Highlights
-
•
For anterior cerebral artery aneurysm clipping dual SSEPs and tcMEPs enhance detection of lower extremity deficits.
-
•
Evoked potentials have limited utility in predicting upper extremity deficits related to subcortical ischemia.
-
•
Four-extremity dual-modality monitoring can also detect pathogenetic mechanisms that are remote from the surgical site.
Abbreviations: ACA, anterior cerebral artery; ACoA, anterior communicating artery; EP, evoked potential; IONM, intraoperative neurophysiological monitoring; MCA, middle cerebral artery; MEP, motor-evoked potential; RAH, recurrent artery of Heubner; SSEP, somatosensory-evoked potential; tcMEP, transcranial motor-evoked potential
Keywords: Anterior cerebral artery, Anterior communicating artery, Cerebral aneurysm, Intraoperative neurophysiological monitoring, Somatosensory-evoked potential, Motor-evoked potential
Abstract
Objective
To investigate the optimal combination of somatosensory- and transcranial motor-evoked potential (SSEP/tcMEP) modalities and monitored extremities during clip reconstruction of aneurysms of the anterior cerebral artery (ACA) and its branches.
Methods
A retrospective review of 104 cases of surgical clipping of ruptured and unruptured aneurysms was performed. SSEP/tcMEP changes and postoperative motor deficits (PMDs) were assessed from upper and lower extremities (UE/LE) to determine the diagnostic accuracy of each modality separately and in combination.
Results
PMDs were reported in 9 of 104 patients; 7 LE and 8 UE (3.6% of 415 extremities). Evoked potential (EP) monitoring failed to predict a PMD in 8 extremities (1.9%). Seven of 8 false negatives had subarachnoid hemorrhage. Sensitivity and specificity in LE were 50% and 97% for tcMEP, 71% and 98% for SSEP, and 83% and 98% for dual-monitoring of both tcMEP/SSEP. Sensitivity and specificity in UE were 38% and 99% for tcMEP, and 50% and 97% for tcMEP/SSEP, respectively.
Conclusions
Combined tcMEP/SSEP is more accurate than single-modality monitoring for LE but is relatively insensitive for UE PMDs.
Significance
During ACA aneurysm clipping, multiple factors may confound the ability of EP monitoring to predict PMDs, especially brachiofacial hemiparesis caused by perforator insufficiency.
1. Introduction
Anterior cerebral artery (ACA) or anterior communicating artery (ACoA) aneurysms make up approximately 12% of all unruptured intracranial aneurysms and up to 50% of all ruptured aneurysms requiring surgical intervention (Molyneux et al., 2002, Wiebers et al., 2003). The rate of symptomatic stroke that manifest as postoperative neurological deficits (PND) is estimated to be around 7.5% following clip reconstruction of brain aneurysms (Li et al., 2018). Somatosensory- (SSEPs) and transcranial motor-evoked potentials (tcMEPs) can be used intraoperatively to help predict and prevent PNDs during clipping (Lin et al., 2011, Thomas and Guo, 2017).
Studies evaluating intraoperative neurophysiological monitoring (IONM) modalities for cerebral aneurysm clipping have found that SSEPs have modest sensitivity (48%-65%) and high specificity (85%-92%) for predicting PNDs (Kashkoush et al., 2020, Thirumala et al., 2016, Thomas and Guo, 2017). tcMEP modalities are reported to be more sensitive (73%-97%), more specific (89%-94%) (Thomas and Guo, 2017) and are also more likely to detect motor deficits related to subcortical ischemia than are SSEPs (Guo and Gelb, 2011, Neuloh and Schramm, 2004). Dual-modality IONM is frequently utilized for certain aneurysmal procedures and has been shown to improve sensitivity (89%-94%) while maintaining specificity (83%-100%) (Thomas and Guo, 2017, Zhu et al., 2019). Studies reporting on diagnostic test accuracy typically pool all cerebral aneurysms without respect to vascular territory of the aneurysm or monitoring set-up (e.g., choice of monitored extremities.) However, surgery for aneurysms of specific vascular territories may put different structures and monitorable pathways of the brain at risk and therefore may create different constellations of PNDs. During ACA and ACoA aneurysm clipping, the blood supply to specific corticospinal and dorsal column medial lemniscus pathways are at risk, both of which are amenable to IONM. Due to complexity and variability of the blood supply to these structures, the optimal choice of monitored extremities and monitoring modalities deserves special considerations.
The accuracy and benefit of monitoring the upper and lower extremity (UE/LE) using both SSEP and tcMEP for ACA and ACoA aneurysm clipping has not been studied. We evaluated the clinical utility and diagnostic accuracy of SSEPs and tcMEPs, monitoring extremities separately and in combination in this retrospective study. We review the neurovascular anatomy of this territory to create an a priori framework that could inform monitoring strategies and IONM limitations for aneurysm clipping of ACA and ACoA aneurysms. Research in this area could aid in the prevention of PNDs and facilitate surgery in individuals with ACA and ACoA aneurysms.
2. Methods
2.1. Study population
A retrospective chart review was conducted of all patients over 18 years of age that underwent craniotomy and clipping of unruptured or ruptured cerebral aneurysms affecting the ACA and ACoA between January 2012 and December 2020 at the UF Health Shands Hospital. The review was conducted in accordance with the University of Florida’s Institutional Review Board regulations (IRB201700329) and was supported by the Department of Anesthesiology. Cases were identified in our institution’s internal IONM database. Inclusion criteria included 1) clipping of an ACA or ACoA aneurysm without clipping of concomitant aneurysms affecting other blood vessels, 2) the use of SSEP or tcMEP or both, and 3) the availability of IONM records, operative reports, and pre- and postoperative progress notes with documentation of neurological status.
2.2. Data collection
Our review included collected information related to the procedure: aneurysm location, Hunt-Hess and Fischer grades for ruptured aneurysms, presence of pre- and postoperative motor deficits, IONM changes from baseline satisfying alert criteria based on institutional protocol (see below), and use of temporary clipping. Basic demographic information was collected on all subjects (age, gender) as well as risk factors for aneurysm rupture, including smoking status, history of substance abuse, weight, diabetes, and hypertension.
IONM records, operative reports, and postoperative progress notes were reviewed to identify and characterize significant IONM changes, their association with intraoperative events, and the presence or absence of early motor PND. PND assessment included only evaluation of motor deficits as these were the most objective neurological examination findings able to be clearly and consistently documented in the medical record. This was based on postoperative clinical examinations performed immediately after emergence from anesthesia and subsequent examinations within the first 24 h as documented in the medical record. Patients were deemed to have a motor PND if abnormal motor function of any extremity was consistently documented in two subsequent postoperative neurosurgical progress notes that was new compared to preoperative status and had not resolved within 24 h. Motor PNDs were categorized as mild (4/5 strength), moderate (3/5 strength), or severe (0–2/5 strength). Two reviewers (FR and CD) performed data collection. All pertinent records and data were reviewed by both reviewers in all cases with IONM changes and motor PNDs, and agreement was reached between the reviewers on classification of findings.
2.3. IONM protocols
IONM was performed using the Cascade Pro® (Cadwell Industries, Inc., Kennewick, WA, USA). The modalities used were tcMEP, SSEP, and electroencephalography (EEG). Surgeries were followed closely by the IONM and anesthesia teams via video feed.
tcMEP monitoring for cerebral aneurysm clipping was introduced at our institution in May 2013. Prior to that period, SSEP and EEG monitoring was standard. For tcMEP monitoring, transcranial electrical stimulation was performed using subdermal corkscrew electrodes placed at C1/C2 or C3/C4 based on the International 10–20 system. Occasionally, stimulation optimization was performed by inserting additional electrodes for quadripolar stimulation. Constant-voltage stimulation was utilized. The stimulus intensity was kept as close to the compound muscle action potential (CMAP) activation threshold, while maintaining a reproducible CMAP from trial-to-trial and ranged from 100 to 400 V (V). Stimuli were delivered using a train of five to nine pulses with a pulse duration of 50 microsec, at an interstimulus interval of 2–4 ms (Legatt et al., 2016). CMAP recordings were obtained using subdermal electrodes placed in the abductor pollicis brevis for the UE and abductor hallucis muscles for the LE. Crossover stimulation was assessed by concurrently recording from the ipsilateral muscles. Stimulation intensities were decreased to eliminate crossover activation and were typically set at 10–20 V above the threshold for tcMEP response. Prior to temporary or permanent clip application, tcMEP baselines were updated. Following clip placement, tcMEPs were obtained approximately every 5 min when possible or when the surgeon requested and were typically continued until surgical wound closure.
