Abstract
As one of the earliest-diverging lineage of the megadiverse beetle suborder Polyphaga, marsh beetles (Scirtidae) are crucial for reconstructing the ancestor of all polyphagan beetles and the ecomorphological underpinnings of their remarkable evolutionary success. The phylogeny of marsh beetles has nonetheless remained challenging to infer, not least because of their fragmentary Mesozoic fossil record. Here we describe a new scirtid beetle genus and species, Varcalium lawrencei gen. et sp. nov., preserving internal tissue, from Albian–Cenomanian Kachin amber (ca 99 Ma), representing the second member of this family known from the deposit. Based on a formal morphological phylogenetic analysis, Varcalium is recovered within the crown-group of Scirtinae, forming a clade with other genera that possess subocular carinae. The finding suggests that the crown-group of Scirtinae has already diversified by the mid-Cretaceous.
Subject terms: Palaeontology, Phylogenetics, Taxonomy, Entomology
Introduction
The marsh beetles (Scirtidae) are a globally distributed group of insufficiently known beetles associated with aquatic habitats. Marsh beetles have been traditionally placed in the superfamily Scirtoidea and regarded as one of the earliest-diverging groups within the megadiverse suborder Polyphaga1. Recent molecular phylogenetic analyses suggest that the classical concept of Scirtoidea (sensu Bouchard et al.2) is not monophyletic and instead the families Scirtidae and Decliniidae form the earliest-diverging clade within Polyphaga3–5. Reflecting these findings and morphological evidence, the concept of Scirtoidea is to be revised to include only Scirtidae, Decliniidae, and poorly-known extinct groups5. In light of these findings, Scirtidae represents a key taxon for reconstructing the last common ancestor of the suborder Polyphaga and hence the origins of its remarkable diversification.
While Decliniidae is a small family with one genus and two described species known from the Northeast Asia only6–8, Scirtidae is cosmopolitan, with around 100 genera and about 1900 described species9. The larvae of Scirtidae are usually aquatic detritus feeders, with adults often short-lived. The internal relationships of Scirtidae are poorly understood. Although recently years have witnessed a growing number of studies on various scirtid groups (e.g.,10–28), generic misplacements within the family seem to remain prevalent. The molecular phylogenetic studies of Scirtidae conducted to date, though with a focus on Australian taxa only, have nevertheless demonstrated that many currently recognised genera are not monophyletic and that scirtid diversity at the generic level is severely underestimated29–32.
Most scirtid fossils are known from Eocene Baltic amber (as listed by Alekseev33). Many of them are well preserved with exposed genitalia and most are placed in extant genera (e.g., Contacyphon Gozis, Elodes Latreille, Microcara Thomson34,35), but several new genera were also described36. Additional scirtids were reported (though some of them were not officially named) from Lower Cretaceous Lebanese amber, Lower Cretaceous deposits in Koonwarra (Australia), and Eocene Fushun and Oise ambers37–39. Though the mid-Cretaceous Kachin amber from northern Myanmar preserved a diverse fauna of insects and other terrestrial arthropods40–42, scirtids appear to be relatively uncommon in Kachin amber. The only previously described scirtid from Kachin amber is Mesernobius anawrahtai Engel, which was poorly illustrated and superficially described, and was originally attributed to the family Ptinidae43. Peris et al.44 transferred Mesernobius Engel to Scirtidae mainly based on the deflexed head, short and broad prothorax, and the bilobed fourth tarsomere. However, the ventral structures of head and thorax of Mesernobius remain unclear and will be crucial for determining its systematic position within Scirtidae.
Recently we have found several scirtids in our collection of Kachin amber. Here, we report a new genus of crown-group Scirtidae from Kachin amber, which, together with other unpublished scirtids, suggests that the family was more diverse in the Kachin amber rainforest than previously thought.
Systematic Palaeontology
Order Coleoptera Linnaeus, 1758.
Suborder Polyphaga Emery, 1886.
Superfamily Scirtoidea Fleming, 1821.
Family Scirtidae Fleming, 1821.
Varcalium Li, Ruta, Tihelka & Cai gen. nov.
Type species. Varcalium lawrencei sp. nov., here designated.
Etymology. The generic name is an anagram of Calvarium Pic, a genus in Scirtidae. The name is masculine in gender.
Diagnosis. Antennae serrate. Subocular carina present, smoothly connecting supraantennal ridge with subgenal ridge. Subgenal ridge without buttonhole configuration (sensu Zwick22), situated close to eye. Clypeus with deep, rectangular emargination. Anterior mesoventral margin without notch for prosternal process. Hind femora not distinctly thickened.
Varcalium lawrencei Li, Ruta, Tihelka & Cai sp. nov.
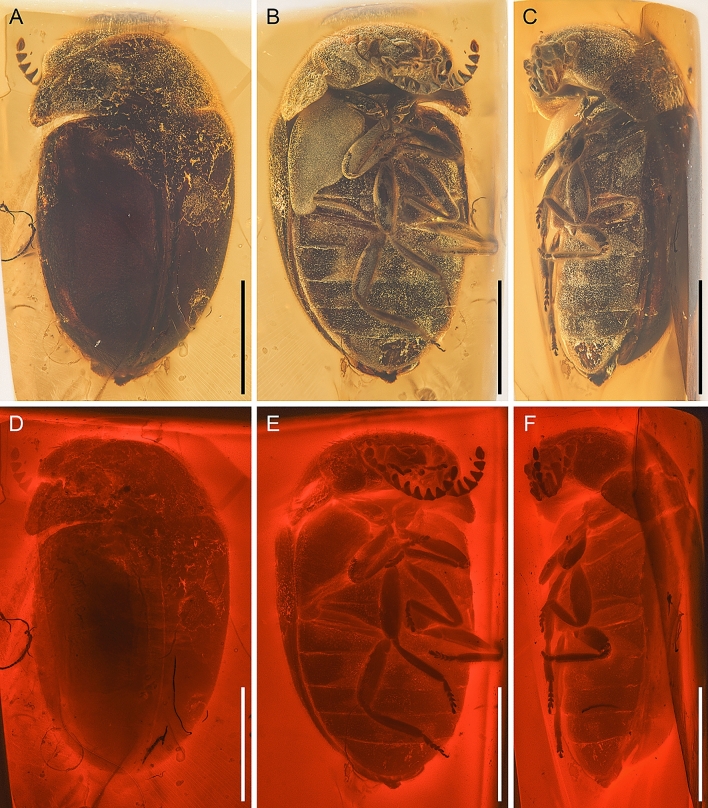
Figure 1.
General habitus of Varcalium lawrencei gen. et sp. nov., holotype, NIGP177336, under incident light (A–C) or widefield fluorescence (D–F). (A, D) Dorsal view. (B, E) Ventral view. (C, F) Lateral view. Scale bars: 1000 μm.
