protein supplement. Hence, in the present work, we examine the effects of pH-shifting and HIU treatment, alone or combined, on the solubility and emulsification of PPI. In addition, structural changes were explored to decode the underlying mechanisms of solubility and emulsification improvements. The goal is to prove this hypothesis and apply these theories to expand the utilization of PPI in food processing systems.
2. Materials and methods
2.1. Materials
The pea protein isolate (PPI, protein ∼87.31 %) was generously donated by Harbin (Heilongjiang, China) and kept at 4 °C prior to experimentation. Soybean oil was purchased from Jinlongyu Co., ltd. (Beijing, China). All other chemicals and reagents used in this study were of analytical grade.
2.2. Combination of pH-shifting and High-intensity ultrasound treatment
The PPI (50 mg/mL) powder was dispersed in 0.01 M phosphate buffer (pH = 7) under stirring at 25 °C for 2 h prior to hydration overnight at 4 °C. The PPI was processed for pH-shifting according to the method described by Jiang et al. [8] with a slight modification. Briefly, the pH of the PPI suspension (50 mg/mL) was slowly adjusted to 12 with 2 M NaOH, followed by stirring for 10 min to make the solution system uniform. Then, the HIU treatment with different powers (0, 200, 300, 400, 500 and 600 W) was carried out according to Liu et al. [11] with some modifications.
For the HIU treatment, the 6-mm (diameter) probe was inserted about 1 cm into the sample solutions, followed by treatment with ultrasound at 20 kHz for 10 min (5 s on and 2 s off) at different powers (200, 300, 400, 500 and 600 W) using a Scientz-II D ultrasound generator (Scientz Biotechnology Co., ltd., Ningbo, China). During the ultrasonic process, an ice-water mixture was circulated in a double-walled beaker to keep the sample temperature below 20 °C, and a temperature probe was integrated for real-time monitoring.
After HIU treatment, the PPI solution was stirred at 25℃ for 1 h before adjusting the pH to 7 using 1 M HCl. The samples processed by pH-shifting and different HIU powers (200, 300, 400, 500 and 600 W) are denoted as U2P, U3P, U4P, U5P, and U6P, respectively. Further, to examine the combined effect, a batch of PPI treated with pH-shifting alone and HIU at different powers (200, 300, 400, 500 and 600 W) alone were prepared as described above and denoted as P, U2, U3, U4, U5, and U6, respectively. The control was a native PPI stirred for only 30 min without pH-shifting and HIU treatment, denoted as C. All samples were stored at 4 °C for further analysis. For samples that need to be stored, sodium azide (3 mM) was added to the emulsion to inhibit the reproduction of microorganisms during storage.
2.3. Solubility
The solubility of PPI was determined using the approach described by Sha et al. [12] with some slight modifications. The sample suspension (50 mg/mL) was diluted to 5 mg/mL in 0.01 M phosphate buffer (pH = 7) and centrifuged at 10,000 g for 10 min. The concentration of supernatant protein was determined using the Biuret method. Protein solubility was calculated as the ratio of the supernatant protein concentration to the total protein concentration.
2.4. Particle size and zeta potential
The samples were diluted to 0.1 mg/mL in 0.01 M phosphate buffer (pH = 7) to avoid multiple scattering effects. The zeta potential and particle size were determined using a particle size and potential analyzer (NANO ZS90, UK) at 25 °C.
2.5. Confocal laser scanning microscopy
Confocal laser scanning microscopy (CLSM; Leica TCS SP8, Heidelberg, Germany) analyzed the size and distribution of PPI with different treatments. After adding 20 μL of Nile blue solution (1 mg/mL) to 1 mL of protein suspension (10 mg/mL), it was mixed evenly and dropped onto a microscope slide with a coverslip. The sample was observed using the 40 × HCPL APO/20 × oil immersion objective (633 nm He-Ne laser) [11].
2.6. Analysis of structural characteristics
2.6.1. Fluorescence spectroscopy
An F-4500 fluorescence spectrophotometer (Hitachi Co., Tokyo, Japan) was used to scan the internal fluorescence of the samples (0.1 mg/mL) in the emission spectrum range of 290 to 400 nm at the excitation wavelength of 280 nm. The excitation and emission slit widths were 10 nm, and the scanning speed was 2400 nm/min.
2.6.2. Surface hydrophobicity
The surface hydrophobicity (H0) of the PPI from different treatments was evaluated using the method by Haskard et al. [13] with a slight modification. The sample was diluted to five concentrations from 0.2 mg/mL to 1.0 mg/mL using 0.01 M phosphate buffer (pH = 7). Then, 4 mL of the diluted sample was added to 10 μL of the ANS solution (8 mM), reacting in the dark for 15 min. The fluorescence intensity was measured (F-4500 fluorescence spectrophotometer, Hitachi Co., Tokyo, Japan) at 340 nm (excitation wavelength) and 410 nm to 500 nm (emission wavelength range). The H0 of PPI was calculated using the slope linear regression analysis.
2.6.3. Sulfhydryl groups (-SH) and disulfide bond (S—S)
According to the method reported by He et al. [14], with some modifications, 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) was used to determine the thiol groups (‑SH) and disulfide bonds (S—S) of the samples. Buffer A (0.086 M Tris, 0.09 M Gly, and 4 mM Na2EDTA) and buffer B (buffer A with 0.5 % sodium dodecyl sulfate and 6 M urea) were prepared for the determination of free -SH and total -SH, respectively. After 1 mL of protein suspension (1 mg/mL) was added to 4 mL of buffer A or buffer B, 50 μL of Ellman’s reagent solution (4 mg/mL of DTNB in buffer A) was added to the sample and mixed uniformly. The resulting solutions were centrifuged at 10,000 g for 10 min, and the supernatant was collected to measure the absorbance at 412 nm using a spectrophotometer. The reaction buffer instead of protein solutions without DTNB was used as the control. The amount of total or free -SH (μmol/g protein) was calculated from the molar extinction coefficient at 13,600. The content of disulfide bonds (μmol/g protein) was calculated as half of the difference between the total -SH and free -SH.
2.6.4. Fitting secondary structure by Fourier transform infrared spectroscopy
The sample solution was freeze-dried for 12 h into powder and mixed with potassium bromide. Fourier transform infrared (FTIR) spectra of PPI with a wavenumber range of 4000–400 cm−1 were determined using a Nicolet iS50 (Thermo Fisher Scientific, China) at 25℃. The relative percentage of secondary structures was obtained by further processing spectral data using PeakFit software [15].
