Abstract
Posttraumatic stress disorder (PTSD) develops in a subset of individuals upon exposure to traumatic stress. In addition to well-defined psychological and behavioral symptoms, some individuals with PTSD also exhibit elevated concentrations of inflammatory markers, including C-reactive protein, interleukin-6, and tumor necrosis factor-α. Moreover, PTSD is often co-morbid with immune-related conditions, such as cardiometabolic and autoimmune disorders. Numerous factors, including lifetime trauma burden, biological sex, genetic background, metabolic conditions, and gut microbiota, may contribute to inflammation in PTSD. Importantly, inflammation can influence neural circuits and neurotransmitter signaling in regions of the brain relevant to fear, anxiety, and emotion regulation. Given the link between PTSD and the immune system, current studies are underway to evaluate the efficacy of anti-inflammatory treatments in those with PTSD. Understanding the complex interactions between PTSD and the immune system is essential for future discovery of diagnostic and therapeutic tools.
Subject terms: Psychiatric disorders, Predictive markers
Introduction
Posttraumatic stress disorder (PTSD) is a debilitating psychiatric disorder characterized by re-experiencing of trauma, avoidance of trauma reminders, and hyperarousal symptoms that cause negative alterations in cognition, mood, and physiologic health [1]. PTSD is unique among other psychiatric disorders, as it requires trauma exposure to develop. Although over 70% of the population is exposed to at least one traumatic event during their lifespan, it is not clear why only some individuals develop PTSD [2]. Given the high comorbidities of inflammatory and metabolic disorders with PTSD [3–9], some studies have focused on the potential for an immune-related or inflammatory etiology for PTSD [10–12], whilst others suggest that PTSD promotes inflammation [13, 14] or that a bidirectional relation between PTSD and inflammation exists [15, 16].
In this review, we first summarize potential mechanisms connecting PTSD and the immune system. We describe studies of peripheral immune markers and mechanisms through which immune alterations affect neurotransmitter systems and brain regions that contribute to PTSD symptomatology. We also highlight the contribution of chronic inflammation in conditions often co-morbid with PTSD. Finally, we explore plausible therapeutic strategies targeting the immune system, based on its interaction with PTSD.
PTSD and inflammation
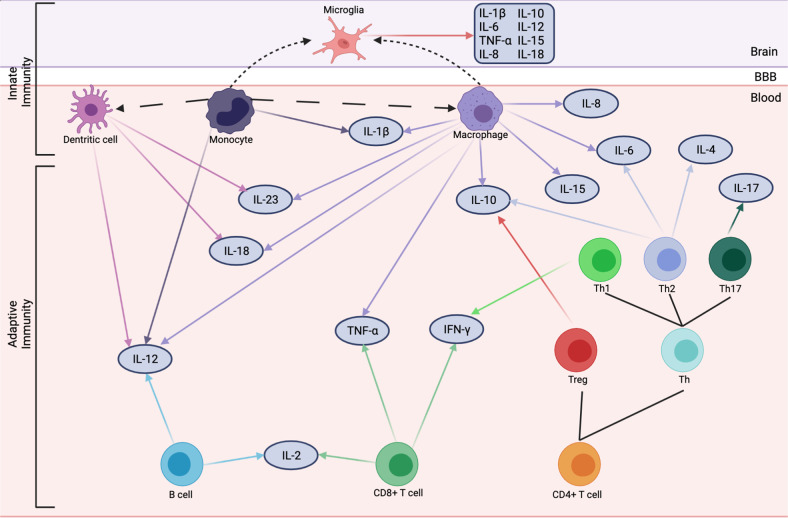
The neuroendocrine, psychophysiological, and neurobiological changes in PTSD etiology and outcome have been extensively studied [17, 18]. Growing evidence in the past two decades points to mechanisms related to the innate (i.e., non-specific first line of defense regulated by innate immune cells, including monocytes, macrophages, dendritic cells, and microglia) and adaptive (i.e., antigen-specific immunity regulated by T and B lymphocytes) immune systems in the pathophysiology of PTSD [17–19]. The initial evidence for the relationship between PTSD and the immune system comes from individual studies and subsequent meta-analyses reporting alterations in peripheral inflammatory markers, such as C-reactive protein (CRP), interferon-gamma (IFN-γ), interleukin-6 (IL-6), interleukin-10 (IL-10), and tumor necrosis factor-alpha (TNF-α) in individuals with PTSD (Fig. 1) [10, 20–23]. Moreover, hypothesis-free genome-wide [24, 25], epigenome-wide [26–30], and transcriptomic studies [31–35] of PTSD have identified multiple genes related to the immune system.
Fig. 1. Immune cells and cytokines implicated in PTSD.
Long dashed lines represent differentiation. Short dashed lines represent trafficking into the brain. BBB blood–brain barrier, IL interleukin, Th T helper cell, Treg regulatory T cell.
Alterations in peripheral immune markers in PTSD
The inflammatory environment in PTSD is characterized by increased levels of pro-inflammatory markers (e.g., CRP, IL-6, IL-1β, IL-2, TNF-α, IFN-γ) and decreased levels of anti-inflammatory markers (e.g., IL-10) (Fig. 1) [10, 20–22]. Elevated inflammatory markers in PTSD may create a positive feedback loop to promote inflammation, such that IL-6, IL-1β, and TNF-α induce CRP to activate the complement system, which triggers a cascade of events to promote inflammation [18]. Nonetheless, it is still not clear whether the inflammatory milieu is the outcome of PTSD, or if pre-existing or trauma-induced inflammation increases the risk of PTSD. Notably, a bidirectional relationship between PTSD and inflammation is supported by recent reports [15, 16, 19], including a large-scale genetic study reporting a bidirectional genetic association between PTSD and CRP [16].
Longitudinal studies investigating whether PTSD development leads to inflammation reported that PTSD caused increases in inflammatory markers, including IL-1β, IL-8, CRP, and tumor necrosis factor receptor II (TNFRII) [36, 37]. On the contrary, Glaus et al. [38] observed lower IL-6 levels following PTSD diagnosis, indicating a decrease in inflammation. Longitudinal studies evaluating whether pre-existing inflammation is a risk factor for PTSD reported that increased levels of CRP and TNFRII predicted PTSD diagnosis [10, 15]. Transcriptomic studies conducted on US Marines revealed that upregulation of immune-related genes and overexpression of genes in networks associated with the innate immune response and interferon signaling at pre-deployment predicted post-deployment PTSD [39, 40]. Here, one can query the possible causes of pre-existing inflammation. Some possible drivers of this pre-existing inflammation (e.g., metabolic conditions, biological sex, and genetics) will be discussed below. Trauma and stress exposure across the lifespan might also contribute to inflammation prior to the incident trauma that results in PTSD [19]. Notably, a transdiagnostic meta-analysis of trauma exposure reported increased peripheral CRP, IL-1β, IL-6, and TNF-α concentrations in participants who experienced traumatic events (e.g., childhood maltreatment, natural disaster, violence) across their lifespan [41]. Specifically, early life adversity, including maltreatment, parental separation, and low socioeconomic status in childhood, is associated with increased CRP, IL-6, and TNF-α levels in adulthood [27, 42, 43]. Concordantly, studies that assessed inflammatory markers in the acute aftermath of trauma showed that increased levels of IL-6, IL-8, and CRP were associated with PTSD at follow-up [11, 12, 44]. In contrast, Michopoulos et al. [45] reported that decreased levels of TNF-α and IFN-γ upon trauma predicted chronic PTSD trajectory. However, since these studies measured inflammatory markers right after the trauma exposure, it is not clear whether the inflammatory response precedes the trauma or is the result of an acute posttraumatic response. The inability to assess the origin of the inflammatory response (i.e., pre- or post-trauma) may account for the inconsistent findings across the studies.
The relationship between inflammation and traumatic experiences is also supported by animal repeated social defeat stress (RSDS) models. For instance, IL-17A secreted from meningeal T cells in the brain was reported to control anxiety-like behavior in mice through neuronal IL-17a receptor subunit (IL-17Ra) signaling [46]. Following RSDS, anxiety-like behaviors are associated with increased levels of peripheral cytokines, including IL-2, IL-10, IL-17A, IL-22, and TNFα [47]. In contrast, Hodes et al. [48] showed increased peripheral cytokines in mice susceptible to social stress after RSDS.
Link between the HPA axis, autonomic nervous system, and inflammation in PTSD
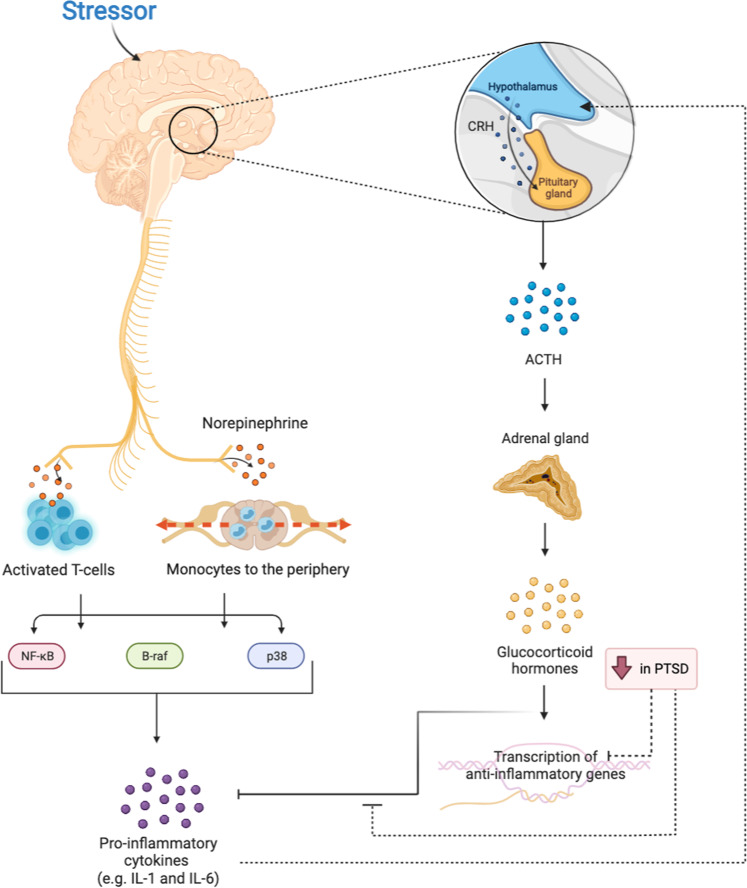
The neuroendocrine stress response is comprised of the autonomic nervous system (ANS) and the hypothalamic–pituitary–adrenal (HPA) axis, which relay signals to the peripheral organs and the immune system (Fig. 2). Upon acute exposure to stress, corticotrophin-releasing hormone (CRH) is secreted from the hypothalamus, thereby activating the HPA axis [49]. The binding of CRH to its receptor on pituitary corticotropes triggers the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary into the systemic circulation, stimulating glucocorticoid (cortisol in humans) synthesis from the adrenal cortex [49]. In parallel, stress exposure also triggers the sympathetic nervous system (SNS) to release catecholamines (e.g., epinephrine and norepinephrine), which are responsible for physiological changes, such as increases in heart rate and blood pressure [50]. In response to norepinephrine, monocytes are mobilized from the bone marrow into the periphery, where they encounter danger-associated molecular patterns (DAMPs), activating nuclear factor kappa B (NF-kB) mediated production of pro-inflammatory cytokines [51, 52]. Indeed, individuals with PTSD exhibited increased peripheral NF-κB activity and NF-κB-mediated transcriptional changes in monocytes, which contribute to the inflammatory environment [34, 35]. Similarly, norepinephrine release from activated SNS fibers further stimulates the NF-κB, B-raf-ERK1/2, and p38 pathways in activated T cells to produce pro-inflammatory cytokines [53–55]. Along with SNS activation, decreased parasympathetic activity in PTSD contributes to the inflammatory milieu [56]. Elevation in these pro-inflammatory cytokines, in turn, leads to HPA axis reactivity [17].
Fig. 2. Relationship between the HPA axis, sympathetic nervous system, and inflammation in PTSD.
Stress exposure stimulates sympathetic nervous system (SNS). Norepinephrine release from activated SNS fibers stimulates proinflammatory cytokines production through the NF-kB, B-Raf, and p38 pathways. The HPA axis is also activated upon exposure to stress, stimulating inflammatory responses that limit HPA reactivity. The reduced ability of glucocorticoids to inhibit inflammatory processes contributes to the proinflammatory environment in PTSD. CRH corticotrophin-releasing hormone, ACTH adrenocorticotropic hormone, NF-κB nuclear factor-κB, IL-1 Interleukin-1, IL-6 Interleukin-6.
