Abstract
Background
Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has proven to be a successful strategy for prevent severe infections. CoronaVac and BNT162b2 are the most used vaccines worldwide, but their use in heterologous vaccination schedules is still subjected to evaluation.
Methods
Fifty healthy individuals who received heterologous prime-boost vaccination with CoronaVac and BNT162b2 were enrolled in a post-vaccination serological follow-up longitudinal prospective study. We evaluated specific serum anti-receptor binding domain (RBD) IgG antibody levels, and their capacity to block RBD-ACE2 interaction with a surrogate neutralization assay. In 20 participants, we assessed antibody binding kinetics by surface plasmon resonance, and Fc-mediated functions by ADCC and ADCP reporter assays.
Results
Our baseline seronegative cohort, displayed seroconversion after two doses of CoronaVac and an important decrease in serum anti-RBD IgG antibodies levels 80 days post-second dose. These levels increased significantly early after the third dose with BNT162b2, but 73 days after the booster we found a new fall. Immunoglobulin functionalities showed a similar behavior.
Conclusions
The heterologous prime-boost vaccination with CoronaVac and BNT162b2 generated an impressive increase in serum anti-RBD specific antibody levels followed by a drop. Nevertheless, these titers remained well above those found in individuals only vaccinated with CoronaVac in the same elapsed time. Serum IgG levels showed high correlation with antibody binding analysis, their capacity to block RBD-ACE2 interaction, and Fc-effectors mechanisms. Our work sheds light on the humoral immune response to heterologous vaccination with CoronaVac and BNT162b2, to define a post-vaccination correlate of protection against SARS-CoV-2 infection and to discuss the scheduling of future vaccine boosters in general population.
Keywords: SARS-CoV-2, Heterologous vaccination, Humoral immune response, Binding kinetics, Fc-mediated functions
1. Introduction
The application of effective vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has proved to be a successful strategy for reducing viral transmission and disease burden. The two most used vaccines worldwide are CoronaVac (Sinovac Life Sciences, Beijing, China), an inactivated SARS-CoV-2 based platform with more than 1.7 billion administered doses, and BNT162b2 (Pfizer/BioNTech), a mRNA-based vaccine with more than 1.5 billion doses [1].
In March 2020, the first cases of Coronavirus Disease 2019 (COVID-19) were diagnosed in Uruguay. By February 24, 2022, of a 3.5 million population more than 830.000 confirmed cases of SARS-CoV-2 infection have been reported and more than 6.900 persons have died [2]. In February 2021, the Ministry of Public Health of Uruguay authorized the emergency use of CoronaVac [3] (600 SU of inactivated virus per dose) and BNT162b2 [4] (30 µg per dose) vaccines in two doses administrated 28 days apart. To date, 77% of the Uruguayan population has been vaccinated with two doses of some of these two vaccines (59.9% received CoronaVac and 38.4% BNT162b2) [2], while only 1.7% was immunized with AstraZeneca vaccine provided by the COVAX initiative (https://vacuna.uy).
Both CoronaVac and BNT162b2 have shown great efficacy to prevent hospitalizations two weeks after second dose (2D) administration [5], [6], simultaneously with the detection of high levels of serum specific antibodies against the SARS-CoV-2 Spike protein [7]. Although immunological parameters required to define a post-vaccination correlate of protection (CoP) against SARS-CoV-2 infection are still under discussion, antibody-mediated viral neutralization has been considered one of the most important contributors [8], along with other antibody-mediated functions like Fc-mediated effector responses [9]. Some studies found that inactivated SARS-CoV-2 vaccines generates lower levels of neutralizing antibodies [1] and a decline over time in the level of specific antibodies has been reported for both, BNT162b2 [10], [11] and CoronaVac vaccines [12].
Faced with this reality, the application of booster doses has become a strategic alternative that must coexist with the demand for a global equitable distribution of vaccines. In this regard, in September 2021 the Ministry of Public Health of Uruguay authorized the administration of a booster dose with BNT162b2 for the subset of the population fully vaccinated with CoronaVac. Nowadays, almost 52% of Uruguayan population has already received this booster.
Here, we analyzed the dynamics of serum IgG antibodies against the receptor binding domain (RBD) of SARS-Cov-2 Spike protein at different times, in a longitudinal prospective study on healthy individuals. We quantified the specific IgG levels in participants who received a two-dose plan of CoronaVac, and after heterologous BNT162b2 third dose (3D) administration. In addition, we analyzed the functionality of specific anti-SARS-CoV-2 antibodies by measuring their neutralizing capacity, Fc-mediated effector functions, and their overall binding kinetics to the RBD. Regarding the measurement of the neutralizing capacity, we must point out that we used an in vitro assay that evidences the blockage of the interaction between the RBD protein and its receptor (ACE2) by specific anti-RBD antibodies, as an accessible experimental approach to address their neutralizing capacity. There is little information regarding the use of a 3D booster with BNT162b on fully vaccinated individuals with 2 doses of CoronaVac [13], [14]. Our work provides new data that help to understand the development and characterization of the humoral immune response against this combination of heterologous vaccines.