2.6.5. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis
The nonreducing and reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) of PPI from different treatments followed the method described by Jiang et al. [16]. Sample solutions (2 mg/mL) were diluted 1:1 with loading buffer (4 % w/v SDS, 20 % v/v glycerol, 0.02 % w/v bromophenol blue, 0.125 M Tris-HCl, with or without 20 % w/v β-mercaptoethanol) and were heated in boiling water for 3 min and cooled for later use. In addition, 12 μL of each sample was injected into the loading area of the gel made of 5 % stacking gel and 12 % separating gel. Bio-Rad Mini-PROTEAN II System Cell apparatus (Bio-Rad Laboratories Inc., Hercules, CA, USA) was used to perform electrophoresis at a voltage from 80 to 120 V. The gel was dyed with Coomassie brilliant blue R-250 and decolorized with a decolorizing solution (7.5 % (v/v) acetic acid and 5 % (v/v) methanol).
2.7. Emulsion preparation
The method of preparing the emulsion is determined based on our previous research with a slight modification [17]. For the sample preparation, the coarse emulsion comprised 10 mL of soybean oil and 90 mL (10 mg/mL) of pretreated PPI suspension (C, U5, P, U5P, and U6P), homogenized at a speed of 11,000 rpm for 2 min. Then, the coarse homogeneous emulsion was passed twice using a high-pressure homogenizer (SPCH-10, Shanghai, China) under the pressure of 70 MPa. Sodium azide (3 mM) was added to the emulsion to inhibit the reproduction of microorganisms during storage.
2.8. Storage stability of PPI emulsions
The storage stability of the PPI emulsion was evaluated by measuring the difference in particle size and polydispersity index (PDI) using a Mastersizer 2000 Laser particle size analyzer instrument (Malvern Instruments ltd., Worcestershire, UK) between the fresh emulsion and the emulsion after seven days of storage at room temperature (25℃). Before measurement, the emulsion was diluted 100-fold with 0.01 M phosphate buffer (pH = 7).
2.9. Zeta potential of PPI emulsions
The zeta potential was measured using a zeta-potential analyzer (Zeta Plus, Malvern, UK). Before measurement, the emulsion was diluted 100-fold with 0.01 M phosphate buffer (pH = 7).
2.10. Morphology of PPI emulsions
The micromorphology of emulsion droplets was visualized using a Leica TCS SP8 confocal laser scanning microscope (Leica, Heidelberg, Germany), as described by Liu et al. [18]. Nile blue (20 μL, 0.1 % w/v in water) for protein staining and Nile red (25 μL, 0.1 % w/v in ethanol) for oil staining were added to 1 mL of emulsion and incubated for 30 min under dark conditions. Observations of the emulsion were conducted by exciting the Nile blue at 633 nm and the Nile red at 488 nm.
2.11. Statistical analysis
Three sample batches were prepared, and measurements were conducted in triplicate. The data were expressed as the average value plus or minus the standard deviation (SD) and were analyzed using the general linear model procedure from the Statistix 8.1 software package (Analytical Software, St. Paul, MN, USA). The significance between the means (p < 0.05) was identified using the one-way analysis of variance with Tukey’s multiple comparisons.
3. Results and discussion
3.1. Solubility
Good solubility is a prerequisite for food protein processing and utilization and is related to various functional properties of proteins. As depicted in Fig. 1A, the solubility of native PPI was relatively low (13.53 %) because the highly ordered rigid structure in the protein tends to aggregate into insoluble particles with relatively poor functional properties [19]. As expected, the solubility of PPI treated with pH-shifting alone significantly increased (p < 0.05) to 63.52 %. For the PPI treated with HIU alone, the solubility significantly increased (p < 0.05) with increased ultrasonic power (200–600 W). In the case of cotreated PPI, however, the solubility further significantly increased (p < 0.05) compared with a single treatment (ultrasound or pH-shifting) and reached the maximum (82.42 %) value in the U5P, indicating the HIU and pH-shifting treatment had a synergistic effect on the increase in PPI solubility.
Fig. 1.
Effects of high-intensity ultrasound (200–600 W) and pH12-shifting treatment, alone (U2, U3, U4, U5, U6, and P) or combined (U2P, U3P, U4P, U5P, and U6P), on the (A) solubility and (B) appearance of pea protein (PPI) in water. Native PPI was used as the control (C). Uppercase (A–F) and lowercase (a–f) letters indicate significant differences in the solubility of PPI with HIU treatment alone and with the combination of pH12-shifting and HIU, respectively (p < 0.05).
Similar to soybean protein isolate, the PPI also undergoes partial protein unfolding during pH-shifting processing and forms a molten globular structure under extremely alkaline conditions [16]. The change in protein structure enhances the ability of protein-water interaction, leading to an increase in the water solubility of PPI [11]. Moreover, the intense mechanical shear forces, local high temperature, and pressure generated by ultrasonic transient cavitation further destroyed the molten globular structure and inhibited protein refolding, reducing the PPI particle size by exacerbating collisions between protein aggregates [20]. The solubility of proteins in the U6P was lower than that in U5P, presumably because the combined treatment under higher power (600 W) might cause an excessive processing effect on the sample, resulting in protein reaggregation to a certain degree. Similar to the findings of Liu et al. [11], myofibrillar proteins over-treated with ultrasound (600 W) produced flocculation, resulting in reduced solubility.
The appearance of PPI (10 mg/mL in 0.01 M phosphate buffer) after 7 days of storage at room temperature (25℃) was observed to visually check the solubility changes for various treatment groups (Fig. 1B). Similar to the solubility results, there were relatively more precipitates at the bottom of the native PPI (C) bottle, corresponding to its insolubility and instability. With the increase in ultrasound power, the precipitation of PPI treated with HIU alone gradually decreased. The PPI suspension treated with the combination was more evenly dispersed in water, and precipitation almost disappeared in the U5P group. However, the precipitation in the U6P group seemed to reappear, confirming the solubility results (Fig. 1A).
3.2. Particle Size, zeta Potential, and Confocal laser scanning microscopy
The particle size and electrostatic interaction forces play an important role in understanding the internal relationship between protein interactions and structural properties. The average particle size volume, PDI, and CLSM results are presented in Fig. 2. The native PPI had irregularly shaped particles with relatively large particles and uneven distribution. As demonstrated by Gao et al [21], most proteins in PPI existed in an aggregated state maintained by strong intermolecular interactions, presenting many macroscopically visible insoluble aggregates in the PPI suspension system [22]. As a result, the CLSM image should develop an irregular and strong aggregation morphology, as seen in C in Fig. 2A.
Fig. 2.
(A) Confocal laser scanning microscopy (CLSM) micrographs, (B) average particle size volume, (C) polydispersity index (PDI), and (D) zeta potential of the aqueous suspensions of pea proteins (PPI) treated with high-intensity ultrasound (200–600 W) and pH12-shifting treatment alone (U2, U3, U4, U5, U6 and P) or combined (U2P, U3P, U4P, U5P and U6P). Native PPI was used as the control (C). Uppercase (A–E) and lowercase (a–e) letters indicate significant differences in average particle size volume and zeta potential of PPI with HIU treatment alone and with a combination of pH12-shifting and HIU, respectively (p < 0.05).