For SSEP monitoring, subdermal needle electrodes were placed to stimulate the median or ulnar nerves at the wrist for UE or the tibial nerves posterior to the medial malleolus for LE bilaterally. Stimuli were delivered using square wave pulses with a duration of 0.2–0.3 ms at a frequency of 1.75–3 Hz. Filter settings were 30–1000 Hz. The stimulus intensity ranged from 20 to 50 mA for the median nerve and 25–60 mA for the tibial nerve. SSEPs were recorded from three channels for both UE and LE. Recording sites included (1) Erb’s point (for UE) and the tibial nerve at the popliteal fossa (for LE), (2) the C5 cervical spinous process, and (3) corkscrew scalp electrodes at C1′, C2′ (for LE) and C3′, C4′ (for UE) referenced to Cz’ or FPz depending on optimized signal-to-noise ratios (MacDonald et al., 2019). SSEP baselines were updated after dural opening and immediately prior to significant aneurysm manipulation (e.g., temporary or permanent clip application), and SSEP monitoring typically continued until the completion of surgical closure.
2.4. IONM alert criteria
For tcMEP, a significant change was considered to be either a disappearance or a reduction of >50% in amplitude, or consistent decrease of amplitude below earlier amplitudes of CMAP when trial-to-trial variability exceeded 50% that persisted on two or more separate tcMEP trials (MacDonald et al., 2013). A significant change for SSEP was considered to be a >50% reduction in amplitude or a 10% increase in latency on two separate SSEP averaging trials (Toleikis and American Society of Neurophysiological Monitoring, 2005). In 2019, we changed our warning criteria to include an obvious amplitude reduction from a recently updated pre-trial baseline value that clearly exceeded trial-to-trial variability, particularly when it was abrupt focal and closely related to surgical manipulation (MacDonald et al., 2019). When a significant IONM change occurred, the surgeon and anesthesia teams were notified. Response to the alert was at the discretion of the surgeon and anesthesiologist depending on intraoperative circumstances and was carried out in closed-loop communication.
Reversible IONM changes were defined as deteriorations that recovered intraoperatively. In contrast, irreversible IONM changes were defined as deteriorations that did not recover by skin closure.
2.5. Anesthetic protocol
All cases were performed under general endotracheal anesthesia. Prior to tcMEP monitoring, neuromuscular train of four stimulation was checked to ensure complete reversal of neuromuscular blockade. Maintenance of anesthesia was typically achieved using total intravenous anesthesia consisting of propofol and narcotic infusions titrated along with phenylephrine infusion to maintain physiologic hemodynamic parameters and adequate depth of anesthesia. Depth of anesthesia was typically assessed by raw EEG monitoring to avoid EEG suppression and increased power of higher frequencies. When volatile inhalational anesthetic agents were used in lieu of propofol infusion, the agents were titrated to a minimum alveolar concentration value of ≤0.5. If inhalational anesthetics interfered with SSEP or tcMEP signals, the anesthetic was converted to total intravenous anesthesia. Hyperventilation and hyperosmolar therapies were often administered to decrease intracranial volume depending on the surgical field based on communication with the surgical team. Serial neurological examinations were conducted by the neurosurgical team upon anesthetic emergence and documented in the progress notes.
2.6. Surgical approach/technique and response to IONM alert
Standard frontotemporal (pterional or orbito-zygomatic) craniotomy and microsurgical technique with arachnoid dissection was used to approach the aneurysm. The need for temporary clipping, choice of vessels to temporary clip, and duration of temporary clipping were performed at the discretion of the surgeon. Indocyanine green video angiography and conventional angiography were used on a case-by-case basis to assess the aneurysm and parent vasculature after clipping was complete. In the event of an angiographic abnormality (e.g., vessel occlusion, clip malposition, residual aneurysm, vasospasm), the surgeon made necessary modifications.
The surgeon and anesthesiologist also had the discretion to act on IONM alert criteria, which typically consisted of increasing blood pressure, removal or adjustments of the clip(s), release and repositioning of retractors, application of warm irrigation to the surgical field, and burst suppression. Response to an IONM alert also involved assessment for technical problems and close communication with the surgeon and IONM team.
2.7. Data analysis
All recorded SSEP and tcMEP modalities were analyzed separately and in combination for the presence or absence of changes satisfying alert criteria (see above) from all monitored extremities separately and in combination by manually reviewing the data collected. We derived sensitivity, specificity, as well as positive and negative predictive values to determine the utility of IONM modalities separately and in combination, including various UE and LE combinations as a diagnostic test in this cohort. We present flow diagrams for diagnostic test evaluation (false negatives, false positives, true negatives and true positives) of intraoperative neuromonitoring modalities by monitored extremities separately (Supplementary Table 1 in Appendix A) and by upper and lower extremity combinations (Supplementary Table 2 in Appendix A).
2.8. Causality linkage analysis of reversible signal changes
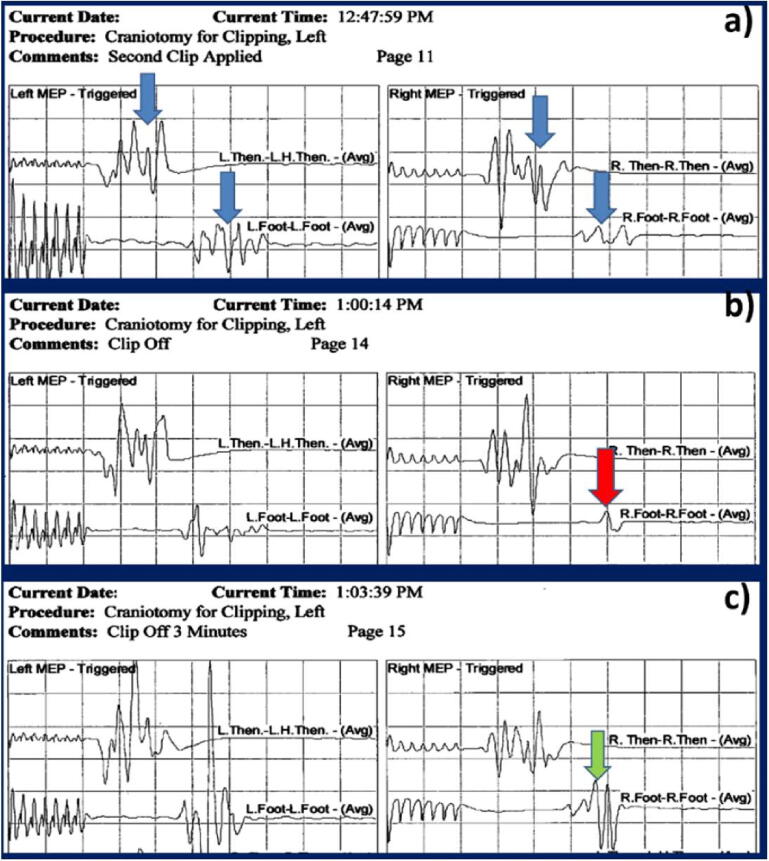
We applied causality linkage analysis to any reversible signal change. When the signal change was resolved by a specific surgical maneuver/intervention (e.g., clip placement and removal), a causality link was established (Skinner and Holdefer, 2014). Whether a reversible signal change was caused by an impending neurological injury that would have led to a PND cannot be established with absolute certainty because a neurological examination correlate cannot be obtained under anesthesia. However, when the resolution of a signal change can be linked to a surgical maneuver, evidence of causation can be established and the reversible signal change can be classified as a true-positive instead of a false-positive alert (Skinner and Holdefer, 2014, Holdefer et al., 2015). (e.g., recovery of signal change immediately after adjusting the aneurysm clip in response to an IONM alert). Causality linkage is illustrated by a representative case in Fig. 1.