2. Materials and methods
2.1. Cohort and study design
Fifty individuals, 60% women, median age 40 years (IQR 30–50) belonging to the staff of the Institut Pasteur de Montevideo, who underwent voluntary heterologous prime-boost vaccination with CoronaVac and BNT162b2, were enrolled in a post-vaccination serological follow-up study. Serum samples were collected at five times: before vaccination (t0); after a median follow-up of 18 days (IQR 16–23) post-2D (t1); 80 days (IQR 78–82) post-2D (t2); 18 days (IQR 16–20) post-3D with BNT162b2 (t3); and finally, 73 days (IQR 72–81) post-3D (for 41 of 50 participants) (t4). All participants declared not to have been diagnosed with COVID-19 prior to or during the development of the study, in two questionnaires performed at t0 and at t3. In addition, local and systemic adverse events within 7 days after 3D were registered. This study was done in accordance with the Helsinki Declaration of the World Medical Association and was approved by the ethical institutional review board (MSP 956,220 – CEI 001-2021). Informed consent was obtained from all participants.
2.2. ELISA, neutralization activity, surface plasmon resonance (SPR), and antibody-dependent cellular cytotoxicity and phagocytosis (ADCC and ADCP) assays
Anti-RBD (Wuhan variant) IgG serum levels were quantified using COVID-19 IgG QUANT ELISA Kit (developed by Universidad de la República, Institut Pasteur de Montevideo and ATGen SRL), according to manufacturer’s instructions. Results were expressed in binding antibody units per milliliter (BAU/mL), by using the First WHO International Standard for anti-SARS-CoV-2 immunoglobulin (NIBSC code: 20/136) for assay calibration (https://www.nibsc.org/documents/ifu/20–136.pdf).
The blockade of RBD-ACE2 interaction by specific anti-SARS-CoV-2 antibodies was analyzed by using an in vitro surrogate virus neutralization test (sVNT; cPass™ SARS-CoV-2 Neutralization Antibody Detection Kit, GenScript®) in serum from individuals who received heterologous vaccination at t1 and t2 (post-2D with CoronaVac) and at t3 and t4 (post-3D with BNT162b2), according to manufacturer’s instructions. Briefly, recombinant RBD conjugated to HRP was pre incubated with different dilutions of serum samples and then added to ACE2 coated ELISA microplates to analyze the capacity of specific serum antibodies to inhibit RBD-ACE2 interaction. A calibrator serum (Cat. No. A02087, GenScript®) of 1.000.000 Arbitrary Units per milliliter (AU/mL) was used according to manufacturer’s instructions to generate a standard curve by serially diluting the calibrator serum from 600 AU/mL to 9.37 AU/mL. The optical densities of serum dilutions measured at 450 nm that fall in the lineal range of the standard curve were used to interpolate a semiquantitative titer value in AU/mL, which were corrected with the corresponding dilution factor to calculate the final value in AU/mL of serum.
SPR assays were conducted with serum samples obtained at four different times (t0, t1, t2, t3) from a sub-group of 20 individuals who received heterologous CoronaVac/BNT162b2 vaccination, to evaluate the binding of total specific antibodies against captured RBD on a SPR sensorchip. A recombinant RBD expressed in S2 insect cells was used for these assays (see Supplementary Material for details, Fig. S1).
ADCC and ADCP were performed on the same 20 serum samples at t1 and t3, using ADCC and ADCP reporter assays (InvivoGen) according to manufacturer’s instructions with minor differences. Jurkat-Lucia™ NFAT cells expressing human FcgRIIIA V158 allotype or human FcgRIIA H131 allotype were used for ADCC and ADCP reporter assays, respectively (see Supplementary Material for details).
2.3. Data analysis
By employing GraphPad Prism 9.2.0.332 we carried out comparisons between groups using Friedmańs test and Dunńs multiple comparison post-hoc test or Wilcoxon test. Although the non-parametric tests used in the multiple comparisons generally yielded significant differences in all the contrasts used, in some cases, the post-hoc comparisons were marginally significant, essentially because of the low number of observations. To overcome this difficulty, we used linear mixed models (R packages “lme4” and “emmeans”) with Tukey correction on the logarithm of the concentrations, modeling the individual as a random effect. Prior to this, we verified the normality and homoscedasticity assumptions through the Shapiro-Wilks test and the studentized Breusch-Pagan test, respectively. In all cases, the post-hoc contrasts were highly significant (p-value < 1 × 10−6). Results from reporter bioassays were obtained in relative luminescence unit (RLUs) and expressed as endpoint titers. To assess consistency in the level of specific antibodies measured by ELISA and SPR, we calculated two-tailed Pearson correlation coefficients with a 95% confidence interval.
