Abstract
Background
Helicobacter pylori (H. pylori) infects ~ 35% of Americans and can lead to serious sequelae if left untreated. Growing evidence indicates that clarithromycin-based therapies (CBT) are becoming increasingly ineffective for treating H. pylori infection. RHB-105 was approved by the US Food and Drug Administration in 2019 for the treatment of H. pylori infection in adults.
Aims
The primary aim of this study was to assess prescribing patterns and associated cure rates of physician-directed therapy for subjects with persistent H. pylori infection after participation in one of two Phase 3 clinical trials (ERADICATE Hp and ERADICATE Hp2).
Methods
We reviewed study reports to identify specific physician-directed regimens selected for subjects whose H. pylori infection was not eradicated. We also conducted a CYP2C19 genotype analysis of subjects who were prescribed CBT. Finally, we analyzed real-world H. pylori retail prescription data and compared these with to the physician-directed therapies in the clinical trials studies.
Results
Following ERADICATE Hp, CBT was prescribed for 27/31 (87%) subjects achieving a 59.3% cure rate. Following ERADICATE Hp2, CBT was prescribed for 48/94 (51%) subjects achieving a 60.4% cure rate. Rapid CYP2C19 metabolizers (2/11) had a cure rate of 18.2% with CBT. Real-world prescription data from IQVIA showed more than 80% of prescriptions for H. pylori infection were for CBT.
Conclusions
Rates of CBT use persist despite sub-optimal eradication rates. Since RHB-105 does not contain clarithromycin, it can be prescribed first-line without concerns about clarithromycin resistance or CYP2C19 status.
Keywords: Helicobacter pylori, Talicia, Rifabutin, RHB-105, Clarithromycin, CYP2C19, Stomach
Introduction
Helicobacter pylori (H. pylori) is the strongest risk factor for peptic ulcer disease and non-cardia gastric cancer and is also associated with dyspepsia, iron deficiency anemia, and vitamin B12 deficiency [1, 5, 6, 13]. Because successful eradication of H. pylori is most likely achieved with the first course of treatment, careful selection of appropriate first-line therapy is vital [4]. Due to limited availability of H. pylori antibiotic susceptibility testing in the USA, most patients are treated empirically with regimens containing two or three antimicrobials and a proton pump inhibitor (PPI). Although resistance of H. pylori to clarithromycin has been estimated to be > 30% domestically [3, 17], clarithromycin-containing regimens are still widely prescribed in the USA.
RHB-105 (Talicia®, RedHill Biopharma, Raleigh, NC)—an all-in-one product containing omeprazole, low-dose rifabutin, and amoxicillin—was approved by the US Food and Drug Administration (FDA) in November 2019 for the treatment of H. pylori infection in adults [19]. It is the first treatment for H. pylori infection approved by the FDA in over a decade. The first Phase 3 study compared RHB-105 to placebo [12]; the second compared it to the equivalent doses of omeprazole and amoxicillin but without rifabutin (active comparator) [8]. The primary endpoint for both studies was eradication of H. pylori following 14 days of double-blinded treatment.
In the pivotal trial “ERADICATE Hp2,” RHB-105 demonstrated a cure rate of 83.8% in treatment-naïve subjects by an intent-to-treat (ITT) analysis (primary endpoint) [8]. Cure rate among subjects with confirmed adherence to the study regimen was 90.3% [8]. No primary or acquired resistance to rifabutin was identified [8], and RHB-105 eradication rates were not significantly influenced by CYP2C19 metabolic status [8]. In the supportive study, “ERADICATE Hp,” RHB-105 demonstrated a cure rate of 89.4% in treatment-naïve subjects by a modified intent-to-treat (mITT) analysis (primary endpoint) [12]. Additionally, there was no statistically significant difference in CYP2C19 status between subjects with and without successful eradication (distribution was assessed over the different genotypes; P value = 0.123) [12]. Most subjects who remained positive for H. pylori infection after completion of the primary treatment had received placebo in ERADICATE Hp, or active comparator in ERADICATE Hp2. Subjects with persistent infection were then treated with physician-directed therapy.