Figure 2.
Details of Varcalium lawrencei gen. et sp. nov., holotype, NIGP177336, under confocal microscopy. (A) Head, anterolateral view. (B) Head, lateral view. (C) Mouthparts, dorsal view. (D) Head, dorsal view. (E) Prothorax, ventral view. (F) Mid legs. (G) Metafemur. (H) Metacoxa. Abbreviations: ac, anteclypeus; an1–11, antennomeres 1–11; cl, clypeus; ey, compound eye; lb, labrum; lbp, labial palp; md, mandible; msf, mesofemur; mstb, mesotibia; mstc, mesotrochanter; msts, mesotarsus; mtc, metacoxal; mtf, metafemur; mxp, maxillary palp; pc, procoxa; pf, profemur; pn, pronotum; ps, prosternum; ptb, protibia; pts, protarsus. Scale bars: 200 μm.
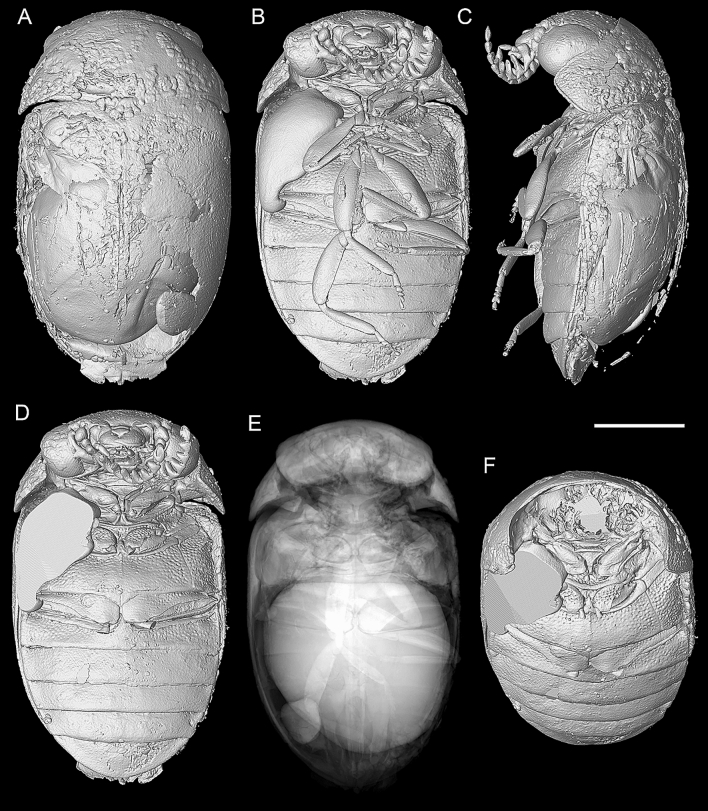
Figure 3.
X-ray microtomographic reconstruction of Varcalium lawrencei gen. et sp. nov., holotype, NIGP177336. (A) Dorsal view. (B) Ventral view. (C) Lateral view. (D) Ventral view, with legs removed. (E) Ventral view, rendered under “Sum along Ray” mode. (F) Anteroventral view, with head removed. Scale bar: 1000 μm.
Etymology. The specific name is a patronym in honour of Dr. John F. Lawrence, an eminent expert in systematics of Coleoptera.
Type material. Holotype, NIGP177336 (field number: HUANG-HP-B-3245), male (NIGP).
Locality and horizon. Amber mine located near Noije Bum Village, Tanai Township, Myitkyina District, Kachin State, Myanmar; unnamed horizon, mid-Cretaceous, Upper Albian to Lower Cenomanian45,46.
Diagnosis. As for the genus.
Description. Adult male. Body compact, elongate oval, 3.0 mm long, 1.8 mm wide, with irregular punctation and short setae.
Head (Fig. 4A–C) about 0.70 times as long as maximum width, with very short tempora, without neck. Distance across eyes about 1.60 times as great as distance between them. Posterior edge of head above occipital foramen with low transverse ridge; upper edge of occipital foramen broadly biemarginate. Eyes (Fig. 2B, D) large; each about as long as half of head width behind eyes, entire and finely facetted, without interfacetal setae. Antennal insertions exposed, widely separated. Antennae (Fig. 2A, Supplementary Fig. S1A, B) about as long as maximum head width, 11-segmented; scape moderately large and broadly ovate, subcylindrical, without sharp ridge on anterior portion; pedicel about half as long and much narrower, subconical, wider at base than at apex; antennomere 3 shorter than 2 and narrow, as wide as long; antennomeres 4–10 uniramose, with the ramus on antennomere 4 thick and club-like; those on the rami of antennomeres 5–7 elongate and flattened (pectinate) and the rami of antennomeres 8–10 gradually shorter and more or less triangular (serrate); antennomeres 4–10 covered with oval sensillae; terminal antennomere elongate and irregularly fusiform. Clypeus transverse, ca. 4.5 times wider than long, deeply emarginate in central portion of anterior margin (Figs. 2C, 4C); frontoclypeal suture absent. Small, transversely rectangular anteclypeus present between clypeus and labrum (Figs. 2C, 4C). Labrum (Figs. 2C, 4C) as long as clypeus and 0.55 times as long as wide, slightly covering mesal portions of mandibles, with straight sides, truncate apex and rounded anterior angles. Mandibles strongly curved, unidentate and overlapping apically (Figs. 2A, C, 4A). Maxilla (Fig. 4B) with relatively small cardo, narrowly elongate stipes and small apical lobe. Maxillary palp with basal palpomere small and globular; second palpomere 2.5 times as long, with oblique apex; third slightly shorter but similar in form and fourth about twice as long as wide, fusiform, widest in the middle of its length and narrowly rounded at apex. Prementum (Fig. 4B) with broadly rounded ligula; labial palpomere 1 long and narrow; palpomere 2 as long as first, much wider and globular; palpomere 3 more than twice as long as second, widest near base and narrowly rounded at apex, arising from apex of preapical palpomere. Mentum (Fig. 4B) subtrapezoidal, about as long as wide, widest posteriorly and gradually narrowing anteriorly, lined with a relatively thick ridge enclosing a flattened depression. Submentum (Fig. 4B) half as long as wide, with curved sides, separated from gula. Gula 0.45 times as long as wide, widest posteriorly and slightly narrowed anteriorly. Subgenal ridges strongly developed, without buttonhole, connected with subocular carinae separating subantennal grooves from eyes (Fig. 4A, B). Subantennal grooves narrowed to maxillary bases (Fig. 4A, B).
Figure 4.