Fig. 1.
Left and right transcranial motor-evoked potential (tcMEP) monitoring during clipping of an anterior communicating artery aneurysm. (Case Nr 112) A) After the clip was applied at 12:47, tcMEP waveforms were preserved in all four extremities (blue arrows). B) At 1:00 pm, a decline in the right foot signal was noted (red arrow) that triggered an alert and the clip was removed by the surgeon. C) Three minutes later, the polyphasic response was regained (green arrow). (Display gain: 100 microV/division, time base: 10 msec/division). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3. Results
3.1. Inclusion/exclusion
One hundred twenty-three surgeries were identified for the predefined study period. After chart review of all cases, 19 cases were excluded. Thirteen cases were excluded because they featured an additional clipping of at least one aneurysm of a vessel other than the ACA or ACoA. An additional 4 cases were excluded due to failure of planned IONM modalities, or incomplete IONM records. One case did not satisfy the inclusion criteria of ACA or ACoA aneurysm, and one case was misidentified as it underwent endovascular coiling of the aneurysm instead of surgical clipping. Ultimately, 104 cases were included in our dataset.
3.2. Demographics and comorbidities
Patients were women (n = 66) and men (n = 38) between 23 and 78 years old (mean 57.1; standard deviation, 12.6). Ninety-nine of the patients included in our dataset had at least one documented comorbidity (diabetes, hypertension, substance abuse, current smoker, or obesity) (Table 1).
Table 1.
Patient risk factors and common comorbidities.
| Risk Factors/Comorbidities | Number of Participants |
|---|---|
| Diabetes | 16 (15%) |
| Hypertension | 76 (73%) |
| Substance Use | 16 (15%) |
| Smoking History | |
| Current Smoker | 29 (28%) |
| Former Smoker | 50 (48%) |
| Body Mass Index | |
| Underweight (<18.5) | 1 (1%) |
| Normal Weight (>18.5, <25) | 34 (37%) |
| Overweight (>25, <30) | 36 (35%) |
| Obese (>30) | 41 (39%) |
Subarachnoid hemorrhage (SAH) was present prior to surgery in 38 cases (37%). Temporary clip placement and occlusion of parent vessels was performed in 50 cases at some point during aneurysm dissection and permanent clip application (48%).
3.3. IONM results
3.3.1. Choice of IONM modalities and extremities
All 104 cases were monitored using bilateral UE (n = 208 extremities) and bilateral LE (n = 207, excluding one amputated LE) SSEPs. SSEP-only monitoring occurred in 28 cases (n = 112 extremities). In addition, UE tcMEPs were monitored in 67 cases (n = 134 extremities) and LE tcMEPs were monitored in 62 cases (n = 123 extremities).
In addition to the 28 SSEP-only cases, 52 cases were monitored using SSEPs and both UE and LE tcMEPs, 14 cases using SSEP and only UE tcMEP monitoring, and 10 cases using SSEP and only LE tcMEP monitoring.
In cases with motor PNDs and IONM changes, SSEP monitoring was obtained successfully in all cases, but there were three instances of persistent crossover CMAP activation during tcMEP monitoring.
3.3.2. Diagnostic accuracy of IONM modalities in combination and in isolation by extremity
There were overall seven new LE motor PNDs and eight new UE motor PNDs (Table 2). All tcMEP and SSEP events by monitored extremity are detailed in Table 3, Table 4, respectively. The majority of tcMEP events occurred without an accompanied SSEP event and vice versa.
Table 2.
All cases with postoperative neurological deficits per extremity.
| Case No. | Motor PND Extremity Involved (n = 15) | Evoked Potential Modality Used on Extremity | Evoked Potential Change | All Monitoring Modalities Used | SAH | Aneurysm location | Motor PND Description | Notes |
|---|---|---|---|---|---|---|---|---|
| 24 | Left LE | SSEP MEP |
None (FN) | UE SSEP LE SSEP UE tcMEP LE tcMEP |
Yes | ACoA | Mild | |
| 34 | Left UE | SSEP (FN) MEP |
MEP | UE SSEP LE SSEP UE tcMEP LE tcMEP |
No | ACoA | -Severe left UE paresis, -transient left LE paresis | Resolved with emergent endovascular thrombectomy that was performed 90 min after the surgery. Related to incidental M4 embolus that caused a perfusion deficit on CT perfusion |
| Left LE | SSEP(FN) MEP |
MEP | ||||||
| 35 | Right UE | SSEPMEP (FN) |
SSEP | UE SSEP LE SSEP UE tcMEP |
Yes | ACoA | Severe, right hemiparesis and left hemiplegia | LE tcMEPs were not monitored due to crossover SAH; evolving severe vasospasm affecting bilateral ACAs diagnosed on postoperative CT angiogram; TcMEP crossover affecting LEs, therefore tcMEP was discontinued |
| Right LE | SSEP | SSEP | ||||||
| Left UE | SSEP MEP |
None (FN) | ||||||
| Left LE | SSEP | None (FN) | ||||||
| 39 | Left LE | SSEP | None (FN) | UE SSEP LE SSEP |
Yes | Right A2 | Severe | A2 (giant A2 aneurysm, treated with staged A3-A3 anastomosis followed by coiling) |
| 50 | Left LE | SSEP | LE SSEP | UE SSEP LE SSEP |
No | ACoA | Mild | |
| 63 | Right UE | SSEP | None (FN) | UE SSEP LE SSEP |
Yes | ACoA | Mild, | Paresis slow gradual improvement over days |
| 69 | Left UE | SSEP MEP |
None (FN) | UE SSEP LE SSEP UE tcMEP LE tcMEP |
Yes | ACoA | Moderate | Presented with TBI patient died 2 weeks postoperatively |
| Right UE | SSEP (FN) MEP |
MEP | ||||||
| 96 | Left UE | SSEP MEP |
None (FN) | UE SSEP LE SSEP UE tcMEP LE tcMEP |
Yes | ACoA | Severe | Giant ruptured ACoA aneurysm. Prolonged temporary clip on right ACA associated with LE SSEP change. Upper extremity deficit presumably caused by perforator occlusion. TcMEP crossover |
| Left LE | SSEPMEP (FN) |
LLE SSEP | ||||||
| 116 | Right UE | SSEP MEP |
None (FN) | UE SSEP LE SSEP UE tcMEP LE tcMEP |
No | ACoA | Mild, 3/5 right deltoid weakness |
TcMEP crossover |
A2 & A3, second and third segments of the anterior cerebral artery; ACA, anterior cerebral artery; ACoA, anterior communicating artery; CT, computed tomography; FN, false negative; ICP, intracranial pressure; IONM, intraoperative neuromonitoring; LE, lower extremity; M4, fourth segment of the middle cerebral artery; tcMEP, motor-evoked potential; N/A, not applicable; RAH, recurrent artery of Heubner; SAH, subarachnoid hemorrhage; PNDs, postoperative neurological deficits; SSEP, somatosensory-evoked potential; TBI, traumatic brain injury; temp, temporary; UE, upper extremity;
Table 3.
