Abstract
Monkeypox–a zoonotic disease caused by the monkeypox virus, an orthopoxviruses family member. Recently monkeypox cases are increasing at an alarming rate in the US and worldwide. Health care professionals should keep a high index of suspicion for the disease in anyone with new onset fever, a vesicular or pustular rash with central umbilication, and lymphadenopathy. Such patients should be isolated at home or the hospital to prevent secondary transmission. The cases are typically self-limited, and most people only need home supportive care. However, as recommended by CDC, immunocompromised patients, pregnant patients, and children younger than eight years should be offered pre- or post-exposure prophylaxis with vaccines. The current outbreak explicitly targets a cohort of homosexual and gay patients. The role of sexual transmission of the virus needs to be explored further. Patients with severe symptoms or respiratory complications can also be treated with antivirals such as tecovirimat (TPOXX) and brincidofovir or with intravenous vaccinia immune globulin (VIGIV).
Keywords: Monkeypox, MSM, Antiviral treatment, Vaccination, Monkeypox rash
Introduction
The monkeypox virus, an orthopoxvirus family member, is the agent that causes the zoonotic disease known as Monkeypox. The variola virus, which induces smallpox, the vaccinia virus, utilized in the smallpox vaccine, and the cowpox virus, are additional family members [1].
According to the Centers for Disease Control and Prevention (CDC), more than 12,500 cases of the current monkeypox outbreak have been identified from more than 50 countries/territories as of July 17th, 2022 [2]. The regions of Europe, Africa, the Americas, the Eastern Mediterranean, and the Western Pacific comprise most of the confirmed cases' geographic distribution [3].
There is 1814 confirmed human monkeypox (HMPX) cases in the US as of July 17th, 2022, with California and New York leading the way [4]. Since we are unable to identify and report every case, the actual number is likely larger. According to preliminary data from the CDC, a significant proportion of patients involve gay, bisexual, and other men who have intercourse with men [5]. Finding appropriate evidence-based strategies to stop secondary transmission is vital, yet the issue is largely overlooked in the United States as case rates climb. Here, we present the case report of Monkeypox in a polygamous, homosexual male with a mini literature review.
Case presentation
A 26-year-old polygamous, homosexual man with a past medical history of syphilis and on tenofovir/emtricitabine for HIV pre-exposure prophylaxis (PrEP) came to the emergency room (ER) for evaluation of progressively worsening rash on his tongue and around the mouth that started five days before presentation. The patient had unprotected sexual intercourse (MSM) one day before the onset of the symptoms. The next morning, he woke up with lesions around his mouth, tongue, and face. The lesions deteriorated one day later, and he also had a low-grade fever. He went to urgent care the same day and was prescribed nystatin oral swish and swallow along with Valtrex for suspected HSV infection. The patient tested negative for COVID-19 at that time. Unfortunately, the patient’s symptoms continued to deteriorate despite being on treatment. Oral lesions increased in numbers, and he developed a sore throat, tongue swelling, burning sensation in his mouth, and difficulty/pain with swallowing solid food. The patient denied having any shortness of breath or difficulty swallowing liquids.
