Abstract
Objectives
To assess the effect of hydroxychloroquine (HCQ) and Tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) as pre-exposure prophylaxis on COVID-19 risk.
Methods
EPICOS is a double-blind, placebo-controlled randomized trial conducted in Spain, Bolivia, and Venezuela. Healthcare workers with negative SARS-CoV-2 IgM/IgG test were randomly assigned to the following: daily TDF/FTC plus HCQ for 12 weeks, TDF/FTC plus HCQ placebo, HCQ plus TDF/FTC placebo, and TDF/FTC placebo plus HCQ placebo. Randomization was performed in groups of four. Primary outcome was laboratory-confirmed, symptomatic COVID-19. We also studied any (symptomatic or asymptomatic) COVID-19. We compared group-specific 14-week risks via differences and ratios with 95% CIs.
Results
Of 1002 individuals screened, 926 (92.4%) were eligible and there were 14 cases of symptomatic COVID-19: 220 were assigned to the TDF/FTC plus HCQ group (3 cases), 231 to the TDF/FTC placebo plus HCQ group (3 cases), 233 to the TDF/FTC plus HCQ placebo group (3 cases), and 223 to the double placebo group (5 cases). Compared with the double placebo group, 14-week risk ratios (95% CI) of symptomatic COVID-19 were 0.39 (0.00–1.98) for TDF + HCQ, 0.34 (0.00–2.06) for TDF, and 0.49 (0.00–2.29) for HCQ. Corresponding risk ratios of any COVID-19 were 0.51 (0.21–1.00) for TDF + HCQ, 0.81 (0.44–1.49) for TDF, and 0.73 (0.41–1.38) for HCQ. Adverse events were generally mild.
Discussion
The target sample size was not met. Our findings are compatible with both benefit and harm of pre-exposure prophylaxis with TDF/FTC and HCQ, alone or in combination, compared with placebo.
Keywords: COVID-19, Pre-exposure prophylaxis, Randomized clinical trial, SARS-CoV-2, Tenofovir
Introduction
Drug repurposing for prophylaxis against COVID-19 started early in the pandemic [1]. Based largely on in vitro evidence, randomized trials of hydroxychloroquine (HCQ) as pre-exposure prophylaxis were among the earliest to be launched [[1], [2], [3], [4], [5]]. However, these trials were small and resulted in imprecise effect estimates [[2], [3], [4], [5]]. Tenofovir disoproxil fumarate (TDF) was another candidate for repurposing based on epidemiological data [6,7], in vitro and in vivo studies [[8], [9], [10], [11], [12], [13]], and its high bioavailability in many tissues [[14], [15], [16]]. However, no randomized trials of TDF for pre-exposure prophylaxis have been completed.
Both HCQ and TDF are generic drugs widely prescribed worldwide with a well-documented safety record [[17], [18], [19]]. HCQ has been used as treatment and prophylaxis of malaria. TDF, in combination with emtricitabine (FTC), has been used for the treatment and prophylaxis of HIV infection. Despite their potential for COVID-19 prophylaxis, these safe and inexpensive drugs have not been studied in randomized trials (TDF) or the randomized trials have been relatively small (HCQ).
We carried out a double-blind placebo-controlled randomized trial to assess the effect of daily HCQ or TDF/FTC, and of their combination, during 12 weeks as pre-exposure prophylaxis against COVID-19 in healthcare workers.
Methods
EPICOS (NCT04334928, EudraCT number 2020-001385-11) was a multicentre, double-blind, placebo-controlled randomized trial to study the effect of TDF/FTC and HCQ as pre-exposure prophylaxis for symptomatic COVID-19 among healthcare workers in Spain, Bolivia, and Venezuela. The trial was designed to recruit 4000 individuals. Assuming a 5% to 10% risk of symptomatic COVID-19 in the placebo group and less than half in the treatment groups, this sample size ensured that the 95% CIs would only include effect values compatible with treatment benefit. However, the start of the vaccination campaign and other factors limited recruitment to 907 participants.
Recruitment into the trial was actively promoted in Spain through regional health authorities and the Ministry of Health, and in Latin America through Esther (Ensemble de Solidarité Thérapeutique Hospitalière En Reseau). Healthcare workers were approached individually and collectively through promotional in-hospital sessions, mailings, and hospital-wide advertisements, and were screened for eligibility after providing informed consent. A mobile phone app was developed for electronic monitoring, weekly reminders of adherence, and side-effects reporting.
Eligibility criteria
Healthcare workers aged 18 to 70 years were eligible if they did not have a prior diagnosis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)f infection, did not have symptoms compatible with SARS-CoV-2 infection, had a negative IgM/IgG test for SARS-CoV-2, had negative HIV and (for women) pregnancy tests, a normal electrocardiogram, and no history of QT interval prolongation, maculopathy, impaired renal function, or immunosuppressive or hematologic conditions. Because women comprised the majority of healthcare workers, we ensured 40% of individuals screened for eligibility were males. Recruitment started in April 2020 in Spain, October 2020 in Bolivia, and March 2021 in Venezuela (Supplementary Fig. 1). The study ended on 30 May 2021.
Randomization and masking
Eligible individuals were randomly assigned to one of four treatment groups: TDF/FTC plus HCQ, TDF/FTC plus HCQ placebo, HCQ plus TDF/FTC placebo and TDF/FTC placebo plus HCQ placebo. Randomization was performed with random permuted blocks using a block size of four. The randomization list was computer-generated by a biostatistician with no clinical involvement in the study and before the study started. Medication was prepared accordingly by an external provider, labelled with a unique consecutive number and assigned in chronological order according to the date of treatment initiation in each centre. Investigators, participants, and data analysts were unaware of their treatment assignment. The allocation concealment was preserved by using identical treatment and placebo tablets. The TDF/FTC placebo was provided by the TDF/FTC manufacturing company who donated the drug and the HCQ was designed ad-hoc for the purpose of this study.
TDF/FTC was administered as a single pill with 245mg of TDF and 200 mg of FTC once daily). HCQ was administered as 200 mg once daily, the minimum dose to reach adequate tissue distribution [20]. Participants received treatment for 12 weeks (or until a SARS-CoV-2 infection was diagnosed), irrespective of symptoms, or the administrative end of the study, whichever occurred first.