Comparison between Varcalium lawrencei gen. et sp. nov. and its extant relatives. (A–C) Varcalium lawrencei gen. et sp. nov., head, mirrored, with antennae (partly) removed. (D, E) Byrrhopsis sp., head, with antennae and mouthparts (partly) removed. (F, G) Perplexacara latusmandibulara, head. (H) Prionocyphon ornatus, head. (I) Macrohelodes sp., head. (J, K) Varcalium lawrencei gen. et sp. nov., aedeagus inside the abdomen, horizontal section (J) or “Sum along Ray” rendering (K), with arrowhead showing the cuticular rings (J). (L) Perplexacara latusmandibulara, aedeagus inside the abdomen. Abbreviations: SAR, supraantennal ridge; SGR, subgenal ridg; SOC, subocular carina; pe, penis; tg, tegmen. Scale bars: 500 μm.
Pronotum about 0.42 times as long as wide, considerably wider than head, widest posteriorly; anterior edge more or less truncate; anterior angles more or less right, not produced forward; sides weakly curved with fine marginal carina; disc moderately convex. Scutellar shield subtriangular. Elytra about 1.09 times as long as combined width and 2.64 times as long as pronotum; sides very slightly curved and apices broadly, conjointly rounded; epipleura widest anteriorly and gradually narrowing posteriorly.
Prosternum (Figs. 2E, 3D, F) subtriangular, very short in front of coxae, with very narrow lateral portions; surface flat but with relatively narrow, declined anterior head rest; prosternal process slender, extending to edges of coxae and narrowly rounded at apex. Procoxae large and oblique, with slender plates. Protrochantin exposed and forming narrow strip. Mesoventrite (Fig. 3D) strongly transverse, lateral portions about one-fourth as long as mesocoxae, which are separated by about a third the coxal length; mesoventral process broad at base, narrowing apically and cleft at apex, touching anterior metaventral process. Mesanepisternum short, about 0.5 times as long as wide, shorter in mesal portion. Mesocoxae transversely oval, with distinct plates, broader mesally. Metaventrite (Fig. 3D) about 0.36 times as long (excluding anterior process) as wide; widest posteriorly and slightly narrower anteriorly, with discrimen and with metakatepisternal suture extending from midline about half way to lateral edges. Metacoxae (Fig. 2H) strongly transverse, reaching elytral epipleura, with plates well developed mesally. Metanepisternum broad, about 2.3 times as long as wide, widest at anterior end.
Femora moderately thickened at middle, with tibial groove on inner edge (Fig. 2F, G). Tibiae (Fig. 2F) slender and subequal in length to femora, not expanded apically, with paired longitudinal carinae and distinct apical spurs. Tarsi 5–5–5 (Fig. 2E, F); tarsomere 1 about twice as long as 2; tarsomeres 2–3 relatively wide, subtriangular; tarsomere 4 bilobed; pretarsal claws simple.
Abdomen (Fig. 3D) about 0.80 times as long as wide; ventrite 1 slightly shorter than 2, with acute intercoxal process; ventrites 1–3 apparently connate; 5 broadly rounded at apex. Terminal segments and aedeagus symmetrical (Fig. 4J, K). Penis tubular, with internal structures visible and paired hooked processes at apex. Tegmen well developed, with wide parameres.
Discussion
Systematic position of Varcalium and comparison with extant relatives
Varcalium shares a typical habitus with extant Scirtidae and can be confidently placed within the family based on the combination of the following characters: head with paired subgenal ridges, protrochantins exposed, mesocoxal cavities laterally open, tarsi 5–5–5, penultimate tarsomere lobed beneath. Varcalium is similar to the sister group of Scirtidae, Decliniidae, in having serrate antennae (Fig. 2A, Supplementary Fig. S1A, B). However, it clearly differs from Decliniidae in having a marginally bordered mentum, very short prosternum in front of procoxae, and a narrow prosternal process47. Besides, a few extant members of Scirtidae also have serrate antennae (e.g., Prionocyphon Redtenbacher19, Macrodascillus Carter20, Prionoscirtes Champion, and Mescirtes Motschulsky12).
Scirtidae is currently divided into three subfamilies, Nipponocyphoninae, Stenocyphoninae, and Scirtinae. Nipponocyphoninae and Stenocyphoninae are basal lineages in the family, and both contain a single genus. Varcalium clearly does not belong to Nipponocyphoninae or Stenocyphoninae based on the absence of a frontoclypeal suture, unidentate mandibles, and tibiae with paired longitudinal carinae (Fig. 2C, F). Within Scirtinae, several genera (Exochomoscirtes Pic, Ora Clark, and Scirtes Illiger) with very thick hind femora for jumping form a distinct and well-supported clade29. Varcalium is excluded from this group by the lack of thickened hind femora (Fig. 2F).
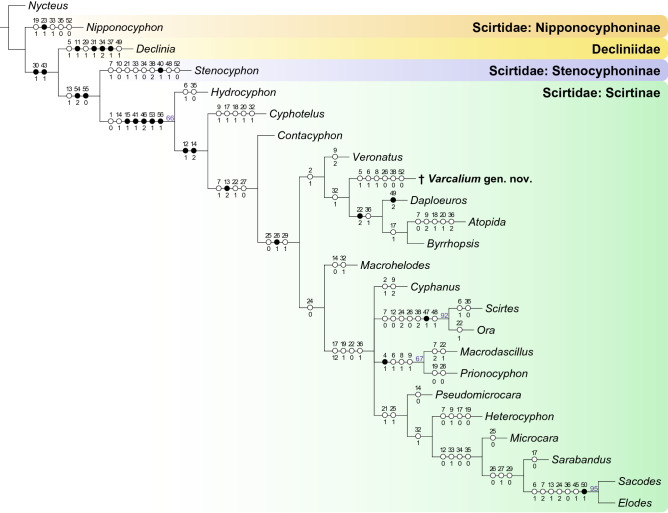
Ridges on head are important characters in scirtid taxonomy (Fig. 4). In the majority of scirtid genera, the supraantennal ridge (SAR) is short, and ends where it meets the medial edge of the eye, and therefore the supraantennal ridge and subgenal ridge (SGR) are not connected (e.g., Fig. 4H, I48). In some other scirtids (e.g., Atopida White, Byrrhopsis Champion, Calvarium, Mucronotus Ruta, Pachycyphon Zwick49–52), the supraantennal ridge turns ventrally in front of eye and becomes the subocular carina (SOC). This subocular carina usually connects the supraantennal ridge with the subgenal ridge, and acts as the outer edge of the subantennal groove (sulcus) in some genera (Fig. 4D–G). In Varcalium, the supraantennal ridge smoothly turns ventrally to become the subocular carina, and then again smoothly turns outwards to become the subgenal ridge (Fig. 4A–C). Thus, Varcalium is likely related to those extant genera possessing subocular carina. The phylogenetic analysis also supports a close relationship for the genera with subocular carina (Fig. 5). All genera coded in the analysis with subocular carina, except for Cyphanus Sharp, form a monophyletic group (Veronatus Sharp, Varcalium, Daploeuros Watts, Atopida, and Byrrhopsis), although only weakly supported. Varcalium is unique in this group in having the subgenal ridge very close to the compound eye (Fig. 4B), while in other members the space between the subgenal ridge and the compound eye is relative wide (Fig. 4E, G) (though in some Scirtes the subgenal ridge could be close to the eye24,53).
