Abstract
The current study aimed to figure out the effect of using a combination of 2% inulin, and 2% Fructo-oligosaccharides (FOS) with Lactobacillus acidophilus and their bacteriocin on some yogurt properties such as coagulation time, extending the shelf life of set yogurt and its microbiological quality, also the acceptance by consumers. The results indicated that coagulation time increased by 22.75% in yogurts prepared with Lactobacillus acidophilus and their bacteriocins compared to the control, and titratable acidity increased gradually in all treatments during storage. Hence control acidity (%) increased from 0.84 ± 0.02A at zero time to 1.23 ± 0.03A after 14 days of cold storage, while treatment (T4) was 0.72 ± 0.01C at zero time and reached 1.20 ± 0.5A after 39 days at the same conditions. The sensory properties showed the superiority of inulin, FOS, and Lactobacillus acidophilus bacteriocin groups. Lactobacillus bulgaricus, Streptococcus thermophiles, and Lactobacillus acidophilus count increased in the treatments compared to the control group, with an extended shelf life to 39 days of storage in the medicines containing lactobacillus acidophilus bacteriocin. Coliforms, Moulds, and yeasts did not detect in the treatments comprising 2% inulin, 2% FOS, and lactobacillus acidophilus bacteriocin for 39 days of refrigerated storage. This study proved that 2% inulin, 2% FOS, and Lactobacillus acidophilus bacteriocin fortification extended the shelf life by more than 5 weeks.
Subject terms: Health care, Microbiology, Antimicrobials, Bacteria, Industrial microbiology
Introduction
One of the most common commercial functional foods is fermented dairy products with probiotics, like milk drinks, yogurt, and cheese1. Yogurt is widely consumed for its therapeutically, nutritional, and sensory properties2, and it is considered the most usual vehicle for delivering functional ingredients3. Yogurt is obtained by fermentation whole, skimmed, or standardized milk through the action of Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus, which can be accompanied by other lactic acid bacteria that confer specific characteristics to the final product4. Probiotics have been defined as live microorganisms that, when administered adequately, consult a health benefit to the host5. Selecting a suitable base product for delivering probiotics is a crucial step toward developing probiotic foods6. Fermented milk has been used for a long time as the primary vehicle for probiotic strains.
Prebiotics were classified as selectively utilized substrates by host microorganisms conferring a health benefit7. Inulin and Fructooligosaccharides (FOS) are the most studied and well-established prebiotics. Inulin consists of a linear chain of fructose, constituted by a monomeric unit of fructose linked by β-(2, 1) glycoside bonds, with a terminal glucose unit linked by an-(1, 2) glycoside bond with a degree of polymerization (DP) more than 10. Fructooligosaccharide (FOS) is a fructose oligosaccharide linked by a β-(2, 1) glycosidic bond and terminated by a glucose molecule linked by α-(1, 2) glycosidic bond to fructose with DP < 108. Prebiotic components can also supplement dairy products, such as cow milk yogurts9,10, resulting in improvements in the quality parameters of the products. Inulin has been described as improving dairy products’ texture11. Furthermore, other Fructo-oligosaccharides are fibers that have been employed together with probiotics in dairy products. Additionally, inulin-type fructans in yogurt may assist customers in consuming the recommended 25 g of dietary fiber daily from the European Food Safety Authority (EFSA). Additionally, consuming inulin has been linked to several health advantages, including a decreased risk of developing diabetes and obesity because of its impact on appetite and caloric intake through many mechanisms, including the synthesis of short-chain fatty acids (SCFA) due to colonic fermentation and the subsequent regulation of gut hormones12. Prebiotic components can also increase probiotic survival in probiotic dairy products, resulting in synbiotic outcomes10. The viability of bacteria is a vital characteristic of using probiotics in dairy products once they survive during the shelf life with minimum viable cells of 106–107 CFU mL−1 to provide health benefits to the host13. Control of pathogenic and spoilage microbes in various foods is crucial to ensuring the quality and safety of food. Bio-preservation has recently gained attention as a subject14; there is an urgent demand for a natural and safe method to boost the shelf life of fresh milk and dairy products; this technique is used to grow food shelf-life by applying a protective microbiota. The main probiotic bacteria that are commercially accessible are lactic acid bacteria (LAB) (Lactobacilli, Streptococci, Lactococci, and Bifidobacteria)15. They generate several metabolites, including organic acids, hydrogen peroxide, diacetyl, antifungal compounds, phenyl lactic acid, and bacteriocins, which guard the products against diseases and bacteria that can cause rotting16–18. In contrast to chemical preservatives, bacteriocins, produced by lactic acid bacteria (LAB), are widely used as safe additive preservatives, extending the shelf-life of food owing to their ability to inhibit a wide variety of pathogens19. Bacteriocins are antimicrobial peptides synthesized by lactic acid bacteria, extending the shelf-life and safety of food items20. Many species of the Lactobacillus genus are used to develop dairy products21. Lactobacillus acidophilus is a homofermentative, microaerophilic, short-chain, gram-positive bacteria with a rod morphology known as an effective bacteriocin-producing strain. Among the main products containing Lactobacillus acidophilus, La-5 are yogurt22 Ice cream23, cheese24, and dairy desserts25. The main objectives of this study were to evaluate the microbiological quality, viability of probiotic starter, physicochemical and sensory parameters of different yogurt formulations made using (inulin and FOS), and incorporation with lactobacillus acidophilus and its bacteriocin during 39-day refrigerated storage.
Materials and methods
Materials
Fresh raw cow and buffalo milk were obtained from the dairy selling outlet of the Faculty of Veterinary Medicine, Menofia University, Egypt, without any interaction with the herd. Lyophilized strains of Lactobacillus acidophilus DSMZ 20079, Lactobacillus bulgaricus (Lb. bulgaricus), and Streptococcus thermophilus were obtained from Cairo-MIRCEN (Microbiological Resource Center) Faculty of Agriculture, Ain Shams University, Cairo, Egypt. MRS broth (De Man, Rogosa, and Sharp) was obtained from Biolife, Italy. Inulin was obtained from Orafti®GR. Fructo-oligosaccharides (FOS) (Orafti®P95) were obtained from Belgium.
