Abstract
In a recent study in Nature Biomedical Engineering, Wei’s group constructed a field-effect transistor (FET) based on the “molecular electromechanical system” (MolEMS). MolEMS enabled FETs to specifically capture viral nucleic acids and efficiently transduce signals, achieving a rapid and ultra-sensitive detection of SARS-CoV-2.
In a recent study in Nature Biomedical Engineering, Wei’s group constructed a field-effect transistor (FET) based on the “molecular electromechanical system” (MolEMS). MolEMS enabled FETs to specifically capture viral nucleic acids and efficiently transduce signals, achieving a rapid and ultra-sensitive detection of SARS-CoV-2.
Main text
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has caused a global outbreak. The COVID-19 pandemic remains severe in most countries, which poses a great threat to the global economy, health care systems, and our lives. Thus, rapid diagnostic methods for SARS-CoV-2 at a large scale are crucial to control the pandemic.1 There are two types of SARS-CoV-2 diagnostic methods, serological and viral nucleic acid tests.2 Serological tests directly detect antibodies or antigenic viral proteins in the infected individuals and obtain results quickly. However, such tests can only detect some infections and can cause false negative diagnoses, especially for the early stage of infections.3 Nucleic acid tests are more sensitive and accurate, including reverse-transcriptase PCR (RT-PCR), isothermal amplification, and clustered regularly interspaced short palindromic repeats (CRISPR).4 At present, RT-PCR5 is the gold standard for the diagnosis and confirmation of SARS-CoV-2 infections. It normally requires nucleic acid extraction and amplification procedures, which are time-consuming (∼2 h) and require a specialized laboratory setting with expensive instruments and trained personnel. Some other technologies, such as CRISPR and isothermal amplification, have been developed but similarly require nucleic acid extraction or amplification. These restrictions become major hurdles for situations with resource scarcity. Therefore, a direct SARS-CoV-2 nucleic acid test with high sensitivity and fast detection is still an immediate need and can be used as a screening tool for the diagnosis of COVID-19.6
A field-effect transistor (FET), directly converting chemical matter to electrical signals, has many advantages, such as easy integration and rapid response.7 Due to its amplification effect on weak signals, the FET is especially suitable for detecting small amounts of analytes in biofluids to construct ultra-sensitive biosensors. In terms of nucleic acid detection, Hwang et al. demonstrated an FET biosensor based on the deformed graphene, achieving a limit of detection down to ∼10–18 mol/L in buffer solutions.8 However, most FET biosensors rarely reach this value and still lack the sensitivity for an unamplified COVID-19 nucleic acid assay without efficient biorecognition and signal transduction.9
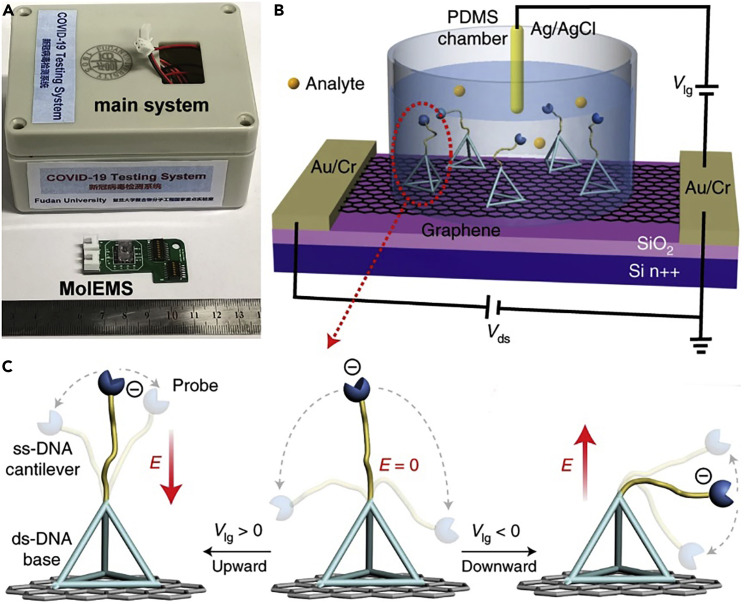
Wei’s group demonstrated a biosensor (Figure 1B) that integrated “molecular electromechanical system” (MolEMS) into a graphene FET.10 MolEMS consisted of an aptamer probe bound to a flexible single-stranded DNA cantilever linked to a self-assembled stiff tetrahedral double-stranded DNA structure. The flexible cantilever of MolEMS was bound to an aptamer probe, used for specific capture segments of nucleic acid of SARS-CoV-2 (i.e., specific biorecognition). Meanwhile, high density of rigid bases served as an anti-fouling layer, which resisted non-target biomolecules, avoiding non-specific adsorption. Under an applied electric field, the flexible cantilever approached the surface of the graphene FET within the Debye length, inducing an obvious electrical signal in the channel due to the field effect (i.e., signal transduction). Eventually, this biosensor was capable of recognizing SARS-CoV-2 accurately and ultra-sensitively. Clinical trials have demonstrated that this biosensor could directly detect nucleic acids of SARS-CoV-2 in less than 4 min in nasopharyngeal samples without the need for nucleic acid extraction or amplification.