In PTSD, HPA axis hyperactivity following repeated trauma may disrupt glucocorticoid signaling, which leads to peripheral and central nervous system inflammation [17]. Normally, following the binding of glucocorticoids to the glucocorticoid receptor (GR), the stress response is dampened via a negative-feedback loop [18]. The glucocorticoid–GR complex also suppresses inflammatory responses either by stimulating the transcription of anti-inflammatory genes in the nucleus or by inhibiting the expression of proinflammatory proteins in the cytosol [18]. However, chronic exposure to stress may result in glucocorticoid resistance, wherein cortisol cannot inhibit NF-κB-mediated pro-inflammatory cytokine release, dampening glucocorticoid negative feedback of the HPA axis [17]. Overall, the inflammatory state in PTSD is propagated through a combination of glucocorticoid resistance along with increased sympathetic and decreased parasympathetic nervous system activity [17].
Chronic exposure to trauma and stress can also stimulate the production and release of DAMPs, such as mitochondrial reactive oxygen species (ROS) [57, 58]. DAMPs are key contributors to local or systemic inflammatory responses in the absence of pathogens or tissue damage [57, 58]. Plasma levels of the astroglial protein S100 calcium-binding protein B (S100b), one of the most studied DAMPs in the field of psychiatry, were reported to be higher in veterans with PTSD compared to healthy veterans [59]. Plasma levels of the nuclear protein high mobility group box 1 protein (HMGB1) were increased in severe blunt chest trauma patients with PTSD compared to those without PTSD [60]. In response to stress exposure, these DAMPs bind pattern recognition receptors (PRRs), including the receptor for advanced glycation end-products (RAGE) and toll-like receptors (TLR) on innate immune cells, activating the NF-kB pathway to produce pro-inflammatory cytokines [57, 58].
Comorbidity between PTSD and immune-related diseases
PTSD can be highly co-morbid with serious physical illnesses, including asthma [61], autoimmune diseases [7, 8], and cardiovascular diseases (CVD) [4, 5]. Multiple studies identified PTSD as a risk factor for CVD and related cardiovascular events, including heart failure and ischemia [62–65], while others propose that CVD symptoms, treatment, and surgery may serve as a trauma that increases PTSD prevalence following acute coronary events [66, 67]. Emerging PTSD symptoms following these cardiovascular events may in turn increase the risk of severe cardiovascular outcomes, including recurrence and mortality [68, 69]. This bidirectional relationship between PTSD and CVD may be due, in part, to contributions from multiple common underlying mechanisms [5, 67, 68], including the ANS [70, 71], HPA axis, oxidative stress [72], and inflammation [5, 73–76].
PTSD is also strongly linked with asthma [3]. The relationship between PTSD and asthma also appears to be bidirectional, as numerous studies report increased asthma prevalence in individuals with PTSD [77–79], while others show a higher odds ratio for PTSD in individuals exhibiting symptoms of asthma [80]. The comorbidity of asthma and PTSD may be explained by shared inflammatory mechanisms. In asthma, the binding of allergens to PRRs triggers both innate and adaptive immune responses [3]. In severe cases of asthma, the T helper 2 (Th2) immune response is augmented by Th17 cells that produce IL-17A, enhancing the pro-inflammatory response [3]. Clinical studies report that those with PTSD do not exhibit differences in Th2 cell proportions [81], but have higher IL-17A levels [82, 83]. Consistent with this finding, those with more severe PTSD symptoms have elevated Th17 cell counts [81]. Overall, this evidence suggests that the link between PTSD and asthma may be driven by an increased Th17 immune response.
PTSD is co-morbid with autoimmune diseases, including inflammatory bowel disease (IBD), rheumatoid arthritis (RA), multiple sclerosis (MS), and psoriasis [7–9]. Notably, the risk of an autoimmune disorder is higher in individuals with PTSD, compared to individuals with other psychiatric disorders [8]. Even though the direction of the association between PTSD and autoimmune diseases is not clear, the fact that this association is not affected by prior trauma and healthy behaviors [7] may suggest that PTSD precedes autoimmune diseases. This hypothesis was supported by a retrospective study of Swedish civilians reporting an increased risk of autoimmune disease development in those with PTSD [84]. Indeed, inflammation is one of the biological mechanisms suspected to link PTSD and autoimmune disorders. Elevated leukocyte, total T-cell counts, and cell-mediated immunity in PTSD may contribute to the development of autoimmune disorders [9, 85, 86].
The inflammatory environment in PTSD may also be exacerbated by co-morbid metabolic conditions [6]. Individuals with PTSD are most likely to suffer from type 2 diabetes mellitus, metabolic syndrome (MetS), and its individual components, including obesity, insulin resistance, and dyslipidemia [8, 87, 88]. This increased comorbidity can be explained by unhealthy lifestyles associated with PTSD (e.g., disrupted sleep patterns, unhealthy diet, tobacco and substance use, physical inactivity), which contribute to inflammation [89–92]. In both MetS and PTSD, the noradrenergic system is activated to trigger an innate immune response [6]. Like PTSD, MetS and obesity are also characterized by an increase in proinflammatory markers, such as CRP, IL-6, and TNF-α [20–22, 93, 94]. Inflammation can promote obesity and insulin resistance, and the resulting fat accumulation, in turn, may lead to elevated levels of proinflammatory cytokines [93, 94]. The connection between PTSD and MetS is supported by a recent hypothesis-free metabolomic study of PTSD that reported dysregulated production and utilization of carbohydrate, lipid, and amino acids, as well as alterations in energy-related pathways [95]. This metabolic evidence indicated inflammation, inefficient energy production, and possibly mitochondrial dysfunction in individuals with PTSD [90]. Mitochondrial dysfunction may lead to increased production of ROS in peripheral organs and immune cells, which contribute to peripheral inflammation. Kusminski and Scherer proposed that inflammation, oxidative stress, and metabolism can be linked together by mitochondrial dysfunction [96]. Overall, a “mitochondrial allostatic load” model may explain the link between these adverse metabolic conditions, inflammation, and PTSD. This model suggests that metabolic dysregulation in PTSD may disrupt mitochondrial activity, resulting in increased ROS production and inflammation [97].
Other drivers of inflammation in PTSD
Gut microbiota plays an important role in the communication between the brain and the gastrointestinal tract, called the “gut-brain axis”. This axis regulates gastrointestinal homeostasis and links areas of the brain with intestinal functions through the vagus nerve, SNS, and both the endocrine and immune networks [98]. The composition of gut microbiota significantly influences the regulation of the gut-brain axis by stimulating immune cells that contribute to neuroinflammation [99]. Stress, diet, and other environmental factors can disrupt the gut microbiome, which signals the intestinal epithelium to produce pro-inflammatory cytokines [99] and may ultimately lead to permeability in the intestinal tract and excessive antigen trafficking and inflammation [99]. Growing evidence implicates dysregulated gut-brain axis signaling in the pathogenesis of stress and mood disorders and reports gut microbiome alterations in individuals with PTSD [100–103]. Gut microbiome alterations may also mediate the association between early life adversity and symptoms of anxiety in adulthood [104]. These data, in conjunction with evidence showing that PTSD is highly co-morbid with inflammatory gastrointestinal diseases (e.g., IBD), [8, 105] may implicate gut microbiota dysbiosis in the inflammatory environment of PTSD.
Since PTSD disproportionally affects women over men, [106] sex may also modulate the immune response in PTSD [107]. The higher prevalence of PTSD in women can be explained by higher trauma vulnerability, dysregulated fear processing, more sensitive HPA axis, and fluctuating HPA axis activity with the menstrual cycle, as well as aberrant immune responses [17, 106, 107]. Multiple studies reported PTSD-associated differences in immune markers based on sex (reviewed in ref. [108]). A gene co-expression study showed upregulation of an IL-12 signaling module in men but not women with PTSD [31]. Neylan et al. [109] reported increased activation of pathways related to the immune response in monocytes of women, but not men with PTSD. In addition, Kim et al. [110] reported sex-specific differences in peripheral blood leukocyte composition, as men but not women with lifetime PTSD have increased monocyte proportions. Evidence from post-mortem brain samples revealed decreased microglia proportions in women with PTSD [108, 111]. The key factor underlying the sex-specific immune response in PTSD may be estrogen, as studies showed that lower estrogen levels are associated with increased PTSD symptoms [112, 113]. A recent study showed the indirect effect of sex on non-remitting PTSD development through pro-inflammatory cytokines [114]. Authors also showed that this indirect effect of sex was moderated by estradiol, such that men with higher estradiol levels have elevated pro-inflammatory cytokine levels, which was associated with a lower risk of non-remitting PTSD [114]. This relationship can be explained by activation of the HPA axis and inhibition of the SNS by elevated estrogen levels that suppress the production of pro-inflammatory cytokines from T cells and macrophages [107, 115].
Another driver of inflammation in PTSD might be genetics, considering SNP-based heritability estimates of 5–20% that vary based on sex [25]. Supporting evidence comes from studies reporting associations between PTSD and polymorphisms in genes involved in the immune system, including the human leukocyte antigen (HLA) locus [25, 116], CRP [117, 118], TNF-α [119], and ankyrin repeat domain-55 (ANKRD55) [24]. The variations in these immune genes may contribute to pleiotropy between PTSD and immune-related disorders, underlying the shared etiology of these complex and co-morbid disorders. The association between PTSD and the HLA locus is of particular interest, as HLA alleles resulting from combinations of different polymorphisms are heavily implicated in autoimmune disorders (reviewed in [120]). For instance, HLA-A*02:01, which was identified as a protective allele for MS, [121] was less frequent in individuals with PTSD [116], suggesting a plausible shared genetic etiology between PTSD and MS. As HLA alleles have distinct antigen-binding properties, PTSD-associated HLA alleles might have enhanced antigen presentation capacity that impacts T-cell activation and inflammation.
Impact of inflammation on brain and behavior
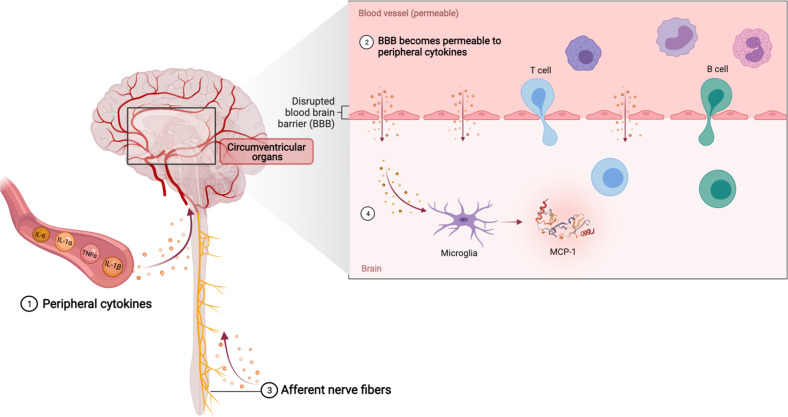
Since PTSD is a brain disorder, there has been a great interest in understanding the mechanisms of neuroinflammation and communication between the brain and the immune system, starting with how peripheral inflammatory responses affect brain function. To date, several mechanisms have been proposed to explain how peripheral inflammatory signals impact the brain (reviewed by [122, 123], Fig. 3): (1) cytokine-specific saturable transporters actively transport some peripheral cytokines (e.g., IL-1α, IL-1β, IL-6, TNF-α), (2) peripheral cytokines pass through the leaky regions in the blood–brain barrier (BBB), (3) activated cytokine receptors on afferent nerve fibers (e.g., vagal nerve) transmit cytokine signals to relevant regions of the brain, and (4) microglial cells that are activated in response to peripheral cytokine signaling produce monocyte chemoattractant protein (MCP-1), which attracts activated peripheral cell types, including monocytes, macrophages and T cells to the brain. Activated microglia and astrocytes also produce cytokines to promote neuroinflammation [18].
Fig. 3. Trafficking of peripheral inflammatory signals to brain.
(1) Active transport of peripheral cytokines. (2) Passage of peripheral cytokines through leaky regions of blood–brain barrier (BBB). (3) Transmission of peripheral cytokine signals to the brain by activated cytokine receptors on afferent nerve fibers. (4) Trafficking of peripheral cell types (e.g., monocytes, macrophages, and T cells) in response to monocyte chemoattractant protein (MCP-1) release by activated microglia.
Microglia are the resident innate immune-cell type in the brain that are responsible for trophic support, chemotaxis, synaptogenesis, and neurogenesis [124]. Peripheral inflammation activates microglia and prevents microglia from exerting their homeostatic functions [124]. Instead, activated microglia produce pro- and anti-inflammatory cytokines that modulate the stress response in the brain, as noted in animal models of stress and PTSD [125–127]. The inflammatory markers released from microglia induce astrocytes to produce cytokines, which, in turn, activate microglia in a positive feedback loop [128]. Multiple studies in animal models demonstrate microglial activation upon stress through increased pro-inflammatory cytokine response [125, 126, 129] or elevated microglial markers [129, 130]; yet studies of depressed patients and controls showed no correlations between microglial activation and peripheral pro-inflammatory cytokine levels [131, 132]. In contrast, a recent study reported lower microglial activation in post-mortem brain samples of PTSD patients [133]. The authors also demonstrated a negative correlation between microglial activation and plasma CRP levels [133]. The inconsistencies in stress-induced microglial activation might be due to differences between species, stress type, or analysis strategies to assess microglial activation (e.g., immunohistochemistry vs. neuroimaging).