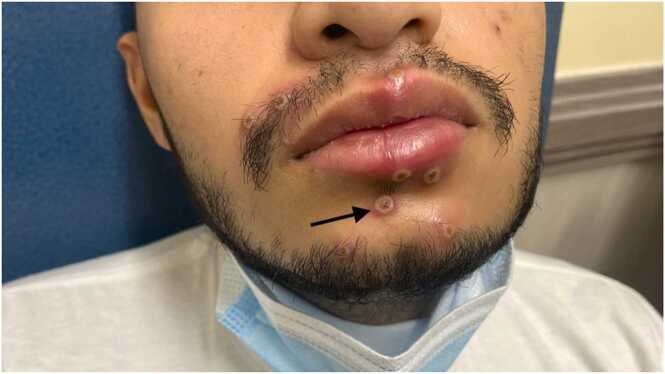
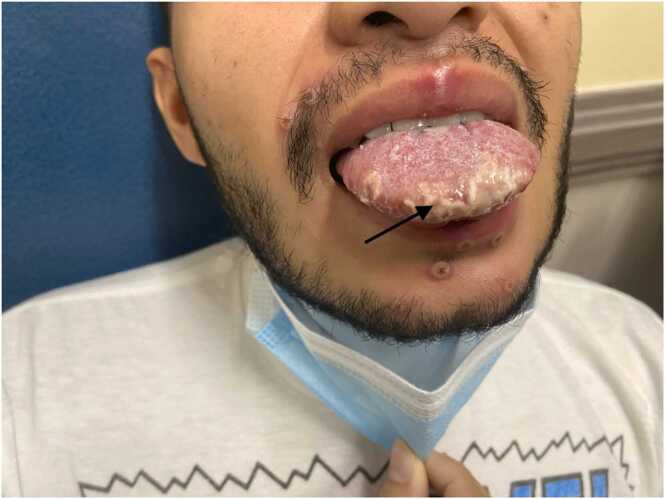
In the ER, the patient’s vital signs revealed a temperature of 100.3 F0, respiratory rate of 21/minute, blood pressure of 131/75 mmHg, and oxygen saturation of 97 % on room air. On physical exam, the patient had multiple umbilicated pox-like perioral and oral lesions with palpable lymphadenopathy in the neck and oral thrush (Figs. 1 and 2). A cardiac examination revealed regular rhythm and tachycardia. The rest of the physical exam remained unremarkable. The blood work is described in Table 1.
Fig. 1.
Arrow depicts tender umbilicated pox-like perioral monkeypox lesions.
Fig. 2.
Arrow depicts oral thrush and tender umbilicated lesions on the tongue.
Table 1.
Blood work on day 0: WBC: White Blood Cell, Hb: Hemoglobin, MCV: Mean Corpuscular Volume, Na: Sodium, K: Potassium, S. creat: Serum Creatinine, GFR: Glomerular Filtration Rate, Ca: calcium, T. Bili: Total Bilirubin, AST: Aspartate Aminotransferase, ALT: Alanine Aminotransferase, Alk Phos: Alkaline Phosphatase.
| Lab Test | Results | Reference Range |
|---|---|---|
| WBC | 10.6 | 4.0–11.0 10 × 3/ul |
| Hemoglobin | 13.9 | 13.0–18.0 g/dL |
| MCV | 86 | 80–95 fl |
| Platelet | 188 | 150–450 10 × 3/uL |
| Sodium | 133 | 136–145 mmol/L |
| Potassium | 3.9 | 3.5–5.1 mmol/L |
| Glucose | 103 | 75–100 mg/dL |
| Serum Creatinine | 0.9 | 0.6–1.3 mg/dL |
| GFR | > 60 | (> 60 mL/min) |
| Calcium | 9.7 | 8.5–10.1 mg/dL |
| T. Bilirubin | 0.8 | 0.2–1.0 mg/dL |
| AST | 16 | 15–37 U/L |
| ALT | 13 | 12–78 U/L |
| Alk Phos | 84 | 45–117 U/L |
| Albumin | 4.9 | 3.4–5.0 g/dL |
An electrocardiogram showed sinus tachycardia with HR 116, PR interval 130 ms, QRS duration 82 ms, QTc interval 418 ms, and no st-t wave elevation or depression. CT scan of head/neck with contrast showed multiple bilateral enlarged posterior jugular and submandibular lymph nodes, most likely reactive. No evidence of soft tissue swelling in the oropharynx, epiglottis, or larynx. No evidence of drainable fluid collection or abscess. The patient triggered a sepsis alert and was started on broad-spectrum antibiotics, including intravenous (IV) vancomycin, piperacillin/tazobactam, IV dexamethasone x1 dose with IV acyclovir, and IV fluconazole in the ER. The patient was admitted, and the ENT and infectious disease (ID) team were consulted for further recommendations. The PCR test was sent out to rule out Monkeypox, HSV, varicella, and repeat COVID-19.