Figure 1.
FET biosensor integration with MolEMS
(A) Prototype of the COVID-19 testing system.
(B) Device configuration of FET biosensor integrated with MolEMS.
(C) Schematic of MolEMS and its electrostatic actuation. A probe is conjugated at the tip of the cantilever for specific biorecognition. E indicates an electric field.
The voltage applied by a liquid gate should be given special care because it hugely affected signal transduction efficiency and thus detection sensitivity. The flexible cantilever used by the authors was negatively charged. When a positive or negative voltage was applied, the cantilever would be lifted up or pushed down, resulting in the cantilever in the upper or lower regions of the MolEMS, respectively (Figure 1C). Specifically, when the gate voltage was positive or zero, the cantilever was limited in the upper region or moved randomly in the wider region, respectively. Thus, there was a high chance that the identified analyte would be far away from the graphene surface. When the gate voltage was negative, the cantilever was limited in the lower region of the MolEMS. Thus, the identified analyte approached the graphene surface within the Debye length, resulting in potential perturbation of the graphene channel followed by current responses in real time. Therefore, a negative gate voltage drove the MolEMS to enhance the current response.
The biosensor was employed to test 33 nasopharyngeal swab samples from RT-PCR-positive patients, 23 samples from RT-PCR-negative patients with fever, and 25 samples from healthy volunteers. All clinical samples were heated to release nucleic acids and were then tested directly without nucleic acid extraction or amplification. The biosensor could detect 33 RT-PCR-positive patients with an electrical signal variation in the range of 0.8%–6.85% while it generated negligible signals (less than 0.08%) for the rest of the samples, which was consistent with RT-PCR results. The biosensor sensitivity was further evaluated by the clinical COVID-19 samples according to Ct values and the RT-PCR standard curve and diluted them serially using viral transport medium (VTM). While RT-PCR detection kits generated negligible signals to low concentration of nucleic acids, the biosensor generated obvious electrical signals even if the nucleic acid concentration decreased to ∼20 copies per mL. The time to COVID-19 diagnosis (total of 26 patients) was 0.1 to 4 min with an average of 1 min. More experiments have shown that the biosensor based on MolEMS had an anti-fouling ability (no response to non-specific species in clinical samples) and long-term stability (up to 21 days stored in VTM at 4°C). These results demonstrated that compared with RT-PCR, this biosensor did not require time-consuming nucleic acid extraction and amplification and had a limit of detection of ∼20 copies per mL and a detection time of less than 4 min, faster than the existing RT-PCR.
In addition, this biosensor could be used to specifically and ultra-sensitively detect other categories of analytes (mercury ion, thrombin, and adenosine 5′-triphosphate) in buffer solution or biological liquid by introducing specific aptamer probe molecules into MolEMS. Besides COVID-19, the authors declared that the development of MolEMS could allow for the precise diagnosis of other diseases in a few minutes without the need for target purification, amplification, or culture. Finally, Wei’s group further developed a portable system (Figure 1A) to enable on-site and point-of-care testing (POCT) of COVID-19 in airports, clinics, and even at home.