3. Results
Early after receiving the 3D of BNT162b2, all individuals reported only mild to moderate adverse events. Injection site pain was by far the most frequent (90%), followed by fatigue, malaise, and chills among other local and systemic adverse events (Table 1 ).
Table 1.
Adverse events after third dose with BNT162b2.
| Participants (%) | ||
|---|---|---|
| Local adverse events | Pain | 45 (90) |
| Swelling | 4 (8) | |
| Skin redness | 2 (4) | |
| Regional lymphadenopathy | 1 (2) | |
| Systemic adverse events | Fatigue | 16 (32) |
| Malaise | 12 (24) | |
| Chills | 10 (20) | |
| Headache | 8 (16) | |
| Myalgia | 8 (16) | |
| Fever | 5 (10) | |
| Arthralgia | 4 (8) | |
| Nausea, vomiting, diharrea | 4 (8) | |
The questionnaire was conducted concomitantly to the blood extraction early after 3D with BNT162b2 by medical staff, and adverse events are classified as local or systemic signs or symptoms. All but two participants presented at least one of the evaluated events, and many of them presented more than one. The table shows the number of participants with the specific sign or symptom and the respective percentage within the cohort (%).
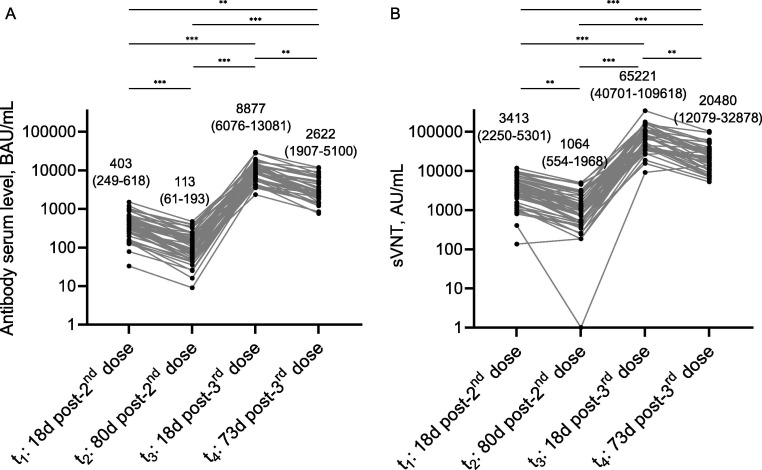
No specific anti-RBD IgG antibodies were found before vaccination (t0) in the serum samples (baseline seronegative). In contrast, all participants displayed seroconversion 18 days post-2D of CoronaVac (t1), with a median of 403 BAU/mL (IQR 249-618) (Fig. 1 ). At t2 all 50 individuals remained seropositive, although with an important decrease in the level of anti-RBD IgG antibodies (median of 113 BAU/mL, IQR 61-193) (Fig. 1). Interestingly, a significant increase of serum anti-RBD IgG antibodies was evidenced after 18 days post-3D with BNT162b2 (t3), reaching a median of 8877 BAU/mL (IQR 6076-13081) (Fig. 1). Finally, after a median of 73 days post-3D (t4) the level of specific anti-RBD IgG antibodies in 41 individuals of our cohort decreased again but towards a median of 2622 BAU/mL (IQR 1907-5100) (Fig. 1). Despite individual differences, all participants showed the same trend in terms of IgG levels (Fig. 2 A). In addition, we observed a concomitant evolution in blocking capacity of RBD-ACE2 interaction in participants who received a heterologous CoronaVac/BNT162b2 vaccination. We detected a drop between t1 and t2 [median of 3413 AU/mL (IQR 2250-5301) vs. 1064 AU/mL (IQR 554-1968), respectively], an important improvement early post-3D with BNT162b2 (t3), and recurrently a reduction between t3 and t4 [median of 65,221 AU/mL (IQR 40701-109618) vs. 20,480 AU/mL (IQR 12079–32878), respectively] (Fig. 2B). According to the sVNT kit specifications, the neutralization titer can be interpreted as low (<1500AU/mL), medium (1500–5000AU/mL) and high (>5000). Besides, these blocking capacities linearly correlates with anti-RBD IgG levels at each time point (Fig. S2).
Fig. 1.
Serum anti-RBD IgG levels at different times in the cohort follow-up. Serum samples of the same 50 individuals that received heterologous prime-boost vaccination with CoronaVac and BNT162b2 were evaluated at different times, except for t4 where 41 of them were studied. Bars and numbers above them represent the median values of each time point and numbers in parentheses are the interquartile ranges. The number of individuals evaluated at each time point is presented on the base of each bar. Bars corresponding to serum samples obtained after the third dose are presented in grey. Comparisons between groups were carried out using Friedman's test and Dunn's multiple comparison post-hoc test. **p = 0.01–0.001, ***p < 0.001. BAU, binding antibody units.