Outcomes
The primary outcome was symptomatic COVID-19, defined as the presence of SARS-CoV-2 infection confirmed by a polymerase chain reaction (PCR) test plus any of the following symptoms: general malaise, fever, cough, joint pain, or breathing difficulty. PCR-confirmed asymptomatic SARS-CoV-2 infection was a secondary outcome. Other secondary outcomes were duration of symptoms and severity, though the later could not be studied. We also studied the outcome “any (symptomatic or asymptomatic) COVID-19 infection”, which had not been pre-specified in the study protocol.
Adherence (number of missed pills) and adverse events were ascertained in each monthly visit and weekly through app reminders. Adverse events were classified as mild (easily tolerated), moderate (interference with normal activities), or severe (incapacitating, with inability to perform normal activities). Regardless of severity, adverse events were classified as serious if they required hospitalization, prolonged an existing hospitalization, or led to major or permanent disability.
Follow-up
Participants attended three monthly visits after randomization. In each visit, they were evaluated for the presence of adverse events, adherence, received standard laboratory tests, IgM/IgG antibody test for SARS-CoV-2, and an electrocardiogram if necessary. A PCR test was performed if the IgM/IgG antibody test was positive or if symptoms were present. A fourth monthly visit was scheduled for the evaluation of adverse events only.
The trial was stopped after recommendations to vaccinate healthcare workers were issued in each country. The decision was made by the trial investigators with the agreement of the Data Safety Monitoring Board.
Statistical analysis
We used the Kaplan-Meier estimator to obtain outcome risks over 14 weeks of follow-up in each treatment group (over 95% of participants had attended their third monthly visit by 14 weeks after randomization). We compared group-specific risks via differences and ratios with the placebo-only group as the reference. Participants were censored if/when they were lost to follow-up. In post hoc analyses, we compared the risk between the two groups containing HCQ and the two groups not containing HCQ, and between the two groups containing TDF/FTC and the two groups not containing TDF/FTC. We calculated 95% CIs using the percentile bootstrap method with 500 repetitions. In sensitivity analyses, we used a Cox model to estimate hazard ratios.
This study was approved by the institutional review boards of University Hospital de La Princesa, Madrid, Spain, Servicio Departamental de Salud de Chuquisaca in Bolivia, and Instituto Nacional de Higiene “Rafael Rangel” in Venezuela. An independent medical monitor and a data safety monitoring board provided oversight of safety and efficacy.
Results
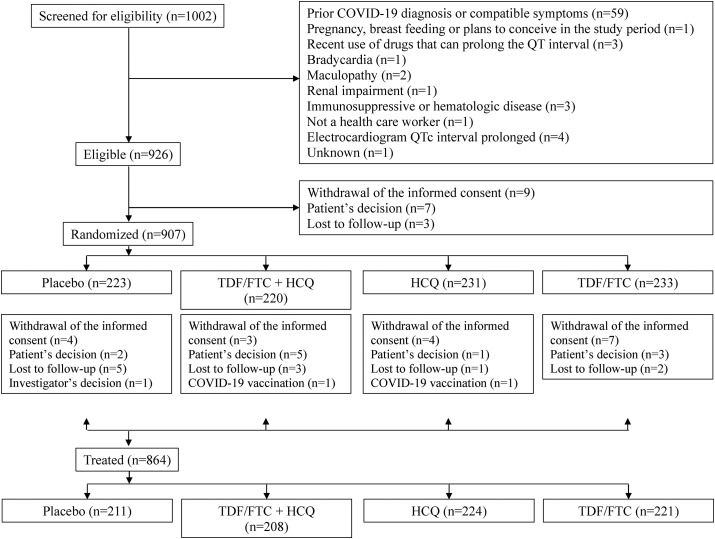
Of 1002 individuals screened for eligibility, 926 (92.4%) were eligible. The main reason for ineligibility was a previous COVID-19 diagnosis or compatible symptoms (Fig. 1 ). Nineteen individuals withdrew or were lost to follow-up before treatment assignment. Of 907 randomized individuals, 220 were assigned to the TDF/FTC plus HCQ group (12 did not start treatment), 231 to the TDF/FTC placebo plus HCQ group (7 did not start treatment), 233 to the TDF/FTC plus HCQ placebo group (12 did not start treatment), and 223 to the double placebo group (12 did not start treatment). Of 696 individuals who completed the scheduled follow-up, 668 completed treatment as indicated in the protocol. The Supplementary materials, Tables S1 and S2, show the reasons for early termination of treatment and incomplete follow-up, respectively, by treatment group.
Fig. 1.
Flowchart of participants, EPICOS randomized trial.
Baseline characteristics of the 907 participants are summarized in Table 1 and the Supplementary material, Table S3. Median age was 38 years (range 18 to 68 years) and 62.5% (567/907) were female. Most participants worked at inpatient care facilities (62.3%; 565/907) and the most frequent occupation was physician (30.8%; 279/907), 64.2% (582/907) of participants were recruited in Spain, 22.3% (202/907) in Bolivia, and 13.6% (123/907) in Venezuela. Comorbidities were rare.
Table 1.
Baseline characteristics of 907 participants, EPICOS randomized trial
| Characteristic | TDF/FTC + HCQ (n = 220) |
TDF/FTC (n = 233) | HCQ (n = 231) | Placebo (n = 223) |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 85 (38.6) | 93 (39.9) | 82 (35.5) | 80 (35.9) |
| Female | 135 (61.4) | 140 (60.1) | 149 (64.5) | 143 (64.1) |
| Age (y), median (range) | 38.0 (18.0, 65.0) | 39.0 (18.0, 68.0) | 38.0 (18.0, 65.0) | 38.0 (18.0, 65.0) |
| Occupation, n (%) | ||||
| Physician | 71 (32.3) | 68 (29.2%) | 74 (32.0%) | 66 (29.6%) |
| Nurse | 63 (28.6) | 77 (33.0%) | 67 (29.0%) | 72 (32.3%) |
| Medical student on clinical rotation | 59 (26.8) | 58 (24.9) | 59 (25.5) | 53 (23.8) |
| Other, with direct patient contact | 13 (5.9) | 13 (5.6) | 11 (4.8) | 11 (4.9) |
| Other, without direct patient contact | 13 (5.9) | 10 (4.3) | 15 (6.5) | 18 (8.1) |
| Unknown | 1 (0.5) | 7 (3.0) | 5 (2.2) | 3 (1.3) |
| Comorbidities, n (%) | ||||
| Cardiac disease | 3 (1.4) | 0 | 1 (0.4) | 2 (0.9) |
| Hypertension | 17 (7.7) | 15 (6.4) | 4 (1.7) | 19 (8.5) |
| Pulmonary disease | 0 | 0 | 0 | 0 |
| Asthma | 17 (7.7) | 8 (3.4) | 20 (8.7) | 9 (4.0) |
| Neoplasia | 4 (1.8) | 4 (1.7) | 2 (0.9) | 1 (0.4) |
| Diabetes | 4 (1.8) | 3 (1.3) | 1 (0.4) | 3 (1.3) |
| Autoimmune disease | 5 (2.3) | 7 (3.0) | 4 (1.7) | 2 (0.9) |
| Country, n (%) | ||||
| Spain | 139 (63.2) | 151 (64.8) | 148 (64.1) | 144 (64.6) |
| Venezuela | 31 (14.1) | 31 (13.3) | 32 (13.9) | 29 (13.0) |
| Bolivia | 50 (22.7) | 51 (21.9) | 51 (22.1) | 50 (22.4) |
HCQ, hydroxychloroquine; FTC, emtricitabine; TDF, tenofovir disoproxil fumarate.