Effect of inflammation on neurocircuitry relevant to fear and anxiety
PTSD is associated with alterations in the brain regions relevant to fear, anxiety, and threat detection, such as the amygdala, hippocampus, medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC), and insula [17, 123]. Hence, evaluating the effect of inflammation on these regions is relevant to understanding the behavioral changes associated with PTSD.
The amygdala is the brain region responsible for fear and anxiety responses and is hyperresponsive in individuals with PTSD [123]. Multiple neuroimaging studies reported an association between heightened amygdala activation upon stress and increased proinflammatory cytokine levels. For instance, increased IL-6 and TNF-α concentrations following endotoxin administration in healthy individuals resulted in increased amygdala activity in response to socially threatening images [134]. Increased amygdala response to congruent and incongruent stimuli was also associated with increased IL-6 levels upon vaccination [135]. Notably, increased pro-inflammatory cytokine levels and amygdala activation are also associated with social disconnection, depressed mood, cognitive disturbance, and fatigue [135, 136].
The hippocampus is involved in fear and memory processing [137, 138]. Importantly, individuals with PTSD have smaller hippocampal volume [139]. In addition, reduced hippocampal volumes are associated with increased inflammation [140]. The effect of inflammation on the hippocampus was assessed in rodent models [141, 142], which suggests that microglial release of cytokines suppresses neurogenesis and stimulates apoptosis of neuronal progenitor cells [143]. Studies also showed the inhibitory effect of IL-1β on long-term potentiation in the hippocampus [144], as well as spatial and contextual memory processing [145]. Hence detrimental effects of inflammation on the hippocampus may be an underlying contributor to cognitive and emotional problems associated with PTSD.
Through their connections to the amygdala and hippocampus, the mPFC regions, including the rostral ACC, subgenual ACC (sgACC, Brodmann’s Area 25), and the medial frontal cortex, play an important role in emotional regulation and fear extinction in PTSD [17]. Multiple studies investigated the effect of peripheral inflammatory markers on mPFC activity in response to stress or upon cytokine inducement [146, 147]. For instance, activation of the ventral mPFC, including the sgACC and the orbitofrontal cortex (OFC), in response to a grief-elicitation task associated with elevated IL-1β and sTNF-RII levels in grieving women [147]. Likewise, elevated IL-6 levels following typhoid vaccination led to increased sgACC activity, which is correlated with mood deterioration, and decreased connectivity of the sACC to the amygdala and mPFC [146]. In addition, increased plasma levels of CRP and IL-6 were correlated with reduced connectivity between the striatum and the ventral mPFC in depressed patients [148]. Finally, exposure to an acute laboratory-based social stressor led to an increase in IL-6 levels, which was associated with stronger functional connectivity between the right amygdala and the dorsomedial PFC [136]. Overall, this evidence links mPFC activation and inflammation in emotion processing following trauma or stress.
The dorsal ACC (dACC, Brodmann’s Area 24) is involved in emotional and physical stress response through threatening social and physical pain stimuli detection and response [123]. Hyperactivation of the dACC is associated with PTSD [137, 149–152] and has been shown to mediate hyperarousal symptoms of PTSD [153]. Neuroimaging studies also demonstrated activation in response to inflammation. For example, IFN-α treatment of hepatitis C patients led to heightened dACC activation, which is associated with visual–spatial-attention errors [154]. Similarly, elevated IL-6 concentrations following typhoid injection are associated with increased dACC activation [135]. Finally, increased IL-6 levels upon endotoxin administration have been shown to be associated with augmented neural activity related to social pain in the dACC of women [155], consistent with sex-specific immune responses in PTSD. Taken together, these data suggest that dACC hyperactivation in response to inflammation may underlie some of the behavioral changes observed in PTSD.
The insula is involved in the emotional distress symptoms of PTSD and plays an important role in interoception (i.e., sense of body’s physiological state) [123]. The insula is activated by peripheral inflammatory stimuli, which is expected considering the role of this region in perceiving the signals from the body [135]. Lower insula activation in response to changes in interceptive responses associated with PTSD in women exposed to intimate partner violence [156]. Likewise, women with PTSD related to intimate partner violence exhibited heightened activation of the insula and the amygdala, as well as weaker functional coupling among the insula, amygdala, and ACC during an emotional face-matching task [157]. Notably, the insula is also a target of the peripheral inflammatory response, such that IL-6 increases following stimulation of innate immunity led to heightened insula activity in response to congruent and incongruent stimuli [135]. Moreover, increased IL-6 and TNF-α levels following endotoxin administration are associated with increased glucose metabolism in the insula, as well as behavioral changes, including fatigue and lower social interest [158]. Similarly, increased IL-6 levels are associated with higher insula activity in response to social pain in women [159]. Hence, pro-inflammatory cytokines may lead to insula hyperactivity and alter the neural circuitry of the amygdala, mPFC, and ACC, thereby contributing to PTSD symptomatology relevant to fear and emotion processing.
Possible mechanism by which inflammation alters neurotransmitter function
Cytokines are critical to maintaining neural homeostasis, by participating in neural plasticity, including neurogenesis, synaptic pruning and remodeling, long-term potentiation, learning, and memory [145]. However, increased inflammatory signaling has detrimental effects on neurotransmitter systems related to the behavior and emotional characteristics of PTSD, such as serotonin, norepinephrine, dopamine, and glutamate [17, 122]. Inflammatory cytokines can alter neurotransmitter functions by influencing synthesis, reuptake, and release of neurotransmitters [122].
Serotonin is a monoamine neurotransmitter that is widely implicated in the etiology and pathophysiology of PTSD [160]. Serotonergic signaling may be influenced by the immune system in PTSD. Rats immunized with Mycobacterium vaccae, which exerts immunoregulatory properties through the production of anti-inflammatory cytokines, showed enhanced fear extinction and altered serotonergic gene expression in the brainstem [161, 162].
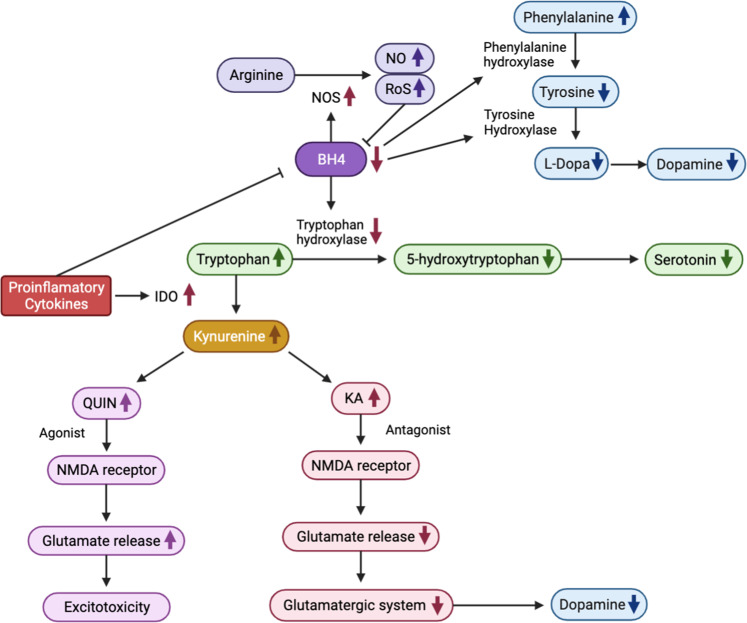
Cytokines can influence serotonin synthesis through the kynurenine pathway (Fig. 4). Pro-inflammatory cytokines increase the activity of indoleamine 2,3-dioxygenase (IDO), which converts tryptophan, the primary amino acid of serotonin, into kynurenine [122]. Hence, increased IDO activity in response to inflammation leads to serotonin depletion in the brain, as observed in animal models [163, 164]. In human studies, IFN-α therapy led to increased kynurenine and decreased tryptophan levels that were associated with symptoms of depression and anxiety, as well as cognitive problems, including memory disturbances and confusion [165, 166]. Another study of IFN-α therapy reported higher TNF-α and lower serotonin concentrations associated with somatic symptoms, including fatigue, loss of appetite, and irritability [167]. These findings suggest that pro-inflammatory cytokines may reduce serotonin concentration by acting on the kynurenine pathway, leading to cognitive and somatic symptoms relevant to PTSD.
Fig. 4. Pro-inflammatory cytokine-induced changes in neurotransmitter systems.
Mechanisms by which proinflammatory cytokines affect the synthesis of monoamine neurotransmitters (i.e., serotonin and dopamine) are illustrated. BH4 tetrahydrobiopterin, IDO indoleamine 2,3-dioxygenase, KA kynurenic acid, NMDA N-methyl-d-aspartate, NO nitric oxide, NOS nitric oxide synthases, QUIN quinolinic acid, ROS reactive oxygen species.
Cytokines also downregulate serotonin synthesis by decreasing the activity of tetrahydrobiopterin (BH4), an enzyme co-factor of tryptophan hydroxylase and a rate-limiting enzyme in serotonin synthesis [122] (Fig. 4). Cytokines can increase the expression and function of serotonin transporters by stimulating the p38 mitogen-activated protein kinase MAPK pathway. Several in vitro studies have shown increased expression and activity of the serotonin transporter through activation of MAPK following TNF-α and IL-1β stimulation [168, 169]. Importantly, fluoxetine, a selective serotonin reuptake inhibitor (SSRI), suppressed the expression of IL-1β, IFN-γ, and TNF-α in the rat hippocampal dentate gyrus and downregulated MAPK signaling [170].
Dopamine plays an important role in PTSD symptom clusters, including re-experiencing symptoms and negative mood and cognition [171]. Cytokines reduce dopamine levels by diminishing the activity of BH4, a co-factor of tyrosine hydroxylase and phenylalanine hydroxylase, the rate-limiting enzymes for dopamine synthesis [122] (Fig. 4). Inflammation can also induce nitric oxide synthase (NOS), the enzyme responsible for converting arginine into nitric oxide (NO), which uses BH4 as a co-factor [122]. Depletion of BH4 in turn leads to NOS uncoupling and production of ROS in the brain. Since BH4 is highly sensitive to oxidative stress, ROS promotes irreversible degradation of BH4, further limiting BH4 availability [123]. In fact, treatment of sympathetic neurons with IL-6 led to lower BH4 levels [172]. Similarly, IFN-α-treated patients exhibited decreased BH4 activity [173, 174], which was associated with lower dopamine levels in their cerebrospinal fluid (CSF) [173].
Glutamate is involved in motivation and motor functions. Inflammation leads to increased glutamate levels, contributing to symptoms of emotional numbness in PTSD [123]. The glutamatergic system partly regulates dopamine release, such that the effect of cytokines on the kynurenine pathway also impacts dopamine synthesis. As discussed above (Fig. 4), cytokines enhance IDO activity, leading to an increase in kynurenine production. Kynurenine can be broken down to kynurenic acid (KA) in astrocytes and quinolinic acid (QUIN) in microglia [123]. Notably, patients undergoing IFN-α treatment showed increased KA and QUIN concentrations in plasma and CSF [122, 123]. KA is an N-methyl-D-aspartate (NMDA) receptor antagonist that inhibits glutamate release and has downstream effects on dopamine [175, 176]. QUIN, an N-NMDA receptor agonist, can activate glutamate release from astrocytes, thereby contributing to excitotoxicity in the brain [123, 175]. Patients undergoing IFN-α treatment showed increased glutamate to creatinine levels in the dACC, which was associated with depressive symptoms [177]. Elevated CRP levels associated with symptoms of depression led to increased basal ganglia glutamate levels [178]. The increased glutamate release from astrocytes reduced brain-derived neurotrophic factor (BDNF), which is essential for neurogenesis and associated with disrupted contextual fear memory in PTSD [179, 180].
The effect of inflammation on other neurotransmitter systems related to PTSD, such as gamma-aminobutyric acid (GABA) and acetylcholine was less studied. PTSD patients were shown to have lower GABA levels in insula [181]. Notably, GABA decreased the production of inflammatory cytokines by suppressing the NF-kB and p38 MAPK pathways in rodents [182]. In addition, the expression and activity of acetylcholinesterase were induced by proinflammatory cytokines, which inhibited the release of acetylcholine from hippocampal neurons [183, 184]. Inhibition of acetylcholine release may, in turn, contribute to inflammation, as acetylcholine can downregulate peripheral cytokine production via the “cholinergic anti-inflammatory reflex.” [122]. These data suggest that cytokines may reduce the release of GABA and acetylcholine, which both have anti-inflammatory effects, thereby leading to an inflammatory environment.
Taken together, studies collectively suggest that trauma may lead to HPA axis and SNS activation that increases proinflammatory cytokine production and subsequent neurotransmitter signaling that increases the risk of fear and anxiety symptomatology. Ultimately, this cascade may contribute to the risk of PTSD onset.
