Abstract
Randomised trials in emergency settings must quickly confirm eligibility and allocate participants to an intervention group without delaying treatment. We report rapid randomisation during two neonatal resuscitation trials using the non-commercial REDCap platform accessed via smartphone. This simple, reliable method has wide applicability for trials in emergency settings.
|
What is Known: • Randomised trials in emergency settings need to rapidly allocate participants to an intervention group. • This process should not delay treatment. | |
|
What is New: • This non-commercial, smartphone-accessible application enabled rapid, accurate randomisation at the bedside. • This has broad applicability for emergency setting trials. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s00431-022-04475-y.
Keywords: Neonatal, Randomised controlled trial
Introduction
Clinicians and researchers conducting randomised trials in emergency settings face the challenge of needing to quickly confirm eligibility and allocate participants to an intervention group without delaying treatment. The urgency is particularly acute in delivery room trials of neonatal resuscitation, which require participant allocation and application of the intervention within seconds of determining eligibility. As a result, trials have historically used methods that are at risk of bias such as sealed envelopes, allocation prior to eligibility confirmation, or quasi-randomisation [1–3]. To ensure validity, the allocation process should be methodologically rigorous, minimise treatment delay, and streamline trial procedures. We report our experience with performing rapid randomisation during neonatal resuscitation using the smartphone-accessible, non-commercial REDCap platform — a database management system [4]. The aim of this study was to evaluate the use of the online randomisation tool for two delivery room randomised controlled trials.
Materials and methods
We used randomisation via REDCap for two unblinded, parallel-arm neonatal resuscitation trials conducted at The Royal Women’s Hospital and Monash Medical Centre in Melbourne, Australia. The Human Research Ethics Committees at each site approved both trials. In the Baby-Directed Umbilical Cord Cutting (BabyDUCC) trial (ACTRN12618000621213) [5], eligible infants ≥ 32+0 weeks’ gestation who required resuscitation within 1 min of birth were randomised to receive respiratory support in the maternal space with the umbilical cord still intact (intervention) or after clamping the umbilical cord and transferring the infant to the warming bed. In the Stabilisation with Nasal High Flow for Intubation of Neonates (SHINE) trial (ACTRN12618001498280) [6], infants undergoing endotracheal intubation were randomised to receive nasal high flow during the intubation attempt, or to standard care (no nasal high flow). The subset of infants in the SHINE trial intubated in the delivery room during neonatal resuscitation was included in the current analysis. Infants intubated in the neonatal unit were excluded as rapid assessment of eligibility and randomisation was not required [5, 6].
A readily accessible, rapid randomisation tool was required to allow treatment allocation at the participant’s bedside without delaying essential resuscitation care. For each trial, a pre-generated randomisation schedule was uploaded to the REDCap randomisation module by an independent statistician. Site investigators accessed the “New Randomisation” page via a password-protected mobile phone weblink that allowed advance selection of strata. The randomisation tool could be accessed by multiple investigators using their unique REDCap login profile on their smartphones, providing additional automatic stratification by site (“Data Access Group”). Enrolment and randomisation of patients could therefore occur simultaneously at the two sites. Following birth, eligibility was assessed. If the infant required resuscitation within 1 min of birth (BabyDUCC trial) or endotracheal intubation (SHINE trial), the researcher or assistant would reveal the allocation via a two-step randomise-and-confirm process (Fig. 1). This involved pressing the “randomise” button on the initial screen page (step 1) and again on a confirmation pop-up window (step 2). For caesarean births in the BabyDUCC trial, a midwife not involved in the study randomised the infant and revealed the allocation as the researcher was scrubbed in the operating field.
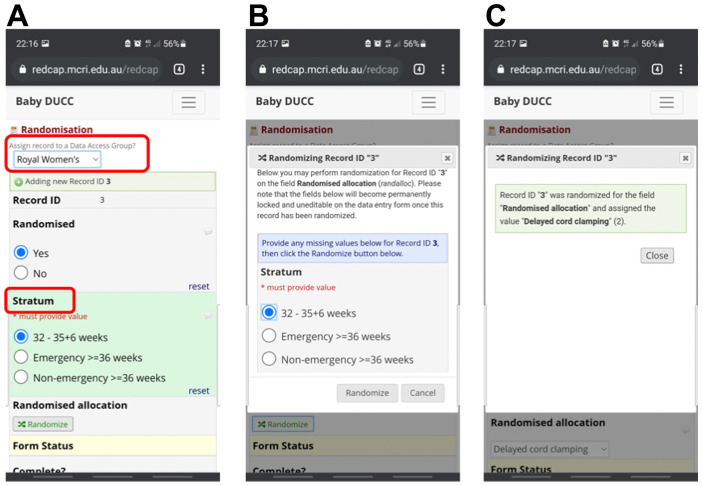
Fig. 1.