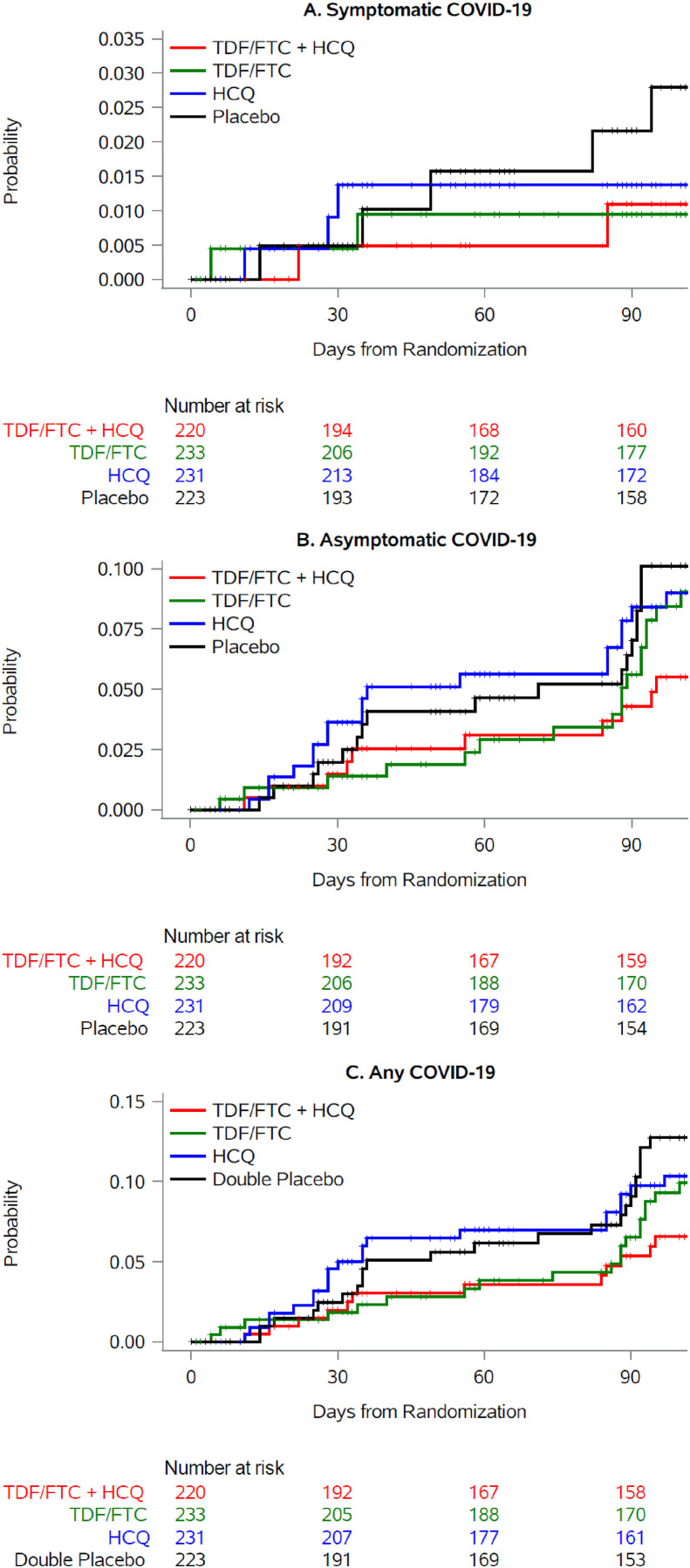
Fig. 2(a) shows the cumulative risk of symptomatic COVID-19 by treatment group. There were 14 cases: 3 in each group with active treatment and 5 in the placebo-only group. All cases had mild symptoms, with variable duration, that did not require hospitalization (see Supplementary material, Table S4). Compared with the placebo-only group, the 14-week risk ratio (95% CI) of symptomatic COVID-19 was 0.39 (0.00–1.98) for TDF + HCQ, 0.34 (0.00–2.06) for TDF, and 0.49 (0.00–2.29) for HCQ (Table 2 ).
Fig. 2.
Cumulative risk of symptomatic and asymptomatic COVID-19 by treatmentgroup, EPICOS randomized trial. (a) Symptomatic COVID-19, (b) Asymptomatic COVID-19, (c) Any COVID-19. Abbreviations: HCQ, hydroxychloroquine; FTC, emtricitabine; TDF, tenofovir disoproxil fumarate.
Table 2.