Fig. 2.
Comparison between serum anti-RBD IgG levels and their capacity to block RBD-ACE2 interaction at different times in the cohort follow-up. Lines show the individual evolution over time of the serum anti-RBD IgG levels (A) and their capacity to block RBD-ACE2 interaction (B) for every participant in the cohort (n = 50). Numbers represents median values with the interquartile range in parentheses. Both datasets show a similar behavior over time in most of the evaluated cases. Comparisons between groups were carried out using Friedman's test and Dunn's multiple comparison post-hoc test. **p = 0.01–0.001, ***p < 0.001. BAU, binding antibody units; AU, arbitrary units.
To evaluate kinetics of binding that could evidence differences in the maturation of humoral response, we selected 20 individuals who received heterologous vaccine scheme and analyzed the response based on total antibodies by SPR. All individuals surpassed the values obtained with pre immunization serum samples (Figs. 3 A and S3) and dissociation proved to be extremely slow, causing that the level of bound antibodies remained almost constant for more than 10 min. By virtue of this, we could not obtain kinetics parameters and registered the level of bound antibodies (in RUs) after 1 min of dissociation, as an arbitrary measurement of the level of total bound anti-RBD antibodies. The values obtained at t2 exhibited a decline with respect to t1, showing a temporal drop in the level of total circulating specific antibodies. Results obtained 18 days post-3D with BNT162b2 (t3) resulted in a sharp increase in the level of total specific antibodies (Fig. 3A). Again, this behavior is in concordance with results obtained by ELISA (Fig. 3B) showing a strong correlation between IgG and total specific antibodies shortly after doses administration (Fig. S4).
Fig. 3.
Comparison of anti-RBD total antibodies measured by SPR and anti-RBD IgG measured by ELISA, in serum samples of 20 individuals selected from the cohort and collected at different times. The levels of total specific antibodies measured by SPR after 1 min dissociation are presented at different times with grey lines showing the individual evolution over time, and the corresponding sensorgrams as stacked insets on the left (A). Sensorgrams are presented after subtracting pre-vaccination data collected at t0 and for clarity, curves are presented with the same color code as in Fig. S5. For comparison, the results obtained by ELISA with the same 20 selected individuals are presented (B) with the same representation as in Fig. 2. Both in SPR and ELISA, pre-vaccination data (at t0) correspond to zero (not shown due to logarithmic representation of y-axis). RU, resonance units; BAU, binding antibody units.
Beyond virus recognition and neutralization by antibodies, Fc-mediated effector functions (like ADCC and ADCP) have been linked to protection against multiple pathogens [15], including SARS-CoV-2 vaccination in animal models [16]. To advance our knowledge on this, we performed in vitro ADCC and ADCP reporter assays on serum samples from 20 participants collected 18 days post-2D with CoronaVac (t1) and 18 days post-3D with BNT162b2 (t3). We found that BNT162b2 booster also increased the ADCC titer from a median of 76 (IQR 37-165) to 548 (IQR 331-834) (Fig. 4 A), and ADCP titer from a median of 28 (IQR 16-51) to 308 (IQR 116-436) (Fig. 4B). The same behavior was shown for blocking capacity of RBD-ACE2 interaction (Fig. 4C) and binding to RBD (Fig. 4D) at t1 and t3. Considering the relevance of Omicron outbreak in our country, we decided to compare the blocking capacity of antibodies developed against SARS-CoV-2 Wuhan and Omicron variants, by performing sVNT with these same 20 sera. We found a clear reduced activity against the Omicron variant at both times, post-2D [median of Wuhan variant, 2371 AU/mL (IQR 1240-4494) vs. median of Omicron variant, 0 AU/mL (IQR 0-264)] and post-3D [median of Wuhan variant, 71,908 AU/mL (IQR 43947-108165), vs. median of Omicron variant, 1510 AU/mL (IQR 0-3728)]. However, the in vitro blocking capacity of RBD-ACE2 interaction showed an increase after booster for both variants, especially for Wuhan one (Fig. 4E).
Fig. 4.
Fc-mediated functions and RBD-ACE2 binding inhibition of specific serum anti-RBD immunoglobulins. Figure shows ADCC (A) and ADCP (B) Fc-mediated functions of serum anti-RBD immunoglobulin in 20 individuals early after two doses of Coronavac (t1) and after heterologous booster with BNT162b2 (t3). Endpoint titers were calculated by interpolating the reciprocal of serum dilutions to baseline, whose values were determined for each plate as the mean signal in absence of serum plus three standard deviations. Inhibition of RBD-ACE2 binding (C) and serum anti-RBD IgG levels (D) were measured in the same samples, showing a similar behavior over time. Comparison of the RBD-ACE2 binding inhibition activity against SARS-CoV-2 Wuhan and Omicron variants were carried out by sVNT in these 20 samples (E). Numbers represents median values and the interquartile range (in parentheses). Comparisons between groups were carried out using Wilcoxon test. *p < 0.05, **p = 0.01–0.001, ***p < 0.001. BAU, binding antibody units; AU, arbitrary units.