Methods
Activation of lactobacillus strain and bacteriocin preparation
Lactobacillus (Lb) acidophilus DSMZ 20079 lyophilized pack was dissolved in MRS broth (de Man, Rogosa, and Sharp) at 37 °C for 24 h26 for activation. The activated culture was inoculated on MRS broth (1 L) aseptically and incubated at 37 °C for16 hrs. Then the culture was boiled in a water bath (WiseBath, Labortechnik, Germany) at 100 °C for 5 min to remove hydrogen peroxide. The cells were harvested by centrifugation at 10,000 rpm (Pro-Centrifuge, Centurion Science Limited, UK) for 20 min at 4 °C twice. The supernatants were collected, and the pH (Martini, Italy) was neutralized to (7) by (1 N) NaOH. The extract was then sterilized using a syringe filter of 0.45 µm pore size to obtain cell-free crude bacteriocin27.
Yogurt manufacture
Conventional lyophilized yogurt starter containing mixed cultures of Lactobacillus bulgaricus (Lb. bulgaricus) and Streptococcus thermophilus (1:1) were activated in sterile reconstituted skimmed milk powder (11%), and incubated at 37 °C for 24 h., then kept refrigerated to be used within in 24 h. Ten kilograms of skimmed milk were obtained by separating the milk fat using a separator and divided into two liters for five treatments; control, two probiotics, and two bacteriocins yogurt treatments supplemented with 2% inulin + 2% FOS, then mixed well28,29. The mixture was pasteurized at 85 °C for 30 min and immediately cooled to 45 °C, then inoculated with activated starter cultures (Lb. bulgaricus and Streptococcus thermophilus) then divided into the following treatments: [C: 2% yogurt starter cultures (Control)]; [T1: 1% yogurt starter cultures + 1% Lb. acidophilus]; [T2: 1% yogurt starter cultures + 1% Lb. acidophilus + 2% inulin + 2% FOS]; [T3: 1% yogurt starter cultures + 1% Lb. acidophilus bacteriocin]; [T4: 1% yogurt starter cultures + 1% Lb. acidophilus bacteriocin + 2% inulin + 2% FOS]. All treatments were mixed well and poured into sterile cups (100 ml), incubated at 42 °C until pH 4.6, then refrigerated at 4 °C.
Yogurt analysis
Coagulation time and Titratable acidity determination
The coagulation time for each yogurt group was calculated from the incubation's starting time until the curd's formation30. Titratable acidity (as % of lactic acid) was determined using the method described in31. The yogurt preparation and examination were repeated three times.
Microbiological analysis
Serial dilution was made to obtain the bacterial count32. Lb. Acidophilus, Lb. bulgaricus, and Streptococcus thermophiles were enumerated using the pouring plate method33. Enumerations of Lb. Acidophilus was carried out in MRS agar under the anaerobic condition at 37 °C for 48 h. Lb. bulgaricus was counted in MRS agar (pH 5.4) under aerobic incubation at 37 °C for 48 h., and Streptococcus thermophilus was counted on M17 agar at 42 °C for 48 h34. Sabouraud Dextrose agar medium supplemented with chloramphenicol (0.01%) was used to determine the total mold and yeast count after incubation at 25 °C for 5–7 days35.
Sensory analysis
The sensory evaluation of the yogurt samples was carried out according to36. The scores used were 60 points for flavor, 30 points for body and texture, and 10 points for appearance, with an overall score of 100.
Statistical analysis
Statistical analyses were performed using the one-way analysis of variance in SPSS 16.0. The results were considered significantly different, with p < 0.05 as37 described.
Results and discussion
Coagulation time and titratable acidity
Coagulation of milk results from the precipitation of milk protein (casein) in acidic conditions at a pH of around 4.638. Table 1 shows that the treatment (T4) was prepared with 1% yogurt starter cultures 1:1 + 1% Lb. acidophilus bacteriocin + 2% inulin + 2% FOS has the longest coagulation time (4 h:10 min) with increasing percentage by 22.75% followed by treatment (T3) prepared by 1% yoghurt starter cultures 1:1 + 1% Lb. acidophilus bacteriocin (4 h.: 5 min) increasing percentage by 21.25%, than treatment (T2) prepared by 1% yoghurt starter cultures 1:1 + 1% Lb. acidophilus + 2% inulin + 2% FOS (3 h:50 min) increasing percentage by 4.79%. The lowest coagulation time was recorded for treatment (T1) which was prepared by 1% yogurt starter cultures 1:1 + 1% Lb. Acidophilus (3 h.:45 min) with an increasing percentage of 3.29% compared to the control treatment (coagulation time of control was 3.29). The variation in the coagulation time may be attributed to the antibacterial activity of probiotic bacteria and prebiotics (inulin and FOS) on the starter culture of yogurt and subsequent acid production39.
Table 1.
Evaluation of the different yogurt treatments during cold storage: coagulation time, acidity, viability of starter bacteria, Lactobacillus acidophilus, and occurrence of yeast and molds.