We have now analyzed prescribing patterns and associated cure rates of physician-directed therapy, specifically CBT, for persistent H. pylori infection. Additionally, subjects’ CYP2C19 genotypes were analyzed to assess the effect of subject CYP2C19 activity on the success of clarithromycin-containing therapy. Finally, we analyzed US retail prescription data from 2018 to 2019 to assess H. pylori therapy prescribing habits by subspecialty to further contextualize the patterns of prescribing observed in the RHB-105 clinical trials.
Methods
As specified in the clinical trial protocols (ERADICATE Hp; NCT03198507 & ERADICATE Hp2; NCT01980095), subjects whose H. pylori infection was not eradicated after receiving the primary study treatment (placebo, RHB-105, or active comparator) received physician-directed therapy based on local standard-of-care. We reviewed clinical trial study reports to identify the specific physician-directed regimen selected for each subject whose H. pylori infection was not eradicated after the primary study treatment. Cure was defined as eradication of H. pylori confirmed by a negative post-treatment urea breath test (UBT) at least 4 weeks post-last dose of therapy. All subjects suspended PPI use at least 2 weeks prior to follow-up testing, per protocol. Subjects whose H. pylori infection persisted after receiving active therapy as the primary study treatment also underwent upper endoscopy with sampling for culture and sensitivity testing. Additionally, subjects’ CYP2C19 genotypes were analyzed to assess metabolic status (i.e., normal vs. rapid metabolism) effect on CBT success, using Fisher’s exact test to calculate the P value between groups.
Longitudinal, patient-level, adjudicated H. pylori prescription data were collected between August 1, 2018, and July 31, 2019 [11]. Prescription claims from various sources including transactional records when claims are submitted for reimbursement to a payer as well as prescriptions captured from pharmacy (third-party payers, Medicare, Medicaid and cash) were analyzed to calculate both percentage of individual subspecialty H. pylori prescriptions that contained clarithromycin, and percentage of total H. pylori prescriptions (all subspecialities) each subspeciality prescribed.
Results
Following completion of “ERADICATE Hp,” 38 subjects remained positive for H. pylori: 33 had been randomized to receive placebo and 5 had received RHB-105. Follow-up data on physician-directed therapy were available for 31 subjects; cure rate was 61.3%. A CBT regimen was prescribed for 27 of these 31 subjects; cure rate with this regimen was 59.3% [12].
Following completion of “ERADICATE Hp2”, 94 subjects had persistent infection: 67 had been randomized to active comparator and 27 to RHB-105. For those with persistent infection following primary study treatment, the overall cure rate with physician-directed treatment was 56.2%. For the 48 subjects who received CBT, the cure rate was 60.4%; for the 22 who received a bismuth-based quadruple regimen, the cure rate was 45.4% [8]. Cure rates based on therapy and initial treatment regimen are shown in Table 1.
Table 1.