Similarly, as the HIU power increases (200–600 W), the particle size decreases in PPI treated with HIU alone (U2–U6 in Fig. 2B), and the PPI treated with pH-shifting alone likewise exhibited a significant reduction (P in Fig. 2B). Interestingly, under the same HIU power, CLSM revealed that the particle size of the combination-treated PPI was smaller than the others, and the dispersion in water was more uniform than that of the HIU and pH-shifting treatments alone. In the U5P, the particle size and PDI of PPI reached the minimum (Fig. 2B and 2C), and the most uniform protein distribution could be observed (p < 0.05), consistent with the solubility analysis (Fig. 1).
A report suggested that ultrasound and alkaline shifting treatment could effectively reduce the particle size of the faba bean protein and improve its solubility [23]. According to Fang et al. [24], their coprocessing reduced the particle size of the soy protein isolate from 147.47 to 114.6 nm because of the strong mechanical force provided by ultrasound treatment (540 W for 5 min) and the electrostatic repulsion caused by pH-shifting.
Thus, the possible action mechanism in this process might be illustrated by the following two aspects. The protein was first induced into a molten globule structure under extreme alkaline conditions, reducing partial protein unfolding results in interactions between side chains (hydrophobic interactions, disulfide bonds, and hydrogen bonds). The strong physical force exerted by HIU further disrupted the intermolecular noncovalent interactions (hydrogen bonding, electrostatic, and hydrophobic interactions), depolymerizing the flexible structure into small protein particles [25].
In contrast, the pH-shifting caused PPI to be far away from its isoelectric point (PI 4.6), resulting in strong electrostatic repulsion, whereas HIU accelerated the collision rate between unstable protein aggregates. The superposition effect of the cotreatment caused the uniform dispersion of protein particles in water [26], [27]. Therefore, the two mechanisms explain how PPI with synergistic treatment could be more uniformly dispersed while reducing particle size.
In addition, a significantly increased particle size caused by protein reaggregation was observed in U6P, similar to the findings reported by Zhu et al. [28], who concluded that overprocessing of walnut protein by ultrasound (600 W for 30 min) caused an increase in particle size. We speculate that excessive HIU power under pH-shifting treatment would produce uncontrollable cavitation effects, generating highly reactive free radicals, intense and excessive particle collisions, and internal overheating, causing the reaggregation of separated aggregates. Therefore, it is necessary to optimize the ultrasound parameters to avoid overprocessing that negatively affects the functional properties of PPI.
The zeta potential represents the surface charge characteristics of a protein. As observed in Fig. 2D, for the control, the net negative charge was relatively low (‑12.2 mV) due to carboxyl deprotonation [29]. Whether it was HIU alone, pH-shifting alone, or both, the net negative potential of the protein was significantly increased (p < 0.05). The maximum net negative potential (-29.8 mV) was observed in U5P. Generally, the high net charge system was not easy to aggregate and was relatively stable because of the strong electrostatic repulsion between protein particles [15], consistent with Fig. 1, Fig. 2A. The combination of HIU and pH-shifting treatment may change the conformation of the protein to a certain extent, exposing the internal polar groups. Thus, with the ionization of the surface polar groups, more charges accumulate on the molecule surface to obtain a higher net negative charge [15].
Nevertheless, the net negative charge of the U6P was significantly lower than U5P, which may be due to the reaggregation of the protein reburying the polar groups that have been exposed on the molecular surface inside the protein. Furthermore, Xiong et al. [30] concluded that the surface charge of proteins was affected by the balance between hydrophilic polar residues and hydrophobic apolar residues. Therefore, the next step was to characterize the PPI structure to verify the hypothesis on this series of action mechanisms.
3.3. Fluorescence Spectroscopy, surface Hydrophobicity, sulfhydryl groups and disulfide bonds
Fluorescence spectroscopy and surface hydrophobicity could be used as indicators to evaluate changes in the tertiary structure of proteins. The fluorescence intensity (Fig. 3A and B) and surface hydrophobicity (Fig. 3C) of PPI were augmented significantly with the rise in HIU power (p < 0.05). Compared with the HIU and pH-shifting treatment alone, the combination treatment had a greater increase, and both reached the maximum value in U5P. Generally, a higher fluorescence intensity increase results in a greater degree of protein unfolding and tryptophan residue exposure [31]. Surface hydrophobicity was influenced by the number of hydrophobic regions on the protein surface in contact with polar media [32]. The increase in surface hydrophobicity may be caused by the protein unfolding, exposing the hydrophobic regions originally embedded in the interior. A stable and uniformly dispersed system was obtained by adjusting the balance of the hydrophobic and hydrophilic groups. These results had a trend similar to the particle size and zeta potential results in Fig. 2, further confirming the hypothesis concerning the mechanism of solubility change.
Fig. 3.
Structural characteristics of pea proteins (PPI) treated with high-intensity ultrasound (HIU; 200–600 W) and pH12-shifting treatment alone or combined. (A and B) Intrinsic emission fluorescence spectra, (C) surface hydrophobicity, and (D) sulfhydryl groups (-SH) and disulfide bond (S—S). Native PPI was used as the control (C). Uppercase (A–F) and lowercase (a-f) letters indicate significant differences in the surface hydrophobicity of PPI with HIU treatment alone and with a combination of pH12-shifting and HIU, respectively (p < 0.05).
However, the fluorescence intensity and surface hydrophobicity of U6P decreased. First, it may be due to oxidation or overheating caused by partially overprocessing, denaturing the protein and leading to repolymerization so that exposed tryptophan residue and hydrophobic groups were reburied. Second, the excessive exposed hydrophobic groups strengthened the connection between protein molecules through hydrophobic interaction, one of the most important forces for maintaining the protein structure, resulting in a decrease in the surface hydrophobicity of the protein [11].
The determination of free -SH was used to further evaluate changes in protein conformation. The total -SH, free -SH and S—S content are presented in Fig. 3D. As HIU power increases, the free -SH and total -SH of PPI increased significantly, whereas the S—S tended to decrease, and the combination treatment presented more marked and extensive changes than single treatments alone (p < 0.05).
There may be two main reasons for the increase in free -SH content. First, the unfolding of the protein structure caused by the combination treatment exposed the internal -SH and converted it into free -SH. Second, the S—S of the PPI was cleaved to form new free -SH, as evidenced by the decrease in S—S and the increase in the total ‑SH [28].
Generally, the legumin of PPI is a hexamer formed by S—S bonding of six subunits via the acidic α-chain and basic β-chain, containing a large amount of sulfur-containing amino acids (mainly methionine and cysteine) [19]. Hence, sulfhydryl-disulfide interchange may also be attributed to legumin. In addition, as shown in Fig. 3D, the significant decrease in free -SH content in U6P may be attributed to the deprotonation of free -SH into reactive and easily oxidized mercaptan ion species during pH-shifting treatment, which was oxidized by the prooxidative factor (hydrogen peroxide, hydroxyl radical, and reactive oxygen species) produced by at excess power (600 W) [33].The decrease in total -SH and the insignificant change in S—S reconfirmed this view (Fig. 3D).