| Parameters | Days | C | T1 | T2 | T3 | T4 | Significance |
|---|---|---|---|---|---|---|---|
| Coagulation time per (hrs.: min) | Zero | 3.34 | 3.45 | 3.50 | 4.05 | 4.10 | |
| Increase (%) | 0.00 | 3.29 | 4.79 | 21.25 | 22.75 | ||
| Acidity as lactic acid (%) | Zero | 0.84 ± 0.02A | 0.80 ± 0.04A | 0.79 ± 0.01B | 0.75 ± 0.03B | 0.72 ± 0.01C | * |
| 7 | 0.95 ± 0.03A | 0.97 ± 0.03A | 0.95 ± 0.03A | 0.83 ± 0.02B | 0.79 ± 0.01C | * | |
| 14 | 1.23 ± 0.03A | 1.10 ± 0.02B | 1.06 ± 0.05BC | 0.90 ± 0.01C | 0.85 ± 0.00D | * | |
| 21 | S | 1.16 ± 0.03A | 1.14 ± 0.02A | 1.06 ± 0.03B | 0.94 ± 0.02C | * | |
| 28 | S | S | 1.22 ± 0.03A | 1.17 ± 0.03B | 1.08 ± 0.02C | * | |
| 32 | S | S | 1.34 ± 0.03A | 1.26 ± 0.05B | 1.18 ± 0.3C | * | |
| 39 | S | S | S | S | 1.20 ± 0.5A | ||
| Lactobacillus delbrueckii sub spp. bulgaricus (log 10 cfu/g) | Zero | 12.75 ± 0.15A | 12.75 ± 0.03A | 12.65 ± 0.05B | 12.73 ± 0.07A | 12.71 ± 0.02A | ns |
| 7 | 10.58 ± 0.04D | 11.35 ± 0.01C | 12.33 ± 0.05B | 12.15 ± 0.11B | 12.47 ± 0.09A | * | |
| 14 | 9.00 ± 0.02D | 10.40 ± 0.02D | 11.86 ± 0.1C | 11.53 ± 0.11B | 12.31 ± 0.03A | * | |
| 21 | S | 9.22 ± 0.03D | 11.13 ± 0.09B | 10.75 ± 0.06C | 12.14 ± 0.05A | * | |
| 28 | S | S | 10.35 ± 0.04B | 10.52 ± 0.04B | 11.24 ± 0.05A | * | |
| 32 | S | S | 9.27 ± 0.12C | 10.12 ± 0.03B | 10.53 ± 0.11A | * | |
| 39 | S | S | S | S | 9.52 ± 0.04A | ||
| Streptococcus thermophilus (log 10 cfu/g) | Zero | 9.77 ± 0.05A | 9.54 ± 0.03A | 9.76 ± 0.03A | 9.62 ± 0.01A | 9.73 ± 0.02A | ns |
| 7 | 8.50 ± 0.05C | 9.11 ± 0.03AB | 9.24 ± 0.01B | 9.35 ± 0.02B | 9.55 ± 0.01A | * | |
| 14 | 7.16 ± 0.06D | 8.16 ± 0.03C | 9.01 ± 0.01A | 8.43 ± 0.01B | 9.00 ± 0.01A | * | |
| 21 | S | 7.24 ± 0.05C | 8.87 ± 0.03A | 8.01 ± 0.03B | 8.73 ± 0.03A | * | |
| 28 | S | S | 7.98 ± 0.03B | 7.68 ± 0.04C | 8.15 ± 0.04A | * | |
| 32 | S | S | 7.65 ± 0.04B | 6.63 ± 0.04C | 7.78 ± 0.04A | * | |
| 39 | S | S | S | S | 7.72 ± 0.04A | ||
| Lactobacillus acidophilus (log 10 cfu/g) | Zero | 10.49 ± 0.11A | 11.65 ± 0.08A | ns | |||
| 7 | 9.68 ± 0.06B | 11.18 ± 0.06AB | * | ||||
| 14 | 8.43 ± 0.06C | 9.43 ± 0.18B | * | ||||
| 21 | 7.54 ± 0.26D | 8.84 ± 0.26C | * | ||||
| 28 | S | 7.52 ± 0.26D | |||||
| 32 | S | 7.32 ± 0.26D | |||||
| 39 | S | S | |||||
| Yeast and molds | Zero | ND | ND | ND | ND | ND | |
| 7 | 2.15 ± 0.01B | ND | ND | ND | ND | ||
| 14 | 3.64 ± 0.04 A | 1.00 ± 0.01 B | ND | ND | ND | * | |
| 21 | S | 2.36 ± 0.03 A | 1.15 ± 0.02C | 1.00 ± 0.05 C | ND | * | |
| 28 | S | S | 1.87 ± 0.01B | 1.35 ± 0.05 B | 1.00 ± 0.03 C | * | |
| 32 | S | S | 2.16 ± 0.03A | 2.14 ± 0.06 A | 1.85 ± 0.01B | * | |
| 39 | S | S | S | S | 2.01 ± 0.05 C |
C: 2% yogurt starter cultures 1:1 (control).
T1: 1% yogurt starter cultures 1:1 + 1% Lb. acidophilus.
T2: 1% yogurt starter cultures 1:1 + 1% Lb. acidophilus + 2% inulin + 2% FOS.
T3: 1% yogurt starter cultures 1:1 + 1% Lb. acidophilus bacteriocin.
T4: 1% yogurt starter cultures 1:1 + 1% Lb. acidophilus bacteriocin + 2% inulin + 2% FOS.
*The values indicated are the mean ± S.E (n = 3). Values in the same raw denoted by different letters (ABCD) differ significantly (p < 0.05) from each other.
S spoilage, ND not detected, ns non-significant.
During refrigerated storage (4 °C), the titratable acidity of yogurt samples in all treatment groups increased gradually as the storage period progressed (Table 1). The addition of inulin and (FOS) insignificantly affected the titratable acidity of yogurt samples. On the other hand, adding inulin and FOS increased the shelf life to 39 days in treatment T 4, which was prepared by 1% yogurt starter cultures 1:1 + 1% Lb. acidophilus bacteriocin + 2% inulin + 2% FOS compared to control treatment (C) which spoiled at 21 day of refrigerated storage. Treatment T2 prepared by 1% yoghurt starter cultures 1:1 + 1% Lb. acidophilus + 2% inulin + 2% FOS and T3: 1% yogurt starter cultures 1:1 + 1% Lb. acidophilus bacteriocin spoiled at 32 days of refrigerated storage. While treatment T1: 1% yoghurt starter cultures 1:1 + 1% Lb. acidophilus was spoiled at 28 days of refrigerated storage. EOSQ40 stated that the yogurt’s titratable acidity did not increase by 1.50%. Guven et al.41 found that yogurt's titratable acidity was not affected by the addition of different ratios of inulin, and it was increased during storage.