4. Discussion
Safety and efficacy of BNT162b2 and CoronaVac vaccines have been previously reported [5], [17], [18], and some studies have directly compared CoronaVac and BNT162b2 vaccines performances [19], [20]. In fact, our group has recently evaluated the humoral response to these vaccines in solid organ transplant recipients [21], [22]. Moreover, in a small number of individuals immunized with BNT162b2, we found similar results when comparing both vaccines over time (Fig. S5). At present, one of the major topics of discussion is the use of vaccine boosters [23], [24], especially heterologous vaccination approaches. In this regard, noteworthy results have recently been published which focuses on heterologous priming with adenoviral-vectored vaccines followed by boosting with mRNA [25], [26] or recombinant protein-based vaccines [27], or viral-vectored vaccine booster after two doses of inactivated vaccine [28]. Information regarding the dynamics of antibody response induced by heterologous prime-boost vaccination with inactivated-virus and mRNA vaccines remains scarce, especially in the medium-term post-booster [13], [14], [29]. Moreover, studies which focuses on functional characteristics of antibodies generated by heterologous prime-boost vaccination with CoronaVac and BNT162b2 are still hard to find.
Our baseline seronegative cohort showed seroconversion 18 days post-2D with CoronaVac, but the level of specific antibodies dropped significantly 80 days post-2D (almost 72%), similarly to the decay overtime reported following the administration of BNT162b2 [10], [30] or CoronaVac [12], [31] vaccines. However, 18 days post-3D with BNT162b2 vaccine, we found an impressive 22-fold increase in serum specific antibody levels if compared with the same period post-2D with CoronaVac. Indeed, the level of antibodies detected in these individuals were almost three times higher than those reached by individuals fully vaccinated with two doses of BNT162b2 (Fig. S5). Our results are in agreement with recent reports analyzing populations that received the prime-boost vaccination with CoronaVac and BNT162b2 [13], [32], as well as with other 3D schedules, by using either homologous [33] or heterologous [34] schemes.
To assess the evolution of specific antibody levels after heterologous booster we compared results obtained 73 days post-3D (t4) with those obtained at t3 and found a drop of 70% considering medians values of each time, similarly to that seen in a comparable span of time post-2D with CoronaVac (between t1 and t2). Despite this, the antibody levels at t4 remained 23-fold higher as compared to levels obtained from participants fully vaccinated with CoronaVac at 80 days post-2D. To the best of our knowledge this is the first study to determine the magnitude of the decline over time in serum specific antibody levels after heterologous vaccination with CoronaVac and BNT162b2 in a healthy population. These results are crucial in the definition of future boosting plans, especially regarding the time between them.
The dynamics of the capacity to block RBD-ACE2 interaction follows the same behavior as anti-RBD antibody levels, showing an important increase early post-3D with BNT162b2 (t3), followed by a new reduction measured 73 days post-3D (t4). Considering a similar period post-vaccination, the blocking of RBD-ACE2 interaction post-3D with BNT162b2 remained significantly higher than those observed post-2D with CoronaVac (t3 vs. t1). Despite the fall observed 73 days post-3D with BNT162b2, the blocking capacity remains almost 20-times higher when compared with that detected 80 days post-2D with CoronaVac (t4 vs. t2). Even more, blocking levels at t4 remained 6-times higher than those detected at t1. sVNT results expressed in AU/mL are very useful for comparing variation between different individuals and over time. If we interpret the results expressed in AU/mL according to the technical specifications of the sVNT kit, we can summarize that the median values of neutralization titers were medium at t1, low at t2, and high at t3 and t4.
The reported dynamics of humoral response highlights the benefits of this heterologous vaccination scheme, considering that the level of serum neutralizing antibodies has been proposed as a highly predictive marker of immune protection for symptomatic SARS-CoV-2 infected patients [35]. Even more, in the context of the emergence of SARS-CoV-2 variants of concern, a higher neutralization capacity achieved by a booster vaccination, can generate an increased protection from severe infection outcomes [8], [36]. However, we found a clear decline in the RBD-ACE2 interaction blocking capacity when we compare the Wuhan variant against the Omicron one, in agree with previous findings of Pérez-Then and col. [13]. Anyway, our results could provide valuable information regarding the temporal behavior of blocking capacity since a CoP for SARS-CoV-2 is crucial in the fight against pandemic [37].
To compare the performance of different vaccine schedules, we urgently need to define efficient methods that serve as CoP for COVID-19 vaccines. These CoPs will also allow assessing the individual and community levels of protection against SARS-CoV-2 infection. Recent studies have shown that antibody levels against the Spike protein and its neutralizing capacity strongly correlates with efficacy across different vaccine platforms [35], [38], [39]. The use of the WHO international standard and the expression of results in BAU/mL, allows its comparison between different laboratories. Recent works suggested an antibody level of approximately 260 BAU/mL as a correlate of protection in the general population [38] and cancer patients [40]. Although this level still needs to be confirmed by other studies, the possibility of having this type of information could be useful for future management of vaccination schedules.