3.4. Fitting secondary structure by Fourier transform infrared spectroscopy
The changes in the content of the secondary structure of PPI were calculated using FTIR. As the FTIR spectra revealed in Fig. 4, different treatments had little effect on the FTIR spectra results of PPI, indicating that no significant change occurred in the protein polypeptide backbone (Fig. 4A and 4B). The amide I band (1700 to 1600 cm−1) related to C O stretching is sensitive to changes in the protein secondary structure. Hence, we deconvoluted the amide I band, as displayed in Fig. 4C and 4D, and calculated the relative content of the secondary structure (Table 1). The spectral ranges of 1692 to 1662, 1662 to 1645, and 1645 to 1638 cm−1 correspond to the β-turn, α-helix, and random coil structures, respectively, and the ranges of 1700 to 1692 and 1638 to 1615 cm−1 correspond to the β-sheet structure [17].
Fig. 4.
(A and B) Fourier transform infrared spectroscopy and (C and D) deconvoluted the amide I band of pea proteins (PPI) treated with high-intensity ultrasound (200–600 W) and pH12-shifting treatment alone (U2, U3, U4, U5, U6, and P) or combined (U2P, U3P, U4P, U5P, and U6P). Native PPI was used as the control (C).
Table 1.
Secondary structure of pea proteins (PPI) treated with high-intensity ultrasound (200–600 W) and pH12-shifting treatments alone (U2, U3, U4, U5, U6, and P) or combined (U2P, U3P, U4P, U5P, and U6P).
| Ultrasound power (W) |
α-Helix(%) |
β-Sheets(%) |
β-Turn(%) |
Random coils(%) |
||||
|---|---|---|---|---|---|---|---|---|
| U | U + pH | U | U + pH | U | U + pH | U | U + pH | |
| 0 | 25.09 ± 0.26A | 20.01 ± 0.08A | 29.99 ± 0.43D | 32.49 ± 0.12F | 28.74 ± 0.33A | 25.43 ± 0.04A | 16.18 ± 0.53C | 22.07 ± 0.09D |
| 200 | 20.47 ± 0.14B | 19.09 ± 0.12B | 32.70 ± 0.22C | 33.08 ± 0.11E | 25.45 ± 0.14B | 25.24 ± 0.06AB | 21.37 ± 0.19B | 22.59 ± 0.12C |
| 300 | 19.23 ± 0.05C | 18.59 ± 0.32C | 32.87 ± 0.13C | 33.78 ± 0.09D | 25.57 ± 0.07B | 25.03 ± 0.10BC | 22.33 ± 0.09A | 22.60 ± 0.08C |
| 400 | 19.10 ± 0.11C | 17.43 ± 0.11D | 33.03 ± 0.08BC | 34.28 ± 0.07C | 25.33 ± 0.08B | 24.94 ± 0.03C | 22.54 ± 0.07A | 23.35 ± 0.14B |
| 500 | 18.55 ± 0.06D | 17.08 ± 0.02D | 33.50 ± 0.11B | 34.57 ± 0.10B | 25.28 ± 0.11B | 24.59 ± 0.09D | 22.66 ± 0.11A | 23.76 ± 0.04A |
| 600 | 18.32 ± 0.03D | 16.55 ± 0.24E | 34.90 ± 0.07A | 35.14 ± 0.03A | 24.15 ± 0.15C | 24.40 ± 0.13D | 22.64 ± 0.13A | 23.91 ± 0.08A |
Values are given as the mean ± SD; Different uppercase letters (A–F) means in the same column with different letters differ significantly (p < 0.05).
Compared with the native PPI, the PPI treated with pH-shifting and HIU alone exhibited varying degrees of reducing α-helix and β-turn content. For HIU-treated PPI, the α-helix and β-turn decrease, and the β-sheets and random coil fractions increase as the HIU power increases, whereas the combination treatment displayed far more extensive structure fraction changes in comparison (p < 0.05). These results are similar to those reported by other researchers who applied ultrasound and pH-shifting to amaranth protein [34] and rapeseed protein [15].
Generally, the α-helix and β-turn are highly ordered and relatively stable secondary structures [35]. Their decrease may be related to the destruction of intra-chain hydrogen bonds formed by imino hydrogen (N—H) and carbonyl oxygen (C O). The β-sheet is a structure that exists in a relatively stretched state, and the structure of the peptide chain that constitutes a random coil has a nonrepetitive and relatively unstable structure [36]. Their increase illustrates the transformation of PPI from a regular stable structure to an out-of-order elastic structure. Furthermore, an increase in the percentage of the β-sheet was accompanied by exposure to hydrophobic areas [26], consistent with the surface hydrophobicity results (Fig. 3C).
3.5. Sodium dodecyl Sulfate-polyacrylamide gel electrophoresis
The protein compositions of PPI in different treatments were discussed by nonreducing and reducing SDS-PAGE. As presented in Fig. 5A, all samples exhibited banding distribution typical of PPI in the absence of β-ME, where the major visualized bands include lipid oxygenase (∼100 kDa), convicilin (∼70–75 kDa), legumin αβ (∼60 kDa), and the three vicilin subunits (∼50 kDa, ∼32–34 kDa, and–18–19 kDa) that comprise trimer vicilin [19]. As previously described, the legumin is a hexamer formed by the acidic α-chain and basic β-chain through S—S bonding; hence, once the S—S inhibitors (β-ME) were added, the legumin αβ was decomposed into legumin α (∼40 kDa) and legumin β (∼20–25 kDa), as demonstrated in Fig. 5B.
Fig. 5.
(A and C) Nonreducing and (B and D) reducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis pattern of (A and B) solutions and (C and D) supernatants of pea proteins (PPI) treated with high-intensity ultrasound (200–600 W) and pH12-shifting treatment alone (U2, U3, U4, U5, U6, and P) or combined (U2P, U3P, U4P, U5P, and U6P). Native PPI was used as the control (C). M: mark.
Moreover, no apparent signs of protein degradation were observed, alone or in the combination treatment, indicating that these treatment processes did not involve fracturing or shortening polypeptide chains. However, in the case of pH-shifting-treated PPI, a prominent decrease in convicilin and legumin αβ levels was observed, particularly in the combination treatment. Neither legumin α nor legumin β bands were observed but reappeared in the case of reducing SDS-PAGE (Fig. 5B). Based on the S‑S content results in Fig. 3D, we speculate that the pH-shifting may induced the depolymerization of legumin αβ and subsequently formed a soluble polymer through a disulfide bridge (high molecular weight accumulation was observed in SDS-PAGE).
The SDS-PAGE of sample supernatant after centrifugation was observed to further identify the protein profile of water-soluble PPI. As illustrated in Fig. 5C, all samples had lower expression levels of the legumin band because legumin is a salt-soluble storage protein with a rigid conformation [8]. Furthermore, the expression level of the bands increased with HIU power both for single and combination treatment, implying an increase in solubility. Notably, a large molecular-weight oligomer was present at the top of the gel in the samples treated either alone or in combination, and its expression level increased with the HIU power increase. When the samples were treated with β-ME (Fig. 5D), the oligomer band disappeared, whereas the legumin α and legumin β bands were recovered and increased with HIU power.