Sensory evaluation
Adding inulin and FOS improved the sensory properties of the resultant yogurt samples. The result revealed that treatment (T2), 1% yogurt starter cultures 1:1 + 1% Lb. acidophilus + 2% inulin + 2% FOS and treatment (T4), 1% yoghurt starter cultures 1:1 + 1% Lb. acidophilus bacteriocin + 2% inulin + 2% FOS) were the highest accepted treatments regarding to sensory evaluation (Figs. 1, 2 and 3). Our results are in line with42, who reported that adding prebiotics could improve the physical and sensory properties of the yogurt. On the other hand, Cruz et al.43 explored that adding inulin may cause changes in yogurt quality attributes due to interactions between the functional ingredient and food matrix components. Inulin may provide yogurt mouth feel and sweet taste44.
Figure 1.
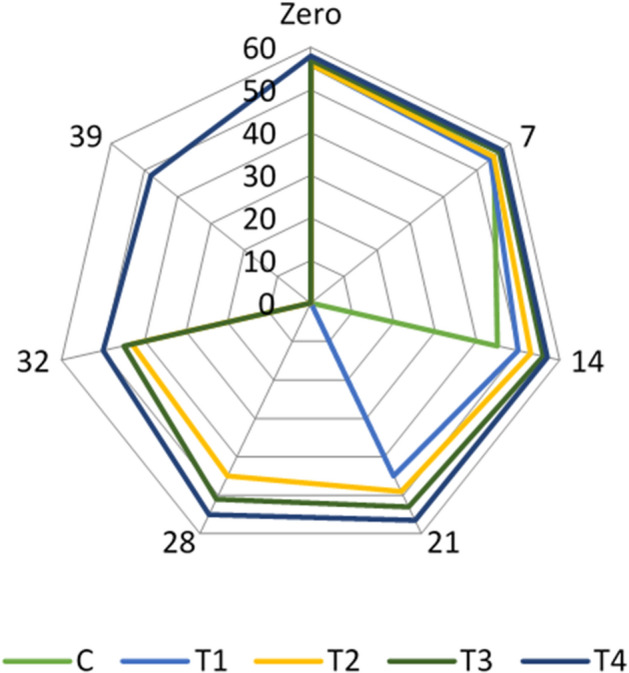
Flavour scores in the examined yogurt treatments throughout their refrigerated storage.
Figure 2.
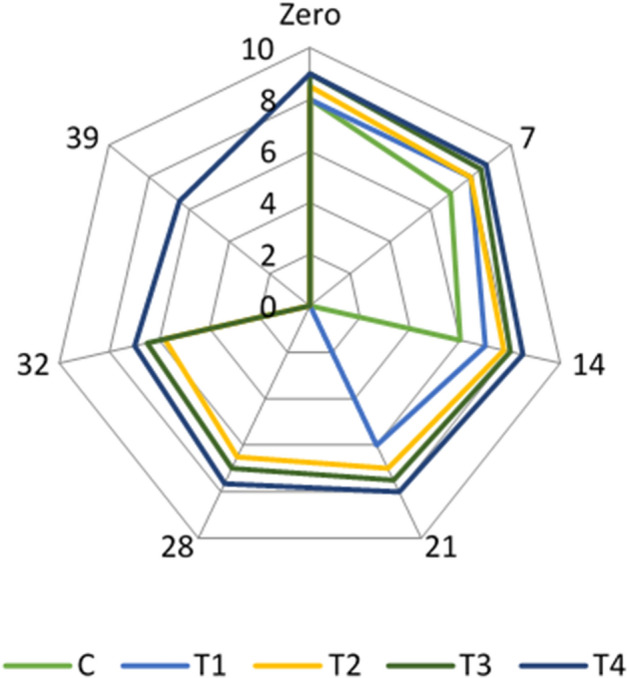
Appearance scores in the examined yogurt treatments throughout their refrigerated storage.
Figure 3.
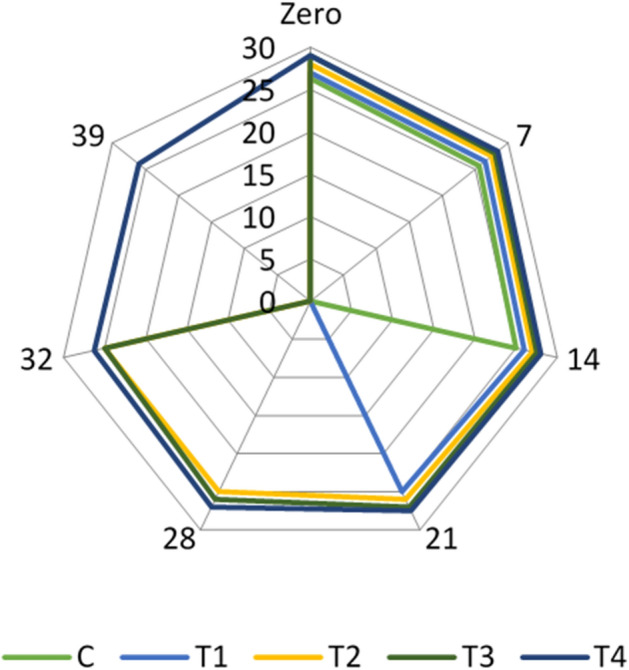
Body and Texture scores in the examined yogurt treatments throughout their refrigerated storage.
Using inulin in yogurt production as carbohydrate fat substitutes can improve color perception45.
Microbiological quality
Yogurt samples were examined microbiologically, as in table (1). There is a decline in the growth rate of Lactobacillus delbrueckii sub spp. bulgaricus in all examined samples. Treatments that contain inulin as in (T2): 1% yoghurt starter cultures 1:1 + 1% Lb. acidophilus + 2% inulin + 2% FOS and (T4): 1% yoghurt starter cultures 1:1 + 1% Lb. acidophilus bacteriocin + 2% inulin + 2% FOS have the highest count at the end of the storage period. The count of Lactobacillus delbrueckii sub spp. bulgaricus in treatment (T2) was 9.27 ± 0.12 CFU/g at 32 days of refrigerated storage, while for treatment (T4), the count was 9.52 ± 0.04 CFU/g at 39 days of refrigerated storage. Our result differs from that, who reported that Lb. bulgaricus in fermented skim milk prepared with 4% inulin showed a stable count during seven days of storage.