Estimated 14-week risks of symptomatic, asymptomatic, and any COVID-19 diagnosis by treatment group, EPICOS randomized trial
| Symptomatic COVID-19 | Cases/n | 14-week risk % (95% CI) |
Risk difference % (95% CI) |
Risk ratio (95% CI) |
|---|---|---|---|---|
| TDF/FTC + HCQ | 3/220 | 1.10 (0.00–2.55) | -1.70 (-4.41–1.09) | 0.39 (0.00–1.98) |
| TDF/FTC | 3/233 | 0.94 (0.00–2.63) | -1.85 (-4.43–1.16) | 0.34 (0.00–2.06) |
| HCQ | 3/231 | 1.37 (0.00–3.12) | -1.42 (-4.48–1.34) | 0.49 (0.00–2.29) |
| Placebo | 5/223 | 2.79 (0.60–5.22) | Reference | Reference |
| Asymptomatic COVID-19 | ||||
| TDF/FTC + HCQ | 10/220 | 5.51 (2.25–9.04) | -4.61 (-10.4–1.30) | 0.54 (0.21–1.19) |
| TDF/FTC | 17/233 | 8.44 (4.70–12.6) | -1.68 (-7.72–4.26) | 0.83 (0.45–1.66) |
| HCQ | 18/231 | 9.01 (5.37–13.3) | -1.11 (-7.06–5.16) | 0.89 (0.49–1.91) |
| Placebo | 18/223 | 10.1 (5.49–14.5) | Reference | Reference |
| Any COVID-19 | ||||
| TDF/FTC + HCQ | 13/220 | 6.56 (2.75–10.27) | -6.17 (-12.32–0.01) | 0.51 (0.21–1.00) |
| TDF/FTC | 20/233 | 9.31 (5.79–13.69) | -3.42 (-9.61–3.32) | 0.81 (0.44–1.49) |
| HCQ | 21/231 | 10.35 (6.23–14.82) | -2.39 (-8.80–4.28) | 0.73 (0.41–1.38) |
| Placebo | 23/223 | 12.74 (7.92–17.44) | Reference | Reference |
HCQ, hydroxychloroquine; FTC, emtricitabine; TDF, tenofovir disoproxil fumarate.
The 14-week risk ratio (95% CI) of symptomatic COVID-19 was 0.68 (0.10–2.04) for the groups assigned to HCQ compared with the two groups not assigned to HCQ (see Supplementary materials, Table S5 and Fig. S2), and 0.49 (0.09–1.70) for the groups assigned to TDF/FTC compared with the two groups not assigned to TDF/FTC (see Supplementary materials, Table S6 and Fig. S3).
Fig. 2(b) shows the cumulative risk of asymptomatic COVID-19 by treatment group. There were 63 cases: 10 in the TDF/FTC + HC group, 17 in the TDF/FTC group, 18 in the HCQ group, and 17 in the placebo only group. Compared with the placebo only group, the 14-week risk ratio (95% CI) of symptomatic COVID-19 was 0.54 (0.21–1.19) for TDF + HCQ, 0.83 (0.45–1.66) for TDF, and 0.89 (0.49–1.91) for HCQ (Table 2).
The 14-week risk ratio (95% CI) of asymptomatic COVID-19 was 0.79 (0.47–1.33) for the groups assigned to HCQ compared with the groups not assigned to HCQ (see Supplementary materials, Table S5 and Fig. S2), and 0.74 (0.43–1.21) for the groups assigned to TDF/FTC compared with the groups not assigned to TDF/FTC (see Supplementary material, Table S6 and Fig. S3).
Fig. 2(c) shows the cumulative risk of any COVID-19 diagnosis by treatment group. There were 77 cases: 13 in the TDF/FTC + HC group, 20 in the TDF/FTC group, 21 in the HCQ group, and 23 in the placebo only group. Compared with the placebo only group, the 14-week risk ratio (95% CI) of any COVID-19 diagnosis was 0.51 (0.21–1.00) for TDF + HCQ, 0.81 (0.44–1.49) for TDF, and 0.73 (0.41–1.38) for HCQ (Table 2).
The 14-week risk ratio (95% CI) of any COVID-19 diagnosis was 0.78 (0.49–1.23) for the groups assigned to HCQ compared with the groups not assigned to HCQ (see Supplementary materials, Table S5 and Fig. S2), and 0.70 (0.43–1.10) for the groups assigned to TDF/FTC compared with the groups not assigned to TDF/FTC (see Supplementary materials, Table S6 and Fig. S3).
The corresponding hazard ratios were similar (see Supplementary materials, Table S7).
The proportion of individuals with adverse events was 45.0% (99/220) in the TDF/FTC + HCQ group, 41.2% (96/233) in the TDF/FTC group, 36.4% (84/231) in the HCQ group, and 36.8% (82/223) in the double placebo group. Most were mild and of gastrointestinal nature (Table 3 ). There were five serious adverse events: 4 in the placebo only group (hospital admission because of a bleeding uterine myoma, hospital admission because of smoke inhalation from a workplace fire, an episode of dizziness and bradypsiquia, and an episode of jaundice and vomiting) and 1 in the TDF/FTC + HCQ group (retinal detachment).
Table 3.
Frequency of adverse events by treatment group, EPICOS randomized trial
| TDF/FTC + HCQ (n = 220) | TDF/FTC (n = 233) | HCQ (n = 231) | Placebo (n = 223) | |
|---|---|---|---|---|
| Severity of adverse event | ||||
| Mild | 78 (35.5) | 77 (33.0) | 63 (27.3) | 63 (28.3) |
| Moderate | 37 (16.8) | 33 (14.2) | 36 (15.6) | 29 (13.0) |
| Severe | 1 (0.5) | 1 (0.4) | 1 (0.4) | 2 (0.9) |
| Adverse event classified as serious | 1 (0.5) | 0 | 0 | 4 (1.8) |
| Adverse event classified as related to study drug | 49 (22.3) | 51 (21.9) | 46 (19.9) | 37 (16.6) |
| Effect of adverse event on study treatment | ||||
| Treatment was interrupted | 28 (12.7) | 27 (11.6) | 14 (6.1) | 19 (8.5) |
| Treatment was delayed | 4 (1.8) | 4 (1.7) | 7 (3.0) | 3 (1.3) |
| Concomitant treatment was prescribed | 23 (10.5) | 26 (11.2) | 23 (10.0) | 21 (9.4) |
| Adverse events by system organ classa | ||||
| Gastrointestinal disorders | 68 (30.9) | 73 (31.3) | 56 (24.2) | 47 (21.1) |
| Blood and lymphatic system disorders | 1 (0.5) | 0 | 0 | 1 (0.4) |
| Cardiac disorders | 1 (0.5) | 2 (0.9) | 1 (0.4) | 3 (1.3) |
| Ear and labyrinth disorders | 1 (0.5) | 2 (0.9) | 0 | 3 (1.3) |
| Eye disorder | 3 (1.4) | 1 (0.4) | 2 (0.9) | 4 (1.8) |
| General disorders | 11 (5.0) | 17 (7.3) | 9 (3.9) | 10 (4.5) |
| Immune system disorder | 0 | 1 (0.4) | 0 | 0 |
| Infections | 4 (1.8) | 0 | 5 (2.2) | 3 (1.3) |
| Injuries | 2 (0.9) | 0 | 1 (0.4) | 2 (0.9) |
| Investigations | 2 (0.9) | 6 (2.6) | 3 (1.3) | 3 (1.3) |
| Metabolism and nutrition disorders | 2 (0.9) | 2 (0.9) | 1 (0.4) | 1 (0.4) |
| Musculoskeletal/connective tissue disorders | 9 (4.1) | 9 (3.9) | 6 (2.6) | 6 (2.7) |
| Nervous system disorders | 22 (10.0) | 31 (13.3) | 26 (11.3) | 19 (8.5) |
| Psychiatric disorders | 3 (1.4) | 3 (1.3) | 4 (1.7) | 8 (3.6) |
| Renal and urinary disorders | 0 | 1 (0.4) | 0 | 1 (0.4) |
| Reproductive system disorder | 1 (0.5) | 0 | 1 (0.4) | 1 (0.4) |
| Respiratory disorders | 1 (0.5) | 3 (1.3) | 3 (1.3) | 2 (0.9) |
| Skin disorders | 14 (6.4) | 6 (2.6) | 6 (2.6) | 4 (1.8) |
| Vascular disorders | 0 | 0 | 1 (0.4) | 3 (1.3) |
More than one adverse event per participant could occur. Data are presented as n (%).