Based on the complexity of the mechanisms associated with the maturation of the humoral response and the effect that different vaccines platforms could have on it, we analyzed differences in the binding kinetics of serum samples by SPR. As opposed to ELISA, where antibodies reach binding equilibrium, using SPR we obtained real-time binding data, allowing kinetic binding evaluation at the expense of a reduction in sensitivity. However, polyclonality and the unknown concentration of specific antibodies prevented us from extracting overall kinetics parameters that could help in elucidating changes associated with longitudinal maturation of humoral response. Even the “apparent koff” (which does not depend on free antibody concentration) could not be properly calculated, probably because of avidity and/or re-binding events of the different isotypes of antibodies which result in an extremely slow dissociation. This slow dissociation (whose differences between doses were inexistent or remained undetectable) is a qualitative feature of anti-RBD antibodies, being compatible with the presence of high affinity IgG produced by plasma cell that underwent the complex process of maturation through successive rounds of somatic hypermutation and selection. On the other hand, this feature of specific IgG antibodies likely favors their ability to trigger effector mechanisms in vivo. Interestingly, the level of specific IgG or total antibodies, measured respectively by ELISA and SPR, showed a similar temporal evolution and a strong correlation early after 2D and 3D (Fig. S4), evidencing the usefulness of both techniques to analyze the dynamics of humoral responses nearly vaccination.
While recent data points to the ability of SARS-CoV-2 mRNA vaccines to evoke robust Fc-effector functions [9], less is known about inactivated virus vaccines and heterologous vaccination. It has been reported that some Fc-dependent pathways of anti-SARS-CoV-2 specific antibodies potentially contribute to COVID-19 severity [41] and/or could play a role in combatting SARS-CoV-2 infections [42]. We determined that these Fc-mediated features show strong correlation with IgG serum levels and blocking capacities, similarly to findings reported in convalescent individuals [43]. This suggests that Fc-mediated functions could be playing an important role in preventing SARS-CoV-2 infections or COVID-19 severity after vaccination, even against emerging virus variants. Moreover, according to our findings, binding of specific antibodies could represent an indirect measurement of Fc-mediated effector functions.
The findings of this study must be seen considering some limitations. The first is the sample size; although, we consider it important to have a five-point IgG levels follow-up, covering pre- and post-vaccination. The second limitation concerns the lack of CoronaVac-boosted group; however, the Ministry of Public Health of Uruguay did not use CoronaVac as a booster option but only the BNT162b2 vaccine. For this reason, there are virtually no people with a third dose of CoronaVac in our country. Third, Uruguay lacks local access to biosafety level 3 (BSL3) facilities for achieving plaque reduction neutralization tests (PRNT), which is the gold standard for detecting and quantifying neutralizing antibodies (NAbs). For this reason, we performed a surrogate neutralization test which clearly has limitations, such as the absence of biological membranes and other proteins or protein domains with a known role in the attachment, binding, and fusion processes of the virus with the host cell. Finally, data on T-cell responses induced during vaccination is a relevant issue to be considered.
5. Conclusion
In this work we provide new evidence regarding heterologous prime-boost vaccination with CoronaVac and BNT162b2 in healthy individuals, showing the dynamic of the levels of specific antibody after approximately 2.5 months. A significant correlation between levels of anti-RBD antibodies, their RBD-ACE2 interaction blocking capacity, and ADCC or ADCP-effectors activities was found, supporting that inexpensive and easily implemented serological tests are important tools for monitoring the immune response after vaccination and define booster doses.
Inactivated virus-based vaccines are useful tools in the global fight against COVID-19, although more data are needed on its efficacy against emerging SARS-CoV-2 variants and on the durability of protection across different age groups, geographical settings and in the presence of comorbidities [44]. In addition, as mRNA-based platform vaccines have shown to be safe and effective over time [17], the use of a heterologous prime-boost vaccination with CoronaVac and BNT162b2 represents a promising and safe strategy.
Authorship
All authors attest they meet the ICMJE criteria for authorship.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We would like to thank all the participants who volunteered for this study at the Institut Pasteur de Montevideo. We especially thank Hugo Naya for his generous support during the preparation of this manuscript. The authors gratefully acknowledge the Cell Biology Unit at the Institut Pasteur de Montevideo for their support and assistance in the present work. To María Teresa Lamaison, Daniela Hirschfeld, and Finance and Purchases Unit members for their dedication and their diligent support during study execution.
Funding
This work was supported by Fondo para la Convergencia Estructural del Mercosur [grant number COF 03/11]; Agencia Nacional de Investigación e Innovación; and Comisión Académica de Posgrado (CAP), Universidad de la República.