Cure rates among subjects in ERADICATE Hp and ERADICATE Hp2 who failed initial treatment and then completed physician-directed therapy and post-treatment UBT (mITT)
| mITT population | ERADICATE Hp | ERADICATE Hp2 | ||||
|---|---|---|---|---|---|---|
| Initial Treatment Group Failures | Initial Treatment Group Failures | |||||
| RHB-105 | Placebo | Combined | RHB-105 | Comparator | Combined | |
| (n = 5) | (n = 33) | (n = 38) | (n = 27) | (n = 67) | (n = 94) | |
| Eradication rate | Eradication rate | Eradication rate | Eradication rate | Eradication rate | Eradication rate | |
| Any PD therapy | 50.0% | 63.0% | 61.3% | 46.2% | 56.7% | 56.2% |
| (n = 4) | (n = 27) | (n = 31) | (n = 26) | (n = 63) | (n = 89) | |
| CLA-based triple | 50.0% | 60.9% | 59.3% | 50.0% | 65.6% | 60.4% |
| (n = 4) | (n = 23) | (n = 27) | (n = 16) | (n = 32) | (n = 48) | |
| Bismuth-based quadruple | 0.0% | 100.0% | 100.0% | 33.3% | 50.0% | 45.4% |
| (n = 0) | (n = 2) | (n = 2) | (n = 6) | (n = 16) | (n = 22) | |
| Other regimens* | 0.0% | 50.0% | 50.0% | 50.0% | 53.3% | 52.6% |
| (n = 0) | (n = 2) | (n = 2) | (n = 4) | (n = 15) | (n = 19) | |
| Missing dataƚ | N/A | N/A | N/A | N/A | N/A | N/A |
| (n = 1) | (n = 6) | (n = 7) | (n = 1) | (n = 4) | (n = 5) | |
*Some other regimens included clarithromycin (CLA) but were not standard CLA-based triple therapy
ƚSubjects who did not complete treatment or did not have a test-of-cure UBT
RHB-105: low-dose rifabutin, amoxicillin, and omeprazole
Comparator: amoxicillin, and omeprazole
Table 2 shows an analysis of the CYP2C19 genotypes of those subjects who received physician-directed CBT and its relationship to eradication success. Subjects with normal CYP2C19 genotypes (*1/*1 and WT/WT) had an eradication rate of 80.0% (16/20) with CBT. Those with rapid CYP2C19 genotypes (*1/*17 and *17/WT) had an eradication rate of 18.2% (2/11) with CBT [P = 0.0017].
Table 2.
Subject CYP2C19 metabolic status versus success of clarithromycin triple therapy
| Phenotype | Genotype | N | Eradicated | % Success |
|---|---|---|---|---|
| Normal |
*1/*1 WT/WT |
20 | 16 | 80.0% |
| Rapid |
*1/*17 *17/WT |
11 | 2 | 18.2% |
| P value | 0.0017 |
Limited susceptibility data were available for subjects given physician-directed treatment with CBT. In subjects for whom there were susceptibility data, those with normal CYP2C19 genotypes were susceptible to both amoxicillin (4/4) and clarithromycin (4/4). Subjects with confirmed rapid metabolizer genotypes were also largely susceptible to both amoxicillin (5/5) and clarithromycin (3/5).
The analysis of 12 months of domestic retail prescribing data for the treatment of H. pylori infection comprising over 1 million prescriptions (Fig. 1) uncovered that over 80% of the total prescriptions from each subspecialty contained clarithromycin. Gastroenterologists prescribed the fewest clarithromycin-containing regimens, with CBTs representing ~ 80% of their total prescriptions for H. pylori. Family practice and general practice prescribed the highest number of CBTs, representing ~ 89% and ~ 86% of their total H. pylori prescriptions, respectively. Further stratification by subspecialty is shown in Fig. 1.
Fig. 1.
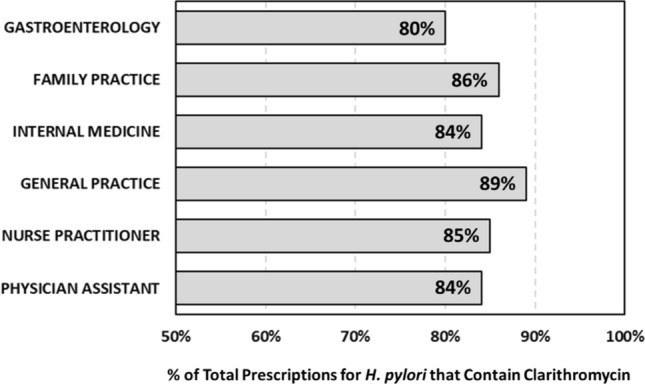
Percent of total prescribed H. pylori regimens containing clarithromycin by subspecialty
Discussion
This analysis of physicians’ management of patients with persistent H. pylori infection reveals some interesting and important findings. The most frequently selected treatments for physician-directed therapy were clarithromycin-containing triple regimens (71.7%). These data, collected prospectively following the two Phase 3 trials which evaluated the combination of PPI, rifabutin, and amoxicillin versus placebo or dual therapy, demonstrate that the overall effectiveness of empiric H. pylori therapies commonly used in the community is sub-optimal. We observed a 63% eradication rate in those subjects who received physician-directed therapy following failure of placebo as the primary study treatment (who could, therefore, be considered treatment-naïve). This is consistent with recently published eradication rates of 58 to 75% [3, 15, 16, 20]. In these Phase 3 studies, investigators did not have access to culture and sensitivity data at the time they prescribed physician-directed therapy since such testing is generally not available in contemporary clinical practice. It is recommended in the American College of Gastroenterology (ACG) guideline that clarithromycin-based treatment regimens for H. pylori infection should be avoided in patients with any prior macrolide use or in regions where the resistance rate is known to be above 15% (or where resistance levels are not known) [4]. Nonetheless, in this study, clarithromycin-containing regimens were the physicians’ most frequent treatment choice, consistent with US commercial usage patterns. Such continued, widespread use of clarithromycin-based treatments without information on local clarithromycin resistance rates is concerning and suggests that there is a strong need for healthcare provider education as well as updates to various practice guidelines.