Potential anti-inflammatory therapeutic approaches
Currently, only two selective SSRIs, paroxetine and sertraline, are approved by the FDA for the treatment of PTSD [18]. However, the response rates of these SSRIs are lower than 65% [185–188], indicating that a large proportion of PTSD patients do not respond to SSRI treatment. Hence, given the inflammatory characteristic of PTSD, strategies that reduce inflammation and/or its effects on the brain may provide new avenues for the development of curative or preventative treatments to be used as an adjuvant to SSRIs or in combination with behavioral approaches.
Monoclonal antibodies to cytokines and their receptors, including TNF, IL-1, IL-6R, IL-12/23, and IL-17, are approved by the FDA for the treatment of autoimmune diseases and cancers. Given the increased IL-1β, IL-6, and TNF-α levels in PTSD, blocking these cytokines may be a straightforward treatment strategy. Although there are no reports of monoclonal antibody use for the treatment of PTSD, multiple studies reported that etanercept (TNF inhibitor), adalimumab (TNF inhibitor), and ustekinumab (IL-12/23 inhibitor) reduced symptoms of depression and anxiety in individuals with psoriasis [189–192]. However, Raison et al. [193] showed that infliximab (TNF inhibitor) was only effective in treatment-resistant depressed patients with higher baseline inflammation. Further, a clinical trial in patients with bipolar depression reported that baseline inflammation moderated the effect of infliximab on reducing anhedonia symptoms [194].
Non-steroidal anti-inflammatory drugs (NSAIDs) and cyclooxygenase 2 (COX-2) inhibitors negatively regulate proinflammatory cytokine production, and thus reduce inflammation. The COX-2 inhibitor celecoxib was shown to improve depression symptoms in patients with the major depressive disorder [195–197], potentially decreasing IL-6 levels [197]. Although no clinical studies evaluated NSAIDs and COX-2 inhibitors for the treatment of PTSD, COX-2 inhibitors were shown to reduce anxiety in mice exposed to stress [198]. In addition, ibuprofen (NSAID) treatment reduced anxiety symptoms in a rat model of PTSD while decreasing expression of TNF-α and IL-1β and increasing BDNF expression in the hippocampus, suggesting that the therapeutic effect of ibuprofen on PTSD was mediated by decreased anti-inflammatory activity and increased BDNF levels in the brain [199].
NACHT domain- leucine-rich repeat- and pyrin domain-containing protein 3 (NLRP3) inflammasome inhibitors block inflammatory cytokine production. Beta-hydroxybutyrate (BHB), an endogenic NLRP3 inflammasome inhibitor, was shown to reduce depressive and anxiety behaviors in rodent models of depression and stress, potentially though decreasing hippocampal TNF-α concentrations [200, 201]. BHB was also effective in reducing anxiety behaviors in rodent models of PTSD and restoring serum TNF-α levels that were elevated in response to single prolonged stress [202].
Glucocorticoids suppress the inflammatory response following stress exposure by promoting the production of anti-inflammatory cytokines and by inhibiting the synthesis of proinflammatory cytokines [18] (Fig. 2). In addition, glucocorticoids participate in transporting protective T cells to the brain during acute trauma [203]. Given the low cortisol concentrations in individuals with PTSD [18], clinical studies examined the effectiveness of synthetic glucocorticoids for treatment of PTSD or preventing PTSD development following acute traumatic stress. Clinical trials showed that stand-alone glucocorticoid treatment or glucocorticoid augmentation combined with psychotherapy improved PTSD symptoms [204–207]. However, a recent clinical trial testing the effectiveness of augmentation of prolonged exposure (PE) with glucocorticoid reported that glucocorticoid augmentation did not significantly ameliorate PTSD symptoms [208]. Still, their exploratory analyses showed that glucocorticoid augmentation improved hyperarousal symptoms in veterans who experienced mild traumatic brain injury and reduced avoidance symptoms in veterans with increased baseline glucocorticoid sensitivity [208]. Moreover, studies investigating the preventative effect of glucocorticoids following exposure to trauma showed that glucocorticoid treatment following acute trauma significantly reduced stress symptoms [209, 210] and decreased the incidence of PTSD [211, 212]. Recently, a large meta-analysis of randomized controlled trials of glucocorticoid treatment reported that, although glucocorticoid treatment alleviated PTSD symptoms, preventative glucocorticoid administration following acute trauma was more effective [213].
Noradrenergic beta-receptor blockers (e.g., propranolol) inhibit norepinephrine signaling that promotes the production of pro-inflammatory cytokines [53, 54]. Studies of animal models showed that propranolol administration following stress exposure reduced pro-inflammatory cytokine levels and abrogated stress-induced changes in the immune-cell composition [214, 215]. Importantly, blockage of noradrenergic beta-receptors has been shown to inhibit reconsolidation of fear memory [216]. Indeed, clinical trials reported that propanol treatment with memory reactivation therapy reduced PTSD symptoms [217, 218]. Beta-blocker administration for the suspected acute coronary syndrome was also shown to reduce PTSD symptoms at 1-month follow-up [219]. However, research on the preventative effects of propranolol is contradictory. Initial studies reported that propanol treatment initiated within 20 hours of the trauma lowered PTSD incidence 2-months after trauma exposure and reduced physiological reactivity to trauma cues after 3 months [220, 221]. In contrast, Stein et al. [222] reported that propanol administration within 48 hours of trauma had no benefits on PTSD symptoms at 1, 4, and 8 months follow-up. Since propanol appears to act by reducing the effect of SNS arousal on trauma memory consolidation, early initiation of propanol treatment seems to be crucial.
Angiotensin-converting enzyme inhibitors (ACE-I) and angiotensin receptor blockers (ARBs) have been evaluated as possible anti-inflammatory therapeutic strategies, considering they are effective in the treatment of cardiometabolic disorders that are highly co-morbid with PTSD [17]. ACE-I and ARBs exert their anti-inflammatory activity in the brain by reducing the expression and secretion of pro-inflammatory cytokines and decreasing microglial activation [223]. Candesartan (ACE-I) was shown to ameliorate impairment of fear extinction in response to inflammatory activity induced by lipopolysaccharide administration [224]. A cross-sectional clinical observation study reported that individuals with PTSD on ACE-I or ARB treatment have lower hyperarousal symptoms compared to patients not taking these medications [225]. This study also showed that ACE-I or ARB use was associated with lower PTSD symptoms in trauma-exposed individuals [225]. However, a recent clinical trial reported that losartan (ARB) did not ameliorate PTSD symptoms [226].
Cannabinoids are also considered for PTSD treatment due to their anti-inflammatory effects. Endocannabinoid (eCB) signaling from macrophage and monocyte cells, including microglia in the brain, participates in inflammatory processes related to PTSD. While deficient eCB signaling promotes inflammation, augmented eCB signaling suppresses inflammation by reducing the secretion of pro-inflammatory cytokines, inhibiting NF-kB-mediated inflammatory gene transcription, decreasing microglial activation, and promoting the release of anti-inflammatory cytokines [227, 228]. Multiple studies showed that nabilone, a synthetic cannabinoid, reduced PTSD-related nightmares and insomnia and improved PTSD symptoms [229–231].
FTY720 (Fingolimod), approved for the treatment of MS, has drawn researchers’ interest for PTSD treatment. Fingolimod is a synthetic analog of sphingosine that non-selectively binds to sphingosine-1-phosphate receptors (S1PRs). Decreased S1PRs activity in response to fingolimod prevents leukocyte migration from lymphocytes to the CNS, thereby suppressing the immune response [232]. Fingolimod was shown to promote neurogenesis, which correlated with improved contextual fear memory in mouse models [233, 234]. Notably, Fingolimod decreased despair and social anxiety-like behavior and reduced blood lymphocyte counts in a rat model of stress by reducing vascular remodeling in the brain [235].
Psychotherapy and behavioral interventions are effective treatments for PTSD and may reduce inflammation by reducing perceived stress and increasing emotion regulation [236]. Currently, there are no studies evaluating the effect of gold-standard PTSD psychotherapies, including PE, on inflammation. Nevertheless, other forms of psychotherapy, including eye movement desensitization, were associated with alterations in TNF-α in soldiers with PTSD [237]. A recent clinical trial of reminder-focused positive psychiatry on attention-deficit hyperactive disorder and PTSD reported a decrease in CRP levels at 6 weeks follow-up [238]. Moreover, other behavioral interventions, including yoga and mindfulness, are associated with decreases in inflammation [236, 239].
Concluding remarks
A growing body of evidence indicates an inflammatory environment in individuals with PTSD. However, many of the epidemiologic studies were limited by the fact that they only investigated specific peripheral cytokines and potentially missed key regulators in the process. Animal models deficient in key inflammatory genes may help identify mechanisms underlying the relationship between inflammation and anxiety-like or social behaviors relevant to PTSD. Similarly, longitudinal epidemiologic studies will be necessary to understand the directionality between PTSD and inflammation as well as immune-related conditions co-morbid with PTSD. For instance, since the gut-brain axis plays an important role in both PTSD and IBD, microbiome studies of PTSD coupled with metabolomics may advance our understanding of this comorbidity.
It is also unclear how peripheral immune alterations are indicative of neuroinflammation. Findings from imaging studies support a link between peripheral inflammation and both cognitive and emotional problems associated with PTSD through alterations in neurocircuitry relevant to fear and anxiety (reviewed in [123]). While this is encouraging, many of the reports of changes in neurotransmitter function in response to inflammation were from studies of depression, and additional research targeting PTSD is required to fully understand how the alterations in neurotransmitter function are relevant to PTSD symptoms.
Finally, the link between PTSD, inflammation, and the brain paves the way for potential anti-inflammatory treatment or preventative therapies. Although multiple anti-inflammatory treatment strategies showed promising results in pre-clinical settings and clinical trials, clinical studies with larger sample sizes and more diverse populations are warranted to fully understand the therapeutic mechanism of action, effectiveness, and possible side effects of these anti-inflammatory treatments.
Acknowledgements
This work was funded by grants from the National Institute of Mental Health R01MH108826 (AKS) and MH115174 (VM). Figures were created with BioRender.com.
Author contributions
SK conceived the idea, designed the review structure, and wrote the manuscript. NCSO contributed to sections on HPA axis and ANS. JCF contributed to sections on neuroimaging and treatment, and reviewed and edited other sections of the paper. VM contributed to sections on immune biomarkers, other drivers of inflammation in PTSD, and treatment, and reviewed and edited other sections of the paper. AKS conceptualized the paper and its organization, comprehensively reviewed all sections, and provided critical edits and revisions on all drafts.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 2013.