AbbreviationsHCQ, hydroxychloroquine; FTC, emtricitabine; TDF, tenofovir disoproxil fumarate.
See supplementary methods for a list of the observed adverse events in each system organ class.
Discussion
EPICOS, a double-blind, placebo-controlled randomized trial, evaluated the effect of treatment with HCQ and TDF/FTC, alone or in combination, as pre-exposure prophylaxis for COVID-19 among healthcare workers. Because the trial recruited approximately a quarter of the intended number of participants, the effect estimates were imprecise: compared with placebo, the risk of symptomatic COVID-19 was lower in the groups assigned to HCQ or TDF/FTC, but effects between a 2-fold risk increase and perfect protection were highly compatible with the data. For any (symptomatic or asymptomatic) COVID-19, the risk in the group assigned to combined HCQ plus TDF/FTC was half the risk in the group assigned to placebo only, and effects between a 79% reduction in risk and no reduction in risk were highly compatible with the data.
HCQ and TDF/FTC were safe, with mostly mild adverse events of gastrointestinal nature, which is consistent with the well-established safety record of both drugs [[17], [18], [19]].
Several placebo-controlled randomized trials have studied HCQ at different doses as pre-exposure prophylaxis for (mostly non-severe) COVID-19 in healthcare workers [2,4,5]. Like EPICOS, five of these trials could not achieve their intended sample size [4,5], partly because potential participants were averse to receive HCQ after poorly conducted observational studies (later retracted) [21] suggested HCQ was not safe, and the “nonsignificant” findings of small randomized trials for prophylaxis were misinterpreted as lack of a beneficial effect. However, a meta-analysis of randomized trials found of pre-exposure prophylaxis estimated a risk ratio of COVID-19 of 0.72 (95% CI, 0.58–0.90) for HCQ compared with no HCQ [3]. The largest trial included in the meta-analysis found similar estimates: a COVID-19 hazard ratio of 0.73 (95% CI, 0.48–1.09) for HCQ vs. placebo after 12 weeks of follow-up in a trial with 1483 participants [5], a COVID-19 OR of 0.75 (95% CI, 0.49–1.15) for HCQ vs. placebo after 29 days follow-up in the HERO-HCQ trial with 1359 participants [4], and relative risk of 0.70 (95% CI, 0.44–0.97) for HCQ vs. ascorbic acid after 42 days of follow-up in a cluster randomized trial of 1051 participants [22]. When taken altogether with the findings from EPICOS, the evidence cannot rule out the possibility that prophylaxis with HCQ offers a modest protection against COVID-19 [3].
No previous randomized trials had studied TDF/FTC as pre-exposure prophylaxis for COVID-19. However, several observational studies have found a lower risk of COVID-19 diagnosis or of hospitalization among individuals who use TDF/FTC compared with those who do not [6,7,[23], [24], [25]]. A study among people with HIV in Spain reported lower risk of COVID-19 hospitalization among individuals treated with TDF/FTC compared with those treated with other antiretrovirals [6,7]. However, the estimates were not adjusted for the potentially different clinical characteristics of individuals receiving each treatment. A second study in over 50 000 persons with HIV and adequate virological control, which adjusted for comorbidities and other factors, also found a lower risk ratio of COVID-19 hospitalization for TDF/FTC compared with TAF (Tenofovir Alafenamide)/FTC. Adjusted and unadjusted estimates were similar [24].
A lower risk of COVID-19 hospitalization or death was also found among HIV-positive individuals who used TDF/FTC for HIV treatment in South Africa [23] and among individuals who used TDF for the treatment of hepatitis B infection [25]. Also, in a study of ferrets infected with SARS-CoV-2, the group treated with TDF/FTC group had lower clinical scores and a shorter duration of symptoms [26]. A phase 2 randomized trial in 60 outpatients with early COVID-19 found reductions in nasopharyngeal shedding of SARS-CoV-2 after initiation of TDF/FTC [27]. On the other hand, a recent in vitro study report could not detect substantial activity of TDF/FTC against SARS-CoV-2 [28].
Even if HCQ and TDF/FTC were effective as pre-exposure prophylaxis for COVID-19, vaccines are a better approach to prevention when available, at least for the variants studied so far and for immunocompetent persons. The efficacy of vaccines seems to be reduced in immunocompromised patients who are in need of other prophylactic strategies [29]. The predominant variants differed by country and period of study [30]. The effectiveness of antivirals, unlike that of monoclonal antibodies, is not expected to vary substantially across variants that differ in surface antigens.
A timelier question is whether HCQ and TDF/FTC could be used for early treatment of COVID-19 in non-hospitalized patients. The question has already been answered for HCQ [31] but not for TDF/FTC, a generic and inexpensive drug combination with the potential for massive worldwide production, and for which the available evidence supports the need for therapeutic trials.
In summary, we conducted a randomized, double-blind, placebo-controlled clinical trial in 907 healthcare workers to compare the risk of COVID-19 after pre-exposure prophylaxis with HCQ and TDF/FTC. Because recruitment had to be ended prematurely, effect estimates were unstable and do not allow to draw definite conclusions. Our findings are compatible with both benefit and harm of pre-exposure prophylaxis with TDF/FTC and HCQ, alone or in combination, compared with placebo.