At the same time, Streptococcus thermophiles growth declined throughout the refrigerated storage. However, inulin and FOS groups have the highest count of Streptococcus thermophiles.
The count of Streptococcus thermophiles treatment (T2) was 7.65 ± 0.04 CFU/g at 32 days of refrigerated storage, and in treatment (T4), the count was 7.72 ± 0.04 CFU/g at 39 days of refrigerated storage (Table 1). These results agree with those obtained by46,47. They mentioned that inulin increases the growth and viability of lactic acid bacteria during fermentation or refrigerated storage. Furthermore, inulin concentrations were perfect for stimulating growth and retaining the viability of probiotic cultures in fermented milk47,48.
Table 1 shows the mean of Lb. acidophilus counts for the yogurt samples examined. Lb. Acidophilus counts declined throughout the storage period in T1 (yogurt with Lb. acidophilus) and T2 (yogurt with Lb. acidophilus, 2% inulin, and 2%FOS) with the final counts of Lb. Acidophilus was 7.54 ± 0.26 CFU/g on day 21 and 7.32 ± 0.26 CFU/g on day 32 and T2, respectively. The addition of inulin effectively increased the Lb. Acidophilus mean count when compared to the group that contains Lb. Acidophilus without inulin. These results agree with Donkor et al.49–51. They found that prebiotic ingredients such as inulin and FOS may exert a protective effect and improve the survival and activity of probiotic bacteria during the storage of probiotic food products.
The viability of probiotics was reported to be affected by many factors such as storage time, oxygen content, fluctuation in temperature, low pH, reduced water activity, and high concentration of salutes52,53.
Our study observed that adding inulin to probiotic yogurt enhances the growth of S. thermophiles, L. bulgaricus, and L. acidophilus until the end of the storage period compared with the treatments without inulin. The increase in probiotic counts of yogurt may be attributed to the action of inulin as a prebiotic substance. Akin et al.54 and48,55 attributed the increase in probiotic counts to the ability of probiotics and yogurt starter cultures to utilize inulin. Similarly, Donkor et al.44 showed that chicory-based inulin was a preferred carbon source for probiotic bacteria by increasing the growth performance and maintaining viability during cold storage. Acidity is one of the most critical factors that affect the viability of S. thermophiles, L. bulgaricus, and L. acidophilus56 as (FOS) act as substrates for the growth of LAB and inhibition of colonic cancer cells growth and putrefactive or pathogenic bacteria present in the colon through the production of short-chain fatty acids (SCFA). Rousseau et al.57 reported that FOS could stimulate the growth of the beneficial strains but not the pathogenic ones. The inhibitory effect against pathogenic bacteria usually results from the reduction in pH as a result of acid production, secretion of hydrogen peroxide, and release of natural antibiotics (bacteriocin) from beneficial microflora selectively stimulated by various prebiotics58.
In this study, (T4) yogurt samples appear to be the treatment that can resist spoilage with yeasts and molds (Table 1). According to EOSQ40, molds and yeasts count must not exceed ten cfu/g in yogurt. T4 was within a permissible limit until 28 days of storage with a mean count of 1.00 ± 0.03 CFU/g, while T2 was within the limit until 14 days of storage. T3 was within the allowable limit until 21 days of storage, with a mean count was 1.00 ± 0.05 CFU/g. T1 was within the permissible limit until 14 days of storage, with a mean count was 1.00 ± 0.01. At the same time, the control, yeast, and mold were not detected on zero-day only. These findings are consistent with the results obtained by Abee et al.59. They mentioned that LAB produces a proteinaceous antimicrobial substance known as bacteriocins that generally act through inactivation of enzymes, depolarization of the target cell membrane, or inhibition of the formation of the cell wall of pathogenic microflora including bacteria, mold, and yeast. Surajit et al.60 reported that inulin has antimicrobial activity against pathogenic microorganisms. Magnusson and Schnürer61,62 mentioned that lactic acid bacteria produced proteinaceous antifungal metabolites. Lactobacillus acidophilus has the most significant activity against Aspergillus flavus and Aspergillus parasiticus. In addition, Lactobacillus acidophilus has been shown to reduce aflatoxin production during 30 days of storage at room temperature in maize kernels63. The antifungal activity appears to be more assertive at lower pH ranges62; this may explain the antifungal activity of Lb. Acidophilus bacteriocin in acidic yogurt conditions. Batish et al.63,64 reported that Lb. acidophilus has antifungal activity. Coliform counts were not detected in any yogurt treatment on either the first day or during the storage period; this may be attributed to the good hygienic conditions during the preparation and storage of yogurts.
Conclusion
This work studies the incorporation of inulin and FOS with (2%) as prebiotics with a traditional starter culture, including Lactobacillus acidophilus and its bacteriocin, to improve the quality of skim milk yogurt. This fortification increases the coagulation time compared with control in treatments containing lb. acidophilus bacteriocin and prebiotics mentioned above (T4) by 22.75%, which also has bacteriocin (T3). Inulin (2%) and FOS (2%) strongly stimulated the growth of Lactobacillus acidophilus, Lactobacillus bulgaricus, and Streptococcus thermophilus. Lactobacillus acidophilus and its bacteriocin with 2% inulin and 2% FOS gave the skim milk yogurt the best scores for flavor, body, texture, and appearance throughout refrigerated storage with an extended shelf life of up to 39 days and inhibit fungal growth without altering the development of the starter.