Figure 5.
Placement of Varcalium gen. nov. within Scirtidae. Black circles indicate nonhomoplasious changes; white circles indicate homoplasious characters. Bootstrap support values lower than 60% are not shown.
The anterior clypeal margin of Varcalium is deeply emarginate in the central portion (Figs. 2C, 4C), which is rarely known in extant Scirtidae, where the anterior clypeal margin is usually straight. A similar deeply emarginate clypeus is known in an unusual member of Perplexacara Watts et al., i.e., P. latusmandibulara (Watts) (Fig. 4F)54. However, it differs from Varcalium in scape distinctly enlarged and flattened, forming sharp anterior ridge, and subocular carina weakened posteriorly and not connected with subgenal ridge. Varcalium also differs from other extant Scirtidae in the position of mesoventral cavity. In extant Scirtidae (and also Decliniidae), if present, the mesoventral cavity would usually extend into the exposed ventral surface of mesoventrite, leaving a v-shaped notch on the anterior margin of mesoventrite (the line between mesoventrite body and procoxal rests). In contrast, the mesoventral cavity of Varcalium is only visible on the surface where procoxal rests develop, and therefore the anterior margin of mesoventrite appears to be intact (Fig. 3D).
Some structures observed in the studied specimen are poorly known in extant Scirtoidea. Very little is known about antennal sensillae (Fig. 2A, Supplementary Fig. S1A,B). Sensillae are present in Eucinetidae55, and relatively large sensilla coeloconica are present on antennomeres 6–11 in Declinia Nikitsky et al.47 (Supplementary Fig. S1D). In Scirtidae sensillar structures were not studied, but are present, especially in genera where modifications of antennae occur, e.g., in Macrodascillus (Supplementary Fig. S1C) and Prionocyphon (Ruta, unpublished).
Cuticular rings (rectal rings; Fig. 4J) present in an oval sac formed by rectum were reported by Lawrence et al.47 in Eucinetidae, Decliniidae, and Nipponocyphon (Scirtidae). Similar structures have been recently reported in Mucronotus velutinus (Solier) although the putative homology of the structures needs to be confirmed52.
Unexposed beetle aedeagus in amber recovered with micro-CT
Amber fossils offer a potential for three-dimensional preservation of internal structures. The most simple and straightforward way to examine the internal structures of an amber inclusion is to cut open the amber piece. Then the exposed internal structures could be observed with electron microscopy or other methods56–59. Alternatively, the amber may be dissolved using chloroform as the solvent, to aid exposing internal structures60,61. However, these methods would irreversibly damage the valuable amber specimens. The development of micro-computed tomography (micro-CT) provides an opportunity to investigate the interior of fossils non-destructively. With the aid of micro-CT, researchers have successfully studied the muscles, brain, and even ingested pollen masses within insects entombed in amber62–66.
The morphology of male genitalia (aedeagus) is of great importance for the classification of many beetle clades. This is true especially in Scirtidae, a group with a remarkable diversity of genital structures and a generally uniform external morphology within individual genera. However, in beetles fossilised in amber, genitalia are mostly retracted in the abdomen and not externally visible. Micro-CT has been successfully deployed to reconstruct the aedeagi of several beetle fossils preserved in Baltic amber62,67–77. By contrast, the unexposed beetle genitalia recovered by micro-CT have been rarely known from Kachin amber, with only one example reported to date65. The structure of the aedeagus is highly variable within Scirtidae. Thus, the reconstruction of the aedeagus in the fossil Varcalium nevertheless provides some important information on this crucial but still relatively poorly understood character system. Two fundamental studies on the scritid aedeagus and copulation were published by Nyholm78,79, and more recently Zwick has revised Nyholm’s hypotheses80. While terminal segments are difficult to identify in the studied specimen, it seems that the aedeagus consists of a tubular penis resembling the one of the extant genus Elodes while the tegmen is typical for numerous extant genera, like Microcara (Fig. 4K).
Materials and methods
Material
The Kachin amber specimen studied here was derived from amber mines near Noije Bum (26°20' N, 96°36′ E), Hukawng Valley, Kachin State, northern Myanmar. Jewellery-grade Kachin amber specimens are commonly carried and sold legally in Ruili, Dehong Prefecture on the border between China and Myanmar. The specimen in this study (NIGP177336) was purchased by C.-Y.C. and D.-Y.H in late 2016 from a Myanmar amber dealer (field number: HUANG-HP-B-3245), and is permanently deposited in the Nanjing Institute of Geology and Palaeontology (NIGP), Chinese Academy of Sciences, Nanjing, China. The amber piece was trimmed with a small table saw, ground with emery papers of different grit sizes, and finally polished with polishing powder.
Imaging
Photographs under incident light were mainly taken with a Zeiss Discovery V20 stereo microscope. Widefield fluorescence images were captured with a Zeiss Axio Imager 2 light microscope combined with a fluorescence imaging system. Confocal images were obtained with a Zeiss LSM710 confocal laser scanning microscope, using the 488 nm Argon laser excitation line. Images under incident light and widefield fluorescence were stacked in Helicon Focus 7.0.2 or Zerene Stacker 1.04. Confocal images were stacked in Helicon Focus 7.0.2. Microtomographic data were obtained with a Zeiss Xradia 520 Versa 3D X-ray microscope at the NIGP micro-CT laboratory and analysed in VGStudio MAX 3.0. Scanning parameters were as follows: isotropic voxel size, 16.916 μm; power, 4 W; acceleration voltage, 50 kV; exposure time, 2 s; projections, 2001. Uncoated specimens of selected species of extant Scirtidae were studied with a S-3400 N Hitachi scanning electron microscope. Images were further processed in Adobe Photoshop CC to adjust brightness and contrast.
Cladistic analysis
To evaluate the systematic placement of Varcalium, we conducted a morphology-based phylogenetic analysis. The data matrix (Supplementary Data S1, S2) was derived from a previously published dataset of Lawrence & Yoshitomi81. The genera Nycteus Latreille (Eucinetidae) and Declinia (Decliniidae, the sister group of Scirtidae) were selected as the outgroup. Since Macrocyphon spencei Armstrong, which the coding for the genus Macrocyphon Pic was based on, has been transferred to Daploeuros20,82, and Contacyphon has been pointed out to be the valid name for Cyphon Paykull83, the names of these taxa were modified accordingly. The state 2 for character 3 in Lawrence & Yoshitomi81 was not present in any of the taxa coded, thus we merged the states 1 and 2. The problematic genus, Amplectopus Sharp, which had been placed Chelonariidae84, was excluded from the analysis. It shares some superficially similar but likely non-homologous features with Decliniidae81, and therefore might interfere the analysis.
