Abstract
We describe a rare case of simultaneous idiopathic right ventricular outflow tract dilatation and idiopathic main pulmonary artery aneurysm. A 59-year-old male presented with complaints of exertional shortness of breath and a cardiac murmur since childhood. CT pulmonary angiogram showed main pulmonary artery dilatation with a diameter of 5.8 cm. Cardiac MRI revealed right ventricular outflow tract dilatation with a diameter of 5.4 cm and a main pulmonary artery aneurysm with a 5.6 cm diameter. Cardiothoracic surgery was consulted for surgical repair. Definitive management of right ventricular outflow tract dilatation and pulmonary artery aneurysms is challenging due to their infrequent diagnosis and lack of established guidelines. The treatment for central aneurysms is surgery which includes aneurysmectomy and right ventricular outflow tract repair or replacement.
Keywords: Aneurysmectomy, Cardiac MRI, Pulmonary artery aneurysm, Rare cardiac anomalies, Right ventricular outflow tract dilatation, Right ventricular outflow tract repair and replacement
Abbreviations: CT-PA, Computed Tomography Pulmonary Angiogram; Echo, Echocardiography; HIV, Human Immunodeficiency Virus; MRI, Magnetic Resonance Imaging; MPA, Main Pulmonary Artery; PAA, Pulmonary Artery Aneurysm; RV, Right Ventricle; RVOT, Right Ventricular Outflow Tract Dilatation; Cm, centimeter
Introduction
The right ventricular outflow tract (RVOT) is the region of blood outflow from RV above the supraventricular crest, defined superiorly by pulmonic valve, inferiorly by RV inflow tract, and tricuspid annulus, and laterally by RV free wall [1]. Idiopathic RVOT dilatation is a rare cardiac condition. Pulmonary artery aneurysms (PAA) defined as focal dilatation involving all the 3-vessel wall layers have been acknowledged to be a rare entity and very infrequently diagnosed [2]. They can be classified as central and peripheral PAA. Central PAA involve the main pulmonary artery (MPA), left pulmonary artery, and right pulmonary artery while the peripheral PAA involve the intrapulmonary arteries [3]. We describe an unusual case of idiopathic RVOT dilatation with simultaneous idiopathic main pulmonary artery aneurysm.
Case report
A 59-year-old male with a medical history of hypertension, diabetes mellitus type 2, and HIV presented to the office with the complaint of exertional shortness of breath on 2 flights of stairs for a few months which was acute, progressive, and not associated with cough, chest pain, paroxysmal nocturnal dyspnea, or orthopnea. He also complained of a cardiac murmur which has been present since childhood. He was vitally stable and his cardiovascular exam showed regular rhythm, and a systolic murmur at the lower left sternal border with a grade of 2/6 characteristic of mitral regurgitation.
Labs including complete blood count, complete metabolic panel, and coagulation profile were unremarkable except for leukopenia, and transaminitis. He had HIV-1 viral load of 35,717 copies per milliliter and a cluster of differentiation 4 (CD4) count of 403 cells/million cubic meters. An electrocardiogram showed sinus bradycardia with a heart rate of 50 beats per minute. Echocardiography revealed a hyper-dynamic left ventricular ejection fraction of 73%, grade 1 impaired relaxation, severe LV concentric hypertrophy, right ventricle outflow tract dilatation of 5.3 cm (Fig. 1), moderate to severe pulmonic regurgitation (Fig. 2), and severe dilation of the main pulmonary artery with a diameter of 5.4 cm.
Fig. 1.
RVOT dilatation on echocardiography: RVOT can be identified on this echocardiogram, the dotted green line measures the length of RVOT as 5.3 cm which represents dilatation (white arrow).
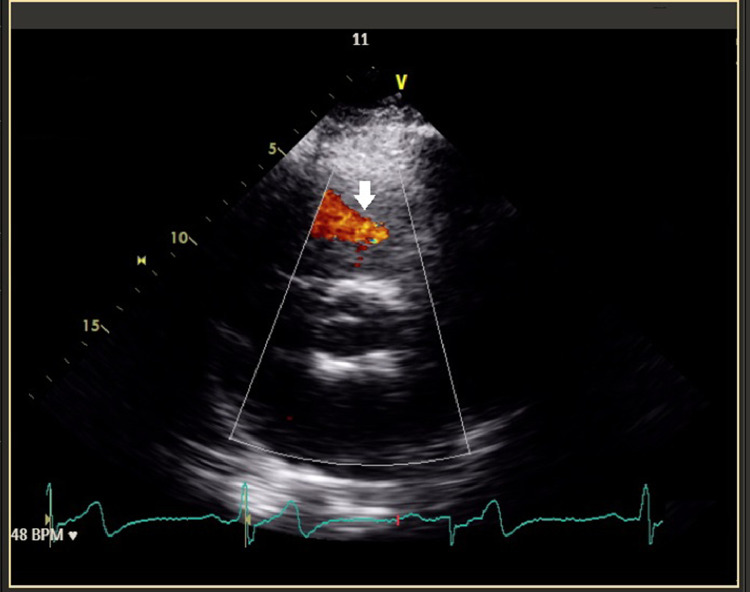
Fig. 2.