Author contributions
H.H and M.S contributed to conceptualization, methodology, formal analysis. H.H, M.S. and H. S. A. contributed to resources and data analysis. H.S. A. contributed to writing original draft preparation, reviewing and editing. All authors read and approved the final manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cassani L, Gomez-Zavaglia A, Simal-Gandara J. Technological strategies ensuring the safe arrival of beneficial micro-organisms to the gut: From food processing and storage to their passage through the gastrointestinal tract. Food Res. Int. 2020;129:1–80. doi: 10.1016/j.foodres.2019.108852. [DOI] [PubMed] [Google Scholar]
- 2.Nyanzi R, Jooste PJ, Buys EM. Invited review: Probiotic yogurt quality criteria, regulatory framework, clinical evidence, and analytical aspects. J. Dairy Sci. 2021;104:1–19. doi: 10.3168/jds.2020-19116. [DOI] [PubMed] [Google Scholar]
- 3.Fabersani E, Grande MV, Aráoz MVC, Zannier ML, Sánchez SS, Grau A, et al. Metabolic effects of goat milk yogurt supplemented with yacon flour in rats on a high-fat diet. J. Funct. Foods. 2018;49:447–457. doi: 10.1016/j.jff.2018.08.042. [DOI] [Google Scholar]
- 4.Mani-López E, Palou E, López-Malo A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J. Dairy Sci. 2014;97:2578–2590. doi: 10.3168/jds.2013-7551. [DOI] [PubMed] [Google Scholar]
- 5.Martín R, Langella P. Emerging health concepts in the probiotics field: Streamlining the definitions. Front. Microbiol. 2019;10:1047. doi: 10.3389/fmicb.2019.01047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boza-Méndez E, López-Calvo R, Cortés-Muñoz M. Innovative dairy products development using probiotics: Challenges and limitations. In Tech. Costarica. 2012;10:213–236. [Google Scholar]
- 7.Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- 8.Parhi P, Song KP, Choo WS. Effect of inulin and fructooligosaccharide supplementation on the growth and survival of Lactobacillus casei in model sugar systems. J. Food Process. Preserv. 2021;45:15228. doi: 10.1111/jfpp.15228. [DOI] [Google Scholar]
- 9.Delgado-Fern Andez P, Hernandez-Hern Andez O, Olano A, Moreno FJ, Corzo N. Probiotic viability in yoghurts containing oligosaccharides derived from lactulose (OsLu) during fermentation and cold storage. Int. Dairy J. 2020;102:104621. doi: 10.1016/j.idairyj.2019.104621. [DOI] [Google Scholar]
- 10.Rosa MC, Carmo MR, Balthazar CF, Guimarães JT, Esmerino EA, Freitas MQ, Silva MC, Pimentel TC, Cruz AG. Dairy products with prebiotics: An overview of the health benefits, technological and sensory properties. Int. Dairy J. 2021;117:105009. doi: 10.1016/j.idairyj.2021.105009. [DOI] [Google Scholar]
- 11.Pimentel TC, Cruz AG, Prudencio SH. Short communication: Influence of long-chain inulin and Lactobacillus paracasei subspecies paracasei on the sensory profile and acceptance of a traditional yogurt. J. Dairy Sci. 2013;96:6233–6241. doi: 10.3168/jds.2013-6695. [DOI] [PubMed] [Google Scholar]
- 12.Van der Beek CM, Canfora EE, Kip AM, et al. The prebiotic inulin improves substrate metabolism and promotes short-chain fatty acid production in overweight to obese men. Metabolism. 2018;87:25–35. doi: 10.1016/j.metabol.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Hussain SA, Raju PN, et al. Packaging material type affects the quality characteristics of Aloe-probiotic lassi during storage. Food Biosci. 2017;19:34–41. doi: 10.1016/j.fbio.2017.05.007. [DOI] [Google Scholar]
- 14.Akhtar S, Sarker MR, Hossain A. Microbiological food safety: A dilemma of developing societies. Crit. Rev. Microbiol. 2013;40:348–359. doi: 10.3109/1040841X.2012.742036. [DOI] [PubMed] [Google Scholar]
- 15.Ranadheera RDCS, Baines SK, Adams MC. Importance of food in probiotic efficacy. Food Res. Int. 2010;43:1–7. doi: 10.1016/j.foodres.2009.09.009. [DOI] [Google Scholar]
- 16.Ryan LA, Zannini E, Dal Bello F, Pawlowska A, Koehler P, et al. Lactobacillus amylovorus DSM 19280 as a novel food-grade antifungal agent for bakery products. Int. J. Food Microbiol. 2011;146:76–283. doi: 10.1016/j.ijfoodmicro.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 17.Ogueke CC, Owuamanam CI, Olawuni IA, Iwouno JO, Peter-Ikechukwu AI. Addition of inulin and manganese chloride increased antibacterial metabolites in yogurt. Pak. J. Nutr. 2014;13:12. doi: 10.3923/pjn.2014.12.16. [DOI] [Google Scholar]
- 18.Anjum N, Maqsood S, Masud T, Ahmad A, Sohail A, Momin A. Lactobacillus acidophilus: Characterization of the species and application in food production. Crit. Rev. Food Sci. Nutr. 2014;54:1241–1251. doi: 10.1080/10408398.2011.621169. [DOI] [PubMed] [Google Scholar]
- 19.Messaoudi S, Manai M, Kergourlay G, Prévost H, Connil N, et al. Lactobacillus salivarius: Bacteriocin and probiotic activity. Food Microbiol. 2013;36:296–304. doi: 10.1016/j.fm.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Salman M, Shahid M, Sahar T, Naheed S, Arif M, et al. Development of regression model for bacteriocin production from local isolate of Lactobacillus acidophilus MS1 using Box-Behnken design. Biocatal. Agric. Biotechnol. 2020;24:101542. doi: 10.1016/j.bcab.2020.101542. [DOI] [Google Scholar]