As previously reported, CYP2C19 status had no significant influence on overall RHB-105 eradication rates. However, patient CYP2C19 status (i.e., normal or rapid metabolism) has been suggested to influence H. pylori eradication success, especially regarding clarithromycin-containing triple therapies. A prior study showed that patients with CYP2C19 genotypes (*17 allele) that are associated with rapid PPI metabolism failed clarithromycin-based triple therapies at higher rates than patients with normal, or slow CYP2C19 metabolizer genotypes [18]. In this study, for patients who received physician-directed CBT, we show that CYP2C19 metabolic status had a significant influence on eradication success. Rapid metabolizers had an 18.2% eradication rate versus 80.0% in normal metabolizers (P = 0.0017). Analysis of limited data on subjects’ antibiotic resistance showed that, generally, subjects had H. pylori strains that were susceptible to both amoxicillin and clarithromycin. Because a recent study of over 2 million direct-to-customer genetic participants showed that over 1/3 of the participants had increased function CYP2C19 genotypes (rapid/ultrarapid) [10], and because few, if any, physicians test for CYP2C19 metabolic status prior to therapy, it is imperative to adopt first-line regimens that are unaffected by CYP2C19 metabolic status.
Our analysis of domestic prescriptions revealed that over 80% of all prescribed therapies for H. pylori infection contained clarithromycin. These data highlight the potential inappropriate use of clarithromycin-containing regimens and help to explain why eradication rates with CBTs appear to be declining. Empirically prescribing a CBT as first-line for the treatment of H. pylori infection, without consideration of a patient’s antibiotic history or CYP2C19 status, may continue to promote increased antibiotic resistance and contribute to treatment failure.
RHB-105 contains rifabutin, an ansamycin class antibiotic that targets the DNA-binding RNA-polymerase of H. pylori, inhibiting RNA synthesis resulting in bacterial cell death. Rifabutin is stable at a pH range of 2–8 and is not readily destroyed by stomach acid [14]. A recent review of 39 studies (9721 patients) concluded a resistance rate to rifabutin of < 1.0% (0.13%), and this rate fell to 0.07% in treatment-naïve patients [7]. Because H. pylori regimens are largely prescribed empirically, rifabutin-triple therapy (RHB-105) can be prescribed without concern for resistance. Since RHB-105 does not contain clarithromycin, it can be recommended as a first-line therapy for patients with H. pylori infection without recourse to pre-treatment antibiotic sensitivity testing, and without concern about local clarithromycin resistance rates or patient CYP2C19 metabolic status. It was proven safe and well tolerated in two Phase 3 clinical trials [8, 12],"TALICIA, [Prescribing Information]; 2019, RedHill Biopharma, INC.,"). The only other regimen that can similarly be used empirically at the current time is bismuth-based quadruple therapy [9] which was one of the primary regimens endorsed in the 2017 ACG practice guideline [4]. It also showed the highest efficacy in a recent retrospective analysis of patients treated empirically at two academic medical centers in Rhode Island [2]. To date, RHB-105 has not been compared directly with a bismuth-based regimen. Because treatment of H. pylori infection remains empiric, and because the ACG guideline states that “first-line therapy generally offers the greatest likelihood of treatment success,” having access to highly reliable first-line treatment regimens is critically important.