Pulmonary regurgitation on echocardiography: pulmonary artery aneurysm can result in pulmonary regurgitation which can be observed as blood flow in the echocardiogram above (white arrow).
CT pulmonary angiogram (Fig. 3) identified MPA dilatation with a diameter of 5.8 cm, right pulmonary artery dilatation with 3.5 cm diameter, left pulmonary artery dilatation with 3.3 cm diameter, right hemidiaphragm elevation, and hepatomegaly.
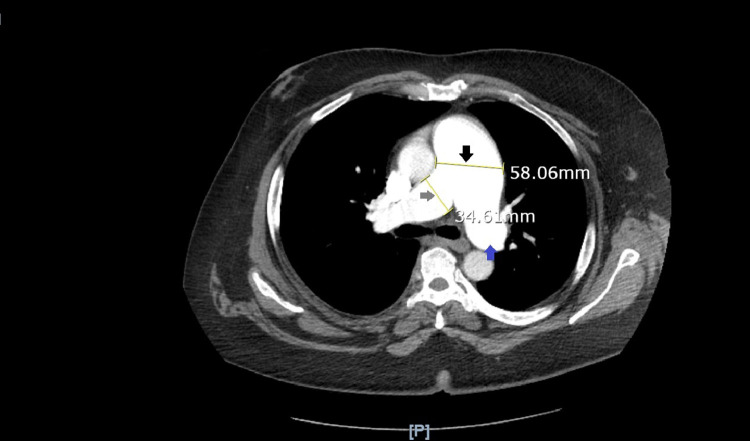
Fig. 3.
Pulmonary artery aneurysm on CT-PA: pulmonary artery is measured as 58.06 mm (5.8 cm) which is greater than the normal diameter of 2.9 cm, hence this CT-PA image confirms pulmonary artery aneurysm formation (black arrow). This image also marks the dilated right pulmonary artery (34.61 mm, grey arrow) and dilated left pulmonary artery (blue arrow).
Cardiac MRI without contrast (Fig. 4) illustrated MPA aneurysm with a 5.6 cm diameter and asymmetric dilation of the left pulmonary artery (3.7 cm) compared to the right pulmonary artery (2.9 cm). It also demonstrated RVOT dilatation which can be seen in Fig. 5 and Video 1. Aneurysmal MPA was found to be in close proximity to the left main and left anterior descending coronary artery. It represented a tri-leaflet pulmonic valve with moderate leaflet thickening and mild regurgitation.
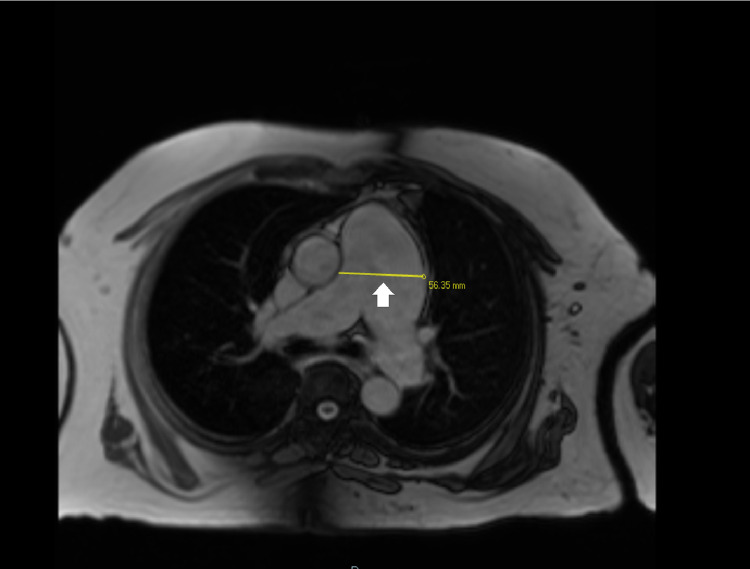
Fig. 4.