The funders did not play any role in the design and conduct of the study; collection, analysis, or interpretation of the data; preparation, approval, or decision to submit the manuscript for publication.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.07.023.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Mallapaty S. China's COVID vaccines have been crucial - now immunity is waning. Nature. 2021;598(7881):398–399. doi: 10.1038/d41586-021-02796-w. [DOI] [PubMed] [Google Scholar]
- 2.Ritchie H, Mathieu E, Rodés-Guirao L, et al. Coronavirus pandemic (COVID-19). Our World in Data; 2020. <https://ourworldindata.org/coronavirus>.
- 3.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jara A., Undurraga E.A., González C., Paredes F., Fontecilla T., Jara G., et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in chile. N Engl J Med. 2021;385(10):875–884. doi: 10.1056/NEJMoa2107715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tenforde M.W., Patel M.M., Ginde A.A., et al. Effectiveness of SARS-CoV-2 mRNA vaccines for preventing covid-19 hospitalizations in the United States. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab687. [DOI] [Google Scholar]
- 7.Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21(8):475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cromer D., Steain M., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3(1):e52–e61. doi: 10.1016/S2666-5247(21)00267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tauzin A., Nayrac M., Benlarbi M., et al. A single dose of the SARS-CoV-2 vaccine BNT162b2 elicits Fc-mediated antibody effector functions and T cell responses. Cell Host Microbe. 2021;29(7):1137–1150 e6. doi: 10.1016/j.chom.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kertes J., Gez S.B., Saciuk Y., Supino-Rosin L., Stein N.S., Mizrahi-Reuveni M., et al. Effectiveness of mRNA BNT162b2 vaccine 6 months after vaccination among patients in large health maintenance organization, Israel. Emerg Infect Dis. 2022;28(2):338–346. doi: 10.3201/eid2802.211834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cucunawangsih C., Wijaya R.S., Lugito N.P.H., Suriapranata I. Antibody response to the inactivated SARS-CoV-2 vaccine among healthcare workers. Indonesia. Int J Infect Dis. 2021;113:15–17. doi: 10.1016/j.ijid.2021.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pérez-Then E., Lucas C., Monteiro V.S., Miric M., Brache V., Cochon L., et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med. 2022;28(3):481–485. doi: 10.1038/s41591-022-01705-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng S.M.S., Mok C.K.P., Leung Y.W.Y., et al. Neutralizing antibodies against the SARS-CoV-2 Omicron variant following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat Med. 2022 doi: 10.1038/s41591-022-01704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu L.L., Suscovich T.J., Fortune S.M., Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2018;18(1):46–61. doi: 10.1038/nri.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorman M.J., Patel N., Guebre-Xabier M., Zhu A.L., Atyeo C., Pullen K.M., et al. Fab and Fc contribute to maximal protection against SARS-CoV-2 following NVX-CoV2373 subunit vaccine with Matrix-M vaccination. Cell Rep Med. 2021;2(9):100405. doi: 10.1016/j.xcrm.2021.100405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas S.J., Moreira E.D., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinc H.O., Saltoglu N., Can G., Balkan I.I., Budak B., Ozbey D., et al. Inactive SARS-CoV-2 vaccine generates high antibody responses in healthcare workers with and without prior infection. Vaccine. 2022;40(1):52–58. doi: 10.1016/j.vaccine.2021.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim W.W., Mak L., Leung G.M., Cowling B.J., Peiris M. Comparative immunogenicity of mRNA and inactivated vaccines against COVID-19. Lancet Microbe. 2021;2(9):e423. doi: 10.1016/S2666-5247(21)00177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mok C.K.P., Cohen C.A., Cheng S.M.S., et al. Comparison of the immunogenicity of BNT162b2 and CoronaVac COVID-19 vaccines in Hong Kong. Respirology. 2021 doi: 10.1111/resp.14191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seija M., Rammauro F., Santiago J., Orihuela N., Zulberti C., Machado D., et al. Comparison of antibody response to SARS-CoV-2 after two doses of inactivated virus and BNT162b2 mRNA vaccines in kidney transplant. Clin Kidney J. 2022;15(3):527–533. doi: 10.1093/ckj/sfab291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prieto J., Rammauro F., López M., Rey R., Fernández A., Bianchi S., et al. Low Immunoglobulin G antibody levels against severe acute respiratory disease coronavirus 2 after 2-dose vaccination among liver transplantation recipients. Liver Transpl. 2022;28(5):891–894. doi: 10.1002/lt.26400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krause P.R., Fleming T.R., Peto R., Longini I.M., Figueroa J.P., Sterne J.A.C., et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet. 2021;398(10308):1377–1380. doi: 10.1016/S0140-6736(21)02046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callaway E. COVID vaccine boosters: the most important questions. Nature. 