According to Jiang et al. [8], the development of the molten sphere structure is usually accompanied by the cleavage of S—S during pH-shifting treatment, leading to the breakdown of legumin αβ to legumin α and legumin β. Simultaneously, the higher pH also promotes the reactivity of sulfur-containing amino acids, resulting in a degree of sulfhydryl-disulfide interchange, which initiates intermolecular cross-linking and forms soluble aggregates [37].
Moreover, the results also reveal that HIU treatment promotes the form of soluble aggregates (Fig. 5C). There have been studies demonstrating that the cavitation and mechanical force of ultrasound lead to the water-splitting reaction and generate highly reactive free radicals, whereas the attack of free radicals on sulfur-containing amino acids can also lead to intermolecular disulfide cross-linking [38]. Overall, both pH-shifting and HIU treatment may dissociate the hexameric legumin, inducing intermolecular disulfide bond cross-linking and molecular rearrangement, and a certain synergistic effect may exist between them. In addition, the formation of soluble aggregates is expected to provide a more steric hindrance and maintain the dispersion distance between protein particles, which may also be the reason for the increase in solubility [11].
3.6. Characterization of PPI emulsions
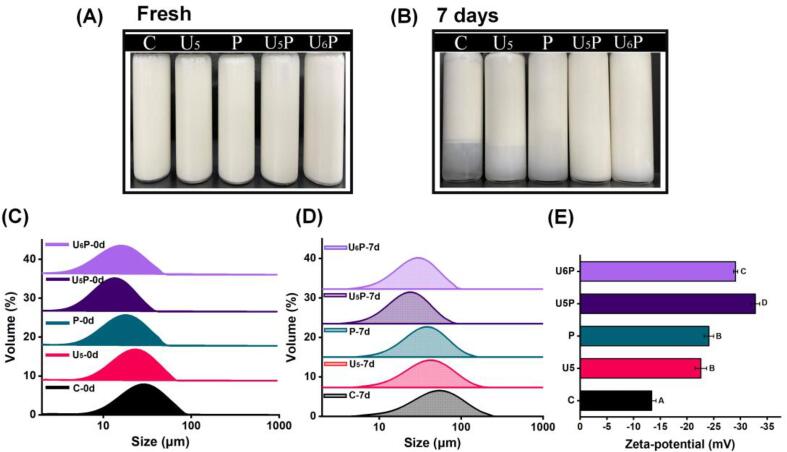
For water-soluble amphiphilic biomacromolecules, its emulsifying properties are closely related to solubility because solubility determines whether molecules can be rapidly unfolded and oriented at the interface. Hence, we selected the control, U5, P, U5P, and U6P to prepare the emulsion based on the above results and analyzed the particle size (d4,3), PDI, and droplet size distribution for fresh and 7-day storage emulsions. As presented in Fig. 6 and Table 2, all fresh emulsions displayed a homogeneous milky white (Fig. 6A), and the emulsion prepared with the control had a larger particle size (29.47 μm) with a wider droplet size distribution (Fig. 6C and Table 2).
Fig. 6.
(A and B) Appearance and (C and D) particle size distribution of fresh and 7-day storage emulsions stabilized by pea proteins (PPI) treated with high-intensity ultrasound and pH12-shifting treatments alone (U5 and P) or combined (U5P and U6P). (E) Zeta potential of fresh emulsions stabilized by pea proteins (PPI) treated with high-intensity ultrasound and pH12-shifting treatments alone (U5 and P) or combined (U5P and U6P). The emulsion stabilized with native PPI was used as the control (C). Letters (A–D) indicate significant differences in the zeta potential of the various treatments (p < 0.05).
Table 2.
Particle size (d4,3) and polydispersity index (PDI) of fresh and 7-day storage emulsions stabilized by pea proteins (PPI) treated with high-intensity ultrasound and pH12-shifting treatments alone (U5 and P) or combined (U5P and U6P). The emulsion stabilized by native PPI was used as the control (C).
| Treatment group |
d4,3 (μm) |
PDI |
||
|---|---|---|---|---|
| day 0 | day 7 | day 0 | day 7 | |
| C | 29.47 ± 0.93A | 59.83 ± 2.02A | 0.47 ± 0.02AB | 0.60 ± 0.03A |
| U5 | 23.31 ± 0.77B | 47.27 ± 1.77B | 0.45 ± 0.02AB | 0.59 ± 0.04A |
| P | 19.55 ± 0.31C | 39.54 ± 1.12C | 0.46 ± 0.03AB | 0.49 ± 0.02B |
| U5P | 13.01 ± 0.11E | 27.68 ± 0.97E | 0.43 ± 0.01B | 0.46 ± 0.01B |
| U6P | 16.38 ± 0.09D | 32.66 ± 1.04D | 0.49 ± 0.02A | 0.47 ± 0.02B |
Values are given as the mean ± SD; Different uppercase letters (A–E) means in the same column with different letters differ significantly (p < 0.05).
Upon treating PPI with HIU at 500 W or pH-shifting alone, the particle size of the emulsion was significantly reduced, and the particle dispersion became more uniform than before (p < 0.05). In the case of the combination treatment, however, the particle size of the emulsion was further significantly reduced, especially at 500 W HIU power (U5P), which reduced to 13.01 μm and had the most uniform droplet distribution of all samples (Table 2), indicating a potential synergistic effect between the HIU and pH-shifting treatment on improving the PPI emulsifying properties. In general, the proteins with high solubility and a small particle size migrated more easily and faster from the aqueous phase to the surface of the oil droplets during homogenization, effectively inhibiting the tendency of re-fusion between droplets [23]. Moreover, the extremely alkaline environment and mechanical shear force generated by ultrasonic cavitation made the protein conformation more flexible, as confirmed by the above results (Fig. 3, Fig. 4). This outcome may provide an opportunity to efficiently cover emulsion droplets during the process of protein rearrangement at the O/W interface [33]. In addition, the exposure of hydrophobic groups promotes the protein-oil interaction and accelerates the instantaneous change process of the reorientation of hydrophilic and hydrophobic groups, leading to the rapid formation of an emulsion system with a small droplet size and relatively uniform distribution [39], [40].
After 7 days of storage, both the control and single treated samples exhibited varying degrees of phase separation, wheres the U5P stabled emulsion still maintained a uniform milky-white appearance (Fig. 6B). The droplet size distribution peak of the emulsion after storage shifted significantly to the right due to increased overall particle size (Fig. 6D). However, the change in particle size exhibited the same trend as that of the fresh emulsion, and the smallest particle size and relatively uniform distribution were still observed in the case of U5P (Table 2), proving that the emulsion stabilized by cotreated PPI had better storage stability.