- 2.Benjet C, Bromet E, Karam EG, Kessler RC, McLaughlin KA, Ruscio AM, et al. The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychol Med. 2016;46:327–43. doi: 10.1017/S0033291715001981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allgire E, McAlees JW, Lewkowich IP, Sah R. Asthma and posttraumatic stress disorder (PTSD): emerging links, potential models and mechanisms. Brain Behav Immun. 2021;97:275–85. [DOI] [PMC free article] [PubMed]
- 4.O’Donnell CJ, Schwartz Longacre L, Cohen BE, Fayad ZA, Gillespie CF, Liberzon I, et al. Posttraumatic stress disorder and cardiovascular disease: state of the science, knowledge gaps, and research opportunities. JAMA Cardiol. 2021;6:1207–16. [DOI] [PubMed]
- 5.Wilson MA, Liberzon I, Lindsey ML, Lokshina Y, Risbrough VB, Sah R, et al. Common pathways and communication between the brain and heart: connecting post-traumatic stress disorder and heart failure. Stress. 2019;22:530–47. doi: 10.1080/10253890.2019.1621283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michopoulos V, Vester A, Neigh G. Posttraumatic stress disorder: a metabolic disorder in disguise? Exp Neurol. 2016;284:220–9. doi: 10.1016/j.expneurol.2016.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bookwalter DB, Roenfeldt KA, LeardMann CA, Kong SY, Riddle MS, Rull RP. Posttraumatic stress disorder and risk of selected autoimmune diseases among US military personnel. BMC Psychiatry. 2020;20:23. doi: 10.1186/s12888-020-2432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Donovan A, Cohen BE, Seal KH, Bertenthal D, Margaretten M, Nishimi K, et al. Elevated risk for autoimmune disorders in iraq and afghanistan veterans with posttraumatic stress disorder. Biol Psychiatry. 2015;77:365–74. doi: 10.1016/j.biopsych.2014.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boscarino JA. Posttraumatic stress disorder and physical illness: results from clinical and epidemiologic studies. Ann N Y Acad Sci. 2004;1032:141–53. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- 10.Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, et al. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71:423–31. doi: 10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen M, Meir T, Klein E, Volpin G, Assaf M, Pollack S. Cytokine levels as potential biomarkers for predicting the development of posttraumatic stress symptoms in casualties of accidents. Int J Psychiatry Med. 2011;42:117–31. doi: 10.2190/PM.42.2.b. [DOI] [PubMed] [Google Scholar]
- 12.Pervanidou P, Kolaitis G, Charitaki S, Margeli A, Ferentinos S, Bakoula C, et al. Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology. 2007;32:991–9. doi: 10.1016/j.psyneuen.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Solomon Z, Levin Y, Assayag EB, Furman O, Shenhar-Tsarfaty S, Berliner S, et al. The Implication of Combat Stress and PTSD trajectories in metabolic syndrome and elevated c-reactive protein levels: a longitudinal study. J Clin Psychiatry. 2017;78:e1180–e1186. doi: 10.4088/JCP.16m11344. [DOI] [PubMed] [Google Scholar]
- 14.Toft H, Bramness JG, Lien L, Abebe DS, Wampold BE, Tilden T, et al. PTSD patients show increasing cytokine levels during treatment despite reduced psychological distress. Neuropsychiatr Dis Treat. 2018;14:2367–78. doi: 10.2147/NDT.S173659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sumner JA, Chen Q, Roberts AL, Winning A, Rimm EB, Gilsanz P, et al. Posttraumatic stress disorder onset and inflammatory and endothelial function biomarkers in women. Brain Behav Immun. 2018;69:203–9. doi: 10.1016/j.bbi.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muniz Carvalho C, Wendt FR, Maihofer AX, Stein DJ, Stein MB, Sumner JA, et al. Dissecting the genetic association of C-reactive protein with PTSD, traumatic events, and social support. Neuropsychopharmacology. 2021;46:1071–7. doi: 10.1038/s41386-020-0655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T. Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology. 2017;42:254–70. doi: 10.1038/npp.2016.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hori H, Kim Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin Neurosci. 2019;73:143–53. doi: 10.1111/pcn.12820. [DOI] [PubMed] [Google Scholar]
- 19.Sumner JA, Nishimi KM, Koenen KC, Roberts AL, Kubzansky LD. Posttraumatic stress disorder and inflammation: untangling issues of bidirectionality. Biol Psychiatry. 2020;87:885–97. doi: 10.1016/j.biopsych.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J, et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry. 2015;2:1002–12. doi: 10.1016/S2215-0366(15)00309-0. [DOI] [PubMed] [Google Scholar]
- 21.Yang JJ, Jiang W. Immune biomarkers alterations in post-traumatic stress disorder: a systematic review and meta-analysis. J Affect Disord. 2020;268:39–46. doi: 10.1016/j.jad.2020.02.044. [DOI] [PubMed] [Google Scholar]
- 22.Yuan N, Chen Y, Xia Y, Dai J, Liu C. Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl Psychiatry. 2019;9:233. doi: 10.1038/s41398-019-0570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teche SP, Rovaris DL, Aguiar BW, Hauck S, Vitola ES, Bau CHD, et al. Resilience to traumatic events related to urban violence and increased IL10 serum levels. Psychiatry Res. 2017;250:136–40. doi: 10.1016/j.psychres.2017.01.072. [DOI] [PubMed] [Google Scholar]
- 24.Stein MB, Chen CY, Ursano RJ, Cai T, Gelernter J, Heeringa SG, et al. Genome-wide association studies of posttraumatic stress disorder in 2 cohorts of US army soldiers. JAMA Psychiatry. 2016;73:695–704. doi: 10.1001/jamapsychiatry.2016.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen CY, Choi KW, et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun. 2019;10:4558. doi: 10.1038/s41467-019-12576-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de Los Santos R, et al. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci USA. 2010;107:9470–5. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, et al. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:700–8. doi: 10.1002/ajmg.b.31212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith AK, Ratanatharathorn A, Maihofer AX, Naviaux RK, Aiello AE, Amstadter AB, et al. Epigenome-wide meta-analysis of PTSD across 10 military and civilian cohorts identifies methylation changes in AHRR. Nat Commun. 2020;11:5965. doi: 10.1038/s41467-020-19615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snijders C, Maihofer AX, Ratanatharathorn A, Baker DG, Boks MP, Geuze E, et al. Longitudinal epigenome-wide association studies of three male military cohorts reveal multiple CpG sites associated with post-traumatic stress disorder. Clin Epigenetics. 2020;12:11. doi: 10.1186/s13148-019-0798-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katrinli S, Zheng Y, Gautam A, Hammamieh R, Yang R, Venkateswaran S, et al. PTSD is associated with increased DNA methylation across regions of HLA-DPB1 and SPATC1L. Brain Behav Immun 2020;91:429–36. [DOI] [PMC free article] [PubMed]
- 31.Breen MS, Tylee DS, Maihofer AX, Neylan TC, Mehta D, Binder EB, et al. PTSD blood transcriptome mega-analysis: shared inflammatory pathways across biological sex and modes of trauma. Neuropsychopharmacology. 2018;43:469–81. doi: 10.1038/npp.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuan PF, Waszczuk MA, Kotov R, Clouston S, Yang X, Singh PK, et al. Gene expression associated with PTSD in World Trade Center responders: an RNA sequencing study. Transl Psychiatry. 2017;7:1297. doi: 10.1038/s41398-017-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuan PF, Yang X, Clouston S, Ren X, Kotov R, Waszczuk M, et al. Cell type-specific gene expression patterns associated with posttraumatic stress disorder in World Trade Center responders. Transl Psychiatry. 2019;9:1. doi: 10.1038/s41398-018-0355-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Donovan A, Sun B, Cole S, Rempel H, Lenoci M, Pulliam L, et al. Transcriptional control of monocyte gene expression in post-traumatic stress disorder. Dis Markers. 2011;30:123–32. doi: 10.1155/2011/560572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pace TW, Wingenfeld K, Schmidt I, Meinlschmidt G, Hellhammer DH, Heim CM. Increased peripheral NF-kappaB pathway activity in women with childhood abuse-related posttraumatic stress disorder. Brain Behav Immun. 2012;26:13–17. doi: 10.1016/j.bbi.2011.07.232. [DOI] [PubMed] [Google Scholar]
- 36.Jergovic M, Bendelja K, Savic Mlakar A, Vojvoda V, Aberle N, Jovanovic T, et al. Circulating levels of hormones, lipids, and immune mediators in post-traumatic stress disorder - a 3-month follow-up study. Front Psychiatry. 2015;6:49. doi: 10.3389/fpsyt.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sumner JA, Chen Q, Roberts AL, Winning A, Rimm EB, Gilsanz P, et al. Cross-sectional and longitudinal associations of chronic posttraumatic stress disorder with inflammatory and endothelial function markers in women. Biol Psychiatry. 2017;82:875–84. doi: 10.1016/j.biopsych.2017.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glaus J, von Kanel R, Lasserre AM, Strippoli MF, Vandeleur CL, Castelao E, et al. The bidirectional relationship between anxiety disorders and circulating levels of inflammatory markers: Results from a large longitudinal population-based study. Depress Anxiety. 2018;35:360–71. doi: 10.1002/da.22710. [DOI] [PubMed] [Google Scholar]
- 39.Breen MS, Maihofer AX, Glatt SJ, Tylee DS, Chandler SD, Tsuang MT, et al. Gene networks specific for innate immunity define post-traumatic stress disorder. Mol Psychiatry. 2015;20:1538–45. doi: 10.1038/mp.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glatt SJ, Tylee DS, Chandler SD, Pazol J, Nievergelt CM, Woelk CH, et al. Blood-based gene-expression predictors of PTSD risk and resilience among deployed marines: a pilot study. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:313–26. doi: 10.1002/ajmg.b.32167. [DOI] [PubMed] [Google Scholar]
- 41.Tursich M, Neufeld RW, Frewen PA, Harricharan S, Kibler JL, Rhind SG, et al. Association of trauma exposure with proinflammatory activity: a transdiagnostic meta-analysis. Transl Psychiatry. 2014;4:e413. doi: 10.1038/tp.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baumeister D, Akhtar R, Ciufolini S, Pariante CM, Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-alpha. Mol Psychiatry. 2016;21:642–9. doi: 10.1038/mp.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104:1319–24. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bielas H, Meister-Langraf RE, Schmid JP, Barth J, Znoj H, Schnyder U, et al. C-reactive protein as a predictor of posttraumatic stress induced by acute myocardial infarction. Gen Hosp Psychiatry. 2018;53:125–30. doi: 10.1016/j.genhosppsych.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Michopoulos V, Beurel E, Gould F, Dhabhar FS, Schultebraucks K, Galatzer-Levy I, et al. Association of prospective risk for chronic PTSD symptoms with low TNFalpha and IFNgamma concentrations in the immediate aftermath of trauma exposure. Am J Psychiatry. 2020;177:58–65. doi: 10.1176/appi.ajp.2019.19010039. [DOI] [PubMed] [Google Scholar]
- 46.Alves de Lima K, Rustenhoven J, Da Mesquita S, Wall M, Salvador AF, Smirnov I, et al. Meningeal gammadelta T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat Immunol. 2020;21:1421–9. doi: 10.1038/s41590-020-0776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elkhatib SK, Moshfegh CM, Watson GF, Case AJ. Peripheral inflammation is strongly linked to elevated zero maze behavior in repeated social defeat stress. Brain Behav Immun. 2020;90:279–85. doi: 10.1016/j.bbi.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 49.Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Neural regulation of the stress response: glucocorticoid feedback mechanisms. Braz J Med Biol Res. 2012;45:292–8. doi: 10.1590/S0100-879X2012007500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–28. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wohleb ES, Powell ND, Godbout JP, Sheridan JF. Stress-induced recruitment of bone marrow-derived monocytes to the brain promotes anxiety-like behavior. J Neurosci. 2013;33:13820–33. doi: 10.1523/JNEUROSCI.1671-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA. 2003;100:1920–5. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tan KS, Nackley AG, Satterfield K, Maixner W, Diatchenko L, Flood PM. Beta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanisms. Cell Signal. 2007;19:251–60. doi: 10.1016/j.cellsig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Elkhatib SK, Case AJ. Autonomic regulation of T-lymphocytes: Implications in cardiovascular disease. Pharm Res. 2019;146:104293. doi: 10.1016/j.phrs.2019.104293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–28. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franklin TC, Xu C, Duman RS. Depression and sterile inflammation: essential role of danger associated molecular patterns. Brain Behav Immun. 2018;72:2–13. doi: 10.1016/j.bbi.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 58.Fleshner M, Frank M, Maier SF. Danger signals and inflammasomes: stress-evoked sterile inflammation in mood disorders. Neuropsychopharmacology. 2017;42:36–45. doi: 10.1038/npp.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brahmajothi MV, Abou-Donia MB. PTSD susceptibility and challenges: pathophysiological consequences of behavioral symptoms. Mil Med. 2020;185:279–85. doi: 10.1093/milmed/usz321. [DOI] [PubMed] [Google Scholar]