Cases with motor-evoked potential event by upper and lower extremity with or without accompanying somatosensory-evoked potential event.
| Case No. | UE tcMEP Change (test interpretation) | LE tcMEP Change (test interpretation) | Concomitant SSEP Change | Aneurysm Location & Hunt Hess/Fisher Grade | Event Related to EP Change | Event Related to Resolution of EP Change | Causality Link Between Surgical Maneuver and Reversible tcMEP Change | Motor PND Severity and Duration |
|---|---|---|---|---|---|---|---|---|
| 30* | Reversible (TP) |
Yes: R (TP) |
Yes: R in LE | ACoA & N/A |
Temp clip | Removal of temp clip | Yes | None |
| 34 | Irreversible (TP) |
Yes: I (TP) |
No | ACoA & N/A | Angiography (M4 branch embolus revealed on postop MRI) | N/A | N/A | Left hemiparesis, severe (Note: resolved with emergent endovascular thrombectomy) |
| 69 | Reversible (TP for LUE/FN for RUE) |
No (TN) |
No | ACoA & 3/4 |
Temp clip | Removal of temp clip | Yes | Left UE and Right UE weakness |
| 74 | No (TN) |
Yes: R (FP) |
No | A1 & 4/4 | Dura opened Note: crossover to left foot | None | No | None |
| 82 | Irreversible (FP) |
No (TN) |
No | ACoA & 3/3 | Aneurysm dissection | None | N/A | None |
| 92 | N/A Note: UE tcMEP not monitored |
Yes: R (TP) |
No | ACoA & 1/3 | Temp clip | Removal of temp clip | Yes | None |
| 94 | N/A Note: UE tcMEP not monitored |
Yes: R (FP) |
No | ACoA & 1/3 | Dura opened | Increased stimulation | No | None |
| 112 | No (TN) |
Yes: R (TP) |
No | ACoA & N/A |
Temp clip | Removal of temp clip | Yes | None |
| 123* | No (TN) |
Yes: R (TP) |
Yes: R in UE | ACoA & 3/3 |
Temp clip | Removal of temp clip | Yes | None |
PND, postoperative neurological deficit; UE, upper extremity; LE, lower extremity; N/A, not applicable; tcMEP, motor-evoked potential; SSEP, somatosensory-evoked potential; TP, true positive; FP, false positive; TN, true negative; FN, false negative; temp, temporary; ACA, anterior cerebral artery; ACoA, anterior communicating artery; MRI, magnetic resonance imaging; A1, first segment of the anterior cerebral artery; M4, 4th segment of the middle cerebral artery; *concomitant change in SSEP and tcMEP modalities.
Table 4.
Cases with SSEP event with or without accompanying tcMEP event.
| Case No. | UE SSEP Change (test interpretation) | LE SSEP Change (test interpretation) | MEP Performed | Concomitant tcMEP Change | Aneurysm Location & Hunt Hess/ Fisher Grade | Event Related to Change | Event Related to Resolution of EP Change | Causality Link Between Surgical Maneuver and Reversible SSEP Change | Motor PND Severity and Duration |
|---|---|---|---|---|---|---|---|---|---|
| 12 | No (TN) |
Reversible (TP) |
UE LE |
No | ACoA & 2/3 | Temp clip | Removal of temp clip | Yes | None |
| 20 | Reversible (FP) | Reversible (FP) Note: LLE SSEP only; RLE amputated |
UE LE Note: LLE tcMEP only; RLE amputated |
No | ACoA & N/A | None | None | No | None |
| 30* | No (TN) |
Reversible (TP) |
UE LE |
Reversible in UE & LE | ACoA & N/A | Temp clip | Removal of temp clip | Yes | None |
| 35 | Irreversible (TP) |
Irreversible (TP) |
UE | No | ACoA & N/A | Occurred during closing of scalp | N/A | N/A | Right hemiparesisLeft hemiplegia (Note: lower tcMEP not monitored due to crossover) |
| 48 | No (TN) |
Reversible (TP) |
None | No | A1 & N/A | Positioning | Re-positioning | Yes (Note: positioning-related injury) | None |
| 50 | Reversible (FP) |
Reversible (TP) |
None | No | ACoA & 2/3 | Adenosine given to control bleed & temp clip | Removal of temp clips | LE, Yes UE, No |
LLE weakness |
| 59 | No (TN) |
Reversible (TP) |
None | No | ACoA with proximal A1 & N/A |
Temp clip | Removal of temp clip | Yes | None |
| 96 | No (FN) |
Reversible (TP) |
UELE (but crossover) |
No | ACoA & 1/2 | Temp clip | Removal of temp clip | Yes | Left hemiplegia |
| 97 | Reversible (FP) | Reversible (FP) |
LE | No | ACoA & 2/3 | Propofol | Propofol Redistribution | No | None |
| 123* | Reversible (TP) |
No (TN) |
UE LE |
Reversible in LE | ACoA & 3/3 | Temp clip | Removal of temp clip | Yes | None |
UE, upper extremity; LE, lower extremity; tcMEP, motor-evoked potential; SSEP, somatosensory-evoked potential; TP, true positive; FP, false positive; TN, true negative; FN, false negative; temp, temporary; ACA, anterior cerebral artery; ACoA, anterior communicating artery; *concomitant change in SSEP and tcMEP modalities.
3.3.2.1. LE
-
•
LE motor PNDs (n = 7) were associated with IONM changes in four instances (true positives). There were two cases with irreversible signal changes (1 SSEP and 1 tcMEP) and two reversible SSEP signal changes that were causally linked to surgical maneuver.
-
•
False negatives occurred in three LEs of which one had dual-modality monitoring with SSEP and tcMEPs, and two were monitored with SSEP only.
-
•
LE tcMEP monitoring had a sensitivity of 50% and specificity of 97%, which was lower than SSEP (71% and 98%, respectively). Combination tcMEP and SSEP on the LE yielded a sensitivity of 83% and specificity of 98% with high positive and negative predictive values (83% and 98%) (Table 5).
Table 5.
Evaluation of diagnostic accuracy of IONM modalities by monitored extremity separately and in combinations for all cases (with or without SAH).
| Lower Extremity |
Upper Extremity |
Upper & Lower Extremity Combinations |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LE tcMEP (n = 123) | LE SSEP (n = 207) | LE SSEP + LE tcMEP (n = 123) | UE tcMEP (n = 134) | UE SSEP (n = 208) | UE SSEP + UE tcMEP (n = 134) | UE & LE SSEP (n = 112 in 28 cases*) | UE & LE SSEP with UE & LE tcMEP (n = 207 in 52 cases) | UE & LE SSEP + UE tcMEP (n = 56 in 14 cases*) | UE & LE SSEP + LE tcMEP (n = 40 in 10 cases*) | |
| Sensitivity | 50% | 71% | 83% | 38% | 12% | 50% | 60%* | 69% | 50%* | 100%* |
| Specificity | 97% | 98% | 98% | 99% | 99% | 97% | 100%* | 98% | 100%* | 97%* |
| PPV | 50% | 83% | 83% | 75% | 33% | 55% | N/A* | 75% | N/A* | N/A* |
| NPV | 97% | 98% | 98% | 96% | 97% | 96% | N/A* | 98% | N/A* | N/A* |
| * Limited number of monitored extremities in category | ||||||||||
UE, upper extremity; LE, lower extremity; tcMEP, transcranial motor-evoked potential; SSEP, somatosensory-evoked potential; PPV, positive predictive value; NPV, negative predictive value.
3.3.2.2. UE
-
•
UE motor PNDs (n = 8) were associated with IONM changes in three instances (true positives), including two cases with irreversible signal change (1 SSEP and 1 tcMEP) and one reversible tcMEP change causally linked to surgical maneuver.
-
•
False negatives occurred in five UE of which four had dual-modality monitoring with SSEP and tcMEP, and one had SSEP monitoring only.
-
•
Sensitivity of tcMEP and SSEP for UE proved low, but tcMEP was better than SSEP (38% vs 12%) and both had equally high specificity. Combination SSEP and tcMEP monitoring increased sensitivity to 50% with preserved specificity (Table 5).
3.3.2.3. LE and UE combinations
-
•
Of the employed monitoring strategies, combined UE and LE SSEP with UE and LE tcMEPs was the IONM strategy most often used in our cohort (52 cases with 207 monitored extremities), with an overall sensitivity of 69% and specificity of 98% (Table 5).
-
•
Other combinations did not have high enough case numbers to characterize their diagnostic utility.
3.3.2.4. Evaluation of false-negative IONM
-
•
New motor PNDs were reported in 9 patients (8.6% of 104 patients) affecting 15 extremities, including 7 LE and 8 UE (3.6% of all 415 monitored extremities, excluding one amputated LE) (Table 2).
-
•
Of these cases, IONM failed to predict a postoperative deficit (false negative) in eight extremities (1.9% of all 415 monitored extremities).
-
•
Seven of these eight (88%) missed extremity deficits presented with SAH, which was disproportionately higher than SAH prevalence (37%) in our cohort.