Parsimony analysis was performed under implied weights using the program TNT 1.585,86. All characters were treated as non-additive. Parsimony analysis has been shown to achieve the highest accuracy under a moderate weighting scheme (e.g., when concavity constants K are between 5 and 20)87,88. Therefore, the concavity constant was set to 12 here, as suggested by Goloboff et al.87. Most parameters were set as default in the “new technology search”, while the value for “find min. length” was changed from 1 to 500. A strict consensus was calculated. The standard bootstrap analysis was implemented by 10,000 pseudoreplicates, where the support values were shown as absolute frequencies. Character states were mapped onto the tree with WinClada 1.00.08. The tree was graphically edited with Adobe Illustrator CC 2017.
Nomenclatural acts
This published work and the nomenclatural acts it contains have been registered in ZooBank. The LSID for this publication is url: lsid:zoobank.org:pub:A3E8EF76-2EF3-4081-9817-8352C2A824C0.
Supplementary Information
Acknowledgements
We are grateful to J.F. Lawrence for the invaluable comments on the morphology of Varcalium gen. nov. We thank Su-Ping Wu for technical help with micro-CT reconstruction, and Rong Huang for technical help with confocal imaging. Three reviewers provided helpful comments on the manuscript. Financial support was provided by the Second Tibetan Plateau Scientific Expedition and Research project (2019QZKK0706), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB26000000 and XDB18000000), the National Natural Science Foundation of China (41688103).
Author contributions
C.-Y.C., R.R. and Y.-D.L. conceived the study. C.-Y.C. processed the fossil. Y.-D.L, R.R. and Z.-H.L. acquired and processed the photomicrographs. Y.-D.L. and C.-Y.C. processed the micro-CT data. Y.-D.L. and R.R. conducted the phylogenetic analysis. Y.-D.L., R.R., C.-Y.C. and E.T. wrote the paper with contributions from the remaining authors.
Data availability
The original confocal and micro-CT data are available from the Zenodo repository (https://doi.org/10.5281/zenodo.6802063).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-16822-y.
References
- 1.Crowson R. The phylogeny of Coleoptera. Annu. Rev. Entomol. 1960;5:111–134. doi: 10.1146/annurev.en.05.010160.000551. [DOI] [Google Scholar]
- 2.Bouchard P, et al. Family-group names in Coleoptera (Insecta) ZooKeys. 2011;88:1–972. doi: 10.3897/zookeys.88.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang S-Q, et al. Evolutionary history of Coleoptera revealed by extensive sampling of genes and species. Nat. Commun. 2018;9:205. doi: 10.1038/s41467-017-02644-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKenna DD, et al. The evolution and genomic basis of beetle diversity. Proc. Natl. Acad. Sci. 2019;116:24729–24737. doi: 10.1073/pnas.1909655116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai C, et al. Integrated phylogenomics and fossil data illuminate the evolution of beetles. Royal Soc. Open Sci. 2022;9:211771. doi: 10.1098/rsos.211771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikitsky N, Lawrence J, Kirejtshuk A, Gratshev V. A new beetle family, Decliniidae fam. n., from Russian Far East and its taxonomic relationships (Coleoptera, Polyphaga) Russ. Entomol. J. 1994;2:3–10. [Google Scholar]
- 7.Sakai M, Satô M. The coleopteran family Decliniidae (Elateriformia, Scirtoidea) new to Japan, with description of its second representative. Elytra, Tokyo. 1996;24:103–111. [Google Scholar]
- 8.Yoshitomi H. New record of Declinia relicta (Coleoptera, Decliniidae) from Korea. Elytra, Tokyo. 2002;30:397–398. [Google Scholar]
- 9.Lawrence, J. F. Scirtidae Fleming, 1821. in Handbook of Zoology, Arthropoda: Insecta, Coleoptera, beetles, Vol. 1: morphology and systematics (Archostemata, Adephaga, Myxophaga, Polyphaga partim), 2nd edn (eds Beutel, R. G. & Leschen, R. A. B.) 215–225 (Walter de Gruyter, 2016).
- 10.Ruta R. Revision of Scirtidae (Insecta: Coleoptera) described by Victor Ivanovitsch Motschulsky. Zootaxa. 2009;2210:26–50. doi: 10.11646/zootaxa.2210.1.2. [DOI] [Google Scholar]
- 11.Ruta R. Chilarboreus gen. nov., a new genus of Chilean Scirtidae (Coleoptera: Scirtoidea), with descriptions of three new species. J. Nat. Hist. 2011;45:1689–1713. doi: 10.1080/00222933.2011.559691. [DOI] [Google Scholar]
- 12.Ruta R. Revision of African Mescirtes Motschulsky, 1863 (Coleoptera: Scirtidae) Afr. Entomol. 2014;22:180–190. doi: 10.4001/003.022.0129. [DOI] [Google Scholar]
- 13.Ruta R. Calvariopsis gen. nov., a new genus of Neotropical Scirtidae (Coleoptera: Scirtoidea) Zootaxa. 2019;4604:1–41. doi: 10.11646/zootaxa.4604.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Ruta R. An overview of Scirtidae (Coleoptera) described by Antoine Joseph Jean Solier (1792–1851) Zootaxa. 2020;4767:563–577. doi: 10.11646/zootaxa.4767.4.6. [DOI] [PubMed] [Google Scholar]
- 15.Yoshitomi H. Scirtidae of the Oriental Region, Part 11. Notes on the Cyphon coarctatus species group (Coleoptera), with descriptions of new species. Jpn. J. Syst. Entomol. 2009;15:101–128. [Google Scholar]
- 16.Yoshitomi H. The Scirtes (Coleoptera: Scirtidae: Scirtinae) of Micronesia. Zootaxa. 2009;1974:1–16. [Google Scholar]
- 17.Yoshitomi H. Scirtidae of the oriental region, part 14. Eight new species of the genus Contacyphon from Malaysia. Jpn. J. Syst. Entomol. 2017;23:5–13. [Google Scholar]