The Monkeypox PCR test returned positive with the health department, while the other tests remained negative. The fluorescent treponemal antibody absorption test (commonly known as the FTA-ABS test) and follow-up RPR test returned positive for syphilis, while the HIV test was negative. IV antibiotics and antiviral were discontinued, and the patient was given magic mouth wash, IV fluconazole for oral thrush, and an intramuscular dose of penicillin × 1 along with supportive care. The patient denied any recent history of travel outside the US. The patient was kept in isolation & airborne precautions. Over the course, the patient’s symptoms did not improve much. With significant swelling of the tongue and an increasing number of lesions on day 3 of hospitalization, the infectious diseases specialist started the patient on Tecovirimat (also known as TPOXX or ST-246), an FDA-approved antiviral for the treatment of human smallpox disease caused by Variola virus in adults and children. The patient’s symptoms started to improve on day 5 of hospitalization. The patient tolerated the diet without difficulties, and his lesions started to crust. He was discharged home in stable condition with instructions to follow up with ID within one week and take tecovirimat 200 mg capsule twice a day for the next two weeks and fluconazole 200 mg one tablet once daily for the next five days. Even though ER provider and admitting provider evaluated the patient with only gloves and a surgical mask in a regular room without negative pressure, fortunately, they remained symptoms-free even after 21 days of exposure.
Discussion
Historically, Monkeypox has mostly been an endemic illness in Africa. Monkeypox virus is divided into two clades, one unique to the Congo Basin, more contagious, and associated with more severe disease. The other one is the West Africa clade, the source of the current monkeypox outbreak in the US and worldwide [6].
Transmission
The double-stranded DNA virus has a wide range of hosts, including monkeys and rodents such as rope squirrels, tree squirrels, Gambian pouched rats, and dormice. The virus primarily transmits from animal to human by contacting infectious fluids while handling sick animals or eating raw/undercooked infected bush meat. The secondary transmission mode is human-to-human via cutaneous-to-cutaneous (skin-to-skin) exposure, via respiratory droplets, or exposure to fomites such as towels, bedding, and sex toys in close contact. The CDC defines close contact as connecting with an individual or animal infected with the monkeypox virus within 6 feet for at least three hours without wearing a mask. A small proportion of cases were also reported in people who live in the same household as an infected person. The CDC Data suggest that most patients are amongst the gay or bisexual population in the current outbreak [7]. It is not entirely clear why the virus is exploiting this particular cohort. This is likely from close skin-to-skin contact; however, additional investigation is required to comprehensively understand if and how genital fluids contribute to the spread of human Monkeypox.
Past outbreaks
Monkeypox started to appear once routine vaccination against the variola virus was discontinued in the 1980s post-smallpox eradication, as smallpox vaccination is thought to provide up to 85 % cross-protection against Monkeypox [8]. Monkeypox was first discovered in laboratory monkeys in the 1950s; however, the first human case was not noted until the 1970s [9]. The first case of Monkeypox beyond Africa was in 2003 in the US [10]. The 2003 outbreak, which caused 71 cases (and 0 deaths) in the US, was linked to the importation of infected African rats housed with other animals, such as pet prairie dogs [11]. Following that, isolated instances were discovered in July and November 2021, most of which were connected to travel to monkeypox-endemic regions.
Clinical symptoms
The monkeypox virus incubation period ranges from 5 days to 3 weeks. Smallpox-like but less severe human Monkeypox is distinguished by a prodromal phase of fever and malaise along with disseminated vesicular or pustular skin rash (2–5 mm in diameter) in crops (mostly at the site of virus inoculation) and lymphadenopathy. The classic rash of Monkeypox is well-circumscribed with central umbilication, i.e., central depression. These lesions eventually crust and fall off in about 1–2 weeks. Other symptoms include fatigue, headache, muscle aches, and sore throat. Symptoms usually resolve in most individuals without complications within 2–4 weeks. However, immunocompromised individuals, pregnant patients, and children younger than eight can get severe complications from the disease, such as pneumonia, ocular infections, airway compromise due to lymphadenopathy, or encephalitis, with a mortality rate of 1–11 % [12]. The severity of symptoms also depends on the mode of transmission as the animal to human transmission will have more severe symptoms [13], [14].
Diagnosis
The recommended laboratory test for Monkeypox is polymerase chain reaction (PCR), which detects virus DNA. The ideal diagnostic samples come from the rash- skin, fluid, crusts, or, in some cases, a biopsy. Methods for detecting antigens and antibodies may be ineffective because they cannot differentiate between orthopoxviruses or acute from chronic.
Prevention
The easiest strategy to stop the spread of viruses is generally to avoid coming in direct contact with infected secretions, bedding, and animals, whether they are living or dead.