- 60.Wang XW, Karki A, Du DY, Zhao XJ, Xiang XY, Lu ZQ. Plasma levels of high mobility group box 1 increase in patients with posttraumatic stress disorder after severe blunt chest trauma: a prospective cohort study. J Surg Res. 2015;193:308–15. doi: 10.1016/j.jss.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 61.Allgire E, McAlees JW, Lewkowich IP, Sah R. Asthma and posttraumatic stress disorder (PTSD): emerging links, potential models and mechanisms. Brain Behav Immun. 2021;97:275–85. doi: 10.1016/j.bbi.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edmondson D, Kronish IM, Shaffer JA, Falzon L, Burg MM. Posttraumatic stress disorder and risk for coronary heart disease: a meta-analytic review. Am Heart J. 2013;166:806–14. doi: 10.1016/j.ahj.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roy SS, Foraker RE, Girton RA, Mansfield AJ. Posttraumatic stress disorder and incident heart failure among a community-based sample of US veterans. Am J Public Health. 2015;105:757–63. doi: 10.2105/AJPH.2014.302342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fudim M, Cerbin LP, Devaraj S, Ajam T, Rao SV, Kamalesh M. Post-traumatic stress disorder and heart failure in men within the veteran affairs health system. Am J Cardiol. 2018;122:275–8. doi: 10.1016/j.amjcard.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Turner JH, Neylan TC, Schiller NB, Li Y, Cohen BE. Objective evidence of myocardial ischemia in patients with posttraumatic stress disorder. Biol Psychiatry. 2013;74:861–6. doi: 10.1016/j.biopsych.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Edmondson D, Richardson S, Falzon L, Davidson KW, Mills MA, Neria Y. Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: a meta-analytic review. PLoS One. 2012;7:e38915. doi: 10.1371/journal.pone.0038915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gander ML, von Kanel R. Myocardial infarction and post-traumatic stress disorder: frequency, outcome, and atherosclerotic mechanisms. Eur J Cardiovasc Prev Rehabil. 2006;13:165–72. doi: 10.1097/01.hjr.0000214606.60995.46. [DOI] [PubMed] [Google Scholar]
- 68.Edmondson D, Cohen BE. Posttraumatic stress disorder and cardiovascular disease. Prog Cardiovasc Dis. 2013;55:548–56. doi: 10.1016/j.pcad.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shemesh E, Yehuda R, Milo O, Dinur I, Rudnick A, Vered Z, et al. Posttraumatic stress, nonadherence, and adverse outcome in survivors of a myocardial infarction. Psychosom Med. 2004;66:521–6. doi: 10.1097/01.psy.0000126199.05189.86. [DOI] [PubMed] [Google Scholar]
- 70.Ulleryd MA, Prahl U, Borsbo J, Schmidt C, Nilsson S, Bergstrom G, et al. The association between autonomic dysfunction, inflammation and atherosclerosis in men under investigation for carotid plaques. PLoS One. 2017;12:e0174974. doi: 10.1371/journal.pone.0174974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fonkoue IT, Marvar PJ, Norrholm S, Li Y, Kankam ML, Jones TN, et al. Symptom severity impacts sympathetic dysregulation and inflammation in post-traumatic stress disorder (PTSD) Brain Behav Immun. 2020;83:260–9. doi: 10.1016/j.bbi.2019.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moshfegh CM, Elkhatib SK, Collins CW, Kohl AJ, Case AJ. Autonomic and redox imbalance correlates with t-lymphocyte inflammation in a model of chronic social defeat stress. Front Behav Neurosci. 2019;13:103. doi: 10.3389/fnbeh.2019.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boscarino JA. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosom Med. 2008;70:668–76. doi: 10.1097/PSY.0b013e31817bccaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Case AJ, Roessner CT, Tian J, Zimmerman MC. Mitochondrial superoxide signaling contributes to norepinephrine-mediated t-lymphocyte cytokine profiles. PLoS One. 2016;11:e0164609. doi: 10.1371/journal.pone.0164609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moshfegh CM, Collins CW, Gunda V, Vasanthakumar A, Cao JZ, Singh PK, et al. Mitochondrial superoxide disrupts the metabolic and epigenetic landscape of CD4(+) and CD8(+) T-lymphocytes. Redox Biol. 2019;27:101141. doi: 10.1016/j.redox.2019.101141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Case AJ, Zimmerman MC. Sympathetic-mediated activation versus suppression of the immune system: consequences for hypertension. J Physiol. 2016;594:527–36. doi: 10.1113/JP271516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Toole BI, Catts SV. Trauma, PTSD, and physical health: an epidemiological study of Australian Vietnam veterans. J Psychosom Res. 2008;64:33–40. doi: 10.1016/j.jpsychores.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 78.Shiratori Y, Samuelson KW. Relationship between posttraumatic stress disorder and asthma among New York area residents exposed to the World Trade Center disaster. J Psychosom Res. 2012;73:122–5. doi: 10.1016/j.jpsychores.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 79.Hung YH, Cheng CM, Lin WC, Bai YM, Su TP, Li CT, et al. Post-traumatic stress disorder and asthma risk: a nationwide longitudinal study. Psychiatry Res. 2019;276:25–30. doi: 10.1016/j.psychres.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 80.Kean EM, Kelsay K, Wamboldt F, Wamboldt MZ. Posttraumatic stress in adolescents with asthma and their parents. J Am Acad Child Adolesc Psychiatry. 2006;45:78–86. doi: 10.1097/01.chi.0000186400.67346.02. [DOI] [PubMed] [Google Scholar]
- 81.Zhou J, Nagarkatti P, Zhong Y, Ginsberg JP, Singh NP, Zhang J, et al. Dysregulation in microRNA expression is associated with alterations in immune functions in combat veterans with post-traumatic stress disorder. PLoS One. 2014;9:e94075. doi: 10.1371/journal.pone.0094075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maloley PM, England BR, Sayles H, Thiele GM, Michaud K, Sokolove J, et al. Post-traumatic stress disorder and serum cytokine and chemokine concentrations in patients with rheumatoid arthritis() Semin Arthritis Rheum. 2019;49:229–35. doi: 10.1016/j.semarthrit.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Z, Mandel H, Levingston CA, Young MRI. An exploratory approach demonstrating immune skewing and a loss of coordination among cytokines in plasma and saliva of Veterans with combat-related PTSD. Hum Immunol. 2016;77:652–7. doi: 10.1016/j.humimm.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Song H, Fang F, Tomasson G, Arnberg FK, Mataix-Cols D, Fernandez de la Cruz L, et al. Association of stress-related disorders with subsequent autoimmune disease. JAMA. 2018;319:2388–2400. doi: 10.1001/jama.2018.7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boscarino JA, Chang J. Higher abnormal leukocyte and lymphocyte counts 20 years after exposure to severe stress: research and clinical implications. Psychosom Med. 1999;61:378–86. doi: 10.1097/00006842-199905000-00019. [DOI] [PubMed] [Google Scholar]
- 86.Ironson G, Wynings C, Schneiderman N, Baum A, Rodriguez M, Greenwood D, et al. Posttraumatic stress symptoms, intrusive thoughts, loss, and immune function after Hurricane Andrew. Psychosom Med. 1997;59:128–41. doi: 10.1097/00006842-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 87.Rosenbaum S, Stubbs B, Ward PB, Steel Z, Lederman O, Vancampfort D. The prevalence and risk of metabolic syndrome and its components among people with posttraumatic stress disorder: a systematic review and meta-analysis. Metabolism. 2015;64:926–33. doi: 10.1016/j.metabol.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 88.Blessing EM, Reus V, Mellon SH, Wolkowitz OM, Flory JD, Bierer L, et al. Biological predictors of insulin resistance associated with posttraumatic stress disorder in young military veterans. Psychoneuroendocrinology. 2017;82:91–97. doi: 10.1016/j.psyneuen.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 89.van den Berk-Clark C, Secrest S, Walls J, Hallberg E, Lustman PJ, Schneider FD, et al. Association between posttraumatic stress disorder and lack of exercise, poor diet, obesity, and co-occuring smoking: a systematic review and meta-analysis. Health Psychol. 2018;37:407–16. doi: 10.1037/hea0000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mellon SH, Gautam A, Hammamieh R, Jett M, Wolkowitz OM. Metabolism, metabolomics, and inflammation in posttraumatic stress disorder. Biol Psychiatry. 2018;83:866–75. doi: 10.1016/j.biopsych.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 91.Winning A, Gilsanz P, Koenen KC, Roberts AL, Chen Q, Sumner JA, et al. Post-traumatic stress disorder and 20-year physical activity trends among women. Am J Prev Med. 2017;52:753–60. doi: 10.1016/j.amepre.2017.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O’Connor MF, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, et al. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun. 2009;23:887–97. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 94.Marsland AL, McCaffery JM, Muldoon MF, Manuck SB. Systemic inflammation and the metabolic syndrome among middle-aged community volunteers. Metabolism. 2010;59:1801–8. doi: 10.1016/j.metabol.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mellon SH, Bersani FS, Lindqvist D, Hammamieh R, Donohue D, Dean K, et al. Metabolomic analysis of male combat veterans with post traumatic stress disorder. PLoS One. 2019;14:e0213839. doi: 10.1371/journal.pone.0213839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kusminski CM, Scherer PE. Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol Metab. 2012;23:435–43. doi: 10.1016/j.tem.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Picard M, Juster RP, McEwen BS. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat Rev Endocrinol. 2014;10:303–10. doi: 10.1038/nrendo.2014.22. [DOI] [PubMed] [Google Scholar]
- 98.Breit S, Kupferberg A, Rogler G, Hasler G. Vagus nerve as modulator of the brain-gut axis in psychiatric and inflammatory disorders. Front Psychiatry. 2018;9:44. doi: 10.3389/fpsyt.2018.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Doney E, Cadoret A, Dion-Albert L, Lebel M, Menard C. Inflammation-driven brain and gut barrier dysfunction in stress and mood disorders. Eur J Neurosci 2021;55:2851–94. [DOI] [PMC free article] [PubMed]
- 100.Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369–78. doi: 10.1016/j.psyneuen.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 101.Hemmings SMJ, Malan-Muller S, van den Heuvel LL, Demmitt BA, Stanislawski MA, Smith DG, et al. The microbiome in posttraumatic stress disorder and trauma-exposed controls: an exploratory study. Psychosom Med. 2017;79:936–46. doi: 10.1097/PSY.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brenner LA, Forster JE, Stearns-Yoder KA, Stamper CE, Hoisington AJ, Brostow DP, et al. Evaluation of an immunomodulatory probiotic intervention for veterans with co-occurring mild traumatic brain injury and posttraumatic stress disorder: a pilot study. Front Neurol. 2020;11:1015. doi: 10.3389/fneur.2020.01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bajaj JS, Sikaroodi M, Fagan A, Heuman D, Gilles H, Gavis EA, et al. Posttraumatic stress disorder is associated with altered gut microbiota that modulates cognitive performance in veterans with cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2019;317:G661–G669. doi: 10.1152/ajpgi.00194.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Coley EJL, Mayer EA, Osadchiy V, Chen Z, Subramanyam V, Zhang Y, et al. Early life adversity predicts brain-gut alterations associated with increased stress and mood. Neurobiol Stress. 2021;15:100348. doi: 10.1016/j.ynstr.2021.100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Taft TH, Bedell A, Craven MR, Guadagnoli L, Quinton S, Hanauer SB. Initial assessment of post-traumatic stress in a US cohort of inflammatory bowel disease patients. Inflamm Bowel Dis. 2019;25:1577–85. doi: 10.1093/ibd/izz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Olff M. Sex and gender differences in post-traumatic stress disorder: an update. Eur J Psychotraumatology. 2017;8:1351204. doi: 10.1080/20008198.2017.1351204. [DOI] [Google Scholar]
- 107.Fonkoue IT, Michopoulos V, Park J. Sex differences in post-traumatic stress disorder risk: autonomic control and inflammation. Clin Auton Res. 2020;30:409–21. doi: 10.1007/s10286-020-00729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang J, Zhao H, Girgenti MJ. Posttraumatic stress disorder brain transcriptomics: convergent genomic signatures across biological sex. Biol Psychiatry. 2021;91:6–13. [DOI] [PubMed]
- 109.Neylan TC, Sun B, Rempel H, Ross J, Lenoci M, O’Donovan A, et al. Suppressed monocyte gene expression profile in men versus women with PTSD. Brain Behav Immun. 2011;25:524–31. doi: 10.1016/j.bbi.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim GS, Smith AK, Xue F, Michopoulos V, Lori A, Armstrong DL, et al. Methylomic profiles reveal sex-specific differences in leukocyte composition associated with post-traumatic stress disorder. Brain Behav Immun. 2019;81:280–91. doi: 10.1016/j.bbi.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Girgenti MJ, Wang J, Ji D, Cruz DA, Traumatic Stress Brain Research G, Stein MB, et al. Transcriptomic organization of the human brain in post-traumatic stress disorder. Nat Neurosci. 2021;24:24–33. doi: 10.1038/s41593-020-00748-7. [DOI] [PubMed] [Google Scholar]
- 112.Sartin-Tarm A, Ross MC, Privatsky AA, Cisler JM. Estradiol modulates neural and behavioral arousal in women with posttraumatic stress disorder during a fear learning and extinction task. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:1114–22. doi: 10.1016/j.bpsc.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Glover EM, Mercer KB, Norrholm SD, Davis M, Duncan E, Bradley B, et al. Inhibition of fear is differentially associated with cycling estrogen levels in women. J Psychiatry Neurosci. 2013;38:341–8. doi: 10.1503/jpn.120129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lalonde CS, Mekawi Y, Ethun KF, Beurel E, Gould F, Dhabhar FS, et al. Sex differences in peritraumatic inflammatory cytokines and steroid hormones contribute to prospective risk for nonremitting posttraumatic stress disorder. Chronic. Chronic stress (Thousand Oaks) 2021;5:24705470211032208. doi: 10.1177/24705470211032208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, et al. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry. 2012;72:19–24. doi: 10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Katrinli S, Lori A, Kilaru V, Carter S, Powers A, Gillespie CF, et al. Association of HLA locus alleles with posttraumatic stress disorder. Brain Behav Immun. 2019;81:655–8. doi: 10.1016/j.bbi.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO, et al. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am J Psychiatry. 2015;172:353–62. doi: 10.1176/appi.ajp.2014.14020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miller MW, Maniates H, Wolf EJ, Logue MW, Schichman SA, Stone A, et al. CRP polymorphisms and DNA methylation of the AIM2 gene influence associations between trauma exposure, PTSD, and C-reactive protein. Brain Behav Immun. 2018;67:194–202. doi: 10.1016/j.bbi.2017.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bruenig D, Mehta D, Morris CP, Harvey W, Lawford B, Young RM, et al. Genetic and serum biomarker evidence for a relationship between TNFalpha and PTSD in Vietnam war combat veterans. Compr Psychiatry. 2017;74:125–33. doi: 10.1016/j.comppsych.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 120.Ghodke Y, Joshi K, Chopra A, Patwardhan B. HLA and disease. Eur J Epidemiol. 2005;20:475–88. doi: 10.1007/s10654-005-5081-x. [DOI] [PubMed] [Google Scholar]