Transparency declaration
Miguel del Toro has received payment for lectures, presentations, speakers bureaus, manuscript writing or educational events from ViiV Healthcare, Gilead Sciences, and Janssen. José Ramón Arribas has received consulting fees from GSK, MSD, Serono, Lilly, Roche, Pfizer, Gilead and payment for lectures, presentations, speakers bureaus, manuscript writing, or educational events from MSD. Miguel A. Hernán has received consultancy fees from ProPublica and Cytel. All authors submitted a COI form to the ESCMID guidelines manager.
EPICOS was sponsored by the Ministry of Health of Spain and supported by research grant COV20/01112, Fondo COVID-19, Instituto de Salud Carlos III, Madrid, Spain. TDF/FTC and TDF/FTC placebos were donated by Gilead Sciences, hydroxychloroquine by Gebro Pharma, hydroxychloroquine placebo by Laboratorios Farmacéuticos Rovi, support for medication packing by Laboratorios Alcalá Farma, and rapid HIV tests by Abbott Laboratories.
Author contributions
RP, XGA, PMS, SM, MAH, and JDA designed the study and prepared the protocol. RP and JDA coordinated the project and supervised field work in Spain and Latin America. All authors were involved in the data collection across centres. Data analysis was done by XGA and MAH. All authors were involved in the interpretation of findings and writing of the manuscript, led by MAH and JDA. The corresponding authors had full access to all the data in the study. All authors have read and approved the final manuscript.
Editor: A. Huttner
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.07.006.
Contributor Information
EPICOS:
Julia del Amo, Rosa Polo, Santiago Moreno, Juan Berenguer, Esteban Martínez, Miguel Hernán, Pablo Martínez de Salazar, and Xabier García de Albéniz
APPENDIX. EPICOS RESEARCH TEAM
The EPICOS randomized trial was sponsored by the Ministry of Health of Spain.
EPICOS investigator team
Julia del amo (principal investigator), Ministry of Health, Madrid, Spain
Rosa Polo, Ministry of Health, Madrid, Spain.
Santiago Moreno, Hospital Ramón y Cajal, Madrid, Spain.
Juan Berenguer, Hospital Gregorio Marañon, Madrid, Spain.
Esteban Martínez, hospital clinic, Barcelona, Spain
Miguel Hernán, CAUSALab and Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA.
Pablo Martínez de Salazar, Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA
Xabier García de Albéniz, RTI Health Solutions, Barcelona, Spain.
Collaborators.
Marieta Iradier, Fundación Estatal, Salud, Infancia y Bienestar Social.
Inma Jarrín, Institute of Health Carlos III, National Center for Epidemiology.
Data safety monitoring board.
Javier Zamora, Hospital Ramón y Cajal, Madrid, Spain.
Antonio Rivero, Hospital Reina Sofía, Córdoba, Spain.
Clara Menéndez, IS Global, Barcelona, Spain.
Contract research organization
Enrique Conde and José Montes, Effice Research, Madrid, Spain.
Participating hospitals
Bolivia.
-
•
Facultad de Medicina Universidad Mayor, Real y Pontificia de San Francisco Xavier de Chuquisaca - Hospital Santa Bárbara, Bolivia. PI: Carolina Terán. Co-investigators: Bettsy Flores, María Elena Choque, Jhaquelin Peñaranda, Gladys Gorena, Mariluz Herrera, Marcela Farfán, David Moya, Jhonny Camacho, Jovanna Ordoñez, José Mayora, Brayan Farfán
Venezuela.
-
•
Hospital Militar Dr. Carlos Arvelo, Centro Clínico María Edelmira Araujo, Instituto Falconiano de Emergencias Médicas, Venezuela. PI: Miguel Morales. Co-investigators: Maryelis Benítez, Rosa Bolaños, Jesús Colina
Spain
-
•
Hospital Doce de Octubre, Madrid. Co-investigators: Federico Pulido, Rafael Rubio, Otilia Bisbal, María de Lagarde, Cristina Epalza, Cristina Lillo-Díaz, Raúl Martínez
-
•
Hospital Nuestra Señora de Sonsoles, Ávila. Co-investigators: Miguel Sebastián Pedrodomingo, César de la Hoz, Demetrio Sánchez, Ana Cristina Antolí, Carmen Grande, Dulce María Astudillo
-
•
Hospital General de Valencia. PI: Miguel García Deltoro. Co-investigators: Jose Ignacio Chirivella
-
•
Hospital de Torrejón, Madrid. PI: César Hita. Co-investigators: María Carmen Montero, Juan Ruíz.