2021;596(7871):178–180. doi: 10.1038/d41586-021-02158-6. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt T., Klemis V., Schub D., Mihm J., Hielscher F., Marx S., et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat Med. 2021;27(9):1530–1535. doi: 10.1038/s41591-021-01464-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Normark J., Vikström L., Gwon Y.-D., Persson I.-L., Edin A., Björsell T., et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 vaccination. N Engl J Med. 2021;385(11):1049–1051. doi: 10.1056/NEJMc2110716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuart A.S.V., Shaw R.H., Liu X., Greenland M., Aley P.K., Andrews N.J., et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2022;399(10319):36–49. doi: 10.1016/S0140-6736(21)02718-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yorsaeng R., Suntronwong N., Phowatthanasathian H., Assawakosri S., Kanokudom S., Thongmee T., et al. Immunogenicity of a third dose viral-vectored COVID-19 vaccine after receiving two-dose inactivated vaccines in healthy adults. Vaccine. 2022;40(3):524–530. doi: 10.1016/j.vaccine.2021.11.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa Clemens S.A., Weckx L., Clemens R., Almeida Mendes A.V., Ramos Souza A., Silveira M.B.V., et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399(10324):521–529. doi: 10.1016/S0140-6736(22)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collier A.-R., Yu J., McMahan K., Liu J., Chandrashekar A., Maron J.S., et al. Differential kinetics of immune responses elicited by Covid-19 vaccines. N Engl J Med. 2021;385(21):2010–2012. doi: 10.1056/NEJMc2115596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng G., Wu Q., Pan H., Li M., Yang J., Wang L., et al. Immunogenicity and safety of a third dose of CoronaVac, and immune persistence of a two-dose schedule, in healthy adults: interim results from two single-centre, double-blind, randomised, placebo-controlled phase 2 clinical trials. Lancet Infect Dis. 2022;22(4):483–495. doi: 10.1016/S1473-3099(21)00681-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Assawasaksakul T., Sathitratanacheewin S., Vichaiwattana P., Wanlapakorn N., Poovorawan Y., Kittanamongkolchai W. Immunogenicity, safety and reactogenicity of a heterogeneous booster following the CoronaVac inactivated SARS-CoV-2 vaccine in patients with SLE: a case series. RMD Open. 2021;7(3):e002019. doi: 10.1136/rmdopen-2021-002019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi A., Koch M., Wu K., Chu L., Ma LingZhi, Hill A., et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med. 2021;27(11):2025–2031. doi: 10.1038/s41591-021-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munro A.P.S., Janani L., Cornelius V., Aley P.K., Babbage G., Baxter D., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398(10318):2258–2276. doi: 10.1016/S0140-6736(21)02717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 36.Hunsawong T., Fernandez S., Buathong R., Khadthasrima N., Rungrojchareonkit K., Lohachanakul J., et al. Limited and short-lasting virus neutralizing titers induced by inactivated SARS-CoV-2 vaccine. Emerg Infect Dis. 2021;27(12):3178–3180. doi: 10.3201/eid2712.211772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krammer F. A correlate of protection for SARS-CoV-2 vaccines is urgently needed. Nat Med. 2021;27(7):1147–1148. doi: 10.1038/s41591-021-01432-4. [DOI] [PubMed] [Google Scholar]
- 38.Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27(11):2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilbert P.B., Montefiori D.C., McDermott A.B., Fong Y., Benkeser D., Deng W., et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science. 2022;375(6576):43–50. doi: 10.1126/science.abm3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrière J., Carles M., Audigier-Valette C., Re D., Adjtoutah Z., Seitz-Polski B., et al. Third dose of anti-SARS-CoV-2 vaccine for patients with cancer: should humoral responses be monitored? A position article. Eur J Cancer. 2022;162:182–193. doi: 10.1016/j.ejca.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adeniji O.S., Giron L.B., Purwar M., Zilberstein N.F., Kulkarni A.J., Shaikh M.W., et al. COVID-19 severity is associated with differential antibody Fc-mediated innate immune functions. mBio. 2021;12(2) doi: 10.1128/mBio.00281-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diez J.M., Romero C., Cruz M., et al. Anti-SARS-CoV-2 hyperimmune globulin demonstrates potent neutralization and antibody-dependent cellular cytotoxicity and phagocytosis through N and S proteins. J Infect Dis. 2021 doi: 10.1093/infdis/jiab540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butler S.E., Crowley A.R., Natarajan H., Xu S., Weiner J.A., Bobak C.A., et al. Distinct features and functions of systemic and mucosal humoral immunity among SARS-CoV-2 convalescent individuals. Front Immunol. 2020;11:618685. doi: 10.3389/fimmu.2020.618685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramasamy M.N., Jessop L.J. CoronaVac: more data for regulators and policy makers. Lancet. 2021;398(10296):186–188. doi: 10.1016/S0140-6736(21)01543-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.