- 121.International Multiple Sclerosis Genetics C, Wellcome Trust Case Control C. Sawcer S, Hellenthal G, Pirinen M, Spencer CC, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–9. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Felger JC. Imaging the role of inflammation in mood and anxiety-related disorders. Curr Neuropharmacol. 2018;16:533–58. doi: 10.2174/1570159X15666171123201142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Butovsky O, Weiner HL. Microglial signatures and their role in health and disease. Nat Rev Neurosci. 2018;19:622–35. doi: 10.1038/s41583-018-0057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Levkovitz Y, Fenchel D, Kaplan Z, Zohar J, Cohen H. Early post-stressor intervention with minocycline, a second-generation tetracycline, attenuates post-traumatic stress response in an animal model of PTSD. Eur Neuropsychopharmacol. 2015;25:124–32. doi: 10.1016/j.euroneuro.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 126.Wilson CB, McLaughlin LD, Nair A, Ebenezer PJ, Dange R, Francis J. Inflammation and oxidative stress are elevated in the brain, blood, and adrenal glands during the progression of post-traumatic stress disorder in a predator exposure animal model. PLoS One. 2013;8:e76146. doi: 10.1371/journal.pone.0076146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Deslauriers J, van Wijngaarde M, Geyer MA, Powell S, Risbrough VB. Effects of LPS-induced immune activation prior to trauma exposure on PTSD-like symptoms in mice. Behav Brain Res. 2017;323:117–23. doi: 10.1016/j.bbr.2017.01.048. [DOI] [PubMed] [Google Scholar]
- 128.Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–87. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- 129.Frank MG, Baratta MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2007;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 130.Tynan RJ, Naicker S, Hinwood M, Nalivaiko E, Buller KM, Pow DV, et al. Chronic stress alters the density and morphology of microglia in a subset of stress-responsive brain regions. Brain Behav Immun. 2010;24:1058–68. doi: 10.1016/j.bbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 131.Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 2015;72:268–75. doi: 10.1001/jamapsychiatry.2014.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sandiego CM, Gallezot JD, Pittman B, Nabulsi N, Lim K, Lin SF, et al. Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc Natl Acad Sci USA. 2015;112:12468–73. doi: 10.1073/pnas.1511003112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bhatt S, Hillmer AT, Girgenti MJ, Rusowicz A, Kapinos M, Nabulsi N, et al. PTSD is associated with neuroimmune suppression: evidence from PET imaging and postmortem transcriptomic studies. Nat Commun. 2020;11:2360. doi: 10.1038/s41467-020-15930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI. Inflammation selectively enhances amygdala activity to socially threatening images. Neuroimage. 2012;59:3222–6. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Dolan RJ, et al. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009;66:415–22. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Muscatell KA, Dedovic K, Slavich GM, Jarcho MR, Breen EC, Bower JE, et al. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav Immun. 2015;43:46–53. doi: 10.1016/j.bbi.2014.06.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, et al. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res. 2009;167:151–60. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, et al. Smaller hippocampal volume in posttraumatic stress disorder: a multisite enigma-pgc study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry. 2018;83:244–53. doi: 10.1016/j.biopsych.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.O’Donovan A, Chao LL, Paulson J, Samuelson KW, Shigenaga JK, Grunfeld C, et al. Altered inflammatory activity associated with reduced hippocampal volume and more severe posttraumatic stress symptoms in Gulf War veterans. Psychoneuroendocrinology. 2015;51:557–66. doi: 10.1016/j.psyneuen.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–7. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–84. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chesnokova V, Pechnick RN, Wawrowsky K. Chronic peripheral inflammation, hippocampal neurogenesis, and behavior. Brain Behav Immun. 2016;58:1–8. doi: 10.1016/j.bbi.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cunningham AJ, Murray CA, O’Neill LA, Lynch MA, O’Connor JJ. Interleukin-1 beta (IL-1 beta) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neurosci Lett. 1996;203:17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- 145.Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 146.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–14. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.O’Connor MF, Irwin MR, Wellisch DK. When grief heats up: pro-inflammatory cytokines predict regional brain activation. Neuroimage. 2009;47:891–6. doi: 10.1016/j.neuroimage.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X, et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry. 2016;21:1358–65. doi: 10.1038/mp.2015.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Felmingham KL, Williams LM, Kemp AH, Rennie C, Gordon E, Bryant RA. Anterior cingulate activity to salient stimuli is modulated by autonomic arousal in posttraumatic stress disorder. Psychiatry Res. 2009;173:59–62. doi: 10.1016/j.pscychresns.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 150.Pannu Hayes J, Labar KS, Petty CM, McCarthy G, Morey RA. Alterations in the neural circuitry for emotion and attention associated with posttraumatic stress symptomatology. Psychiatry Res. 2009;172:7–15. doi: 10.1016/j.pscychresns.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50:932–42. doi: 10.1016/S0006-3223(01)01215-X. [DOI] [PubMed] [Google Scholar]
- 152.Shin LM, Bush G, Whalen PJ, Handwerger K, Cannistraro PA, Wright CI, et al. Dorsal anterior cingulate function in posttraumatic stress disorder. J Trauma Stress. 2007;20:701–12. doi: 10.1002/jts.20231. [DOI] [PubMed] [Google Scholar]
- 153.Hamner MB, Lorberbaum JP, George MS. Potential role of the anterior cingulate cortex in PTSD: review and hypothesis. Depress Anxiety. 1999;9:1–14. doi: 10.1002/(SICI)1520-6394(1999)9:1<1::AID-DA1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 154.Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, et al. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry. 2005;58:190–6. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Eisenberger NI, Lieberman MD, Satpute AB. Personality from a controlled processing perspective: an fMRI study of neuroticism, extraversion, and self-consciousness. Cogn Affect Behav Neurosci. 2005;5:169–81. doi: 10.3758/CABN.5.2.169. [DOI] [PubMed] [Google Scholar]
- 156.Simmons A, Strigo IA, Matthews SC, Paulus MP, Stein MB. Initial evidence of a failure to activate right anterior insula during affective set shifting in posttraumatic stress disorder. Psychosom Med. 2009;71:373–7. doi: 10.1097/PSY.0b013e3181a56ed8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry. 2010;68:433–41. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hannestad J, Subramanyam K, Dellagioia N, Planeta-Wilson B, Weinzimmer D, Pittman B, et al. Glucose metabolism in the insula and cingulate is affected by systemic inflammation in humans. J Nucl Med. 2012;53:601–7. doi: 10.2967/jnumed.111.097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47:881–90. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kim YK, Amidfar M, Won E. A review on inflammatory cytokine-induced alterations of the brain as potential neural biomarkers in post-traumatic stress disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2019;91:103–12. doi: 10.1016/j.pnpbp.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 161.Fox JH, Hassell JE, Jr., Siebler PH, Arnold MR, Lamb AK, Smith DG, et al. Preimmunization with a heat-killed preparation of Mycobacterium vaccae enhances fear extinction in the fear-potentiated startle paradigm. Brain Behav Immun. 2017;66:70–84. doi: 10.1016/j.bbi.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 162.Hassell JE, Jr., Fox JH, Arnold MR, Siebler PH, Lieb MW, Schmidt D, et al. Treatment with a heat-killed preparation of Mycobacterium vaccae after fear conditioning enhances fear extinction in the fear-potentiated startle paradigm. Brain Behav Immun. 2019;81:151–60. doi: 10.1016/j.bbi.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Corona AW, Norden DM, Skendelas JP, Huang Y, O’Connor JC, Lawson M, et al. Indoleamine 2,3-dioxygenase inhibition attenuates lipopolysaccharide induced persistent microglial activation and depressive-like complications in fractalkine receptor (CX(3)CR1)-deficient mice. Brain Behav Immun. 2013;31:134–42. doi: 10.1016/j.bbi.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu S, Cheng Y, Chen WZ, Lv JX, Zheng BS, Huang DD, et al. Inflammation Disturbed the Tryptophan Catabolites in Hippocampus of Post-operative Fatigue Syndrome Rats via Indoleamine 2,3-Dioxygenas Enzyme and the Improvement Effect of Ginsenoside Rb1. Front Neurosci. 2021;15:652817. doi: 10.3389/fnins.2021.652817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 166.Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, et al. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54:906–14. doi: 10.1016/S0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 167.Loftis JM, Patterson AL, Wilhelm CJ, McNett H, Morasco BJ, Huckans M, et al. Vulnerability to somatic symptoms of depression during interferon-alpha therapy for hepatitis C: a 16-week prospective study. J Psychosom Res. 2013;74:57–63. doi: 10.1016/j.jpsychores.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–31. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- 169.Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005;280:15649–58. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- 170.Zhao Y, Shang P, Wang M, Xie M, Liu J. Neuroprotective effects of fluoxetine against chronic stress-induced neural inflammation and apoptosis: involvement of the p38 activity. Front Physiol. 2020;11:351. doi: 10.3389/fphys.2020.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ney LJ, Akhurst J, Bruno R, Laing PAF, Matthews A, Felmingham KL. Dopamine, endocannabinoids and their interaction in fear extinction and negative affect in PTSD. Prog Neuropsychopharmacol Biol Psychiatry. 2021;105:110118. doi: 10.1016/j.pnpbp.2020.110118. [DOI] [PubMed] [Google Scholar]
- 172.Li W, Knowlton D, Woodward WR, Habecker BA. Regulation of noradrenergic function by inflammatory cytokines and depolarization. J Neurochem. 2003;86:774–83. doi: 10.1046/j.1471-4159.2003.01890.x. [DOI] [PubMed] [Google Scholar]
- 173.Felger JC, Li L, Marvar PJ, Woolwine BJ, Harrison DG, Raison CL, et al. Tyrosine metabolism during interferon-alpha administration: association with fatigue and CSF dopamine concentrations. Brain Behav Immun. 2013;31:153–60. doi: 10.1016/j.bbi.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zoller H, Schloegl A, Schroecksnadel S, Vogel W, Fuchs D. Interferon-alpha therapy in patients with hepatitis C virus infection increases plasma phenylalanine and the phenylalanine to tyrosine ratio. J Interferon Cytokine Res. 2012;32:216–20. doi: 10.1089/jir.2011.0093. [DOI] [PubMed] [Google Scholar]
- 175.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharm Exp Ther. 2002;303:1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 176.Wu HQ, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J Neural Transm (Vienna) 2007;114:33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- 177.Haroon E, Woolwine BJ, Chen X, Pace TW, Parekh S, Spivey JR, et al. IFN-alpha-induced cortical and subcortical glutamate changes assessed by magnetic resonance spectroscopy. Neuropsychopharmacology. 2014;39:1777–85. doi: 10.1038/npp.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Haroon E, Fleischer CC, Felger JC, Chen X, Woolwine BJ, Patel T, et al. Conceptual convergence: increased inflammation is associated with increased basal ganglia glutamate in patients with major depression. Mol Psychiatry. 2016;21:1351–7. doi: 10.1038/mp.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11:682–96. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Notaras M, van den Buuse M. Neurobiology of BDNF in fear memory, sensitivity to stress, and stress-related disorders. Mol Psychiatry. 2020;25:2251–74. doi: 10.1038/s41380-019-0639-2. [DOI] [PubMed] [Google Scholar]
- 181.Rosso IM, Weiner MR, Crowley DJ, Silveri MM, Rauch SL, Jensen JE. Insula and anterior cingulate GABA levels in posttraumatic stress disorder: preliminary findings using magnetic resonance spectroscopy. Depress Anxiety. 2014;31:115–23. doi: 10.1002/da.22155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lee M, Schwab C, McGeer PL. Astrocytes are GABAergic cells that modulate microglial activity. Glia. 2011;59:152–65. doi: 10.1002/glia.21087. [DOI] [PubMed] [Google Scholar]
- 183.Li Y, Liu L, Kang J, Sheng JG, Barger SW, Mrak RE, et al. Neuronal-glial interactions mediated by interleukin-1 enhance neuronal acetylcholinesterase activity and mRNA expression. J Neurosci. 2000;20:149–55. doi: 10.1523/JNEUROSCI.20-01-00149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Rada P, Mark GP, Vitek MP, Mangano RM, Blume AJ, Beer B, et al. Interleukin-1 beta decreases acetylcholine measured by microdialysis in the hippocampus of freely moving rats. Brain Res. 1991;550:287–90. doi: 10.1016/0006-8993(91)91330-4. [DOI] [PubMed] [Google Scholar]