The main instability mechanisms during emulsion storage are coalescence and flocculation [3]. A protein with a flexible structure tends to form a viscoelastic interface film, creating a steric hindrance between the oil droplets to inhibit the flocculation of droplets during storage. A moderate increase in surface hydrophobicity promotes protein–protein interaction at the O/W interface, enhancing the rigidity of the interface membrane [41]. This finding is similar to the finding by Ma et al. [31]. They proposed that the cross-linking of the disulfide bonds and the increase in surface hydrophobicity of the interface protein during the rearrangement of interfacial proteins had a positive effect on the compactness of the interface membrane, improving the emulsion stability. In addition, the particle size and PDI of the U6P stabilized emulsion were significantly increased before and after storage compared to those of U5P (p < 0.05). This outcome may be due to the excessive exposure of hydrophobic groups and re-aggregation of proteins, leading to a strong association of interfacial protein molecules and reducing the emulsion storage stability [39].
Higher electrostatic repulsion is a key factor in improving the storage stability of emulsions and uniformly dispersing the droplets [42]. As depicted in Fig. 6E, the zeta potential of the emulsion coated by PPI treated with HIU or pH-shifting alone increased significantly compared to the control emulsion (p < 0.05), consistent with previous results (Fig. 2D). The emulsion coated by U5P exhibited the highest net negative charge value (–32.8 mV), indicating its higher electrostatic repulsion. After the protein with a net negative charge completely covers the droplet surface, a sufficiently strong electrostatic repulsion force generates to disperse the droplet uniformly and maintains a certain distance between the droplets during storage to avoid unstable behavior, such as flocculation.
In contrast, an increase in emulsion droplet size and decrease in net negative potential occurred in U6P, perhaps because of the overexposure of hydrophobic sites, as discussed in Section 3.3 and presented in Fig. 3C. Likewise, Arredondo-Parada et al. [43] stated that the cavitation-mediated charge reduction of squid proteins is related to the migration of hydrophobic apolar sites, since most proteins have non-polar hydrophobic residues (alkyls and aromatic groups) and hydrophilic polar groups (–OH and –NH2), whose balance can change the surface charge distribution to some extent.
3.7. Morphology of PPI emulsions
To visualize oil droplets and protein distribution in the PPI emulsion, CLSM was used to observe the microscopic morphology of the fresh emulsion. As depicted in Fig. 7, large oil droplets with irregular spherical shapes were observed. A possible explanation for this phenomenon is that the protein aggregates with rigid structures could not be quickly adsorbed on the surface of the oil droplets and spread to form a dense interfacial film during homogenization, leading to the fusion of the closer droplets [44].
Fig. 7.
Confocal laser scanning microscopy (CLSM) micrographs and schematic diagram of the emulsions stabilized by pea proteins (PPI) treated with high-intensity ultrasound and pH12-shifting treatments alone (U5 and P) or combined (U5P and U6P). The emulsion stabilized by native PPI was used as the control (C).
In addition, the image of the control indicated that, although numerous proteins (dyed green) exist around the oil droplets (dyed red), the interface of the protein membrane structure is not continuous, and some oil droplets are not fully coated. This result could also be demonstrated by the protein phase image, in which only some irregular voids and a few incomplete ring structures were observed, and many proteins form clusters with proteins attached to the surfaces of larger droplets (Fig. 7). Therefore, we propose that the formation of the unstable emulsion structure in the control was mostly contributed by the simple superposition and accumulation of aggregated protein and oil droplets of different sizes.
Similarly, a decrease in droplet size and increase in distribution uniformity were observed after various treatments in PPI, and the smallest particles with the most uniform distribution were observed in the combination treatment (U5P in Fig. 7). Additionally, the clustered protein aggregates disappeared, and the proteins were uniformly localized on the droplet surface. These results indicate a greater coating efficiency of U5P on oil droplets due to higher emulsifying activity, contributing to the emulsion stability. According to Jiang et al.[8], the molten globular structure formed during pH-shifting treatment has higher surface activity, which can be effectively loaded at the O/W interface. In addition, the larger hydrodynamic volume of the interfacial molten globule provides more steric hindrance, maintaining the separation distance between droplets. In the present case, the partially unfolded pea peptides under the action of HIU and pH-shifting co-treatment were more readily loaded onto the droplet surface via hydrophobic interactions than PPI treated with pH-shifting alone, as illustrated in the schematic diagram in Fig. 7. Furthermore, strong electrostatic repulsion between droplets was expected to counteract the van der Waals attraction between nonpolar groups, and a thicker interfacial layer compensates for hydrophobic interactions between hydrophobic groups, facilitating the emulsion stability based on the DLVO theory [45]. However, the excessive HIU power reduced the emulsion stability, as illuminated by the reassembly of the protein clusters (U6P in Fig. 7), which may arise due to the protein oxidation and increased associations of the exposed hydrophobic core induced by the cavitation.
4. Conclusions
This study demonstrates that the ultrasound and pH-shifting treatments have a synergistic effect in effectively improving the solubility and emulsion stability of PPI. This effect may be attributed to the disintegration of highly ordered rigid polymers caused by pH-shifting. The intense physical force applied by ultrasound further unfolds the protein, causing the internal polar and hydrophobic groups to be exposed, increasing solubility. In addition, during the emulsification process, the synergistic treatment promotes the formation of a strong viscoelastic interface film by accelerating the adsorption and rearrangement of the protein at the oil–water interface, improving the emulsion stability. The PPI treated with the combination at 500 W of HIU power and its coated emulsion have the smallest particle size, highest zeta potential, and most uniform distribution. Overall, the combination treatment with ultrasound and pH-shifting is a potential method to effectively improve the solubility and emulsion stability of PPI by modifying the protein structure, allowing the modified PPI to function as surfactants, bioactive compounds, and other food or pharmaceutical additives.
CRediT authorship contribution statement
Jingnan Zhang: Conceptualization, Formal analysis, Investigation, Writing – original draft, Visualization. Qian Liu: Investigation, Software. Qian Chen: Formal analysis, Investigation, Visualization. Fangda Sun: Writing – review & editing. Haotian Liu: Supervision, Project administration. Baohua Kong: Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was funded by the Major Science and Technology Projects in Heilongjiang province (2021ZX12B05, 2020ZX07B72).
Contributor Information
Haotian Liu, Email: liuht920@neau.edu.cn.
Baohua Kong, Email: kongbh63@hotmail.com.
Data availability
Data will be made available on request.