This POCT of nucleic acid has the advantages of simple operation, fast detection, and no need for a professional laboratory, which compensate for the deficiencies of RT-PCR. However, many efforts need to be made in at least two aspects. On one hand, more clinical trials are expected by increasing the number of samples to fully verify the clinical performance of biosensors. On the other hand, it is necessary to establish relevant industry standards or technical specifications to formulate the production, storage, and use of biosensors. In the long run, this biosensor has specific application scenarios and necessities, such as rapid diagnosis of suspected patients and on-site screening of people in close contact with patients. It is believed that this technology will be gradually improved to meet the application needs.
Acknowledgments
Declaration of interests
The authors declare no competing interests.
References
- 1.Zhang Z.W., Ma P., Ahmed R., Wang J., Akin D., Soto F., Liu B.F., Li P.W., Demirci U. Advanced point-of-care testing technologies for Human acute respiratory Virus detection. Adv. Mater. 2022;34:2103646. doi: 10.1002/adma.202103646. [DOI] [PubMed] [Google Scholar]
- 2.Weissleder R., Lee H., Ko J., Pittet M.J. COVID-19 diagnostics in context. Sci. Translational Med. 2020;12:eabc1931. doi: 10.1126/scitranslmed.abc1931. [DOI] [PubMed] [Google Scholar]
- 3.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, Transmission, diagnosis, and Treatment of coronavirus disease 2019 (COVID-19) A Review. JAMA-Journal Am. Med. Assoc. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 4.Udugama B., Kadhiresan P., Kozlowski H.N., Malekjahani A., Osborne M., Li V.Y.C., Chen H.M., Mubareka S., Gubbay J.B., Chan W.C.W. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14:3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- 5.Vandenberg O., Martiny D., Rochas O., van Belkum A., Kozlakidis Z. Considerations for diagnostic COVID-19 tests. Nat. Rev. Microbiol. 2021;19:171–183. doi: 10.1038/s41579-020-00461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torres M.D.T., de Araujo W.R., Araujo W R d, de Lima L.F., Lima L F d, Ferreira A.L., de la Fuente-Nunez C., Fuente-Nunez C d l. Low-cost biosensor for rapid detection of SARS-CoV-2 at the point of care. Matter. 2021;4:2403–2416. doi: 10.1016/j.matt.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadighbayan D., Hasanzadeh M., Ghafar-Zadeh E. Biosensing based on field-effect transistors (FET): recent progress and challenges. Trac-Trends Anal. Chem. 2020;133:116067. doi: 10.1016/j.trac.2020.116067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang M.T., Heiranian M., Kim Y., You S., Leem J., Taqieddin A., Faramarzi V., Jing Y.H., Park I., van der Zande A.M., et al. Ultrasensitive detection of nucleic acids using deformed graphene channel field effect biosensors. Nat. Commun. 2020;11:1543. doi: 10.1038/s41467-020-15330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X., Kong D., Guo M., Wang L., Gu C., Dai C., Wang Y., Jiang Q., Ai Z., Zhang C., et al. Rapid SARS-CoV-2 nucleic acid testing and Pooled assay by tetrahedral DNA Nanostructure transistor. Nano Lett. 2021;21:9450–9457. doi: 10.1021/acs.nanolett.1c02748. [DOI] [PubMed] [Google Scholar]
- 10.Wang L.Q., Wang X.J., Wu Y.G., Guo M.Q., Gu C.J., Dai C.H., Kong D.R., Wang Y., Zhang C., Qu D., et al. Rapid and ultrasensitive electromechanical detection of ions, biomolecules and SARS-CoV-2 RNA in unamplified samples. Nat. Biomed. Eng. 2022;6:276–285. doi: 10.1038/s41551-021-00833-7. [DOI] [PubMed] [Google Scholar]