Acknowledgments
We would like to acknowledge Carol B. Rockett, PharmD, for valuable editorial contributions to the manuscript.
Author's contribution
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Dr. CWH and Dr. KLS. The first draft of the manuscript was written by Dr. CWH and Dr. WDC. Substantial alterations to the manuscript were made by Dr. KLS and Dr. JSA. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
There was no financial support for the preparation of this article.
Declarations
Conflict of interest
Dr. CH is a Professor Emeritus, Department of Medicine, University of Tennessee College of Medicine, Memphis, TN. He serves as a consultant to RedHill Biopharma, Phathom, Allakos, Ironwood, EndoStim. Speaker for RedHill Biopharma, Alnylam, Sanofi / Genzyme. Stockholder of Antibe Therapeutics. Dr. WC is a Nostrant Professor of Gastroenterology & Nutrition Sciences, Director of the GI Physiology Laboratory, Director of the Digestive Disorders Nutrition & Lifestyle Program, and Co-Director of the Michigan Bowel Control Program. He serves as a Board Member for the American College of Gastroenterology, International Foundation of GI Disorders, Rome Foundation. He serves as a consultant to Abbvie, Allakos, Alnylam, Arena, Biomerica, Gemelli, Ironwood, Nestle, QOL Medical, Phathom, Progenity, Redhill Biopharma, Salix/Valeant, Takeda, Urovant, and Vibrant. His research is supported by the NIH, FDA, Biomerica, Commonwealth Diagnostics, QOL Medical, and Salix. He is a shareholder of GI on Demand and Modify Health. Drs. KS and JA are employees of RedHill Biopharma, Inc., Raleigh, NC USA and are stockholders of RedHill Biopharma. Dr. Howden is a consultant for RedHill Biopharma, Phathom Pharmaceuticals, Ironwood, Clexio, Allakos, and Alfasigma. He is a speaker for RedHill Biopharma, Alfasigma, and Alnylam. He owns stock in Antibe Therapeutics. Dr. Sheldon is an employee of RedHill Biopharma, Inc., 8045 Arco Corporate Drive, Suite 200, Raleigh, NC 27617, USA. Dr. Almenoff is an employee of RedHill Biopharma, Inc., 8045 Arco Corporate Drive, Suite 200, Raleigh, NC 27617, USA. Dr. Chey, Board member: American College of Gastroenterology, GI on Demand, International Foundation of Functional GI Disorders, Rome Foundation; Consultant: AbbVie, Alfasigma, Allakos, Alnylam, Bayer, Biomerica, Cosmo, IM Health, Ironwood, QOL Medical, Phathom, RedHill Biopharma, Salix/Valeant, Takeda, Urovant, Vibrant; Grant/Research Support: Bioamerica, Commonwealth Diagnostics International, QOL Medical, Salix; Stock/Stock options: GI on Demand, Modify Health.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adamu MA, Weck MN, Rothenbacher D, Brenner H. Incidence and risk factors for the development of chronic atrophic gastritis: five year follow-up of a population-based cohort study. Int J Cancer. 2011;128:1652–1658. doi: 10.1002/ijc.25476. [DOI] [PubMed] [Google Scholar]
- 2.Alsamman MA, Vecchio EC, Shawwa K, et al. Retrospective analysis confirms tetracycline quadruple as best Helicobacter pylori regimen in the USA. Dig Dis Sci. 2019;64:2893–2898. doi: 10.1007/s10620-019-05694-4. [DOI] [PubMed] [Google Scholar]