-
•
Five of eight (63%) false negatives occurred in UEs of which four had dual-modality monitoring with SSEP and tcMEPs, and one had SSEP only monitoring. All of these were AcoA.
3.3.2.5. Evaluating IONM in cases without SAH
-
•
Sensitivity and specificity for lower extremity tcMEP and SSEP are higher when excluding cases of SAH.
-
•
Sensitivity and specificity for upper extremity tcMEP is higher but SSEP performed similarly poorly in predicting postoperative motor deficits in this cohort as well.
-
•
The diagnostic utility of IONM in patients without SAH is summarized in Table 6.
Table 6.
Evaluation of diagnostic accuracy of IONM modalities by monitored extremity separately and in combinations for cases only without SAH.
| Lower Extremity |
Upper Extremity |
|||||
|---|---|---|---|---|---|---|
| LE tcMEP (n = 76) | LE SSEP (n = 129) | LE SSEP + LE tcMEP (n = 79) | UE tcMEP (n = 84) | UE SSEP (n = 125) | UE SSEP + UE tcMEP (n = 81) | |
| Sensitivity | 100% | 80% | 100% | 50% | 0% | 75% |
| Specificity | 97% | 99% | 99% | 100% | 99% | 100% |
| PPV | 33% | 80% | 83% | 100% | 0% | 100% |
| NPV | 100% | 99% | 100% | 99% | 98% | 99% |
UE, upper extremity; LE, lower extremity; tcMEP, transcranial motor-evoked potential; SSEP, somatosensory-evoked potential; PPV, positive predictive value; NPV, negative predictive value.
4. Discussion
4.1. Neurovascular anatomy and IONM implications
There are a significant number of anatomical variations of the ACA–ACoA complex, including asymmetry and morphological distortion of the anterior Circle of Willis, unilateral hypoplastic ACA, and hypoplastic or absent ACoA (Perlmutter and Rhoton, 1976). Surgical maneuvers that may lead to ischemia during cerebral aneurysm surgery include permanent clip malposition, prolonged temporary clip occlusion, brain retraction, and direct vascular injury, among others (Sasaki et al., 2007). A detailed understanding of the at-risk arteries that supply both cortical and subcortical structures of motor (corticospinal tract) and sensory (dorsal column medial lemniscus tract) pathways is essential in choosing an IONM strategy both in terms of modalities and monitored extremities.
4.1.1. ACA and branch anatomy
The ACA originates from the internal carotid artery and by communicating with the contralateral ACA through the ACoA forms the anterior portion of the Circle of Willis. The ACA continues as the pericallosal artery as it arches anteromedially along the corpus callosum (Maga et al., 2013). Cortical branches of the ACA supply the medial and superomedial parts of the hemisphere, including the paracentral lobule where the primary motor cortex and primary somatosensory cortex for the LE are located (Kakou et al., 2000).
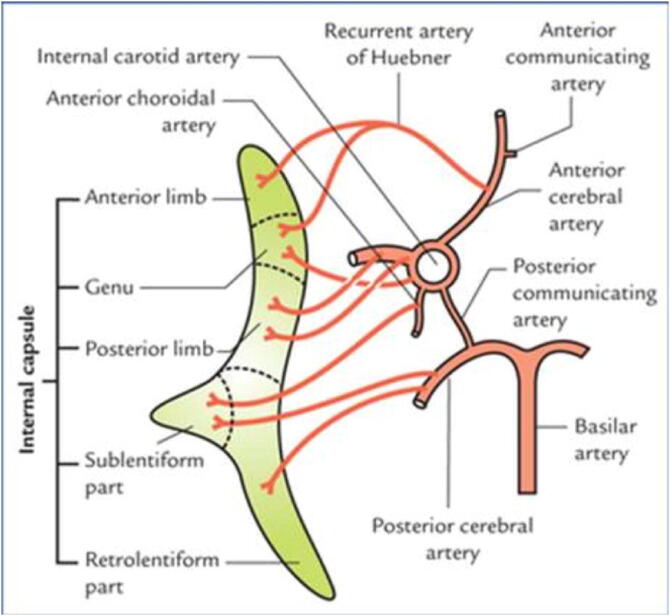
Importantly, the ACA also contributes blood supply to subcortical structures via several perforating branches along its course and the recurrent artery of Huebner (RAH) (Fig. 2). Perforators that arise from the proximal ACA supply the genu of the internal capsule that contain the pyramidal tract corresponding to the upper extremity and face (Dunker and Harris, 1976). The largest of the perforators, the RAH typically arises from the ACA at the junction between the ACA and ACoA in approximately 40% to 76% of cases according to cadaveric studies (Maga et al., 2013, Matsuda et al., 2018). The RAH may rarely originate from other Circle of Willis vessels, be duplicate, or not exist (Matsuda et al., 2018). Identification of the RAH and more proximal ACA-derived perforators during surgical manipulation may be challenging because of distorted anatomy and an unusual rate of anatomical variations, particularly in patients with SAH (Perlmutter and Rhoton, 1976). Occlusion or damage to the perforators contributing to the genu of the internal capsule can cause infarct of the pyramidal tract. Additionally, damage to the RAH near its origin can cause infarct of the basal ganglia including the anteromedial part of the caudate nucleus, anterior putamen and globus pallidus, which collectively are components of the corpus striatum. Infarction of the mediobasal striatum, manifests as brachiofacial hemiparesis (Zunon-Kipré et al., 2012, Maga et al., 2013). This is more likely when there is a limited contribution to these structures from the lateral lenticulostriate arteries as the number of perforating branches from the proximal ACA is inversely related to the number of perforators from the MCA (Zunon-Kipré et al., 2012).
Fig. 2.
A schematic illustration of blood supply of the internal capsule. The recurrent artery of Heubner supplies the anterior genu, which typically houses corticospinal tract fibers corresponding to the upper extremity and face. Reprinted from Textbook of Clinical Neuroanatomy, 2nd ed., Singh V (ed.), Fig. 14.12, 2010, with permission from Elsevier.
4.1.2. Subcortical blood supply and IONM implications
Within the internal capsule, the corticospinal tract is somatotopically arranged such that the fibers corresponding to the UE are anterior to LE fibers and therefore are more likely to be affected by an insufficiency of blood supply through the RAH. In contrast, LE corticospinal tract fibers – as they are more posterior within the internal capsule – are more likely to receive blood supply by other perforators such as those arising from the MCA.
Because of these neuroanatomical considerations, monitoring UE tcMEPs may detect subcortical ischemia specifically caused by interruption of blood flow within the RAH. In our study, UE tcMEP monitoring predicted only two cases of seven new UE deficits (including one reversible change that resolved with intervention) and therefore did not prove to be clinically reliable in predicting UE neurological deficits. In three cases there was evidence of intermittent crossover activation of the ipsilateral side during tcMEP stimulation indicating inappropriately deep penetration of current (Gonzalez et al., 2019). Increased activation threshold in the setting of SAH is a likely culprit.
4.1.3. Cortical blood supply and IONM implications
Surgical manipulation of the ACA at the Circle of Willis can interrupt blood flow within the cortical branches of the pericallosal ACA supplying the LE of the cortical homunculus. Sako et al. (1998) evaluated the sensitivity of LE SSEPs to temporary clip occlusion of ACA in 15 patients and found that SSEP changes were detected in four of seven cases for unilateral A1 occlusion (mean occlusion time, 23 mins) and in six of eight cases for bilateral A1 occlusion (mean occlusion time, 12 min). Although SSEP proved to be sensitive to interruption of blood flow through the ACAs, interestingly, none of the patients had a new neurological deficit or computed tomographic evidence of new infarct in the ACA distribution. The authors concluded that leptomeningeal collateral circulation likely plays an important role in sustaining these areas during prolonged temporary occlusions of the proximal ACA even when SSEP changes occur (Sako et al., 1998). Nevertheless, it remains unclear how long temporary clip occlusion can be safely maintained and SSEP changes during temporary clip occlusion have been found to be predictive of perioperative stroke particularly in unruptured aneurysms (Kashkoush et al., 2017). Of note, cortical representations of the UEs derive their blood supply from the MCA and are unlikely to be affected by ACA insufficiency.