- 21.Gebara C, Ribeiro MCE, Chaves KS, Gandara ALN, Gigante ML. Effectiveness of different methodologies for the selective enumeration of Lactobacillus acidophilus La5 from yoghurt and Prato cheese. LWT-Food Sci. Technol. 2015;64:508–513. doi: 10.1016/j.lwt.2015.04.061. [DOI] [Google Scholar]
- 22.Savard P, Lamarche B, Paradis ME, Thiboutot H, Laurin É, et al. Impact of Bifidobacterium animalis subsp. lactis BB-12 and Lactobacillus acidophilus LA-5-containing yoghurt, on fecal bacterial counts of healthy adults. Int. J. Food Microbiol. 2011;149:50–57. doi: 10.1016/j.ijfoodmicro.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Magariños H, Selaive S, Costa M, Flores M, Pizarro O. Viability of probiotic micro-organisms (Lactobacillus acidophilus la-5 and Bifidobacterium animalis subsp. Lactis bb-12) in ice cream. Int. J. Dairy Technol. 2007;60:128–134. doi: 10.1111/j.1471-0307.2007.00307.x. [DOI] [Google Scholar]
- 24.Alves LL, Richards NS, Mattanna P, Andrade DF, S Rezer AP, Milani LI, Cruz AG, Faria JA. Cream cheese as a symbiotic food carrier using Bifidobacterium animalis B b-12 and Lactobacillus acidophilus L a-5 and inulin. Int. J. Dairy Technol. 2013;66:63–69. doi: 10.1111/j.1471-0307.2012.00880.x. [DOI] [Google Scholar]
- 25.Xavier-Santos D, Bedani R, Perego P, Converti A, Saad SMI. L. acidophilus La-5, fructo-oligosaccharides and inulin may improve sensory acceptance and texture profile of a synbiotic diet mousse. LWT. 2019;105:329–335. doi: 10.1016/j.lwt.2019.02.011. [DOI] [Google Scholar]
- 26.Okuro PK, Thomazini M, Balieiro JC, Liberal RD, Fávaro-Trindade CS. Co-encapsulation of Lactobacillus acidophilus with inulin or polydextrose in solid lipid microparticles provides protection and improves stability. Food Res. Int. 2013;53:96–103. doi: 10.1016/j.foodres.2013.03.042. [DOI] [Google Scholar]
- 27.da Silva Sabo S, Converti A, Todorov SD, Domínguez JM, de Souza Oliveira RP. Effect of inulin on growth and bacteriocin production by Lactobacillus plantarum in stationary and shaken cultures. Int. J. Food Sci. Technol. 2015;50:864–870. doi: 10.1111/ijfs.12711. [DOI] [Google Scholar]
- 28.Akalın AS, Erişir D. Effects of inulin and oligofructose on the rheological characteristics and probiotic culture survival in low-fat probiotic ice cream. J. Food Sci. 2008;73:M184–M188. doi: 10.1111/j.1750-3841.2008.00728.x. [DOI] [PubMed] [Google Scholar]
- 29.Oliveira RPDS, Perego P, De Oliveira MN, Converti A. Effect of inulin as a prebiotic to improve growth and counts of a probiotic cocktail in fermented skim milk. LWT-Food Sci Technol. 2011;44:520–523. doi: 10.1016/j.lwt.2010.08.024. [DOI] [Google Scholar]
- 30.Hassan A, Amjad I. Nutritional evaluation of yoghurt prepared by different starter cultures and their physiochemical analysis during storage. Afr. J. Biotechnol. 2010;9:2913–2917. doi: 10.5897/AJB09.1483. [DOI] [Google Scholar]
- 31.AOAC (Association of Official Analytical chemists). Official methods of analysis, 17th ed. (AOAC International, 2000).
- 32.APHA (American Public Health Association). Compendium Methods for the Microbiological Examination of Foods, 4th ed. (APHA, 2001).
- 33.Kodaka H, Mizuochi S, Teramura H, Nirazuka T. Comparison of the compact dry TC method with the standard pour plate method (AOAC Official Method 966.23) for determining aerobic colony counts in food samples: Performance-tested method SM. J. AOAC Int. 2005;88:1702–1713. doi: 10.1093/jaoac/88.6.1702. [DOI] [PubMed] [Google Scholar]
- 34.do Espírito Santo AP, et al. Fibers from fruit by-products enhance probiotic viability and fatty acid profile and increase CLA content in yoghurts. Int. J. Food Microbiol. 2012;154:135–144. doi: 10.1016/j.ijfoodmicro.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 35.IDF (International Dairy Federation): Milk and Milk Products: Enumeration of Yeast and Molds IDF: 94B. (1990).
- 36.Mehanna NM, Saleh TM, Mehanna AS, El-Asfory SMA. The quality of low-calorie buffalo Zabady. Egypt. J Dairy Sci. 2000;28:59–72. [Google Scholar]
- 37.Clarke, G. M. & Kempson, R. E. Introduction to the design and analysis of experiments. Arnold, a Member of the Holder Headline Group, 1st ed. (1997).
- 38.Lee WJ, Lucey JA. Formation and physical properties of yogurt. Asian-Australas. 2010;23:1127–1136. doi: 10.5713/ajas.2010.r.05. [DOI] [Google Scholar]
- 39.Akabanda F, Owusu-Kwarteng J, Tano-Debrah K, Parkouda C, Jespersen L. The use of lactic acid bacteria starter culture in the production of Nunu, a spontaneously fermented milk product in Ghana. Int. J. Food Sci. 2014;2014:1–11. doi: 10.1155/2014/721067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.EOSQ (Egyptian Organization Standardization and Quality). Egyptian Organization for Standardization and Quality No. 1000 (2005).