Cardiac MRI imaging for pulmonary artery aneurysm: this cardiac MRI scan specifies the diameter of pulmonary artery aneurysm as 56.35 mm (5.6 cm) with the yellow line (white arrow).
Fig. 5.
Cardiac MRI imaging for RVOT dilatation: the measured length of 54.33 mm (5.4 cm) characterizes the right ventricular outflow tract dilatation (white arrow). The left ventricle can be seen below (black arrow) RVOT.
Cardiothoracic surgery was consulted for definitive surgical repair for RVOT dilatation and MPA aneurysm. The consulted surgeons are deciding the preferred surgical procedure due to the advanced expertise required for a successful repair.
Discussion
Both idiopathic RVOT dilatation and idiopathic PAA are rare, a co-occurrence is even rarer. RVOT dilatation/aneurysm can develop due to complications of surgical procedures like ventriculotomy for the repair of tetralogy of Fallot (ToF) and creation of aorta to main pulmonary artery shunt but idiopathic etiology has been rarely reported [4]. Our patient never had cardiac surgery and was found to have RVOT dilatation on imaging for dyspnea workup.
The Framingham Heart Study established the mean MPA diameter as 2.51 + 0.28 cm, sex-specific reference values were 2.9 cm in men and 2.7 cm in women [5]. Cardiac MRI measured MPA diameter to be 5.6 cm which is greater than 2.9 cm, hence it confirmed MPA aneurysm formation in our patient. PAA can have a wide variety of etiologies including acquired conditions like pulmonary hypertension, vasculitis, infections, neoplasm, and trauma [2]. Congenital causes include heart defects like patent ductus arteriosus, ventricular septal defect, atrial septal defect, and connective tissue disorders [2,6]. Our patient had a childhood murmur but none of the associated congenital disorders were seen on imaging. Hence, it is most likely idiopathic PAA for which Greene and Baldwin outlined four pathological criteria, (a) pulmonary trunk dilatation, (b) lack of intracardiac or extracardiac shunts, (c) no history of chronic cardiac or pulmonary conditions and (d) absence of arterial disorders like arteriosclerosis or syphilis among others [7]. Even though they have a low incidence, PAA can represent life-threatening medical conditions and necessitate prompt diagnosis and management. CT pulmonary angiogram (CT-PA) is the gold standard for diagnosis of PAA as it can establish the diagnosis and describes the size, number, location, and extent of PAA [2]. For optimal imaging, echocardiography and cardiac MRI should be included.
Determining the definitive management for PAA has proven to be challenging due to its rarity, inadequate literature, and absence of established treatment guidelines. Management options include conservative, surgical and endovascular therapies [6], the choice depends on the location of PAA. For central PAA or MPA aneurysm, the only viable treatment option is surgery, but the evidence supporting the absolute diameter threshold for surgery is lacking. However, it has been suggested, to operate on the patients with MPAA >5.5 cm in concordance with guidelines available for aortic disease [2]. Other indications for surgery include an increase in the diameter of the aneurysm of ≥0.5 cm in 6 months, compression of adjacent structures, thrombus formation in the aneurysm sac, presence of clinical symptoms, and signs of rupture or dissection [2]. Some authors have indicated that MPA aneurysms should be operated on irrespectively after diagnosis because of fatal outcomes including failure and rupture of the right side of the heart [8]. Surgical techniques that have been described for MPAA include aneurysmectomy, RVOT repair/replacement, and aneurysmorrhaphy or arterioplasty [2,3]. The method of choice is aneurysmectomy and RVOT repair/replacement which can be performed by replacement of the pulmonary artery and pulmonary trunk with a conduit starting in the RVOT using Gore-Tex or Dacron tubes, homografts, or xenografts (porcine aortic grafts or bovine jugular conduit) [2]. If the pulmonary valve is not involved, a valve-sparing procedure can be used [2].
Treatment modalities for peripheral PAA are conservative, surgical, and endovascular therapies. Conservative management can be done in asymptomatic cases [2]. Surgical procedures include aneurysmorrhaphy, aneurysmectomy, lobectomy, bilobectomy, and pneumonectomy [6]. Lung resection is a high-risk surgical procedure and is often fatal [9] so endovascular procedures can be considered as first-line therapy for small arteries, however, there is no consensus to prefer surgical vs endovascular management. Endovascular therapy includes coil embolization of PAA and the use of vascular plugs [6]. There also have been rare use of balloon embolization, stent-graft, and glue embolization with n-butyl cyanoacrylate (NBCA) [6]. These are experimental procedures for PAA carrying risks like aneurysm rupture, nontarget embolization, arterial dissection, and arterial thrombosis, and long-term complications haven't been established.