- 3.Argueta EA, Alsamman MA, Moss SF, D’Agata EMC. Impact of antimicrobial resistance rates on eradication of Helicobacter pylori in a US population. Gastroenterology. 2021;160:2181–2183. doi: 10.1053/j.gastro.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG clinical guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Kao JY, Kanwal F, et al. Houston consensus conference on testing for helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol. 2018;16:992–1002. doi: 10.1016/j.cgh.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eslick GD, Lim LL, Byles JE, Xia HH, Talley NJ. Association of Helicobacter pylori infection with gastric carcinoma: a meta-analysis. Am J Gastroenterol. 1999;94:2373–2379. doi: 10.1111/j.1572-0241.1999.01360.x. [DOI] [PubMed] [Google Scholar]
- 7.Gisbert JP. Rifabutin for the treatment of Helicobacter pylori infection: a review. Pathogens. 2020 doi: 10.3390/pathogens10010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham DY, Canaan Y, Maher J, et al. Rifabutin-based triple therapy (RHB-105) for Helicobacter pylori eradication: a double-blind, randomized, controlled trial. Ann Intern Med. 2020;172:795–802. doi: 10.7326/M19-3734. [DOI] [PubMed] [Google Scholar]
- 9.Howden CW, Graham DY. Recent developments pertaining to H. pylori infection. Am J Gastroenterol. 2021;116:1–3. doi: 10.14309/ajg.0000000000001031. [DOI] [PubMed] [Google Scholar]
- 10.Ionova Y, Ashenhurst J, Zhan J, et al. CYP2C19 allele frequencies in over 2.2 million direct-to-consumer genetics research participants and the potential implication for prescriptions in a large health system. Clin Transl Sci. 2020;13:1298–1306. doi: 10.1111/cts.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IQVIA Real World Data: Longitudinal Prescription (LRx) Asset. (2019).
- 12.Kalfus IN, Graham DY, Riff DS, Panas RM. Rifabutin-containing triple therapy (RHB-105) for eradication of Helicobacter pylori: randomized ERADICATE Hp trial. Antibiotics (Basel) 2020 doi: 10.3390/antibiotics9100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato S, Matsukura N, Tsukada K, et al. Helicobacter pylori infection-negative gastric cancer in Japanese hospital patients: incidence and pathological characteristics. Cancer Sci. 2007;98:790–794. doi: 10.1111/j.1349-7006.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunin CM. Antimicrobial activity of rifabutin. Clin Infect Dis. 1996;22:S3–13. doi: 10.1093/clinids/22.supplement_1.s3. [DOI] [PubMed] [Google Scholar]
- 15.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the maastricht IV/florence consensus report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 16.O'Connor A, Gisbert JP, O'Morain C, Ladas S. Treatment of Helicobacter pylori Infection 2015. Helicobacter. 2015;20:54–61. doi: 10.1111/hel.12258. [DOI] [PubMed] [Google Scholar]
- 17.Park JY, Dunbar KB, Mitui M, et al. Helicobacter pylori clarithromycin resistance and treatment failure are common in the USA. Dig Dis Sci. 2016;61:2373–2380. doi: 10.1007/s10620-016-4091-8. [DOI] [PubMed] [Google Scholar]
- 18.Sugimoto M, Furuta T. Efficacy of tailored Helicobacter pylori eradication therapy based on antibiotic susceptibility and CYP2C19 genotype. World J Gastroenterol. 2014;20:6400–6411. doi: 10.3748/wjg.v20.i21.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.TALICIA (omeprazole magnesium, amoxicillin and rifabutin) delayed release capsules, for oral use [Prescribing Information]. Raleigh, NC. RedHill Biopharma, INC.; 2019.
- 20.Venerito M, Krieger T, Ecker T, Leandro G, Malfertheiner P. Meta-analysis of bismuth quadruple therapy versus clarithromycin triple therapy for empiric primary treatment of Helicobacter pylori infection. Digestion. 2013;88:33–45. doi: 10.1159/000350719. [DOI] [PubMed] [Google Scholar]