When evaluating the sensitivity of SSEPs and tcMEPs to ischemia affecting the LE of the cortical homunculus, it is important to realize that the sensory cortical field is more peripheral than the motor field in relation to the blood supply from the ACA through the cortical branches. As such, sensory areas are more likely to receive adequate collateral blood flow via the leptomeningeal branches and may be more resistant than motor areas to interruption of blood flow through the ACA to the paracentral lobule. When monitoring sensory and motor function through SSEPs and tcMEPs, the a priori position is that tcMEPs are more sensitive and more affected by ACA occlusion. SSEPs are imperfect surrogates for LE motor function in the event of cortical ischemia. Nevertheless, we found that for LEs, SSEPs performed better in detecting ischemia and predicting motor deficits than tcMEP both in terms of sensitivity (78% vs 50%) and specificity (99% vs 97%) and higher positive and negative predictive values. Combination SSEP and tcMEP for LEs was even more sensitive at 83% than either modality alone.
Notwithstanding some of the limitations of tcMEP especially relating to its inability to accurately aim stimulus at a relatively small and shallow desired target area, according to our dataset, tcMEPs appear to play an important role in complementing SSEP in monitoring for cortical ischemia affecting the LEs. For example, there was only one instance of simultaneous change in both tcMEPs and SSEPs in the LEs during temporary clipping (Table 2). Most of the time, tcMEP or SSEP change occurred in isolation and happened during temporary clipping.
Finally, cortical ischemia may affect other areas of motor control, including the supplementary motor area, which can manifest as pseudoparalysis, especially in the early postoperative period. As tcMEP is specific to the corticospinal tract, compromise of other areas participating in integrated motor control could masquerade as false negatives. As such, we excluded transient weakness of <24-hour duration from our analysis, which could be attributed to pseudoparalysis.
4.2. Transcranial MEP stimulation technique and impact on false negatives
Successful transcranial stimulation requires delivery of electrical charge to motor areas identified as being at risk for injury. The choice of stimulation technique (constant voltage vs constant current) can influence current penetration and charge delivery and ultimately the accuracy of tcMEP monitoring in supratentorial surgeries.
Constant-voltage (or voltage-controlled) simulation delivers short duration electrical pulses (50 msec) at a set voltage, and impedance (tissue and electrode) influences the amount of current delivered to the tissues. Constant-current stimulation on the other hand delivers constant current pulses of longer duration (e.g. 200–500 msec) to the tissues by adjusting the voltage between stimulating electrodes depending on system impedance. Constant-voltage stimulation may result in very high current, when impedance is low, on the other hand current has an upper limit in the constant-current paradigm.
Higher success rates of eliciting tcMEP response in spine surgery has been shown with constant-voltage stimulation technique when using maximal stimulator output settings. This may represent an overall higher current, and more complete recruitment of corticospinal tract fibers when using the voltage-controlled stimulation paradigm (Shigematsu et al., 2017, Hausmann et al., 2002). In supratentorial surgery a recent study demonstrated higher CMAP amplitudes both at threshold and maximal intensity stimulation, and also more efficient charge delivery (resulting in less risk of thermal injury or excitotoxicity) to brain tissue when using constant-voltage stimulation. However, threshold currents were consistently higher with constant-voltage stimulation to elicit CMAP response for both UE and LE. Higher threshold currents observed during constant-voltage stimulation raises the possibility of deeper current penetration, but authors did not report false negatives in their limited sample size of 16 patients undergoing tumor resection (Lettieri et al., 2021).
An important caveat to monitoring both UE and LE tcMEPs in supratentorial surgery is that greater intensity and charge delivery is typically required to elicit LE tcMEP response compared with UE (Lettieri et al., 2021). It is not uncommon in this context that current penetrates deeper than areas at risk for ischemia. Such deep penetration of stimulating current would render monitoring blind to selective cortical ischemia as would occur during manipulation of the ACA or ACoA. Penetration of current deeper into the brain parenchyma potentially also leads to a failure to detect impending brachiofacial paresis from subcortical ischemia by RAH compromise. Crossover activation of the ipsilateral muscles may help to determine if overstimulation is occurring (Gonzalez et al., 2019). Even in the absence of crossover activation, current penetration may still be unacceptably deep and may cause false-negative monitoring. While near-threshold stimulation may result in lower CMAP amplitudes, achieving a stable polyphasic waveform with minimal trial-to-trial variability prior to critical phases of aneurysm manipulation is therefore likely to reduce false negatives compared with the use of intensities that produce maximal CMAP amplitudes (also called supramaximal stimulation). Constant-current pulses using long pulse duration may allow the lowest stimulation threshold to produce monitorable CMAPs (Szelényi et al., 2007a). Use of constant-voltage stimulators in our patient population may have contributed to a higher false-negative rate.
In any stimulation paradigm, optimizing stimulus delivery by placing scalp electrodes close to the surgical incision or using quadripolar stimulation to allow more localized charge delivery with lower energy may reduce this problem. Alternatively, direct cortical stimulation tcMEPs have the advantage of more localized current penetration and increased sensitivity (Szelényi et al., 2005, Szelényi et al., 2007b, Szelényi et al., 2007a, Li et al., 2011).
4.3. IONM in the context of subarachnoid hemorrhage
The presence of a SAH introduces additional layers of difficulty not just for the conduct of surgery, but also for obtaining and interpreting IONM. Given the high proportion of SAH in the false-negative cohort (7 of 8, compared to 38 of 104 for our entire cohort) we speculate that various mechanisms related to SAH could be contributory to a higher number of IONM false negatives.
First, the presence of subarachnoid blood products can contribute to impairment of microcirculation through increased ICP and brain compression, which may persist intraoperatively because of the need for increased brain retraction. Impaired autoregulation, and hypoperfusion may interfere with neuronal function and excitability. This may manifest in an increased stimulation threshold for tcMEPs, which is therefore more likely to bypass the operative site even at near-threshold stimulus delivery (Kanaya et al., 2019). The presence of crossover activation may be evidence of deeper current penetration (case 35 and 96 -Table 2), but the absence of crossover activation does not rule out deeper than optimal penetration. Vasospasm can develop during manipulation of perforators (Kuroda et al, 2013). Vascular insufficiency caused by direct injury to small perforators or transient vasospasm may go completely undetected even after video or conventional angiography. This coupled with increased stimulation thresholds of tcMEPs in this cohort is a setup for false negatives.
Second, vasospasm or other causes of vascular insufficiency such as clip torsion may also evolve during surgical closure and the immediate postoperative period after the cessation of monitoring. Such mechanism could result in a neurological deficit on immediate postoperative exam, which will also classify as a false negative. As an example, in case number 35 (Table 2), the presence of severe bilateral vasospasm in the ACA distribution was detected on computed tomographic angiogram when evaluating the cause for delayed emergence. Similarly, SSEPs may be degraded and not be as sensitive in detecting ischemia during temporary clip occlusion compared with non-ruptured aneurysm clipping (Kashkoush et al., 2017). There are a myriad of factors that could alter the conditions for IONM in SAH, therefore interpretation of IONM results in patients with ruptured aneurysm should be done with caution.
In order to eliminate confounders specific to SAH we also evaluated IONM as a diagnostic test separately in patients not affected by SAH (Table 6). Not surprisingly, for UE tcMEP we found higher sensitivity and specificity than that observed in the entire cohort (50% and 100% vs 38% and 98%). Likewise, LEs tcMEP and SSEP performed better both in terms of sensitivity and specificity than for the entire cohort. Combination tcMEP and SSEP for LE had a 100% sensitivity and 99% sensitivity, which are higher than the entire cohort (Table 6). However, because of a relatively low number of cases and even lower number of IONM events and motor deficits, caution is advised when trying to make conclusions regarding these findings.
4.4. Accounting for reversible signal changes
In our cohort, the majority of EP changes were reversible and most often occurred at the time of temporary clip occlusion. In cases without motor PND, four of five tcMEP reversible signal changes could be causally linked to temporary clip occlusion. Likewise, 7 of 11 SSEP reversible signal changes were causally linked to an inciting event: 6 to temporary clipping and 1 to a positioning-related change. These monitoring events likely signaled neuronal compromise and potentially trigged a course correction during surgery and merit classification as true positives in contrast to those signal changes where evidence of causation could not be identified (Skinner and Holdefer, 2014).