- 18.Yoshitomi H. Oriental Hydrocyphon (Coleoptera: Scirtidae: Scirtinae): Seven new species from Indonesia, Thailand, Malaysia, and India. Psyche. 2012;2012:603875. [Google Scholar]
- 19.Watts C. Revision of Australian Prionocyphon Redtenbacher (Scirtidae: Coleoptera) Trans. R. Soc. S. Aust. 2010;134:53–88. [Google Scholar]
- 20.Watts C. Revision of Australian Scirtidae of the Genera Chameloscyphon gen. nov., Daploeuros gen. nov., Dasyscyphon gen. nov., Eurycyphon gen. nov., Macrodascillus Carter, Petrocyphon gen. nov. and Spaniosdascillus gen. nov. (Coleoptera) Trans. R. Soc. South Aust. 2011;135:66–110. [Google Scholar]
- 21.Zwick P. Some Scirtidae (Coleoptera) from Palawan (the Philippines), mainly from phytotelmata. Aquat. Insects. 2011;33:233–252. doi: 10.1080/01650424.2011.614257. [DOI] [Google Scholar]
- 22.Zwick P. Australian marsh beetles. 3. A restricted concept of genus Cyphon, Australian species of Cyphon s. str., and the new Australasian genus Nanocyphon (Coleoptera: Scirtidae) Genus. 2013;24:163–189. [Google Scholar]
- 23.Zwick P. Three new marsh beetles (Col.: Scirtidae) from New Guinea and Java. Linz. Biol. Beitr. 2015;47:1885–1895. [Google Scholar]
- 24.Zwick P. Australian marsh beetles (Coleoptera: Scirtidae). 9. The relations of Australasian Ypsiloncyphon species to their Asian congeners, additions, mainly to Petrocyphon and Prionocyphon, and a key to Australian genera of Scirtinae. Zootaxa. 2016;4085:151–198. doi: 10.11646/zootaxa.4085.2.1. [DOI] [PubMed] [Google Scholar]
- 25.Libonatti ML, Ruta R. Review of the Argentinean species of Pseudomicrocara Armstrong (Coleoptera: Scirtidae) Zootaxa. 2013;3718:137–157. doi: 10.11646/zootaxa.3718.2.3. [DOI] [PubMed] [Google Scholar]
- 26.Ruta R, Yoshitomi H, Klausnitzer B. Review of the genus Dermestocyphon (Coleoptera: Scirtidae: Scirtinae) Acta Entomol. Mus. Natl. Pragae. 2013;53:253–285. [Google Scholar]
- 27.Ruta R, Libonatti ML. Redescriptions of Scirtidae (Coleoptera: Scirtoidea) described by Carl Henrik Boheman (1796–1868) with notes on Scirtes adustus iversenotatus Pic, 1930. Zootaxa. 2016;4072:203–216. doi: 10.11646/zootaxa.4072.2.4. [DOI] [PubMed] [Google Scholar]
- 28.Kiałka A, Ruta R. An illustrated catalogue of the New Zealand marsh beetles (Coleoptera: Scirtidae) Zootaxa. 2017;4366:1–76. doi: 10.11646/zootaxa.4366.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Cooper SJ, Watts CH, Saint KM, Leijs R. Phylogenetic relationships of Australian Scirtidae (Coleoptera) based on mitochondrial and nuclear sequences. Invertebr. Syst. 2014;28:628–642. doi: 10.1071/IS13046. [DOI] [Google Scholar]
- 30.Watts CH, Cooper SJ, Saint KM. Review of Australian Scirtes Illiger, Ora Clark and Exochomoscirtes Pic Coleoptera: Scirtidae) including descriptions of new species, new groups and a multi-gene molecular phylogeny of Australian and non-Australian species. Zootaxa. 2017;4347:511–532. doi: 10.11646/zootaxa.4347.3.5. [DOI] [PubMed] [Google Scholar]
- 31.Watts C, Cooper S, Libonatti M. New genera, species and combinations in the Pseudomicrocara Armstrong group (Coleoptera: Scirtidae) based on morphology supported by mitochondrial and nuclear gene sequence data. Zootaxa. 2020;4831:1–66. doi: 10.11646/zootaxa.4831.1.1. [DOI] [PubMed] [Google Scholar]
- 32.Bradford TM, Ruta R, Cooper SJ, Libonatti ML, Watts CH. Evolutionary history of the Australasian Scirtinae (Scirtidae; Coleoptera) inferred from ultraconserved elements. Invertebr. Syst. 2022;36:291–305. doi: 10.1071/IS21053. [DOI] [Google Scholar]
- 33.Alekseev VI. The beetles (Insecta: Coleoptera) of Baltic amber: the checklist of described species and preliminary analysis of biodiversity. Zool. Ecol. 2013;23:5–12. doi: 10.1080/21658005.2013.769717. [DOI] [Google Scholar]
- 34.Klausnitzer B. Neue Arten der Familie Scirtidae (Coleoptera) aus Baltischem Bernstein (Teil 1) (106. Beitrag zur Kenntnis der Scirtidae) Entomol. Nachr. Ber. 2004;48:99–104. [Google Scholar]
- 35.Klausnitzer B. Neue Arten der Familie Scirtidae (Coleoptera) aus Baltischem Bernstein (Teil 3) (172. Beitrag zur Kenntnis der Scirtidae) Linz. Biol. Beitr. 2012;44:313–318. [Google Scholar]
- 36.Iablokoff-Khnzorian S. Predstawiteli semeystva Helodidae (Coleoptera) iz baltiyskogo yantarya. Paleontol. Zhurn. 1961;1961:109–116. [Google Scholar]
- 37.Kirejtshuk AG, Nel A. New beetles of the suborder Polyphaga from the Lowermost Eocene French amber (Insecta: Coleoptera) Ann. Soc. Entomol. Fr. 2008;44:419–442. doi: 10.1080/00379271.2008.10697578. [DOI] [Google Scholar]
- 38.Kirejtshuk AG, Azar D. New taxa of beetles (Insecta, Coleoptera) from Lebanese amber with evolutionary and systematic comments. Alavesia. 2008;2:15–46. [Google Scholar]
- 39.Jell PA, Duncan PM. Invertebrates, mainly insects, from the freshwater, lower Cretaceous, Koonwarra fossil bed (Korumburra Group), south Gippsland, Victoria. Mem. Assoc. Australas. Palaeontol. 1986;3:111–205. [Google Scholar]
- 40.Ross AJ. Burmese (Myanmar) amber checklist and bibliography 2018. Palaeoentomology. 2019;2:22–84. doi: 10.11646/palaeoentomology.2.1.5. [DOI] [Google Scholar]
- 41.Ross AJ. Supplement to the Burmese (Myanmar) amber checklist and bibliography, 2019. Palaeoentomology. 2020;3:103–118. doi: 10.11646/palaeoentomology.3.1.14. [DOI] [Google Scholar]
- 42.Ross AJ. Supplement to the Burmese (Myanmar) amber checklist and bibliography, 2020. Palaeoentomology. 2021;4:57–76. doi: 10.11646/palaeoentomology.4.1.11. [DOI] [Google Scholar]