- 185.Brady K, Pearlstein T, Asnis GM, Baker D, Rothbaum B, Sikes CR, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA. 2000;283:1837–44. doi: 10.1001/jama.283.14.1837. [DOI] [PubMed] [Google Scholar]
- 186.Davidson JR, Rothbaum BO, van der Kolk BA, Sikes CR, Farfel GM. Multicenter, double-blind comparison of sertraline and placebo in the treatment of posttraumatic stress disorder. Arch Gen Psychiatry. 2001;58:485–92. doi: 10.1001/archpsyc.58.5.485. [DOI] [PubMed] [Google Scholar]
- 187.Marshall RD, Beebe KL, Oldham M, Zaninelli R. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatry. 2001;158:1982–8. doi: 10.1176/appi.ajp.158.12.1982. [DOI] [PubMed] [Google Scholar]
- 188.Stein DJ, Davidson J, Seedat S, Beebe K. Paroxetine in the treatment of post-traumatic stress disorder: pooled analysis of placebo-controlled studies. Expert Opin Pharmacother. 2003;4:1829–38. doi: 10.1517/14656566.4.10.1829. [DOI] [PubMed] [Google Scholar]
- 189.Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- 190.Menter A, Augustin M, Signorovitch J, Yu AP, Wu EQ, Gupta SR, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812–8. doi: 10.1016/j.jaad.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 191.Langley RG, Feldman SR, Han C, Schenkel B, Szapary P, Hsu MC, et al. Ustekinumab significantly improves symptoms of anxiety, depression, and skin-related quality of life in patients with moderate-to-severe psoriasis: Results from a randomized, double-blind, placebo-controlled phase III trial. J Am Acad Dermatol. 2010;63:457–65. doi: 10.1016/j.jaad.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 192.Kohler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–91. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 193.Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lee Y, Mansur RB, Brietzke E, Carmona NE, Subramaniapillai M, Pan Z, et al. Efficacy of adjunctive infliximab vs. placebo in the treatment of anhedonia in bipolar I/II depression. Brain Behav Immun. 2020;88:631–9. doi: 10.1016/j.bbi.2020.04.063. [DOI] [PubMed] [Google Scholar]
- 195.Akhondzadeh S, Jafari S, Raisi F, Nasehi AA, Ghoreishi A, Salehi B, et al. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress Anxiety. 2009;26:607–11. doi: 10.1002/da.20589. [DOI] [PubMed] [Google Scholar]
- 196.Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–4. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]
- 197.Abbasi SH, Hosseini F, Modabbernia A, Ashrafi M, Akhondzadeh S. Effect of celecoxib add-on treatment on symptoms and serum IL-6 concentrations in patients with major depressive disorder: randomized double-blind placebo-controlled study. J Affect Disord. 2012;141:308–14. doi: 10.1016/j.jad.2012.03.033. [DOI] [PubMed] [Google Scholar]
- 198.Gamble-George JC, Baldi R, Halladay L, Kocharian A, Hartley N, Silva CG, et al. Cyclooxygenase-2 inhibition reduces stress-induced affective pathology. Elife 2016;5:e14137. [DOI] [PMC free article] [PubMed]
- 199.Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm DH. Effects of systemic administration of ibuprofen on stress response in a rat model of post-traumatic stress disorder. Korean J Physiol Pharm. 2016;20:357–66. doi: 10.4196/kjpp.2016.20.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yamanashi T, Iwata M, Kamiya N, Tsunetomi K, Kajitani N, Wada N, et al. Beta-hydroxybutyrate, an endogenic NLRP3 inflammasome inhibitor, attenuates stress-induced behavioral and inflammatory responses. Sci Rep. 2017;7:7677. doi: 10.1038/s41598-017-08055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Kajitani N, Iwata M, Miura A, Tsunetomi K, Yamanashi T, Matsuo R, et al. Prefrontal cortex infusion of beta-hydroxybutyrate, an endogenous NLRP3 inflammasome inhibitor, produces antidepressant-like effects in a rodent model of depression. Neuropsychopharmacol Rep. 2020;40:157–65. doi: 10.1002/npr2.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yamanashi T, Iwata M, Shibushita M, Tsunetomi K, Nagata M, Kajitani N, et al. Beta-hydroxybutyrate, an endogenous NLRP3 inflammasome inhibitor, attenuates anxiety-related behavior in a rodent post-traumatic stress disorder model. Sci Rep. 2020;10:21629. doi: 10.1038/s41598-020-78410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lewitus GM, Cohen H, Schwartz M. Reducing post-traumatic anxiety by immunization. Brain Behav Immun. 2008;22:1108–14. doi: 10.1016/j.bbi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 204.Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry. 2004;161:1488–90. doi: 10.1176/appi.ajp.161.8.1488. [DOI] [PubMed] [Google Scholar]
- 205.Suris A, North C, Adinoff B, Powell CM, Greene R. Effects of exogenous glucocorticoid on combat-related PTSD symptoms. Ann Clin Psychiatry. 2010;22:274–9. [PMC free article] [PubMed] [Google Scholar]
- 206.Yehuda R, Bierer LM, Pratchett LC, Lehrner A, Koch EC, Van Manen JA, et al. Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: Randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology. 2015;51:589–97. doi: 10.1016/j.psyneuen.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 207.Delahanty DL, Gabert-Quillen C, Ostrowski SA, Nugent NR, Fischer B, Morris A, et al. The efficacy of initial hydrocortisone administration at preventing posttraumatic distress in adult trauma patients: a randomized trial. CNS Spectr. 2013;18:103–11. doi: 10.1017/S1092852913000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lehrner A, Hildebrandt T, Bierer LM, Flory JD, Bader HN, Makotkine I, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of Prolonged Exposure for PTSD in U.S. combat veterans. Behav Res Ther. 2021;144:103924. doi: 10.1016/j.brat.2021.103924. [DOI] [PubMed] [Google Scholar]
- 209.Weis F, Kilger E, Roozendaal B, de Quervain DJ, Lamm P, Schmidt M, et al. Stress doses of hydrocortisone reduce chronic stress symptoms and improve health-related quality of life in high-risk patients after cardiac surgery: a randomized study. J Thorac Cardiovasc Surg. 2006;131:277–82. doi: 10.1016/j.jtcvs.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 210.Schelling G, Roozendaal B, Krauseneck T, Schmoelz M, Dominique DEQ, Briegel J. Efficacy of hydrocortisone in preventing posttraumatic stress disorder following critical illness and major surgery. Ann N. Y Acad Sci. 2006;1071:46–53. doi: 10.1196/annals.1364.005. [DOI] [PubMed] [Google Scholar]
- 211.Schelling G, Briegel J, Roozendaal B, Stoll C, Rothenhausler HB, Kapfhammer HP. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol Psychiatry. 2001;50:978–85. doi: 10.1016/S0006-3223(01)01270-7. [DOI] [PubMed] [Google Scholar]
- 212.Zohar J, Yahalom H, Kozlovsky N, Cwikel-Hamzany S, Matar MA, Kaplan Z, et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. Eur Neuropsychopharmacol. 2011;21:796–809. doi: 10.1016/j.euroneuro.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 213.Kothgassner OD, Pellegrini M, Goreis A, Giordano V, Edobor J, Fischer S, et al. Hydrocortisone administration for reducing post-traumatic stress symptoms: a systematic review and meta-analysis. Psychoneuroendocrinology. 2021;126:105168. doi: 10.1016/j.psyneuen.2021.105168. [DOI] [PubMed] [Google Scholar]
- 214.Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain Behav Immun. 2012;26:1150–9. doi: 10.1016/j.bbi.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Haffner-Luntzer M, Foertsch S, Fischer V, Prystaz K, Tschaffon M, Modinger Y, et al. Chroynic psychosocial stress compromises the immune response and endochondral ossification during bone fracture healing via beta-AR signaling. Proc Natl Acad Sci USA. 2019;116:8615–22. doi: 10.1073/pnas.1819218116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–8. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- 217.Brunet A, Saumier D, Liu A, Streiner DL, Tremblay J, Pitman RK. Reduction of PTSD symptoms with pre-reactivation propranolol therapy: a randomized controlled trial. Am J Psychiatry. 2018;175:427–33. doi: 10.1176/appi.ajp.2017.17050481. [DOI] [PubMed] [Google Scholar]
- 218.Roullet P, Vaiva G, Very E, Bourcier A, Yrondi A, Dupuch L, et al. Traumatic memory reactivation with or without propranolol for PTSD and comorbid MD symptoms: a randomised clinical trial. Neuropsychopharmacology. 2021;46:1643–9. doi: 10.1038/s41386-021-00984-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Meli L, Chang BP, Shimbo D, Swan BW, Edmondson D, Sumner JA. Beta blocker administration during emergency department evaluation for acute coronary syndrome is associated with lower posttraumatic stress symptoms 1-month later. J Trauma Stress. 2017;30:313–7. doi: 10.1002/jts.22195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, et al. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51:189–92. doi: 10.1016/S0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- 221.Vaiva G, Ducrocq F, Jezequel K, Averland B, Lestavel P, Brunet A, et al. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54:947–9. doi: 10.1016/S0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- 222.Stein MB, Kerridge C, Dimsdale JE, Hoyt DB. Pharmacotherapy to prevent PTSD: Results from a randomized controlled proof-of-concept trial in physically injured patients. J Trauma Stress. 2007;20:923–32. doi: 10.1002/jts.20270. [DOI] [PubMed] [Google Scholar]
- 223.Benicky J, Sanchez-Lemus E, Honda M, Pang T, Orecna M, Wang J, et al. Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology. 2011;36:857–70. doi: 10.1038/npp.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Quinones MM, Maldonado L, Velazquez B, Porter JT. Candesartan ameliorates impaired fear extinction induced by innate immune activation. Brain Behav Immun. 2016;52:169–77. doi: 10.1016/j.bbi.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Khoury NM, Marvar PJ, Gillespie CF, Wingo A, Schwartz A, Bradley B, et al. The renin-angiotensin pathway in posttraumatic stress disorder: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers are associated with fewer traumatic stress symptoms. J Clin Psychiatry. 2012;73:849–55. doi: 10.4088/JCP.11m07316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Stein MB, Jain S, Simon NM, West JC, Marvar PJ, Bui E, et al. Randomized, placebo-controlled trial of the angiotensin receptor antagonist losartan for posttraumatic stress disorder. Biol Psychiatry. 2021;90:473–81. doi: 10.1016/j.biopsych.2021.05.012. [DOI] [PubMed] [Google Scholar]
- 227.Hill MN, Campolongo P, Yehuda R, Patel S. Integrating endocannabinoid signaling and cannabinoids into the biology and treatment of posttraumatic stress disorder. Neuropsychopharmacology. 2018;43:80–102. doi: 10.1038/npp.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Steenkamp MM, Blessing EM, Galatzer-Levy IR, Hollahan LC, Anderson WT. Marijuana and other cannabinoids as a treatment for posttraumatic stress disorder: a literature review. Depress Anxiety. 2017;34:207–16. doi: 10.1002/da.22596. [DOI] [PubMed] [Google Scholar]
- 229.Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD) CNS Neurosci Ther. 2009;15:84–88. doi: 10.1111/j.1755-5949.2008.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Jetly R, Heber A, Fraser G, Boisvert D. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51:585–8. doi: 10.1016/j.psyneuen.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 231.Cameron C, Watson D, Robinson J. Use of a synthetic cannabinoid in a correctional population for posttraumatic stress disorder-related insomnia and nightmares, chronic pain, harm reduction, and other indications: a retrospective evaluation. J Clin Psychopharmacol. 2014;34:559–64. doi: 10.1097/JCP.0000000000000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Brinkmann V, Billich A, Baumruker T, Heining P, Schmouder R, Francis G, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat Rev Drug Discov. 2010;9:883–97. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 233.Hait NC, Wise LE, Allegood JC, O’Brien M, Avni D, Reeves TM, et al. Active, phosphorylated fingolimod inhibits histone deacetylases and facilitates fear extinction memory. Nat Neurosci. 2014;17:971–80. doi: 10.1038/nn.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Efstathopoulos P, Kourgiantaki A, Karali K, Sidiropoulou K, Margioris AN, Gravanis A, et al. Fingolimod induces neurogenesis in adult mouse hippocampus and improves contextual fear memory. Transl Psychiatry. 2015;5:e685. doi: 10.1038/tp.2015.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Corbett B, Luz S, Sotuyo N, Pearson-Leary J, Moorthy GS, Zuppa AF, et al. FTY720 (Fingolimod), a modulator of sphingosine-1-phosphate receptors, increases baseline hypothalamic-pituitary adrenal axis activity and alters behaviors relevant to affect and anxiety. Physiol Behav. 2021;240:113556. doi: 10.1016/j.physbeh.2021.113556. [DOI] [PubMed] [Google Scholar]
- 236.Bower JE, Irwin MR. Mind-body therapies and control of inflammatory biology: a descriptive review. Brain Behav Immun. 2016;51:1–11. doi: 10.1016/j.bbi.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Himmerich H, Willmund GD, Zimmermann P, Wolf JE, Buhler AH, Kirkby KC, et al. Serum concentrations of TNF-alpha and its soluble receptors during psychotherapy in German soldiers suffering from combat-related PTSD. Psychiatr Danub. 2016;28:293–8. [PubMed] [Google Scholar]
- 238.Ahmadi N, Chaudhry S, Salam T, Rodriguez J, Kase M, Olango G, et al. A randomized controlled feasibility trial of reminder-focused positive psychiatry in adolescents with comorbid attention-deficit/hyperactivity disorder and posttraumatic stress disorder. Prim Care Companion CNS Disord 2020;22:19m02579. [DOI] [PubMed]
- 239.Sanada K, Montero-Marin J, Barcelo-Soler A, Ikuse D, Ota M, Hirata A, et al. Effects of mindfulness-based interventions on biomarkers and low-grade inflammation in patients with psychiatric disorders: a meta-analytic review. Int J Mol Sci 2020;21:2484. [DOI] [PMC free article] [PubMed]