Screenshots of the randomisation process. A weblink to the “New Randomisation” (A) is accessed through a weblink shortcut saved on the site investigator’s smartphone home screen. This page requires the user to log in. Strata are selected as shown in the red boxes. Once participant eligibility is confirmed, the investigator presses the “Randomise” button (step 1). A window (B) appears to confirm the strata, and the decision to randomise is confirmed by pressing the “Randomise” button again (step 2). The group allocation is then revealed (C) and a record simultaneously created for the participant. The allocation is permanently locked into the participant’s REDCap data entry form and cannot be edited
Both trials incorporated video and audio recordings (GoPro, USA) of resuscitation that captured the randomisation events (Supplementary Fig. 1). A single researcher who was not present at the births (GS) reviewed the recordings to determine the time between the decision to randomise and the disclosure of group allocation. Decision to randomise was defined as the time when the researcher confirmed eligibility in conjunction with the clinical team. In the BabyDUCC trial, this was a call to randomise the infant following an assessment that the infant required resuscitation to establish breathing. In the SHINE trial, the decision to randomise was when the clinical team confirmed to the researcher the intention to intubate the infant. Allocation revelation was defined as the time when the study allocation was verbalised to the clinical team. Where the allocation was not immediately verbalised, for instance when the clinical team was busy with other resuscitation tasks, allocation revelation was defined as the time when the allocation appeared on the smartphone device as captured by the recording.
Supplementary Fig. 1. Infants enrolled in the Baby-Directed Umbilical Cord Cutting (BabyDUCC) trial and the Stabilisation with Nasal High Flow for Intubation of Neonates (SHINE) trial with available data for evaluation of the randomisation tool.
*The number of births attended for the SHINE trial was unavailable, but no infants were accidentally randomised prior to confirmation of eligibility.
Results
In the BabyDUCC trial (N = 123, Supplementary Fig. 1), the decision to randomise occurred at a mean (SD) of 25.2 (11.9) seconds after birth. Group allocation was revealed 5.9 (2.9) seconds after the decision to randomise. Two randomisation errors occurred: in each case, an assistant had progressed through the initial “Randomise” step (step 1) followed by accidental confirmation (step 2) before birth. We subsequently enforced the planned approach of waiting until after birth to progress past step 1. Following enforcement of the two-step randomise-and-confirm process after birth and assessment of eligibility, no further errors occurred (N = 94).
In the SHINE trial (N = 68, Supplementary Fig. 1), the two-step randomise-and-confirm process after confirmation of eligibility was employed throughout. Group allocation was revealed at a mean (SD) of 6.8 (4.9) seconds after the decision to randomise. One post-randomisation exclusion occurred when a randomisation was appropriately confirmed in a patient that was initially planned for endotracheal intubation but subsequently was not intubated.
All eligible infants were successfully randomised in each trial. There were no instances of technical difficulties related to internet access or REDCap server downtime. The supplementary online videos provide representative examples of the randomisation tool being used (Supplementary videos 1 & 2).
Discussion
We have described our experience with the REDCap randomisation tool accessed using a smartphone browser. The tool was reliable, easy to use, and facilitated group assignment within seconds of confirming patient eligibility. We recommend adhering to the two-step randomise-and-confirm process to avoid accidental randomisation.
Other scenarios where the provider must quickly confirm eligibility and allocate participants to an intervention group without delaying treatment include trials of airway interventions and out-of-hospital cardiac arrest. Recent major trials in these fields used sealed envelopes [7], randomisation of the provider [8], opening of a pre-randomised trial drug kit [9], and randomisation of the day of the week [10]. The method we report here allows methodologically rigorous randomisation of the individual participant following confirmation of eligibility. A previously reported mobile app for rapid randomisation [11] is no longer available (personal communication) and we are not aware of other similar platforms.
The advantages of the method we used include centralised, stratified randomisation that can be performed at the bedside using any internet- or mobile data–enabled smartphone. Multiple investigators can use the tool across different recruitment sites including locations worldwide. Allocation concealment is rigorous and there is automatic lock of the group allocation within the study database. The system also supports blinding of group allocation for trials where this is required. To enable others to replicate this method, we include the REDCap ODM XML “metadata-only” file for the BabyDUCC trial (Supplemental File) that can be easily modified to suit the individual trial’s requirements. Although not used in our trials, dynamic randomisation by minimisation can be implemented in REDCap using a custom module developed by one of the study authors (LS). This tool has broad applicability for trials in different emergency settings.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1: RandTool_SupplVid_1 (MP4 67 MB)
Supplementary file2: RandTool_SupplVid_2 (MP4 56 MB)
Authors’ contributions
SB, KH, DB, and PD contributed to the study conception and design. Data collection and analysis were performed by SB, KH, GS, LS, DB, SD, CR, BM, GP, and SH. The first draft of the manuscript was written by SB and KH, and all authors revised the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. The study authors receive funding from the National Health and Medical Research Council (NH&MRC) Program Grant (#606789), NH&MRC Centre for Research Excellence in Newborn Medicine (#1153176), NH&MRC Fellowships (CR, GP, SH, PD), Medical Research Future Fund (BM), and Australian Government Research Training Program Scholarships (SB, KH). The funders had no role in the study design, in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Availability of data and material
Data will be available upon reasonable request following completion and publication of each of the main trials.