Conclusion
RVOT dilatation and PAA are extremely rare and are infrequently diagnosed. Hitherto, there are no clear guidelines to institute optimal treatment for these patients. CT-PA is the imaging of choice to make the diagnosis. PAA complications include right heart failure, right heart rupture, aneurysmal rupture, and dissection. PAA are classified as peripheral and central PAA. Peripheral PAA can be managed conservatively, surgically, or with endovascular intervention. Central PAA involving MPA can only be managed surgically by aneurysmectomy and repair or replacement of the RVOT, specifically for MPAA >5.5 cm. Since no large series of PAA patients have been published, surgical outcome parameters like morbidity and mortality data can't be summarized. Extensive research involving RVOT dilatation and PAA patients needs to be conducted to outline ideal management guidelines, and prognostic outcomes of different surgical approaches but the rarity of these cardiac conditions certainly makes it challenging.
Video Legends: Video 1: RVOT Dilatation on Cine Cardiac MRI: This cine sequence of cardiac MRI illustrates blood flow through the dilated RVOT. Left ventricle can be seen in systolic and diastolic phases of the cardiac cycle below RVOT.
Patient Consent
Informed consent was obtained from the subject involved in the study. The well-being of the subject takes precedence over the interests of science and society. No human experimentation was involved.
Footnotes
Competing Interests: All authors declare no conflict of interest.
Disclosures: The authors have nothing to disclose.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Acknowledgement: I appreciate the input from Dr. Dishang Bhavsar and Dr. Samir Garyali for the case report and thank them for their guidance. They have given full permission to be co-authors for this project.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2022.07.003.
Appendix. Supplementary materials
References
- 1.Mond HG, Hillock RJ, Stevenson IH, Mcgavigan AD. The right ventricular outflow tract: the road to septal pacing. Pacing Clin Electrophysiol. 2007;30(4):482–491. doi: 10.1111/j.1540-8159.2007.00697.x. [DOI] [PubMed] [Google Scholar]
- 2.Kreibich M, Siepe M, Kroll J, Höhn R, Grohmann J, Beyersdorf F. Aneurysms of the pulmonary artery. Circulation. 2015;131(3):310–316. doi: 10.1161/CIRCULATIONAHA.114.012907. [DOI] [PubMed] [Google Scholar]
- 3.Theodoropoulos P, Ziganshin BA, Tranquilli M, Elefteriades JA. Pulmonary artery aneurysms: four case reports and literature review. Int J Angiol. 2013;22(3):143–148. doi: 10.1055/s-0033-1347907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peer SM, Bhat PS, Furtado AD, Chikkatur R. Right ventricular outflow tract aneurysm with thrombus. Interact Cardiovasc Thorac Surg. 2012;14(4):488–490. doi: 10.1093/icvts/ivr151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Truong QA, Massaro JM, Rogers IS, et al. Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography: the Framingham Heart Study. Circ Cardiovasc Imaging. 2012;5(1):147–154. doi: 10.1161/CIRCIMAGING.111.968610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park HS, Chamarthy MR, Lamus D, Saboo SS, Sutphin PD, Kalva SP. Pulmonary artery aneurysms: diagnosis & endovascular therapy. Cardiovasc Diagn Ther. 2018;8(3):350–361. doi: 10.21037/cdt.2018.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greene DG, Baldwin ED, et al. Pure congenital pulmonary stenosis and idiopathic congenital dilatation of the pulmonary artery. Am J Med. 1949;6:24–40. doi: 10.1016/0002-9343(49)90004-2. [DOI] [PubMed] [Google Scholar]
- 8.Metras D, Ouattara K, Quezzin-Coulibaly A. Aneurysm of the pulmonary artery with cystic medial necrosis and massive pulmonary valvular insufficiency. Report of two successful surgical cases. Eur J Cardio-Thoracic Surg. 1987;1(2):119–124. doi: 10.1016/1010-7940(87)90023-6. [DOI] [PubMed] [Google Scholar]
- 9.Ramakrishna G, Sprung J, Ravi BS, Chandrasekaran K, McGoon MD. Impact of pulmonary hypertension on the outcomes of noncardiac surgery: predictors of perioperative morbidity and mortality. J Am Coll Cardiol. 2005;45(10):1691–1699. doi: 10.1016/j.jacc.2005.02.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.