- 41.Guven M, Yasar K, Karaca OB, Hayaloglu AA. The effect of inulin as a fat replacer on the quality of set-type low-fat yogurt manufacture. Int. J. Dairy Technol. 2005;58:180–184. doi: 10.1111/j.1471-0307.2005.00210.x. [DOI] [Google Scholar]
- 42.Srisuvor N, Chinprahast N, Prakitchaiwattana C, Subhimaros S. Effects of inulin and polydextrose on physicochemical and sensory properties of low-fat set yoghurt with probiotic-cultured banana purée. LWT-Food Sci. Technol. 2013;51:30–36. doi: 10.1016/j.lwt.2012.10.018. [DOI] [Google Scholar]
- 43.Cruz AG, et al. Sensory analysis: Relevance for prebiotic, probiotic, and synbiotic product development. Compr. Rev. Food Sci. Food Saf. 2010;9:358–373. doi: 10.1111/j.1541-4337.2010.00115.x. [DOI] [PubMed] [Google Scholar]
- 44.Donkor ON, Henriksson A, Vasiljevic T, Shah NP. Rheological properties and sensory characteristics of set-type soy yogurt. J. Agric. Food Chem. 2007;55:9868–9876. doi: 10.1021/jf071050r. [DOI] [PubMed] [Google Scholar]
- 45.Brennan CS, Tudorica CM. Carbohydrate-based fat replacers in the modification of the rheological, textural and sensory quality of yoghurt: Comparative study of the utilisation of barley beta-glucan, guar gum and inulin. Int. J. Food Sci. Technol. 2008;43:824–833. doi: 10.1111/j.1365-2621.2007.01522.x. [DOI] [Google Scholar]
- 46.El-Nagar G, Clowes G, Tudoricǎ CM, Kuri V, Brennan CS. Rheological quality and stability of yog-ice cream with added inulin. Int. J. Dairy Technol. 2002;55:89–93. doi: 10.1046/j.1471-0307.2002.00042.x. [DOI] [Google Scholar]
- 47.Akalin AS, Gönç S, Ünal G, Fenderya SERAP. Effects of fructooligosaccharide and whey protein concentrate on the viability of starter culture in reduced-fat probiotic yogurt during storage. J. Food Sci. 2007;72:M222–M227. doi: 10.1111/j.1750-3841.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 48.Aryana KJ, Plauche S, Rao RM, McGrew P, Shah NP. Fat-free plain yogurt manufactured with inulins of various chain lengths and Lactobacillus acidophilus. J. Food Sci. 2007;72:M79–M84. doi: 10.1111/j.1750-3841.2007.00302.x. [DOI] [PubMed] [Google Scholar]
- 49.Donkor ON, Nilmini SLI, Stolic P, Vasiljevic T, Shah NP. Survival and activity of selected probiotic organisms in set-type yoghurt during cold storage. Int. Dairy J. 2007;17:657–665. doi: 10.1016/j.idairyj.2006.08.006. [DOI] [Google Scholar]
- 50.Buriti FC, Castro IA, Saad SM. Viability of Lactobacillus acidophilus in synbiotic guava mousses and its survival under in vitro simulated gastrointestinal conditions. Int. J. Food Microbiol. 2010;137:121–129. doi: 10.1016/j.ijfoodmicro.2009.11.030. [DOI] [PubMed] [Google Scholar]
- 51.Hernández-Hernández O, Muthaiyan A, Moreno FJ, Montilla A, Sanz ML, Ricke SC. Effect of prebiotic carbohydrates on the growth and tolerance of Lactobacillus. Food Microbiol. 2012;30:355–361. doi: 10.1016/j.fm.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 52.Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P. Relevant factors for the preparation of freeze-dried lactic acid bacteria. Int. Dairy J. 2004;14:835–847. doi: 10.1016/j.idairyj.2004.02.001. [DOI] [Google Scholar]
- 53.Capela P, Hay TKC, Shah NP. Effect of cryoprotectants, prebiotics and microencapsulation on survival of probiotic organisms in yoghurt and freeze-dried yogurt. Food Res. Int. 2006;39:203–211. doi: 10.1016/j.foodres.2005.07.007. [DOI] [Google Scholar]
- 54.Akın MB, Akın MS, Kırmacı Z. Effects of inulin and sugar levels on the viability of yogurt and probiotic bacteria and the physical and sensory characteristics in probiotic ice-cream. Food Chem. 2007;104:93–99. doi: 10.1016/j.foodchem.2006.11.030. [DOI] [Google Scholar]
- 55.Sendra E, Fayos P, Lario Y, Fernández-López J, Sayas-Barberá E, Pérez-Alvarez JA. Incorporation of citrus fibers in fermented milk containing probiotic bacteria. Food Microbiol. 2008;25:13–21. doi: 10.1016/j.fm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Dave RI, Shah NP. Viability of yoghurt and probiotic bacteria in yoghurts made from commercial starter cultures. Int. Dairy J. 1997;7:31–41. doi: 10.1016/S0958-6946(96)00046-5. [DOI] [Google Scholar]
- 57.Rousseau V, Lepargneur JP, Roques C, Remaud-Simeon M, Paul F. Prebiotic effects of oligosaccharides on selected vaginal lactobacilli and pathogenic microorganisms. Anaerobe. 2005;11:145–153. doi: 10.1016/j.anaerobe.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Manning TS, Gibson GR. Prebiotics. Best Pract. Res. Clin. Gastroenterol. 2004;18:287–298. doi: 10.1016/j.bpg.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 59.Abee T, Krockel L, Hill C. Bacteriocins: Modes of action and potentials in food preservation and control of food poisoning. Int. J. Food Microbiol. 1995;28:169–185. doi: 10.1016/0168-1605(95)00055-0. [DOI] [PubMed] [Google Scholar]
- 60.Mandal S, Patra AD, Hati S. Effect of inulin on growth and antimicrobial activity of Lactobacillus spp. Int. J. Ferment. Foods. 2016;5:47–52. doi: 10.5958/2321-712X.2016.00006.5. [DOI] [Google Scholar]
- 61.Magnusson J, Schnürer J. Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ. 2001;67:1–5. doi: 10.1128/AEM.67.1.1-5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rouse S, Harnett D, Vaughan A, Sinderen DV. Lactic acid bacteria with potential to eliminate fungal spoilage in foods. J. Appl. Microbiol. 2008;104:915–923. doi: 10.1111/j.1365-2672.2007.03619.x. [DOI] [PubMed] [Google Scholar]
- 63.Elsanhoty RM. Screening of some lactobacillus strains for their antifungal activities against aflatoxin producing aspergilli in vitro and maize. J. Food Agric. Environ. 2008;6:35–40. [Google Scholar]
- 64.Batish VK, Lai R, Grover S. Studies on environmental and nutritional factors on production of antifungal substance by Lactobacillus acidophilus R. Food Microbiol. 1990;7:199–206. doi: 10.1016/0740-0020(90)90025-D. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.