Declarations
Ethics approval
The Human Research Ethics Committees at each site (Royal Women’s Hospital and Monash Health) approved both trials.
Consent to participate
Written consent was obtained from parents/guardians for participation in each trial.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shiraz Badurdeen, Email: shiraz.badurdeen@thewomens.org.au.
Kate A. Hodgson, Email: kate.hodgson@thewomens.org.au
Georgia A. Santomartino, Email: georgia.santomartino@thewomens.org.au
Luke Stevens, Email: luke.stevens@mcri.edu.au.
Susan Donath, Email: susan.donath@mcri.edu.au.
Calum T. Roberts, Email: calum.roberts@monash.edu
Brett J. Manley, Email: brett.manley@thewomens.org.au
Graeme R. Polglase, Email: graeme.polglase@monash.edu
Stuart B. Hooper, Email: stuart.hooper@monash.edu
Peter G. Davis, Email: pgd@unimelb.edu.au
Douglas A. Blank, Email: douglas.blank@monashhealth.org
References
- 1.Higgins J, Thomas J, Chandler J et al (2020) Cochrane handbook for systematic reviews of interventions. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. Cochrane. www.training.cochrane.org/handbook
- 2.Corbett MS, Moe-Byrne T, Oddie S, McGuire W. Randomization methods in emergency setting trials: a descriptive review. Res Synth Methods. 2016;7(1):46–54. doi: 10.1002/jrsm.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirpalani H, Ratcliffe SJ, Keszler M, et al. Effect of sustained inflations vs intermittent positive pressure ventilation on bronchopulmonary dysplasia or death among extremely preterm infants: the SAIL randomized clinical trial. JAMA - J Am Med Assoc. 2019;321(12):1165–1175. doi: 10.1001/jama.2019.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris PA, Taylor R, Minor BL et al (2019) The REDCap consortium: building an international community of software platform partners. J Biomed Inform 95. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed]
- 5.ANZCTR - Registration. The Baby-Directed Umbilical Cord Cutting (Baby-DUCC) trial. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=374884. Accessed 20 Nov 2019
- 6.Hodgson K, Owen L, Kamlin C et al (2020) A multicentre, randomised trial of stabilisation with nasal high flow during neonatal endotracheal intubation (the SHINE trial): a study protocol. BMJ Open 10(10). 10.1136/BMJOPEN-2020-039230 [DOI] [PMC free article] [PubMed]
- 7.Casey JD, Janz DR, Russell DW, et al. Bag-mask ventilation during tracheal intubation of critically ill adults. N Engl J Med. 2019;380(9):811. doi: 10.1056/NEJMOA1812405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benger JR, Kirby K, Black S, et al. Effect of a strategy of a supraglottic airway device vs tracheal intubation during out-of-hospital cardiac arrest on functional outcome: the AIRWAYS-2 randomized clinical trial. JAMA. 2018;320(8):779. doi: 10.1001/JAMA.2018.11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kudenchuk PJ, Brown SP, Daya M, et al. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med. 2016;374(18):1711–1722. doi: 10.1056/NEJMOA1514204/SUPPL_FILE/NEJMOA1514204_DISCLOSURES.PDF. [DOI] [PubMed] [Google Scholar]
- 10.Pejovic NJ, Myrnerts Höök S, Byamugisha J, et al. A randomized trial of laryngeal mask airway in neonatal resuscitation. N Engl J Med. 2020;383(22):2138–2147. doi: 10.1056/NEJMOA2005333/SUPPL_FILE/NEJMOA2005333_DATA-SHARING.PDF. [DOI] [PubMed] [Google Scholar]
- 11.Deserno TM, Keszei AP. Mobile access to virtual randomization for investigator-initiated trials. Clin Trials. 2017;14(4):396–405. doi: 10.1177/1740774517706509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1: RandTool_SupplVid_1 (MP4 67 MB)
Supplementary file2: RandTool_SupplVid_2 (MP4 56 MB)
Data Availability Statement
Data will be available upon reasonable request following completion and publication of each of the main trials.