References
- 1.Almeida A.G., Moreno Y.M.F., Carciofi B.A.M. Plant proteins as high-quality nutritional source for human diet, Trends. Food Sci. Technol. 2020;97:170–184. doi: 10.1016/j.tifs.2020.01.011. [DOI] [Google Scholar]
- 2.Arteaga V.G., Guardia M.A., Muranyi I., Eisner P., Weisz U.S. Effect of enzymatic hydrolysis on molecular weight distribution, techno-functional properties and sensory perception of pea protein isolates. Innov. Food Sci. Emerging. 2020;65 doi: 10.1016/j.ifset.2020.102449. [DOI] [Google Scholar]
- 3.Burger T.G., Zhang Y. Recent progress in the utilization of pea protein as an emulsifier for food applications. Trends Food Sci. Tech. 2019;86:25–33. doi: 10.1016/j.tifs.2019.02.007. [DOI] [Google Scholar]
- 4.Zhang S., Huang W., Roopesh M.S., Chen L. Pre-treatment by combining atmospheric cold plasma and pH-shifting to prepare pea protein concentrate powders with improved gelling properties. Food Res. Int. 2022;154 doi: 10.1016/j.foodres.2022.111028. [DOI] [PubMed] [Google Scholar]
- 5.Zha F., Dong S., Rao J., Chen B. Pea protein isolate-gum Arabic Maillard conjugates improves physical and oxidative stability of oil-in-water emulsions. Food Chem. 2019;285:130–138. doi: 10.1016/j.foodchem.2019.01.151. [DOI] [PubMed] [Google Scholar]
- 6.Klost M., Drusch S. Functionalisation of pea protein by tryptic hydrolysis – Characterisation of interfacial and functional properties. Food Hydrocoll. 2019;86:134–140. doi: 10.1016/j.foodhyd.2018.03.013. [DOI] [Google Scholar]
- 7.Bu F., Nayak G., Bruggeman P., Annor G., Ismail B.P. Impact of plasma reactive species on the structure and functionality of pea protein isolate. Food Chem. 2022;371 doi: 10.1016/j.foodchem.2021.131135. [DOI] [PubMed] [Google Scholar]
- 8.Jiang J., Zhu B., Liu Y., Xiong Y.L. Interfacial structural role of pH-shifting processed pea protein in the oxidative stability of oil/water emulsions. J. Agric. Food Chem. 2014;62:1683–1691. doi: 10.1021/jf405190h. [DOI] [PubMed] [Google Scholar]
- 9.Jiang J., Wang Q., Xiong Y.L. A pH shift approach to the improvement of interfacial properties of plant seed proteins. Curr. Opin. Food Sci. 2018;19:50–56. doi: 10.1016/j.cofs.2018.01.002. [DOI] [Google Scholar]
- 10.Sun Q., Zhao X., Zhang C., Xia X., Sun F., Kong B. Ultrasound-assisted immersion freezing accelerates the freezing process and improves the quality of common carp (Cyprinus carpio) at different power levels, LWT-Food. Sci. Technol. 2019;108:106–112. doi: 10.1016/j.lwt.2019.03.042. [DOI] [Google Scholar]
- 11.Liu H., Zhang H., Liu Q., Chen Q., Kong B. Solubilization and stable dispersion of myofibrillar proteins in water through the destruction and inhibition of the assembly of filaments using high-intensity ultrasound. Ultrason. Sonochem. 2020;67 doi: 10.1016/j.ultsonch.2020.105160. [DOI] [PubMed] [Google Scholar]
- 12.Sha L., Koosis A.O., Wang Q., True A.D., Xiong Y.L. Interfacial dilatational and emulsifying properties of ultrasound-treated pea protein. Food Chem. 2021;350 doi: 10.1016/j.foodchem.2021.129271. [DOI] [PubMed] [Google Scholar]
- 13.Haskard C.A., Li-Chan E.C.Y. Hydrophobicity of bovine serum albumin and ovalbumin determined using uncharged (PRODAN) and anionic (ANS\r, -\r,) fluorescent probes. J. Agr. Food Chem. 1998;46:2671–2677. doi: 10.1021/jf970876y. [DOI] [Google Scholar]
- 14.He X., Chen J., He X., Feng Z., Li C., Liu W., Dai T., Liu C. Industry-scale microfluidization as a potential technique to improve solubility and modify structure of pea protein. Innov. Food Sci. Emerging. 2021;67 doi: 10.1016/j.ifset.2020.102582. [DOI] [Google Scholar]
- 15.Li Y., Cheng Y., Zhang Z., Wang Y., Mintah B.K., Dabbour M., Jiang H., He R., Ma H. Modification of rapeseed protein by ultrasound-assisted pH shift treatment: Ultrasonic mode and frequency screening, changes in protein solubility and structural characteristics. Ultrason. Sonochem. 2020;69 doi: 10.1016/j.ultsonch.2020.105240. [DOI] [PubMed] [Google Scholar]
- 16.Jiang J., Chen J., Xiong Y.L. Structural and emulsifying properties of soy protein isolate subjected to acid and alkaline pH-shifting processes. J. Agric. Food Chem. 2009;57:7576–7583. doi: 10.1021/jf901585n. [DOI] [PubMed] [Google Scholar]
- 17.Liu H., Zhang J., Wang H., Chen Q., Kong B. High-intensity ultrasound improves the physical stability of myofibrillar protein emulsion at low ionic strength by destroying and suppressing myosin molecular assembly. Ultrason. Sonochem. 2021;74 doi: 10.1016/j.ultsonch.2021.105554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H., Han G., Zhang H., Liu Q., Kong B. Improving the physical and oxidative stability of emulsions based on the interfacial electrostatic effects between porcine bone protein hydrolysates and porcine bone protein hydrolysate-rutin conjugates. Food Hydrocoll. 2019;94:418–427. doi: 10.1016/j.foodhyd.2019.03.037. [DOI] [Google Scholar]
- 19.Boukid F., Rosell C.M., Castellari M. Pea protein ingredients: a mainstream ingredient to (re)formulate innovative foods and beverages. Trends Food Sci. Technol. 2021;110:729–742. doi: 10.1016/j.tifs.2021.02.040. [DOI] [Google Scholar]
- 20.Wang Y., Wang S., Li R., Wang Y., Xiang Q., Li K., Bai Y. Effects of combined treatment with ultrasound and pH shifting on foaming properties of chickpea protein isolate. Food Hydrocoll. 2022;124 doi: 10.1016/j.foodhyd.2021.107351. [DOI] [Google Scholar]
- 21.Gao K., Zha F., Yang Z., Rao J., Chen B. Structure characteristics and functionality of water-soluble fraction from high-intensity ultrasound treated pea protein isolate. Food Hydrocoll. 2022;125 doi: 10.1016/j.foodhyd.2021.107409. [DOI] [Google Scholar]
- 22.Zhu P., Huang W., Guo X., Chen L. Strong and elastic pea protein hydrogels formed through pH-shifting method. Food Hydrocoll. 2021;117 doi: 10.1016/j.foodhyd.2021.106705. [DOI] [Google Scholar]
- 23.Alavi F., Chen L., Djomeh Z.E. Effect of ultrasound-assisted alkaline treatment on functional property modifications of faba bean protein. Food Chem. 2021;354 doi: 10.1016/j.foodchem.2021.129494. [DOI] [PubMed] [Google Scholar]