- 43.Engel MS. A primitive anobiid beetle in mid-Cretaceous amber from Myanmar (Coleoptera: Anobiidae) Alavesia. 2010;3:31–34. [Google Scholar]
- 44.Peris D, Philips TK, Delclòs X. Ptinid beetles from the Cretaceous gymnosperm-dominated forests. Cretac. Res. 2015;52:440–452. doi: 10.1016/j.cretres.2014.02.009. [DOI] [Google Scholar]
- 45.Shi G, et al. Age constraint on Burmese amber based on U-Pb dating of zircons. Cretac. Res. 2012;37:155–163. doi: 10.1016/j.cretres.2012.03.014. [DOI] [Google Scholar]
- 46.Mao Y, et al. Various amberground marine animals on Burmese amber with discussions on its age. Palaeoentomology. 2018;1:91–103. doi: 10.11646/palaeoentomology.1.1.11. [DOI] [Google Scholar]
- 47.Lawrence J, Nikitsky N, Kirejtshuk A. Phylogenetic position of Decliniidae and comments on the classification of Elateriformia (sensu lato) In: Pakaluk J, Ślipiński SA, editors. Biology, Phylogeny, and Classification of Coleoptera. Papers Celebrating the 80th Birthday of Roy A. Crowson. Muzeum i Instytut Zoologii Polska Akademia Nauk; 1995. pp. 375–410. [Google Scholar]
- 48.Ruta R. Anticyphon gen. nov., a new genus of Scirtidae (Coleoptera: Scirtoidea) inhabiting high altitude Andean cloud forests and páramo formation. Zootaxa. 2016;4175:301–318. doi: 10.11646/zootaxa.4175.4.1. [DOI] [PubMed] [Google Scholar]
- 49.Ruta R. Revision of the genus Calvarium Pic, 1918 (Coleoptera: Scirtidae). Part 1. Redescription of the genus and catalogue of described taxa. Ann. Zool. 2010;60:341–350. doi: 10.3161/000345410X535343. [DOI] [Google Scholar]
- 50.Zwick P. Australian marsh beetles (Coleoptera: Scirtidae). 2. Pachycyphon, a new genus of presumably terrestrial Australian Scirtidae. Zootaxa. 2013;3626:326–344. doi: 10.11646/zootaxa.3626.3.2. [DOI] [PubMed] [Google Scholar]
- 51.Kiałka A, Ruta R. Meatopida gen. nov., a new genus to accommodate two species originally described in Atopida White, 1846 (Coleoptera: Scirtoidea: Scirtidae) Zootaxa. 2018;4382:242–260. doi: 10.11646/zootaxa.4382.2.2. [DOI] [PubMed] [Google Scholar]
- 52.Ruta R. Three new genera of large marsh beetles (Coleoptera: Scirtidae) from Valdivian temperate rain forests of southern South America. Zootaxa. 2021;5048:451–485. doi: 10.11646/zootaxa.5048.4.1. [DOI] [PubMed] [Google Scholar]
- 53.Libonatti ML. Notes on Some South American Species of Scirtes Illiger, 1807 (Coleoptera: Scirtidae) Ann. Zool. 2017;67:349–368. doi: 10.3161/00034541ANZ2017.67.2.012. [DOI] [Google Scholar]
- 54.Watts C, Bradford T, Cooper S. A new genus, Perplexacara, and new generic placements of species of Australian marsh beetles (Coleoptera: Scirtidae) based on morphology and molecular genetic data. Zootaxa. 2021;4927:539–548. doi: 10.11646/zootaxa.4927.4.4. [DOI] [PubMed] [Google Scholar]
- 55.Lawrence JF. New species of Eucinetus and Noteucinetus from Australia (Coleoptera: Scirtoidea: Eucinetidae) Zootaxa. 2019;4668:151–182. doi: 10.11646/zootaxa.4668.2.1. [DOI] [PubMed] [Google Scholar]
- 56.Poinar GO, Jr, Hess R. Ultrastructure of 40-million-year-old insect tissue. Science. 1982;215:1241–1242. doi: 10.1126/science.215.4537.1241. [DOI] [PubMed] [Google Scholar]
- 57.Poinar GO, Jr, Hess R. Preservative qualities of recent and fossil resins: Electron micrograph studies on tissue preserved in Baltic amber. J. Balt. Stud. 1985;16:222–230. doi: 10.1080/01629778500000141. [DOI] [Google Scholar]
- 58.Grimaldi D, Bonwich E, Delannoy M, Doberstein S. Electron microscopic studies of mummified tissues in amber fossils. Am. Mus. Novit. 1994;3097:1–31. [Google Scholar]
- 59.Koller B, Schmitt JM, Tischendorf G. Cellular fine structures and histochemical reactions in the tissue of a cypress twig preserved in Baltic amber. Proc. R. Soc. B: Biol. Sci. 2005;272:121–126. doi: 10.1098/rspb.2004.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azar D. A new method for extracting plant and insect fossils from Lebanese amber. Palaeontology. 1997;40:1027–1029. [Google Scholar]
- 61.Nascimbene P, Silverstein H. The preparation of fragile Cretaceous ambers for conservation and study of organismal inclusions. In: Grimaldi D, editor. Studies on Fossils in Amber, with Particular Reference to the Cretaceous of New Jersey. Backhuys Publishers; 2000. pp. 93–102. [Google Scholar]
- 62.Perreau M, Tafforeau P. Virtual dissection using phase-contrast X-ray synchrotron microtomography: Reducing the gap between fossils and extant species. Syst. Entomol. 2011;36:573–580. doi: 10.1111/j.1365-3113.2011.00573.x. [DOI] [Google Scholar]
- 63.Van de Kamp T, Dos Santos Rolo T, Baumbach T, Krogmann L. Scanning the past–synchrotron X-ray microtomography of fossil wasps in amber. Entomol. heute. 2014;26:151–160. [Google Scholar]
- 64.Grimaldi DA, Peñalver E, Barrón E, Herhold HW, Engel MS. Direct evidence for eudicot pollen-feeding in a Cretaceous stinging wasp (Angiospermae; Hymenoptera, Aculeata) preserved in Burmese amber. Commun. Biol. 2019;2:408. doi: 10.1038/s42003-019-0652-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y-D, Yamamoto S, Huang D-Y, Cai C-Y. New species of Paraodontomma from mid-Cretaceous Burmese amber with muscle tissue preservation (Coleoptera: Archostemata: Ommatidae) Pap. Avulsos Zool. 2021;61:e20216153. doi: 10.11606/1807-0205/2021.61.53. [DOI] [Google Scholar]
- 66.Pohl H, Wipfler B, Grimaldi D, Beckmann F, Beutel RG. Reconstructing the anatomy of the 42-million-year-old fossil †Mengea tertiaria (Insecta, Strepsiptera) Naturwissenschaften. 2010;97:855–859. doi: 10.1007/s00114-010-0703-x. [DOI] [PubMed] [Google Scholar]