4.5. IONM as a tool to monitor for unusual conditions
Furthermore, combination IONM involving UE tcMEPs may have utility beyond simply monitoring for subcortical ischemia as illustrated by case number 34 (Table 2). After a routine intraoperative course and during angiography, a sudden disappearance of UE and a decreased amplitude of LE tcMEPs were noted. Increased stimulation returned the LE tcMEPs to baseline. The UE tcMEPs retained a decreased amplitude. There were no SSEP changes. The patient woke up from surgery with severe hemiparesis, plegic UE, and decreased strength of LE. A postoperative CT perfusion showed a small perfusion defect in the right parietal lobe. The patient was taken for emergent endovascular therapy about 90 min after surgery, where angiogram revealed a distal MCA branch embolus, which was successfully retrieved. Patient made a full recovery. Putatively an embolic phenomenon may have been triggered by intraoperative angiography. This could explain the transient LE weakness as well.
4.6. Limitations
This study has important limitations. First, it was done retrospectively, based on information collected during routine care, therefore, it may not have fully captured transient deficits in this population. As tcMEP modality was not available for the entire study period and in the absence of guidelines on recommended IONM modalities for ACA aneurysm clipping a consistent protocol for EP modalities and extremities was not established. This may have affected the interpretation of EP changes and the intraoperative response to such changes, ultimately impacting outcomes. Nevertheless, the majority of cases used all four extremity SSEP and tcMEP monitoring. Our study focused on IONM as a diagnostic test for postoperative motor deficits, and silent infarcts were not measured given the lack of consistent imaging protocols. SSEP alert criteria changes were introduced in 2019, based on more conservative guidelines, which may have increased sensitivity at the expense of decreased specificity and more frequent interference with the conduct of surgery. Response to IONM alerts were not protocolized and thus the impact of IONM alerts on the conduct of surgery and postoperative outcomes remains elusive. Likewise, the application of causality linkage guidelines to classify reversible signal changes retrospectively is limited by available information that could be gleaned from review of the medical records. The approach to monitoring and anesthesia was done thoughtfully, with an eye toward facilitating monitoring, but not in a strictly standardized fashion. This captures real-world clinical practice. Given the sizeable study population, the conclusions are nonetheless well supported.
5. Conclusions
Neither SSEPs nor tcMEPs for LE monitoring appear to be sensitive enough to be useful as a sole monitoring modality. However, dual-modality monitoring of the LEs improved sensitivity and may provide benefit to the preservation of the LEs during surgical clipping of ACA and ACoA aneurysms. Surprisingly, UE tcMEPs appeared to have limited use in identifying injury secondary to insufficiency of the RAH and perforating vessels originating from the proximal ACA in our cohort.
Our data suggests that more information is indeed better, i.e., four extremities for both SSEPs and tcMEPs during surgical clipping of aneurysms of the ACA and ACoA. The reason is the variability in vascular supply and collateral circulation, technical limitations of IONM during craniotomy (e.g., electrode placement site and incision, impedance from subarachnoid air), as well as the many potential pathogenetic mechanisms that may lead to motor deficits in these complex surgeries. Despite full monitoring, there are deficits that will be missed and there will be deficits that are identified, but cannot be reversed. IONM is therefore best used as a component of a multimodality monitoring strategy such as in combination with angiography and doppler sonography. The effectiveness of IONM as an intervention to minimize deficits depends on robust performance and diligent interpretation while recognizing inherent and operator-dependent limitations in a collaborative atmosphere between neurophysiologists, neurosurgeons, and anesthesiologists.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank our neuromonitoring technicians William E. Kretzman, R. EPT, Linda L. Moss and Steven A. Finlay, R.EEG for their technical expertise, and Corey Astrom, ELS, for her editorial expertise and assistance with this manuscript. This research received support from the Department of Anesthesiology, University of Florida College of Medicine, Gainesville, FL.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cnp.2022.07.001.
Contributor Information
Ferenc Rabai, Email: rabai.ferenc@mayo.edu.
Claire M. Dorey, Email: cdorey@ufl.edu.
W. Christopher Fox, Email: fox.chris@mayo.edu.
Christoph N. Seubert, Email: cseubert@anest.ufl.edu.
Steven A. Robicsek, Email: robicsek@ufl.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Dunker R.O., Harris A.B. Surgical anatomy of the proximal anterior cerebral artery. J. Neurosurg. 1976;44(3):359–367. doi: 10.3171/jns.1976.44.3.0359. [DOI] [PubMed] [Google Scholar]
- Gonzalez A.A., Akopian V., Lagoa I., Shilian P., Parikh P. Crossover phenomena in motor evoked potentials during intraoperative neurophysiological monitoring of cranial surgeries. J. Clin. Neurophysiol. 2019;36(3):236–241. doi: 10.1097/WNP.0000000000000570. [DOI] [PubMed] [Google Scholar]
- Guo L., Gelb A.W. The use of motor evoked potential monitoring during cerebral aneurysm surgery to predict pure motor deficits due to subcortical ischemia. Clin. Neurophysiol. 2011;122(4):648–655. doi: 10.1016/j.clinph.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Hausmann O.N., Min K., Boos N., Ruetsch Y.A., Erni T., Curt A. Transcranial electrical stimulation: significance of fast versus slow charge delivery for intra-operative monitoring. Clin. Neurophysiol. 2002;113(10):1532–1535. doi: 10.1016/s1388-2457(02)00213-4. [DOI] [PubMed] [Google Scholar]
- Holdefer R.N., MacDonald D.B., Skinner S.A. Somatosensory and motor evoked potentials as biomarkers for post-operative neurological status. Clin. Neurophysiol. 2015;126(5):857–865. doi: 10.1016/j.clinph.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Kakou M., Destrieux C., Velut S. Microanatomy of the pericallosal arterial complex. J. Neurosurg. 2000;93(4):667–675. doi: 10.3171/jns.2000.93.4.0667. [DOI] [PubMed] [Google Scholar]
- Kanaya K., Goto T., Horiuchi T., Hongo K. Threshold variation of transcranial motor evoked potential with threshold criterion in frontotemporal craniotomy. Clin. Neurophysiol. Pract. 2019;4:184–189. doi: 10.1016/j.cnp.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashkoush A.I., Jankowitz B.T., Gardner P., Friedlander R.M., Chang Y.F., Crammond D.J., Balzer J.R., Thirumala P.D. Somatosensory evoked potentials during temporary arterial occlusion for intracranial aneurysm surgery: predictive value for perioperative stroke. World Neurosurg. 2017;104:442–451. doi: 10.1016/j.wneu.2017.05.036. [DOI] [PubMed] [Google Scholar]
- Kashkoush A.I., Nguyen C., Balzer J., Habeych M., Crammond D.J., Thirumala P.D. Diagnostic accuracy of somatosensory evoked potentials during intracranial aneurysm clipping for perioperative stroke. J. Clin. Monit. Comput. 2020;34(4):811–819. doi: 10.1007/s10877-019-00369-x. [DOI] [PubMed] [Google Scholar]
- K. Kuroda H. Kinouchi K. Kanemaru Y. Nishiyama M. Ogiwara H. Yoshioka T. Horikoshi Intra-arterial injection fluorescein videoangiography in aneurysm surgery Neurosurgery. 2013;72(2 Suppl Operative):ons141-50; discussion ons150 10.1227/NEU.0b013e3182752f32. [DOI] [PubMed]