The FDA has approved two smallpox vaccines (Table 2) to prevent Monkeypox in the United States. CDC guidelines suggest against mass vaccinations and recommend limiting the vaccine use for prEP in high-risk individuals and post-exposure prophylaxis (PEP) [15].
Table 2.
Characteristics of available vaccines against Monkeypox.
| Vaccine name | JYNNEOS (Imvamune or Imvanex) | ACAM2000. |
|---|---|---|
| Type | Replication-deficient Live virus vaccine | Live cowpox Vaccinia virus vaccine |
| Doses | 2 doses, 28 days apart | 1 dose |
| Immune Response | 2 weeks after the second dose | 4 weeks post-vaccination |
| Major Side Effects | injection site reactions, Muscle pain, Headache, Fatigue. | Injection site wound (Take) may require 6 weeks to heal, inadvertent inoculation with vaccinia, Myopericarditis. |
| Pregnancy | Not contraindicated in pregnancy and breastfeeding based on animal studies. | Contraindicated in Pregnancy. |
| Contraindication | Severe Allergy to components (gentamicin, ciprofloxacin, egg protein) | Cardiac disease, Congenital or acquired immune deficiency disorders, e.g. HIV, skin conditions like atopic dermatitis/eczema; Eye disorder treated with topical steroids |
| Availability | Limited | Ample |
Further studies are needed to specify the efficacy of the smallpox vaccines against the current outbreak of monkeypox infection [15]. Patients with severe monkeypox infection or exposed people with T-cell immunodeficiency, for whom smallpox immunization is contraindicated, may benefit from the administration of intravenous vaccinia immune globulin (VIGIV) prophylactically [16], [17]. At the moment, it's unclear if VIGIV has any proven efficacy in treating monkeypox.
-
–
PrEP: The CDC advises giving the vaccine to any individual at high risk of contracting the disease, i.e., laboratory workers or researchers working on the monkeypox virus [15].
-
–
PEP: For the maximum chance of symptom prevention, the CDC advises administering the vaccination within four days after exposure in close contact. The vaccination can only lessen symptoms severity if administered between 4 and 14 days from the date of exposure [15].
Prevention in Health Care Personnel (HCP)
There seems to be a low possibility that exposure in healthcare settings will result in transmission. One healthcare worker gets infected for every 100 confirmed monkeypox cases approximately [18]. Clinicians should wear personal protective equipment, such as a gown, gloves, eye protection, and a fitting N95 mask when caring for patients with active skin lesions. A person with monkeypox infection should also be covered in a mask, have any lesions covered with a gown or sheet, and keep isolated. CDC recommends patients admitted to the hospital should be put in a negative-pressure room if one is available. PrEP or PEP should be offered to HCP who care for such patients [19]. HCP can work while actively watching for signs like fever or rash for at least 21 days after exposure if exposed to infected patients without protection (i.e., without donning PPE).
Treatment
Treatment is primarily supportive as most cases are mild and self-limiting. For severe disease, disease in pregnant patients, in children less than eight years of age, or in immunocompromised individuals, the FDA has approved antiviral drugs to treat smallpox, such as tecovirimat (TPOXX) and brincidofovir, to treat monkeypox, however, no antiviral medications are specifically made to treat or prevent monkeypox [20].
Conclusion
Monkeypox infection is spreading at an alarming rate, and it is critical to understand the constantly shifting epidemiology of the disease. International cooperation for better surveillance and case identification of monkeypox patients is required to boost epidemic preparedness and mitigation efforts. The recent COVID-19 pandemic has effectively helped health care professionals employ routine use of PPEs like gloves and surgical masks. Still, it is crucial not to undervalue the need for greater awareness of this disease in the general public. The global outbreak of the highly contagious monkeypox virus predominantly affects men who have intercourse with other men. Additional research needs to be done on the prospect of sexual transmission of the virus. Young individuals with monkeypox symptoms who have a history of MSM or HIV/AIDS should also get tested simultaneously for other STIs like syphilis and herpes.
Funding
The author has not declared receiving any grant/funding for this research from any funding agency in the public, commercial or not-for-profit sectors.
The article submitted is neither published nor under consideration by any other journal.
Consent
Informed written patient consent was obtained for this case report.