-
•
Hospital Universitario de Tenerife. Canarias. Co-investigators: Ma Mar Alonso, Ma Remedios Alemán, Ana Ma López, Dácil García, Ricardo Pelazas
-
•
Hospital Universitario de Burgos. Co-investigators: Pablo González Recio
-
•
Hospital Puerta de Hierro, Madrid. Co-investigators: Fernando Martínez-Vera, Alejandro Muñoz, Sara De la Fuente, Ana Muñoz, José Manuel Vázquez
-
•
Hospital Universitario Insular de Gran Canaria. Co-investigators: Cristina Carranza, Michele Hernández, Nieves Jaén, Carmen Lavilla, Elena Pisos, Laura Suárez
-
•
Hospital La Princesa, Madrid. Co-investigators: Ignacio de los Santos, Lucio García-Fraile, Ángela Gutiérrez, Azucena Bautista
-
•
Hospital General de Segovia. Co-investigators: Sara Muñoz, José María Alonso de los Santos, Eva María Ferreira, Ana Carrero
-
•
Complejo Hospitalario Universitario de Albacete. Co-investigators: Fernando Mateos, José Javier Blanch, Julian Eloy Solis García
-
•
Parc Sanitari Sant Joan de Déu. Sant Boi del Llobregat, Barcelona. Co-investigators: Montserrat Sanmartí, Raquel Gómez, Encarna Moreno, María Carmen Álvarez
-
•
Hospital General Universitario de Elche. Co-investigators: Javier García-Abellán. Félix Gutiérrez, Sergio Padilla, Gabriel Estañ
-
•
Hospital Clínico Universitario Virgen de la Arrixaca. Murcia. Co-investigators: Encarnación Moral, Sonia Marín, Aychel Elena Roura, Ana Pareja
-
•
Hospital Universitario de León. Co-investigators: Manuel Martín Regidor, Esperanza Gutiérrez, Luis Jorge Valdivia, Patricia Capón
-
•
Hospital Universitario Virgen de la Victoria de Málaga. Co-investigators: Rosario Palacios, Cristina Gomez-Ayerbe, Eva Cabrera César, Alejandro Galán Romero
-
•
Hospital Universitario Miguel Servet. Zaragoza. Co-investigators: Álvaro Cecilio, Rosa Fenoll
-
•
Hospital Clínico San Cecilio (Complejo Hospitalario Universitario de Granada). Co-investigators: José Peregrina, Francisco Anguita, Laura Martín
-
•
Hospital Universitario Ramón y Cajal. Madrid. PI: Fernando Dronda. Co-investigators: Johannes Häemmerle, Clara Crespillo
-
•
Hospital Arnau de Vilanova – Llíria. Valencia. PI: Juan Flores. Co-investigators: Lidia Castellano
-
•
Hospital Clínico Universitario de Valladolid. PI: Carlos Dueñas. Co-investigators: Laura Rodríguez Fernández, Genoveva Zapico
-
•
Hospital La Fe. Valencia. PI: María Tasias. Co-investigators: Pablo Berrocal, Cristina Campo
-
•
Hospital Universitario Virgen Macarena/Instituto de Biomedicina de Sevilla. PI: Jesús Rodríguez Baño. Co-investigators: Ángel Domínguez-Castellano, María José Ríos-Villegas
-
•
Hospital Sant Joan de Déu. Barcelona. PI: Antoni Noguera-Julian. Co-investigators: Clàudia Fortuny, María Ríos-Barnés
-
•
Hospital Universitario de Salamanca. PI: Guillermo Hernández-Pérez. Co-investigators: María Sánchez-Ledesma, Cristina Carbonell
-
•
Complejo Asistencial Universitario de Palencia. PI: Jacinto Sánchez-Navarro. Co-investigators: Cristina Sánchez del Hoyo, Yolanda Morán
-
•
Fundación Jiménez Díaz, Madrid. PI: Alfonso Cabello. Co-investigators: Irene Carrillo, Miguel Górgolas
-
•
Hospital Clínico de Santiago de Compostela. PI: Antonio Antela. Co-investigators: Elena Losada, Maria Jesús Domínguez
-
•
Hospital Universitario de Ferrol. PI: Ana Isabel Mariño. Co-investigators: Sabela Sánchez-Trigo, Silvia Martínez-Varela
-
•
Hospital Infanta Margarita, Córdoba. PI: Eduardo Aguilar. Co-investigators: Jesús González-Lama, Alejandro Plata
-
•
Hospital Reina Sofía Tudela. Navarra. PI: Ma Teresa Rubio. Co-investigators: Marta Marín, Lucía Zardoya
-
•
Hospital Universitari Sagrat Cor - Grupo Quirónsalud. Barcelona. PI: Diego de Mendoza. Co-investigators: Antonio Gutiérrez, Rosa Coll
-
•
Hospital Clínico Universitario Lozano Blesa, Zaragoza. PI: Isabel Sanjoaquín. Co-investigators: Silvia Loscos
-
•
Hospital Virgen de la Luz, Cuenca. PI: MP Geijo. Co-investigators: O Belinchon
-
•
Hospital Reina Sofía, Murcia. PI: Enrique Bernal
-
•
Hospitales Universitarios Rey Juan Carlos, Infanta Elena y General de Villalba, Madrid. PI: Ámbar Deschamps-Perdomo
-
•
Hospital Universitario Príncipe de Asturias, Madrid. PI: José Alberto Arranz
-
•
Hospital Universitario La Paz, Madrid. PI: Alberto Borobia
-
•
Hospital del Mar, Barcelona. PI: Hernando Knobel
-
•
Hospital Universitario Doctor Peset, Valencia. PI: Arturo Artero
-
•
Complejo Hospitalario de Navarra, Pamplona. PI: María Rivero
-
•
Hospital Clínico de Valencia. PI: María José Galindo
-
•
Hospital Universitario de Móstoles. Madrid. PI: Concepción Cepeda
-
•
Hospital San Pedro, Logroño. PI: José Ramón Blanco
-
•
Hospital Clínico San Carlos, Madrid. PI: Vicente Estrada
-
•
Hospital Universitario Araba-Txagorritxu. Vitoria. PI: Ainhoa Lecuona
-
•
Hospital Río Hortega de Valladolid. PI: Julia Gómez
-
•
Hospital Universitario Central de Asturias. PI: Víctor Asensi
-
•
Hospital Universitario Severo Ochoa, Madrid. PI: Miguel Cervero
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Davis J.S., Ferreira D., Denholm J.T., Tong S.Y. Clinical trials for the prevention and treatment of COVID-19: current state of play. Med J Aust. 2020;213:86–93. doi: 10.5694/mja2.50673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abella B.S., Jolkovsky E.L., Biney B.T., Uspal J.E., Hyman M.C., Frank I., et al. Efficacy and safety of hydroxychloroquine vs placebo for pre-exposure SARS-CoV-2 prophylaxis among health care workers: a randomized clinical trial. JAMA Intern Med. 2021;181:195–202. doi: 10.1001/jamainternmed.2020.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-Albéniz X., Del Amo J., Polo R., Morales-Asencio J.M., Hernán M.A. Systematic review and meta-analysis of randomized trials of hydroxychloroquine for the prevention of COVID-19. Eur J Epidemiol. 2022:1–8. doi: 10.1007/s10654-022-00891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naggie S., Milstone A., Castro M., Collins S.P., Seetha L., Anderson D.J., et al. Hydroxychloroquine for pre-exposure prophylaxis of COVID-19 in health care workers: a randomized, multicenter, placebo-controlled trial (HERO-HCQ) medRxiv. 2021 doi: 10.1101/2021.08.19.21262275. 2021.08.19.21262275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajasingham R., Bangdiwala A.S., Nicol M.R., Skipper C.P., Pastick K.A., Axelrod M.L., et al. Hydroxychloroquine as pre-exposure prophylaxis for coronavirus disease 2019 (COVID-19) in healthcare workers: a randomized trial. Clin Infect Dis. 2021;72:e835–e843. doi: 10.1093/cid/ciaa1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Amo J., Polo R., Moreno S., Díaz A., Martínez E., Arribas J.R., et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy : a cohort study. Ann Intern Med. 2020;173:536–541. doi: 10.7326/M20-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Amo J., Polo R., Moreno S., Díaz A., Martínez E., Arribas J.R., et al. Antiretrovirals and risk of COVID-19 diagnosis and hospitalization in HIV-positive persons. Epidemiology. 