- 67.Perreau M. Description of a new genus and two new species of Leiodidae (Coleoptera) from Baltic amber using phase contrast synchrotron X-ray microtomography. Zootaxa. 2012;3455:81–88. doi: 10.11646/zootaxa.3455.1.5. [DOI] [Google Scholar]
- 68.Perreau M, Perkovsky EE. Further description of Catops nathani Perkovsky 2001 from Late Eocene Baltic amber (Coleoptera: Leiodidae: Cholevinae: Cholevini) using phase contrast synchrotron X-ray microtomography. Ann. Soc. Entomol. Fr. 2014;50:414–417. doi: 10.1080/00379271.2014.984957. [DOI] [Google Scholar]
- 69.Schmidt J, Belousov I, Michalik P. X-ray microscopy reveals endophallic structures in a new species of the ground beetle genus Trechus Clairville, 1806 from Baltic amber (Coleoptera, Carabidae, Trechini) ZooKeys. 2016;614:113–127. doi: 10.3897/zookeys.614.9283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmidt J, Goepel T, Will K. Description of the first flightless platynine ground beetle preserved in Baltic amber (Coleoptera: Carabidae) Zootaxa. 2017;4318:110–122. doi: 10.11646/zootaxa.4318.1.4. [DOI] [Google Scholar]
- 71.Schmidt J, Michalik P. The ground beetle genus Bembidion Latreille in Baltic amber: Review of preserved specimens and first 3D reconstruction of endophallic structures using X-ray microscopy (Coleoptera, Carabidae, Bembidiini) ZooKeys. 2017;662:101–126. doi: 10.3897/zookeys.662.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmidt J, Scholz S, Kavanaugh DH. Unexpected findings in the Eocene Baltic amber forests: Ground beetle fossils of the tribe Nebriini (Coleoptera: Carabidae) Zootaxa. 2019;4701:350–370. doi: 10.11646/zootaxa.4701.4.2. [DOI] [PubMed] [Google Scholar]
- 73.Reike H-P, Bukejs A, Arlt T, Kardjilov N, Manke I. Phase-contrast synchrotron microtomography reveals the internal morphology of a new fossil species of the Corticaria-sylvicola-group (Coleoptera: Latridiidae) Zootaxa. 2017;4242:578–590. doi: 10.11646/zootaxa.4242.3.9. [DOI] [PubMed] [Google Scholar]
- 74.Reike H-P, Alekseev VI, Gröhn C, Arlt T, Manke I. First extinct species of the genus Holoparamecus (Coleoptera: Merophysiidae: Holoparamecinae) from Eocene amber deposits. Stud. Rep. Taxon. Ser. 2020;16:241–255. [Google Scholar]
- 75.Alekseev VI, Kupryjanowicz J, Kairišs K, Bukejs A. The first described fossil species of Litargus Erichson (Coleoptera: Mycetophagidae) from Eocene Baltic amber examined with X-ray microtomography, and new records of Crowsonium succinium Abdullah, 1964. Zootaxa. 2020;4768:405–414. doi: 10.11646/zootaxa.4768.3.7. [DOI] [PubMed] [Google Scholar]
- 76.Nabozhenko MV, Kairišs K, Bukejs A. The oldest fossil darkling beetle of the genus Neomida Latreille, 1829 (Coleoptera: Tenebrionidae) from Eocene Baltic amber examined with X-ray microtomography. Zootaxa. 2020;4768:435–442. doi: 10.11646/zootaxa.4768.3.10. [DOI] [PubMed] [Google Scholar]
- 77.Bukejs A, Bezděk J, Alekseev VI, Kairišs K, McKellar RC. Description of the male of fossil Calomicrus eocenicus Bukejs et Bezděk (Coleoptera: Chrysomelidae: Galerucinae) from Eocene Baltic amber using X-ray microtomography. Foss. Rec. 2020;23:105–115. doi: 10.5194/fr-23-105-2020. [DOI] [Google Scholar]
- 78.Nyholm T. Uber Bau und Funktion der Kopulationsorgane bei den Cyphones (Col. Helodidae). studien uber die Familie Helodidae. X. Entomol. Tidskr. 1969;90:233–271. [Google Scholar]
- 79.Nyholm T. Zur Morphologie und Funktion des Helodiden-Aedoeagus (Col.) Entomol. Scand. 1972;3:81–119. doi: 10.1163/187631272X00193. [DOI] [Google Scholar]
- 80.Zwick P. To the knowledge of Sarabandus robustus (LeConte)(Col.: Scirtidae: Scirtinae), and on the groundplan of male marsh beetle genitalia. Linz. Biol. Beitr. 2015;47:1439–1449. [Google Scholar]
- 81.Lawrence JF, Yoshitomi H. Nipponocyphon, a new genus of Japanese Scirtidae (Coleoptera) and its phylogenetic significance. Elytra, Tokyo. 2007;35:507–527. [Google Scholar]
- 82.Ruta R. A new species of Daploeuros Watts (Coleoptera: Scirtidae) Zootaxa. 2020;4728:334–340. doi: 10.11646/zootaxa.4728.3.2. [DOI] [PubMed] [Google Scholar]
- 83.Zwick P, Klausnitzer B, Ruta R. Contacyphon Gozis, 1886 removed from synonymy (Coleoptera: Scirtidae) to accommodate species so far combined with the invalid name, Cyphon Paykull, 1799. Entomol. Bl. Col. 2013;109:337–353. [Google Scholar]
- 84.Kasap H, Crowson R. A comparative anatomical study of Elateriformia and Dascilloidea (Coleoptera) Trans. R. Entomol. Soc London. 1975;126:441–495. doi: 10.1111/j.1365-2311.1975.tb00858.x. [DOI] [Google Scholar]
- 85.Goloboff PA, Catalano SA. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics. 2016;32:221–238. doi: 10.1111/cla.12160. [DOI] [PubMed] [Google Scholar]
- 86.Goloboff PA, Farris JS, Nixon KC. TNT, a free program for phylogenetic analysis. Cladistics. 2008;24:774–786. doi: 10.1111/j.1096-0031.2008.00217.x. [DOI] [Google Scholar]
- 87.Goloboff PA, Torres A, Arias JS. Weighted parsimony outperforms other methods of phylogenetic inference under models appropriate for morphology. Cladistics. 2018;34:407–437. doi: 10.1111/cla.12205. [DOI] [PubMed] [Google Scholar]
- 88.Smith MR. Bayesian and parsimony approaches reconstruct informative trees from simulated morphological datasets. Biol. Lett. 2019;15:20180632. doi: 10.1098/rsbl.2018.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original confocal and micro-CT data are available from the Zenodo repository (https://doi.org/10.5281/zenodo.6802063).