Disclosure
The author reports no conflicts of interest (financial or non-financial) in this work.
References
- 1.(ICTV) ICoToV. Virus Taxonomy: 2020 Release. Available from: 〈https://talk.ictvonline.org/taxonomy〉. Last Accessed on 07/17/2022.
- 2.The Centers for Disease Control and Prevention (CDC). 2022 Monkeypox Outbreak Global Map. Last accessed on 07/17/2022. Available at: 〈https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html〉.
- 3.World Health Organization. Multi-country monkeypox outbreak: situation update. Last accessed on 07/17/2022. Available at: 〈https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON396〉.
- 4.The Centers for Disease Control and Prevention (CDC). 2022 U.S. Map & Case Count. Last accessed on 07/17/2022. Available at: 〈https://www.cdc.gov/poxvirus/monkeypox/response/2022/us-map.html〉.
- 5.The Centers for Disease Control and Prevention (CDC). About Monkeypox, 2022 U.S. Monkeypox Outbreak. Last accessed on 07/17/2022. Available at: 〈https://www.cdc.gov/poxvirus/monkeypox/about.html〉.
- 6.Likos A.M., Sammons S.A., Olson V.A., et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86(Pt 10):2661–2672. doi: 10.1099/vir.0.81215-0. [PMID: 16186219] [DOI] [PubMed] [Google Scholar]
- 7.Di Giulio D.B., Eckburg P.B. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4(4):15–25. doi: 10.1016/s1473-3099(03)00856-9. [PMID: 14720564] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fine P.E., Jezek Z., Grab B., Dixon H. The transmission potential of monkeypox virus in human populations. Int J Epidemiol. 1988;17(3):643–650. doi: 10.1093/ije/17.3.643. [PMID: 2850277] [DOI] [PubMed] [Google Scholar]
- 9.Breman J.G., Kalisa-Ruti, Steniowski M.V., Zanotto E., Gromyko A.I., Arita I. Human monkeypox, 1970–79. Bull World Health Organ. 1980;58(2):165–182. [PMID: 6249508; PMCID: PMC2395797] [PMC free article] [PubMed] [Google Scholar]
- 10.Adalja A., Inglesby T. A novel international monkeypox outbreak. Ann Intern Med. 2022 doi: 10.7326/M22-1581. [Epub ahead of print. PMID: 35605243] [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). Update: multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep., Vol. 52; 2003, p. 642–6. PMID: 12855947. [PubMed]
- 12.Centers for Disease Control and Prevention. Monkeypox in multiple countries. Last accessed on 07/17/2022. Available at: 〈https://wwwnc.cdc.gov/travel/notices/alert/monkeypox〉.
- 13.Jezek Z., Grab B., Szczeniowski M., Paluku K.M., Mutombo M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull World Health Organ. 1988;66(4):459–464. [PMID: 2844428; PMCID: PMC2491168] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds M.G., Yorita K.L., Kuehnert M.J., et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194(6):773–780. doi: 10.1086/505880. [Epub 2006 Aug 8. PMID: 16941343] [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Considerations for Monkeypox Vaccination. Last accessed on 07/17/2022. Available at: 〈https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html〉.
- 16.Guarner J., Del Rio C., Malani P.N. Monkeypox in 2022–what clinicians need to know. JAMA. 2022;328(2):139–140. doi: 10.1001/jama.2022.10802. [PMID: 35696257] [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Clinical Recognition. Last accessed on 07/17/2022. Available at: 〈www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html〉.
- 18.Petersen B.W., Kabamba J., McCollum A.M., et al. Vaccinating against monkeypox in the Democratic Republic of the Congo. Antivir Res. 2019;162:171–177. doi: 10.1016/j.antiviral.2018.11.004. [Epub 2018 Nov 14. PMID: 30445121; PMCID: PMC6438175] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao A.K., Petersen B.W., Whitehill F., et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the advisory committee on immunization practices – United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(22):734–742. doi: 10.15585/mmwr.mm7122e1. [PMID: 35653347; PMCID: PMC9169520] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petersen B. Orthopoxvirus work group background and considerations. Advisory Committee on Immunization Practices (ACIP); 2021. Last accessed on 07/17/2022. Available at: 〈https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/24-25/02-OPX-Petersen.pdf〉.