2020;31:e49–e51. doi: 10.1097/EDE.0000000000001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chien M., Anderson T.K., Jockusch S., Tao C., Li X., Kumar S., et al. Nucleotide analogues as inhibitors of SARS-CoV-2 polymerase, a key drug target for COVID-19. J Proteome Res. 2020;19:4690–4697. doi: 10.1021/acs.jproteome.0c00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clososki G.C., Soldi R.A., da Silva R.M., Guaratini T., Lopes J.N., Pereira P.R., et al. Tenofovir disoproxil fumarate: new chemical developments and encouraging in vitro biological results for SARS-CoV-2. J Braz Chem Soc. 2020;31:1552–1556. doi: 10.21577/0103-5053.20200106. [DOI] [Google Scholar]
- 10.Copertino D.C., Casado Lima B.C., Duarte R.R.R., Powell T.R., Ormsby C.E., Wilkin T., et al. Antiretroviral drug activity and potential for pre-exposure prophylaxis against COVID-19 and HIV infection. J Biomol Struct Dyn. 2021:1–14. doi: 10.1080/07391102.2021.1901144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elfiky A.A. Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jockusch S., Tao C., Li X., Anderson T.K., Chien M., Kumar S., et al. A library of nucleotide analogues terminate RNA synthesis catalyzed by polymerases of coronaviruses that cause SARS and COVID-19. Antivir Res. 2020;180 doi: 10.1016/j.antiviral.2020.104857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanella I., Zizioli D., Castelli F., Quiros-Roldan E. Tenofovir, another inexpensive, well-known and widely available old drug repurposed for SARS-COV-2 infection. Pharmaceuticals (Basel) 2021;14:454. doi: 10.3390/ph14050454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson K.B., Prince H.A., Kraft E., Jenkins A.J., Shaheen N.J., Rooney J.F., et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3:112re4. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seifert S.M., Chen X., Meditz A.L., Castillo-Mancilla J.R., Gardner E.M., Predhomme J.A., et al. Intracellular tenofovir and emtricitabine anabolites in genital, rectal, and blood compartments from first dose to steady state. AIDS Res Hum Retroviruses. 2016;32:981–991. doi: 10.1089/aid.2016.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Twigg H.L., Schnizlein-Bick C.T., Weiden M., Valentine F., Wheat J., Day R.B., et al. Measurement of antiretroviral drugs in the lungs of HIV-infected patients. HIV Ther. 2010;4:247–251. doi: 10.2217/hiv.10.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponticelli C., Moroni G. Hydroxychloroquine in systemic lupus erythematosus (SLE) Expert Opin Drug Saf. 2017;16:411–419. doi: 10.1080/14740338.2017.1269168. [DOI] [PubMed] [Google Scholar]
- 18.Pilkington V., Hill A., Hughes S., Nwokolo N., Pozniak A. How safe is TDF/FTC as PrEP? A systematic review and meta-analysis of the risk of adverse events in 13 randomised trials of PrEP. J Virus Erad. 2018;4:215–224. doi: 10.1016/S2055-6640(20)30312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernández-Díaz S., Bateman B.T., Straub L., Zhu Y., Mogun H., Fischer M., et al. Safety of tenofovir disoproxil fumarate for pregnant women facing the coronavirus disease 2019 pandemic. Am J Epidemiol. 2021;190:2339–2349. doi: 10.1093/aje/kwab109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehra M.R., Desai S.S., Ruschitzka F., Patel A.N. RETRACTED: hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Seet R.C.S., Quek A.M.L., Ooi D.S.Q., Sengupta S., Lakshminarasappa S.R., Koo C.Y., et al. Positive impact of oral hydroxychloroquine and povidone-iodine throat spray for COVID-19 prophylaxis: an open-label randomized trial. Int J Infect Dis. 2021;106:314–322. doi: 10.1016/j.ijid.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases SA Risk factors for coronavirus disease 2019 (COVID-19) death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2021;73:e2005–e2015. doi: 10.1093/cid/ciaa1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Del Amo J., Polo R., Moreno S., Martínez E., Cabello A., Iribarren J., et al. 29th conference on retroviruses and opportunistic infections (CROI) 2022. Tenofovir disoproxil fumarate and severity of COVID-19 in people with HIV infection. Abstract 00867. [DOI] [Google Scholar]
- 25.Muñoz-Mateos B., Buti M., Fernández I., Hernández M., Bernal V.F.D. Tenofovir reduces the severity of COVID-19 infection in chronic hepatitis B patients. J Hepatol. 2021;75:S746–S747. doi: 10.1097/QAD.000000000000337225. [DOI] [Google Scholar]
- 26.Park S., Yu K., Kim Y., Kim S., Kim E., Kim S., et al. Antiviral efficacies of FDA-approved drugs against SARS-CoV-2 infection in ferrets. mBio. 2020;11:e01114–e01120. doi: 10.1128/mBio.01114-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parienti J.J., Prazuck T., Peyro-Saint-Paul L., Fournier A., Valentin C., Brucato S., et al. Effect of tenofovir disoproxil fumarate and emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: a pilot, randomized, open-label phase 2 trial. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng J., Bilello J., Babusis D., Gordon C., Tchesnokov E., Perry J., et al. 18th European AIDS Conference (EACS); October 27-30, 2021. NRTIs tenofovir, TAF, TDF, and FTC are inactive against SARS-CoV-2. Virtual & London, United Kingdom. [Google Scholar]
- 29.Embi P.J., Levy M.E., Naleway A.L., Patel P., Gaglani M., Natarajan K., et al. Effectiveness of 2-dose vaccination with mRNA COVID-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults - nine states, January-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1553–1559. doi: 10.15585/mmwr.mm7044e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.López M.G., Chiner-Oms A., García de Viedma D., Ruiz-Rodriguez P., Bracho M.A., Cancino-Muñoz I., et al. The first wave of the COVID-19 epidemic in Spain was associated with early introductions and fast spread of a dominating genetic variant. Nat Genet. 2021;53:1405–1414. doi: 10.1038/s41588-021-00936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skipper C.P., Pastick K.A., Engen N.W., Bangdiwala A.S., Abassi M., Lofgren S.M., et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Intern Med. 2020;173:623–631. doi: 10.7326/M20-4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.