- Legatt A.D., Emerson R.G., Epstein C.M., MacDonald D.B., Deletis V., Bravo R.J., López J.R. ACNS guideline: transcranial electrical stimulation motor evoked potential monitoring. J. Clin. Neurophysiol. 2016;33(1):42–50. doi: 10.1097/WNP.0000000000000253. [DOI] [PubMed] [Google Scholar]
- Lettieri C., Pauletto G., Valiante G., Ius T., Verriello L., Valente M., Skrap M., Gigli G.L., Budai R. Fast or slow? A comparison between two transcranial electrical stimulation techniques for eliciting motor-evoked potentials during supratentorial surgery. J. Clin. Neurophysiol. 2021 doi: 10.1097/WNP.0000000000000902. [DOI] [PubMed] [Google Scholar]
- Li F., Deshaies E.M., Allott G., Canute G., Gorji R. Direct cortical stimulation but not transcranial electrical stimulation motor evoked potentials detect brain ischemia during brain tumor resection. Am J Electroneurodiagnostic Technol. 2011;51(3):191–197. [PubMed] [Google Scholar]
- Li M., Wu J., Chen X., Jiang P., Yang F., Ma Y., Li Z., Cao Y., Wang S. Symptomatic and silent cerebral infarction following surgical clipping of unruptured intracranial aneurysms: incidence, risk factors, and clinical outcome. Neurosurg. Rev. 2018;41(2):675–682. doi: 10.1007/s10143-017-0913-1. [DOI] [PubMed] [Google Scholar]
- Lin J., Zhao J., Zhao Y., Zhang D., Wang R., Qiao H., Wang S. Multiple intraoperative monitoring-assisted microneurosurgical treatment for anterior circulation cerebral aneurysm. J. Int. Med. Res. 2011;39(3):891–903. doi: 10.1177/147323001103900323. [DOI] [PubMed] [Google Scholar]
- MacDonald D.B., Dong C., Quatrale R., Sala F., Skinner S., Soto F., Szelényi A. Recommendations of the International Society of Intraoperative Neurophysiology for intraoperative somatosensory evoked potentials. Clin. Neurophysiol. 2019;130(1):161–179. doi: 10.1016/j.clinph.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Macdonald D.B., Skinner S., Shils J., Yingling C. American Society of Neurophysiological Monitoring. Intraoperative motor evoked potential monitoring - a position statement by the American Society of Neurophysiological Monitoring. Clin. Neurophysiol. 2013;124(12):2291–2316. doi: 10.1016/j.clinph.2013.07.025. [DOI] [PubMed] [Google Scholar]
- Maga P., Tomaszewski K.A., Skrzat J., Tomaszewska I.M., Iskra T., Pasternak A., Walocha J.A. Microanatomical study of the recurrent artery of Heubner. Ann Anat. 2013;195(4):342–350. doi: 10.1016/j.aanat.2013.03.011. [DOI] [PubMed] [Google Scholar]
- Matsuda W., Sonomura T., Honma S., Ohno S., Goto T., Hirai S., Itoh M., Honda Y., Fujieda H., Udagawa J., Ueda S. Anatomical variations of the recurrent artery of Heubner: number, origin, and course. Anat Sci Int. 2018;93(3):317–322. doi: 10.1007/s12565-017-0415-9. [DOI] [PubMed] [Google Scholar]
- Molyneux A., Kerr R., Stratton I., Sandercock P., Clarke M., Shrimpton J., Holman R. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- Neuloh G., Schramm J. Monitoring of motor evoked potentials compared with somatosensory evoked potentials and microvascular Doppler ultrasonography in cerebral aneurysm surgery. J. Neurosurg. 2004;100(3):389–399. doi: 10.3171/jns.2004.100.3.0389. [DOI] [PubMed] [Google Scholar]
- Perlmutter D., Rhoton A.L. Microsurgical anatomy of the anterior cerebral-anterior communicating-recurrent artery complex. J. Neurosurg. 1976;45(3):259–272. doi: 10.3171/jns.1976.45.3.0259. [DOI] [PubMed] [Google Scholar]
- Sako K., Nakai H., Kawata Y., Takizawa K., Satho M., Yonemasu Y. Temporary arterial occlusion during anterior communicating or anterior cerebral artery aneurysm operation under tibial nerve somatosensory evoked potential monitoring. Surg. Neurol. 1998;49(3):316–322. doi: 10.1016/s0090-3019(97)00225-5. discussion 322. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Kodama N., Matsumoto M., Suzuki K., Konno Y., Sakuma J., Endo Y., Oinuma M. Blood flow disturbance in perforating arteries attributable to aneurysm surgery. J. Neurosurg. 2007;107(1):60–67. doi: 10.3171/JNS-07/07/0060. [DOI] [PubMed] [Google Scholar]
- Shigematsu H., Kawaguchi M., Hayashi H., Takatani T., Iwata E., Tanaka M., Okuda A., Morimoto Y., Masuda K., Tanaka Y., Tanaka Y. Higher success rate with transcranial electrical stimulation of motor-evoked potentials using constant-voltage stimulation compared with constant-current stimulation in patients undergoing spinal surgery. Spine J. 2017;17(10):1472–1479. doi: 10.1016/j.spinee.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Skinner S.A., Holdefer R.N. Intraoperative neuromonitoring alerts that reverse with intervention: treatment paradox and what to do about it. J. Clin. Neurophysiol. 2014;31(2):118–126. doi: 10.1097/WNP.0000000000000030. [DOI] [PubMed] [Google Scholar]
- Szelényi A., Kothbauer K.F., Deletis V. Transcranial electric stimulation for intraoperative motor evoked potential monitoring: Stimulation parameters and electrode montages. Clin. Neurophysiol. 2007;118(7):1586–1595. doi: 10.1016/j.clinph.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Szelényi A., Langer D., Beck J., Raabe A., Flamm E.S., Seifert V., Deletis V. Transcranial and direct cortical stimulation for motor evoked potential monitoring in intracerebral aneurysm surgery. Clin. Neurophysiol. 2007;37(6):391–398. doi: 10.1016/j.neucli.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Szelényi A., Kothbauer K., de Camargo A.B., Langer D., Flamm E.S., Deletis V. Motor evoked potential monitoring during cerebral aneurysm surgery: technical aspects and comparison of transcranial and direct cortical stimulation. Neurosurgery. 2005;57(4 Suppl):331–338. doi: 10.1227/01.neu.0000176643.69108.fc. [DOI] [PubMed] [Google Scholar]
- Thirumala P.D., Udesh R., Muralidharan A., Thiagarajan K., Crammond D.J., Chang Y.F., Balzer J.R. Diagnostic value of somatosensory-evoked potential monitoring during cerebral aneurysm clipping: a systematic review. World Neurosurg. 2016;89:672–680. doi: 10.1016/j.wneu.2015.12.008. [DOI] [PubMed] [Google Scholar]
- B. Thomas D. Guo The diagnostic accuracy of evoked potential monitoring techniques during intracranial aneurysm surgery for predicting postoperative ischemic damage: a systematic review and meta-analysis World Neurosurg 103 2017 829 40.e3 10.1016/j.wneu.2017.04.071. [DOI] [PubMed]
- Toleikis J.R. American Society of Neurophysiological Monitoring. Intraoperative monitoring using somatosensory evoked potentials. A position statement by the American Society of Neurophysiological Monitoring. J. Clin. Monit. Comput. 2005;19(3):241–258. doi: 10.1007/s10877-005-4397-0. [DOI] [PubMed] [Google Scholar]
- Wiebers D.O., Whisnant J.P., Huston J., Meissner I., Brown R.D., Jr, Piepgras D.G., Forbes G.S., Thielen K., Nichols D., O'Fallon W.M., Peacock J., Jaeger L., Kassell N.F., Kongable-Beckman G.L., Torner J.C. International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103–110. doi: 10.1016/s0140-6736(03)13860-3. [DOI] [PubMed] [Google Scholar]
- Zhu F., Chui J., Herrick I., Martin J. Intraoperative evoked potential monitoring for detecting cerebral injury during adult aneurysm clipping surgery: a systematic review and meta-analysis of diagnostic test accuracy. BMJ Open. 2019;9(2):e022810. doi: 10.1136/bmjopen-2018-022810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunon-Kipré Y., Peltier J., Haïdara A., Havet E., Kakou M., Le Gars D. Microsurgical anatomy of distal medial striate artery (recurrent artery of Heubner) Surg. Radiol. Anat. 2012;34(1):15–20. doi: 10.1007/s00276-011-0888-50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.