- 24.Fang Z., Cai X., Wu J., Zhang L., Fang Y., Wang S. Effect of simultaneous treatment combining ultrasonication and pH-shifting on SPI in the formation of nanoparticles and encapsulating resveratrol. Food Hydrocoll. 2021;111 doi: 10.1016/j.foodhyd.2020.106250. [DOI] [Google Scholar]
- 25.Wang Y., Wang Y., Li K., Bai Y., Li B., Xu W. Effect of high intensity ultrasound on physicochemical, interfacial and gel properties of chickpea protein isolate. LWT-Food Sci. Technol. 2020;129 doi: 10.1016/j.lwt.2020.109563. [DOI] [Google Scholar]
- 26.Tian Y., Zhang Z., Zhang P., Taha A., Hu H., Pan S. The role of conformational state of pH-shifted β-conglycinin on the oil/water interfacial properties and emulsifying capacities. Food Hydrocoll. 2020;108 doi: 10.1016/j.foodhyd.2020.105990. [DOI] [Google Scholar]
- 27.O’sullivan J.J., Park M., Beevers J., Greenwood R.W., Norton I.T. Applications of ultrasound for the functional modification of proteins and nanoemulsion formation: a review. Food Hydrocoll. 2017;71:299–310. doi: 10.1016/j.foodhyd.2016.12.037. [DOI] [Google Scholar]
- 28.Zhu Z., Zhu W., Yi J., Liu N., Cao Y., Lu J., Decker E.A., McClements D.J. Effects of sonication on the physicochemical and functional properties of walnut protein isolate. Food Res. Int. 2018;106:853–861. doi: 10.1016/j.foodres.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 29.Carpentier J., Conforto E., Chaigneau C., Vendeville J.E., Maugard T. Complex coacervation of pea protein isolate and tragacanth gum: Comparative study with commercial polysaccharides. Innov. Food Sci. Emerg. 2021;69 doi: 10.1016/j.ifset.2021.102641. [DOI] [Google Scholar]
- 30.Xiong W., Wang Y., Zhang C., Wan J., Shah B.R., Pei Y., Zou B., Li J., Li B. High intensity ultrasound modified ovalbumin: Structure, interface and gelation properties. Ultrason. Sonochem. 2016;31:302–309. doi: 10.1016/j.ultsonch.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Ma W., Wang J., Xu X., Qin L., Wu C., Du M. Ultrasound treatment improved the physicochemical characteristics of cod protein and enhanced the stability of oil-in-water emulsion. Food Res. Int. 2019;121:247–256. doi: 10.1016/j.foodres.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 32.Lee H., Yildiz G., dos Santos L.C., Jiang S., Andrade J.E., Engeseth N.J., Feng H. Soy protein nano-aggregates with improved functional properties prepared by sequential pH treatment and ultrasonication. Food Hydrocoll. 2016;55:200–209. doi: 10.1016/j.foodhyd.2015.11.022. [DOI] [Google Scholar]
- 33.Gharibzahedi S.M.T., Smith B. The functional modification of legume proteins by ultrasonication: a review. Trends Food Sci. Tech. 2020;98:107–116. doi: 10.1016/j.tifs.2020.02.002. [DOI] [Google Scholar]
- 34.Gonzalez J.J.F., Calleros C.L., Carter E.J.V., Mandujano E.A., Ramirez J.A., Velasco A.M. Modifying the structure, physicochemical properties, and foaming ability of amaranth protein by dual pH-shifting and ultrasound treatments. LWT-Food Sci. Technol. 2022;153 doi: 10.1016/j.lwt.2021.112561. [DOI] [Google Scholar]
- 35.Dong X., Wang J., Raghavan V. Impact of microwave processing on the secondary structure, in-vitro protein digestibility and allergenicity of shrimp (Litopenaeus vannamei) proteins. Food Chem. 2021;337 doi: 10.1016/j.foodhyd.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 36.Zhu Y., Fu S., Wu C., Qi B., Teng F., Wang Z., Li Y., Zhou L. The investigation of protein flexibility of various soybean cultivars in relation to physicochemical and conformational properties. Food Hydrocoll. 2020;103 doi: 10.1016/j.foodhyd.2020.105709. [DOI] [Google Scholar]
- 37.Deleu L.J., Lambrecht M.A., Vondel J.V.D., Delcour J.A. The impact of alkaline conditions on storage proteins of cereals and pseudo-cereals. Curr. Opin. Food Sci. 2019;25:98–103. doi: 10.1016/j.cofs.2019.02.017. [DOI] [Google Scholar]
- 38.Kang D., Gao X., Ge Q., Zhou G., Zhang W. Effects of ultrasound on the beef structure and water distribution during curing through protein degradation and modification. Ultrason. Sonochem. 2017;38:317–325. doi: 10.1016/j.ultsonch.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 39.Hu Y., Wu Z., Sun Y., Cao J., He J., Dang Y., Pan D., Zhou C. Insight into ultrasound-assisted phosphorylation on the structural and emulsifying properties of goose liver protein. Food Chem. 2022;373 doi: 10.1016/j.foodchem.2021.131598. [DOI] [PubMed] [Google Scholar]
- 40.Yan C., Zhou Z. Solubility and emulsifying properties of phosphorylated walnut protein isolate extracted by sodium trimetaphosphate. LWT-Food Sci. Technol. 2021;143 doi: 10.1016/j.lwt.2021.111117. [DOI] [Google Scholar]
- 41.Yan S., Xu J., Zhang S., Li Y. Effects of flexibility and surface hydrophobicity on emulsifying properties: Ultrasound-treated soybean protein isolate. LWT-Food Sci. Technol. 2021;142 doi: 10.1016/j.lwt.2021.110881. [DOI] [Google Scholar]
- 42.Lu Y., Pan D., Xia Q., Cao J., Zhou C., He J., Sun Y., Xu S. Impact of pH-dependent succinylation on the structural features and emulsifying properties of chicken liver protein. Food Chem. 2021;358 doi: 10.1016/j.foodchem.2021.129868. [DOI] [PubMed] [Google Scholar]
- 43.Arredondo-Parada I., Torres-Arreola W., Suárez-Jiménez G.M., Ramírez-Suárez G.C., Juárez-Onofre G.E., Rodríguez-Félix F., Marquez-Rios E. Effect of ultrasound on physicochemical and foaming properties of a protein concentrate from giant squid (Dosidicus gigas) mantle. LWT-Food Sci. Technol. 2020;121 doi: 10.1016/j.lwt.2019.108954. [DOI] [Google Scholar]
- 44.Kim W., Wang Y., Selomulya C. Dairy and plant proteins as natural food emulsifiers. Trends Food Sci. Technol. 2020;105:261–272. doi: 10.1016/j.tifs.2020.09.012. [DOI] [Google Scholar]
- 45.Dickinson E. Strategies to control and inhibit the flocculation of protein-stabilized oil-in-water emulsions. Food Hydrocoll. 2019;96:209–223. doi: 10.1016/j.foodhyd.2019.05.021. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.